Heritability of Morphophysiological Traits in Popcorn for Drought Tolerance and Their Use as Breeding Indicators of Superior Genotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Genotypes
2.2. Growing Conditions
2.3. Morphological Traits
2.4. Water Status
2.5. Root Traits
2.6. Statistical Analysis
3. Results
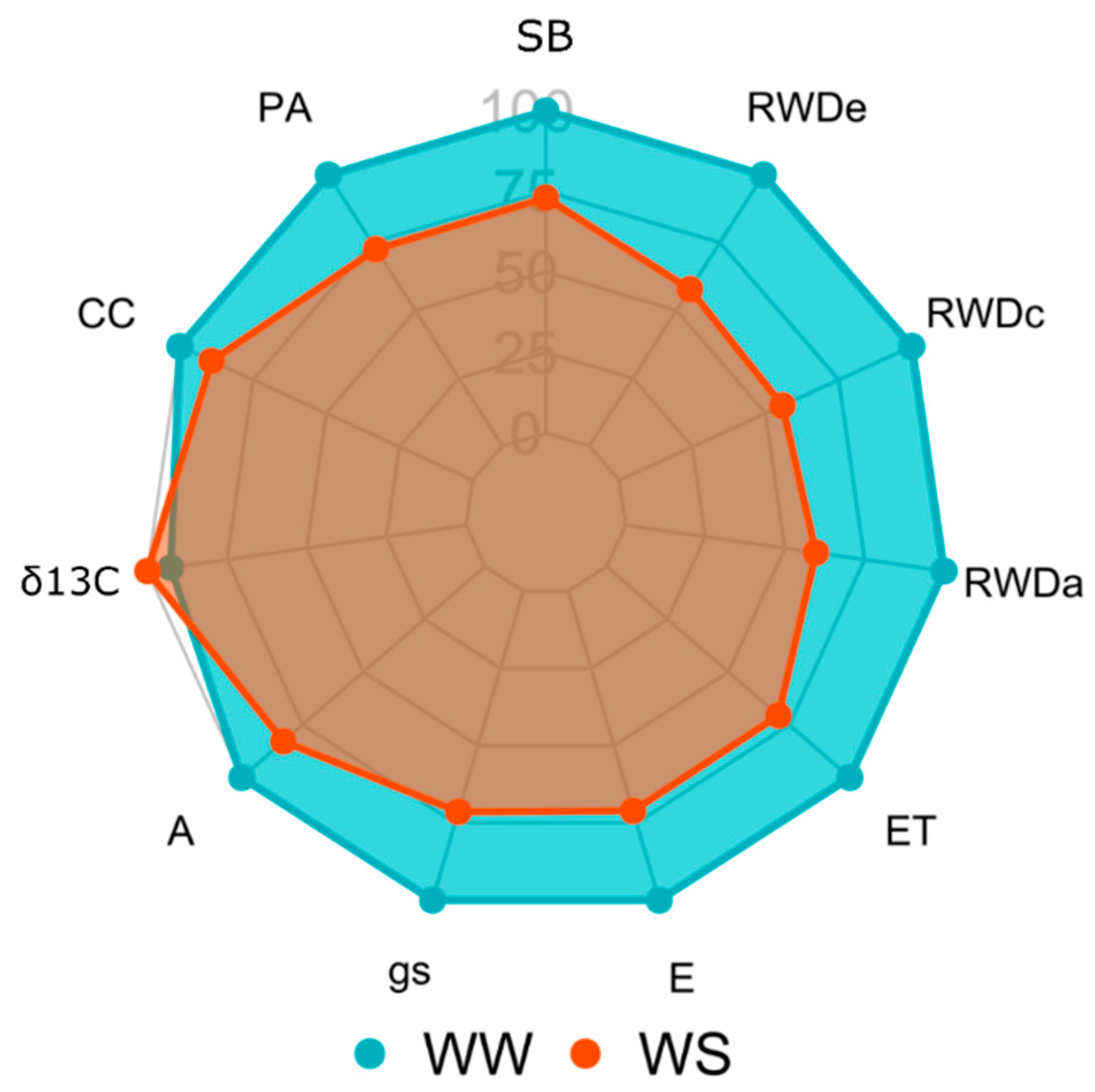
3.1. Genetic Variability in the Different WCs and the Impact of Applied Water Stress
3.2. Importance of Quadratic Components
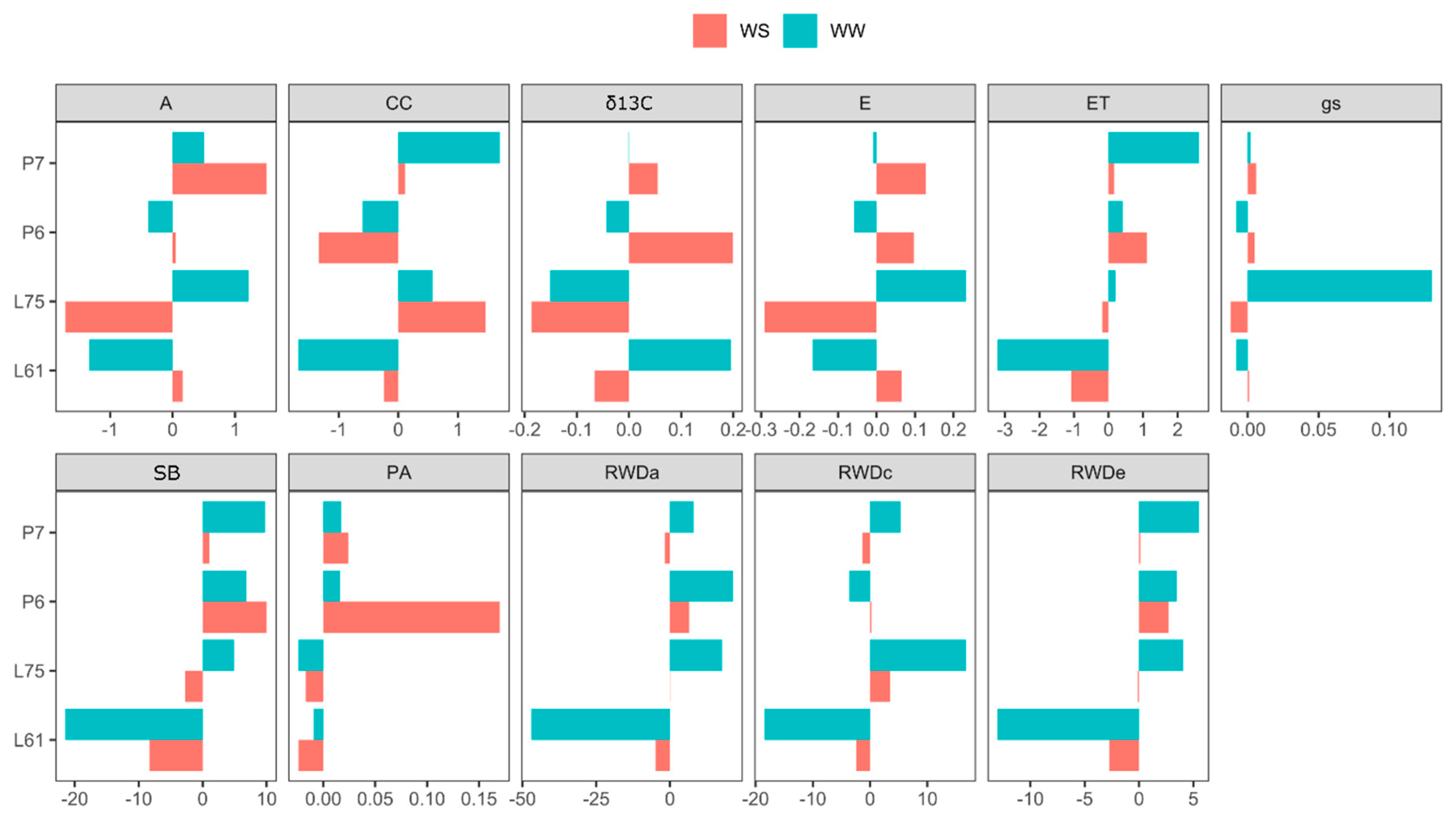
3.3. Effects of General Combining Ability (GCA) in Different Water Conditions
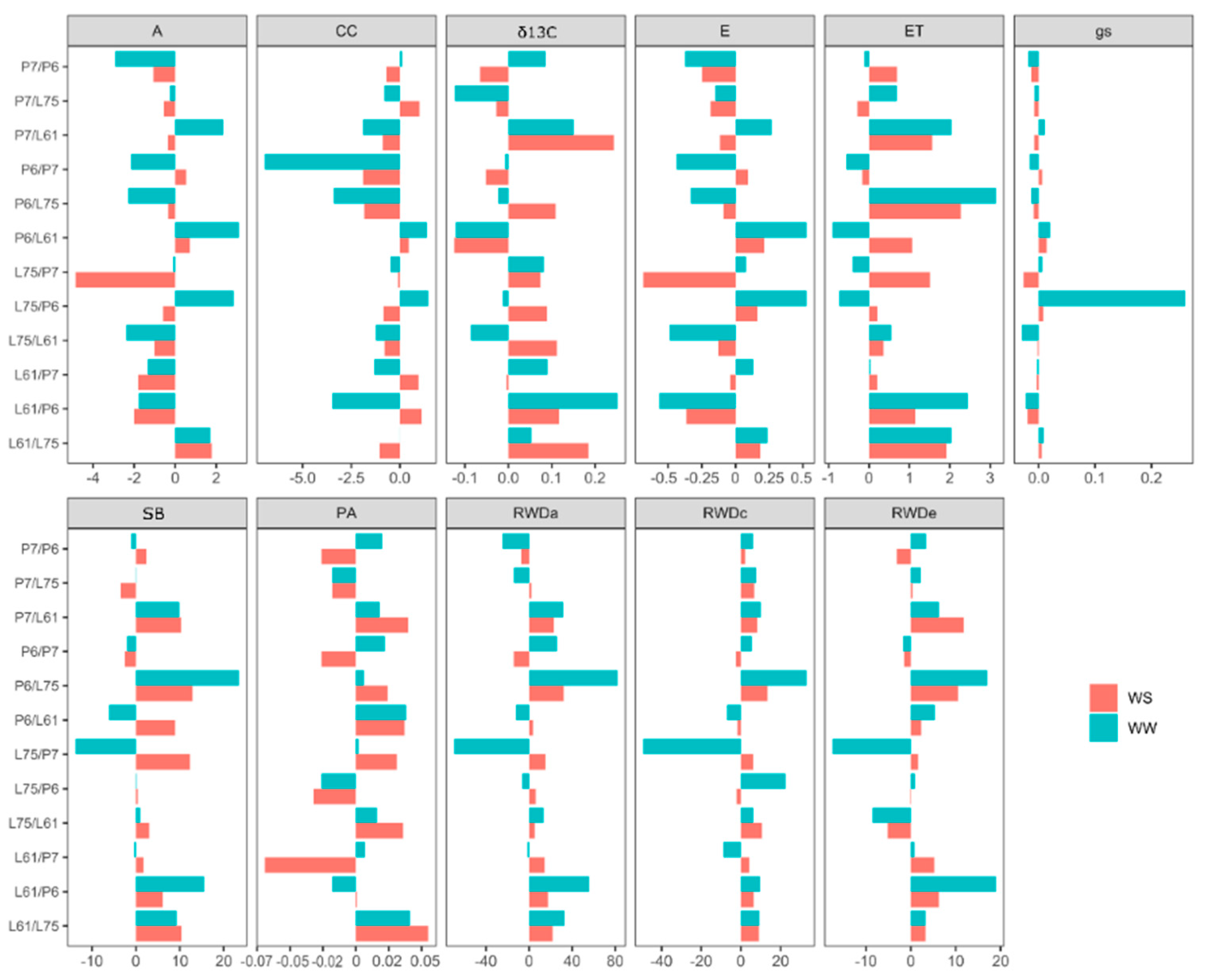
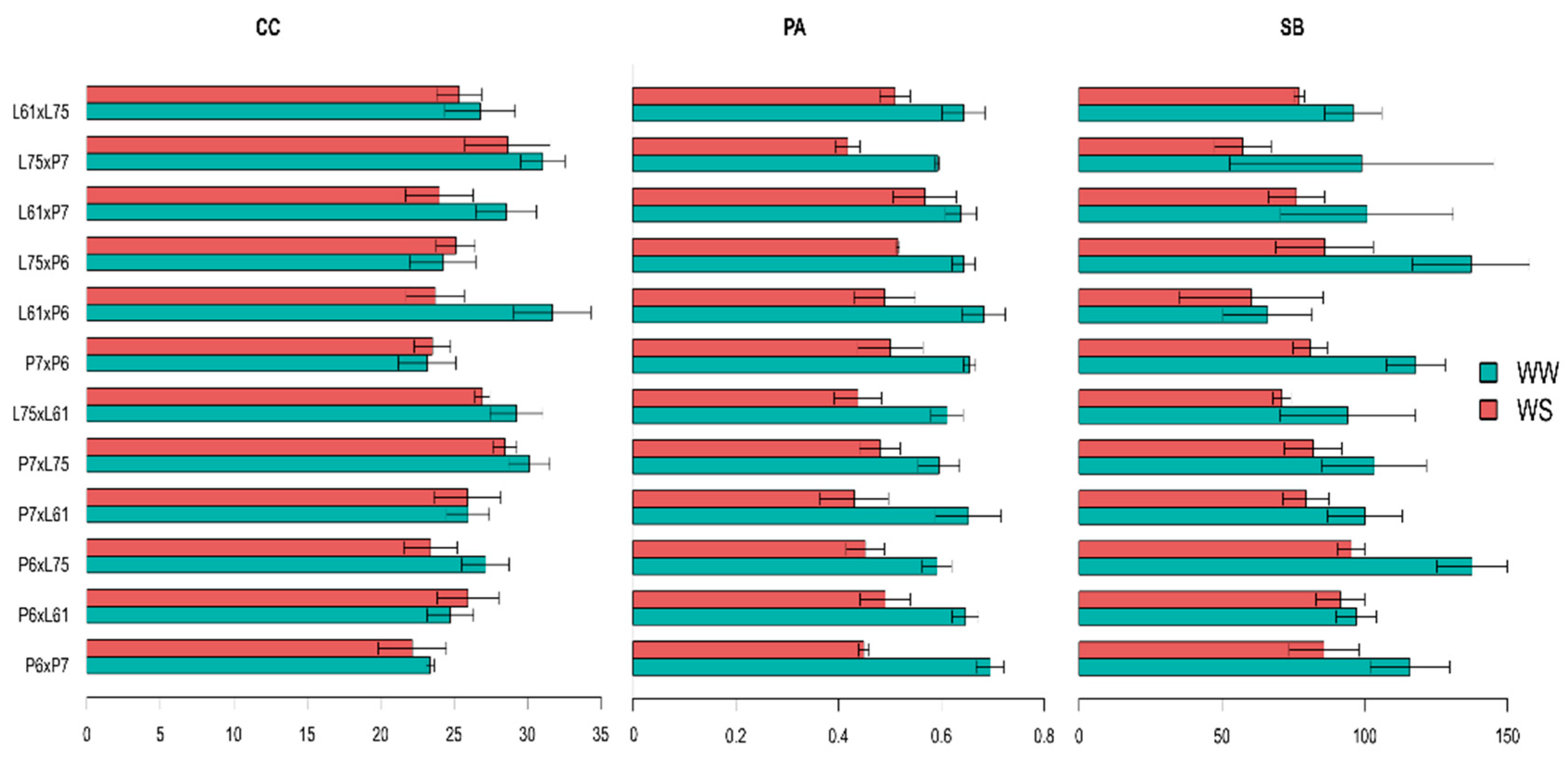
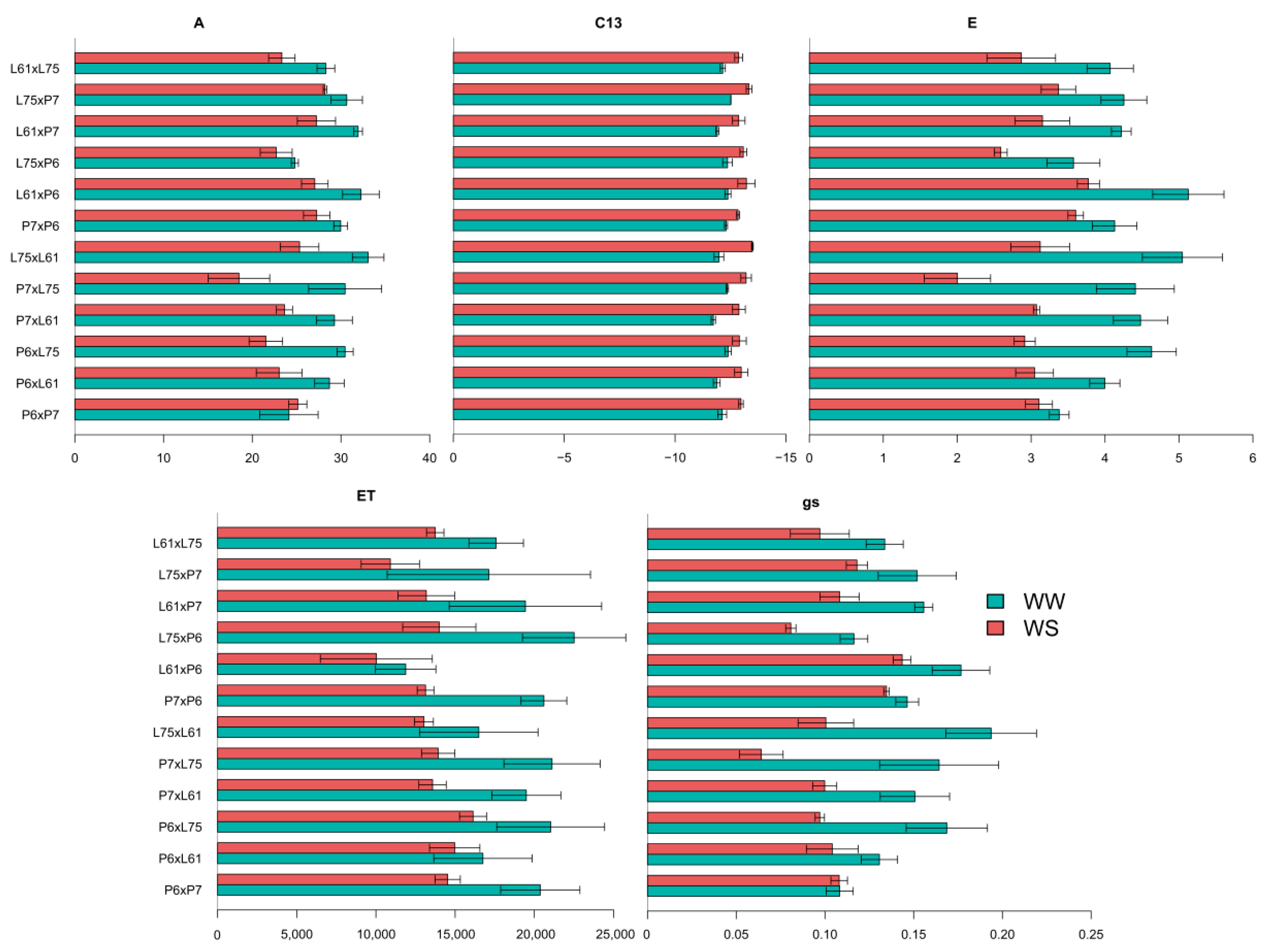
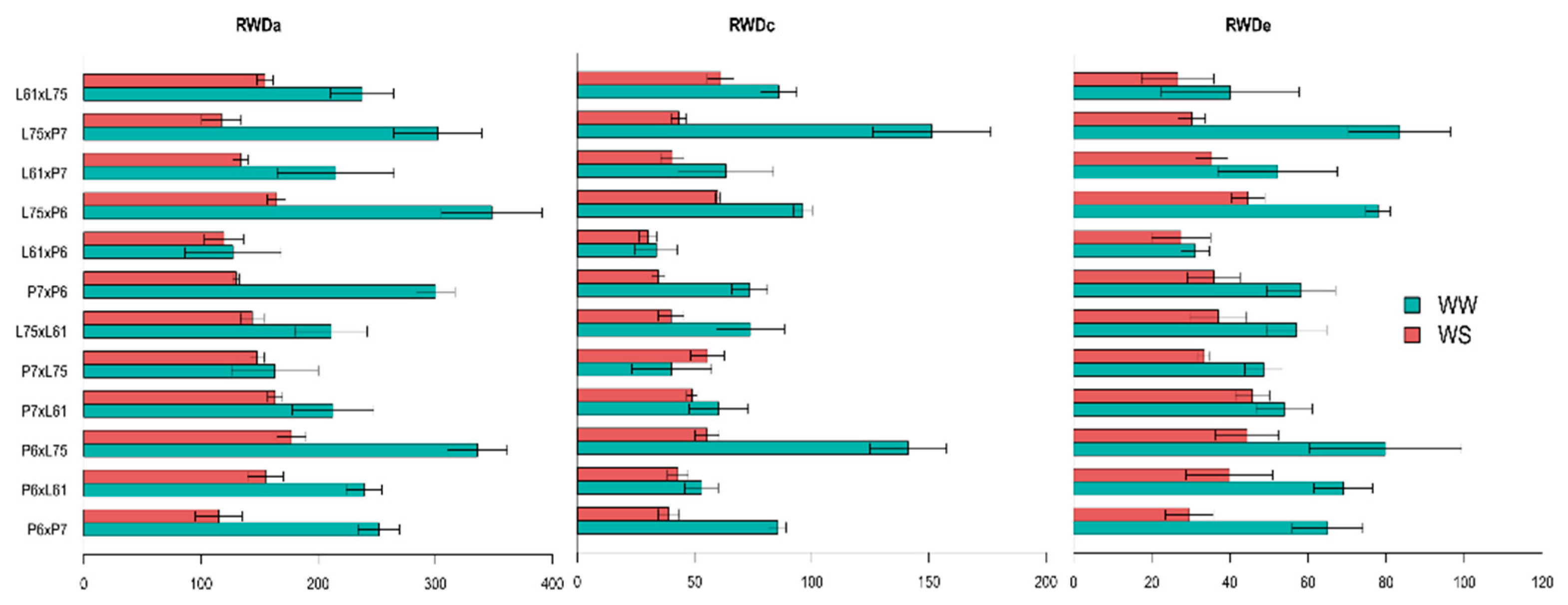
3.4. Effects of Specific Combining Ability and Estimates of Means of the Hybrids under Different Water Conditions
4. Discussion
4.1. Morphological Traits
4.2. Water Status
4.3. Traits Related to the Length of the Roots
4.4. Implications in Popcorn Breeding Programs for Water-Limited Soil Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamphorst, S.H.; do Amaral Júnior, A.T.; Vergara-Diaz, O.; Gracia-Romero, A.; Fernandez-Gallego, J.A.; Chang-Espino, M.C.; Buchaillot, M.L.; Rezzouk, F.Z.; de Lima, V.J.; Serret, M.D.; et al. Heterosis and Reciprocal Effects for Physiological and Morphological Traits of Popcorn Plants under Different Water Conditions. Agric. Water Manag. 2022, 261, 107371. [Google Scholar] [CrossRef]
- Leite, J.T.; do Amaral Junior, A.T.; Kamphorst, S.H.; de Lima, V.J.; dos Santos Junior, D.R.; Schmitt, K.F.M.; de Souza, Y.P.; Santos, T.D.O.; Bispo, R.B.; Mafra, G.S.; et al. Water Use Efficiency in Popcorn (Zea mays L. Var. Everta): Which Physiological Traits Would Be Useful for Breeding? Plants 2021, 10, 1450. [Google Scholar] [CrossRef] [PubMed]
- Araus, J.L.; Sánchez, C.; Cabrera-Bosquet, L. Is Heterosis in Maize Mediated through Better Water Use? New Phytol. 2010, 187, 392–406. [Google Scholar] [CrossRef] [PubMed]
- Chairi, F.; Elazab, A.; Sanchez-Bragado, R.; Araus, J.L.; Serret, M.D. Heterosis for Water Status in Maize Seedlings. Agric. Water Manag. 2016, 164, 100–109. [Google Scholar] [CrossRef]
- Rockenbach, M.F.; Corrêa, C.C.G.; Heringer, A.S.; Freitas, I.L.J.; Santa-Catarina, C.; do Amaral-Júnior, A.T.; Silveira, V. Differentially Abundant Proteins Associated with Heterosis in the Primary Roots of Popcorn. PLoS ONE 2018, 13, e0197114. [Google Scholar] [CrossRef]
- Cairns, J.E.; Sanchez, C.; Vargas, M.; Ordoñez, R.; Araus, J.L. Dissecting Maize Productivity: Ideotypes Associated with Grain Yield under Drought Stress and Well-Watered Conditions. J. Integr. Plant Biol. 2012, 54, 1007–1020. [Google Scholar] [CrossRef]
- Earl, H.J.; Tollenaar, M. Using Chlorophyll Fluorometry to Compare Photosynthetic Performance of Commercial Maize (Zea mays L.) Hybrids in the Field. Field Crops Res. 1999, 61, 201–210. [Google Scholar] [CrossRef]
- Birchler, J.A.; Yao, H.; Chudalayandi, S.; Vaiman, D.; Veitia, R.A. Heterosis. Plant Cell Online 2010, 22, 2105–2112. [Google Scholar] [CrossRef]
- Tollenaar, M.; Lee, E.A. Physiological Dissection of Grain Yield in Maize by Examining Genetic Improvement and Heterosis. Maydica 2006, 51, 399–408. [Google Scholar]
- Ahmadzadeh, A.; Lee, E.A.; Tollenaar, M. Heterosis for Leaf CO Exchange Rate during the Grain-Filling Period in Maize. Crop Sci. 2004, 44, 2095. [Google Scholar] [CrossRef]
- Tollenaar, M.; Ahmadzadeh, A.; Lee, E.A. Physiological Basis of Heterosis for Grain Yield in Maize. Crop Sci. 2004, 44, 2086. [Google Scholar] [CrossRef]
- Vittorazzi, C.; Júnior, A.T.A.; Guimarães, A.G.; Silva, F.H.L.; Pena, G.F.; Daher, R.F.; Gerhardt, I.F.S.; Oliveira, G.H.F.; Santos, P.H.A.D.; Souza, Y.P.; et al. Evaluation of Genetic Variability to Form Heterotic Groups in Popcorn. Genet. Mol. Res. 2018, 17, 18083. [Google Scholar] [CrossRef]
- de Lima, V.J.; do Amaral Júnior, A.T.; Kamphorst, S.H.; Bispo, R.B.; Leite, J.T.; de Oliveira Santos, T.; Medeiros Schmitt, K.F.; Chaves, M.M.; de Oliveira, U.A.; Araújo Diniz Santos, P.H.; et al. Combined Dominance and Additive Gene Effects in Trait Inheritance of Drought-Stressed and Full Irrigated Popcorn. Agronomy 2019, 9, 782. [Google Scholar] [CrossRef]
- Kamphorst, S.H.; do Amaral Júnior, A.T.; de Lima, V.J.; Guimarães, L.J.M.; Schmitt, K.F.M.; Leite, J.T.; Santos, P.H.A.D.; Chaves, M.M.; Mafra, G.S.; dos Santos Junior, D.R.; et al. Can Genetic Progress for Drought Tolerance in Popcorn Be Achieved by Indirect Selection? Agronomy 2019, 9, 792. [Google Scholar] [CrossRef]
- De Lima, V.J.; do Amaral Júnior, A.T.; Kamphorst, S.H.; dos Santos, A.; Schmidt, K.F.M.; Azeredo, V.C.; Leite, J.T.; dos Santos Junior, D.R.; Santos, T.D.O.; Bispo, R.B.; et al. UENF WS01: Popcorn Hybrid with Water Use Efficiency for the State of Rio de Janeiro. Crop Breed. Appl. Biotechnol. 2021, 21, e375821411. [Google Scholar] [CrossRef]
- Romano, G.; Zia, S.; Spreer, W.; Sanchez, C.; Cairns, J.; Luis, J.; Müller, J. Use of Thermography for High Throughput Phenotyping of Tropical Maize Adaptation in Water Stress. Comput. Electron. Agric. 2011, 79, 67–74. [Google Scholar] [CrossRef]
- Adebayo, M.A.; Menkir, A.; Blay, E.; Gracen, V.; Danquah, E.; Hearne, S. Genetic Analysis of Drought Tolerance in Adapted × Exotic Crosses of Maize Inbred Lines under Managed Stress Conditions. Euphytica 2014, 196, 261–270. [Google Scholar] [CrossRef]
- Mendes, W.D.S.; Drews, T.A.; Medeiros, J.C.; Rosa, J.D.; Gualberto, A.V.S.; Mielezrski, F. Development and Productivity of Maize in Response to Spatial Arrangement under Semiarid Condition of Northeastern Brazil. Aust. J. Crop Sci. 2017, 11, 313–321. [Google Scholar] [CrossRef]
- Dalal, M.; Sharma, T.R. Biotechnological Applications for Improvement of Drought Tolerance. In Abiotic Stress Management for Resilient Agriculture; Minhas, P.S., Rane, J., Pasala, R.K., Eds.; Springer: Singapore, 2017; pp. 299–312. [Google Scholar]
- Kamphorst, S.H.; do Amaral Junior, A.T.; de Lima, V.J.; Carena, M.J.; Azeredo, V.C.; Mafra, G.S.; Santos, P.H.A.D.; Leite, J.T.; Schmitt, K.F.M.; dos Santos Junior, D.R.; et al. Driving Sustainable Popcorn Breeding for Drought Tolerance in Brazil. Front. Plant Sci. 2021, 12, 2285. [Google Scholar] [CrossRef]
- Giaveno, C.D.; Ribeiro, R.V.; Souza, G.M.; Oliveira, R.F. Screening of Tropical Maize for Salt Stress Tolerance. Cropp Breed. Appl. Biotechnol. 2007, 7, 304–313. [Google Scholar] [CrossRef][Green Version]
- Peters, G.P.; Marland, G.; le Quéré, C.; Boden, T.; Canadell, J.G.; Raupach, M.R. Rapid Growth in CO2 Emissions after the 2008–2009 Global Financial Crisis. Nat. Clim. Chang. 2011, 2, 2–4. [Google Scholar] [CrossRef]
- Kamphorst, S.H.; de Lima, V.J.; Leite, J.T.; Carvalho, C.M.; Xavier, K.B.; Campostrini, E. Popcorn Breeding for Water-Stress Tolerance or for Agronomic Water-Use Efficiency? Genet. Mol. Res. 2018, 17, 1–18. [Google Scholar] [CrossRef]
- Kamphorst, S.H.; de Lima, V.J.; Schimitt, K.F.M.; Leite, J.T.; Azeredo, V.C.; Pena, G.F.; Santos, P.H.A.D.; Júnior, D.R.S.; Júnior, S.B.D.S.; Bispo, R.B.; et al. Water Stress Adaptation of Popcorn Roots and Association with Agronomic Traits. Genet. Mol. Res. 2018, 17, 1–14. [Google Scholar] [CrossRef]
- Kamphorst, S.H.; Gonçalves, G.M.B.; do Amaral Júnior, A.T.; de Lima, V.J.; Leite, J.T.; Schmitt, K.F.M.; dos Santos Junior, D.R.; Santos, J.S.; de Oliveira, F.T.; Corrêa, C.C.G.; et al. Screening of Popcorn Genotypes for Drought Tolerance Using Canonical Correlations. Agronomy 2020, 10, 1519. [Google Scholar] [CrossRef]
- Kamphorst, S.H.; do Amaral Júnior, A.T.; de Lima, V.J.; Santos, P.H.A.D.; Rodrigues, W.P.; Vivas, J.M.S.; Gonçalves, G.M.B.; Schmitt, K.F.M.; Leite, J.T.; Vivas, M.; et al. Comparison of Selection Traits for Effective Popcorn (Zea mays L. Var. Everta) Breeding Under Water Limiting Conditions. Front. Plant Sci. 2020, 11, 1289. [Google Scholar] [CrossRef]
- Kamphorst, S.H.; Gonçalves, G.M.B.; do Amaral Júnior, A.T.; de Lima, V.J.; Schmitt, K.F.M.; Leite, J.T.; Azeredo, V.C.; Gomes, L.P.; Silva, J.G.D.S.; Carvalho, C.M.; et al. Supporting Physiological Trait for Indirect Selection for Grain Yield in Drought-Stressed Popcorn. Plants 2021, 10, 1510. [Google Scholar] [CrossRef]
- Araus, J.L.; Kefauver, S.C.; Zaman-Allah, M.; Olsen, M.S.; Cairns, J.E. Translating High-Throughput Phenotyping into Genetic Gain. Trends Plant Sci. 2018, 23, 451–466. [Google Scholar] [CrossRef]
- Araus, J.L.; Kefauver, S.C. Breeding to Adapt Agriculture to Climate Change: Affordable Phenotyping Solutions. Curr. Opin. Plant Biol. 2018, 45, 237–247. [Google Scholar] [CrossRef]
- Elazab, A.; Serret, M.D.; Araus, J.L. Interactive Effect of Water and Nitrogen Regimes on Plant Growth, Root Traits and Water Status of Old and Modern Durum Wheat Genotypes. Planta 2016, 244, 125–144. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Coplen, T.B. Explanatory Glossary of Terms Used in Expression of Relative Isotope Ratios and Gas Ratios. IUPAC Recommendations 2008. International Union of Pure and Applied Chemistry Inorganic Chemistry Division, Commission on Isotopic Abundances and Atomic Weights: Research Triangle Park, NC, USA, 2008. Available online: https://old.iupac.org/reports/provisional/abstract08/coplen_310508.html (accessed on 24 May 2022).
- Farquhar, G.D.; Ehleringer, J.R.; Hubick, K.T. Carbon Isotope Discrimination and Photosynthesis. Annu. Rev. Plant Biol. 1989, 40, 503–537. [Google Scholar] [CrossRef]
- Elazab, A.; Molero, G.; Serret, M.D.; Araus, J.L. Root Traits and Δ13C and Δ18O of Durum Wheat under Different Water Regimes. Funct. Plant Biol. 2012, 39, 379. [Google Scholar] [CrossRef]
- Griffing, B. Concept of General and Specific Combining Ability in Relation to Diallel Crossing Systems. Aust. J. Biol. Sci. 1956, 9, 462–493. [Google Scholar] [CrossRef]
- Cruz, C.D. GENES—A Software Package for Analysis in Experimental Statistics and Quantitative Genetics. Acta Scientiarum. Agron. 2013, 35, 271–276. [Google Scholar] [CrossRef]
- de Castro, F.A.; Campostrini, E.; Netto, A.T.; de Menezes De Assis Gomes, M.; Ferraz, T.M.; Glenn, D.M. Portable Chlorophyll Meter (PCM-502) Values Are Related to Total Chlorophyll Concentration and Photosynthetic Capacity in Papaya (Carica papaya L.). Theor. Exp. Plant Physiol. 2014, 26, 201–210. [Google Scholar] [CrossRef]
- Avramova, V.; AbdElgawad, H.; Zhang, Z.; Fotschki, B.; Casadevall, R.; Vergauwen, L.; Knapen, D.; Taleisnik, E.; Guisez, Y.; Asard, H.; et al. Drought Induces Distinct Growth Response, Protection, and Recovery Mechanisms in the Maize Leaf Growth Zone. Plant Physiol. 2015, 169, 1382–1396. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The Role of the Plant Antioxidant System in Drought Tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Caverzan, A.; Casassola, A.; Brammer, S.P. Antioxidant Responses of Wheat Plants under Stress. Genet. Mol. Biol. 2016, 39, 1–6. [Google Scholar] [CrossRef]
- Cabrera-Bosquet, L.; Sánchez, C.; Araus, J.L. How Yield Relates to Ash Content, Δ13C and Δ18O in Maize Grown under Different Water Regimes. Ann. Bot. 2009, 104, 1207–1216. [Google Scholar] [CrossRef]
- Liu, S.; Qin, F. Genetic Dissection of Maize Drought Tolerance for Trait Improvement. Mol. Breed. 2021, 41, 8. [Google Scholar] [CrossRef]
- Ali, M.L.; Luetchens, J.; Singh, A.; Shaver, T.M.; Kruger, G.R.; Lorenz, A.J. Greenhouse Screening of Maize Genotypes for Deep Root Mass and Related Root Traits and Their Association with Grain Yield under Water-Deficit Conditions in the Field. Euphytica 2016, 207, 79–94. [Google Scholar] [CrossRef]
- Gao, Y.; Lynch, J.P. Reduced Crown Root Number Improves Water Acquisition under Water Deficit Stress in Maize (Zea mays L.). J. Exp. Bot. 2016, 67, 4545–4557. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Atkin, O.; Millenaar, F. Respiratory Patterns in Roots in Relation to Their Functioning. In Plant Roots; CRC Press: Boca Raton, FL, USA, 2002; pp. 521–552. ISBN 9780203909423. [Google Scholar]
- Cruz, C.D.; Carneiro, P.C.S.; Regazzi, A.J. Modelos Biométricos Aplicados Ao Melhoramento Genético—Volume II; UFV: Viçosa, Brazil, 2014; ISBN 8572691510. [Google Scholar]
- Hallauer, A.R.; Carena, M.J.; Miranda Filho, J.B. Quantitative Genetics in Maize Breeding; Springer: New York, NY, USA, 2010; ISBN 978-1-4419-0765-3. [Google Scholar]
- Chun, L.; Mi, G.; Li, J.; Chen, F.; Zhang, F. Genetic Analysis of Maize Root Characteristics in Response to Low Nitrogen Stress. Plant Soil 2005, 276, 369–382. [Google Scholar] [CrossRef]
- Cortés, A.J.; Blair, M.W. Genotyping by Sequencing and Genome–Environment Associations in Wild Common Bean Predict Widespread Divergent Adaptation to Drought. Front. Plant Sci. 2018, 9, 128. [Google Scholar] [CrossRef]
- López-Hernández, F.; Cortés, A.J. Last-Generation Genome–Environment Associations Reveal the Genetic Basis of Heat Tolerance in Common Bean (Phaseolus vulgaris L.). Front. Genet. 2019, 10, 954. [Google Scholar] [CrossRef]
- Cortés, A.J.; López-Hernández, F. Harnessing Crop Wild Diversity for Climate Change Adaptation. Genes 2021, 12, 783. [Google Scholar] [CrossRef]
- Mhlaba, Z.B.; Amelework, B.; Shimelis, H.; Modi, A.T.; Mashilo, J. Genetic Differentiation among Selected Tepary Bean Collections Revealed by Morphological Traits and Simple Sequence Repeat Markers. Acta Agric. Scand. Sect. B Soil Plant Sci. 2018, 68, 608–618. [Google Scholar] [CrossRef]

| SV/Traits | WW | WS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G | GCA | SCA | REC | G | GCA | SCA | REC | ||||
| QM | QM | QM | QM | QM | QM | ||||||
| Morphological traits | Shoot Biomass (g) | 1795.93 ** | 5009.76 ** | 1553.88 ** | 431.05 ns | 102.28 | 891.35 ** | 1423.28 ** | 1309.28 * | 207.46 ns | 74.73 |
| Whole Plant area (m2) | 0.007 ** | 0.009 ** | 0.011 ** | 0.001 * | 0.62 | 0.014 ** | 0.013 ** | 0.019 ** | 0.009 ns | 0.46 | |
| Chlorophyll concentration (Dualex index) | 81.79 * | 51.16 ** | 161.24 * | 17.65 ns | 29.11 | 15.85 ** | 32.04 ** | 19.62 ** | 3.97 * | 25.96 | |
| Water status | Carbon isotope discrimination (δ13C) | 0.168 ** | 0.504 ** | 0.075 * | 0.093 ns | −12.16 | 0.270 * | 0.656 ** | 0.223 ns | 0.125 ns | −13.1 |
| Net CO2 assimilation rate (µmol CO2 m−2 s−1) | 38.61 ** | 29.18 ** | 54.79 ns | 27.15 ** | 29.08 | 24.74 ** | 41.90 * | 7.63 ** | 33.27 ** | 24.12 | |
| Stomatal conductance (mol H2O m−2 s−1) | 0.0022 ** | 0.0024 ** | 0.0019** | 0.0025 ** | 0.14 | 0.0011 ** | 0.0016 * | 0.0006 * | 0.001 ** | 0.1 | |
| Transpiration (mmol H2O m−2 s−1) | 1.083 ** | 0.678 ** | 1.368 ns | 1.001 ** | 4.26 | 0.559 ** | 0.916 ns | 0.233 ** | 0.705 ** | 3.03 | |
| Cumulative plant transpiration (dm3 plant−1) | 48.89 ** | 138.89 ** | 45.82** | 6.97 ns | 18.03 | 20.08 ** | 19.56 ** | 36.14 * | 4.29 ns | 12.73 | |
| Root traits | RWDa (cm) | 17,114.45 ** | 24,279.33 ns | 21,879.75 ** | 8766.70 ** | 220.91 | 2602.32 ** | 555.36 ** | 5339.91 ** | 888.19 ** | 132.07 |
| RWDc (cm) | 3739.59 ** | 5285.68 ** | 3541.11 ** | 3165.02 ** | 72.34 | 539.77 ** | 162.64 ** | 1050.24 ** | 217.85 ** | 40.39 | |
| RWDe (cm) | 1052.96 ** | 1825.53 ns | 971.83 ** | 747.81 ns | 54.33 | 319.71 ** | 118.40 ** | 635.11 ** | 104.97 ** | 31.43 | |
| SV/Traits | WW | WS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GCA | SCA | REC | GCA | SCA | REC | ||||||||
| Φg | % | Φs | % | Φrc | % | Φg | % | Φs | % | Φrc | % | ||
| Morphological traits | Shoot biomass | 189.57 | 13 | 364.64 | 78 | −4.81 | 0 | 54.33 | 4 | 396.64 | 94 | 14.68 | 3 |
| Whole plant area | 0.0003 | 43 | 0.0033 | 40 | −0.00002 | 0 | 0.0005 | 6 | 0.0053 | 59 | 0.001 | 36 | |
| Chlorophyll concentration (Dualex index) | 1.86 | 3 | 51.6 | 93 | 1.86 | 3 | 1.04 | 20 | 4.18 | 80 | −0.52 | 0 | |
| Water status | Carbon isotope discrimination (δ13C) | 0.013 | 58 | 0.003 | 30 | 0.004 | 12 | 0.024 | 36 | 0.047 | 53 | 0.008 | 11 |
| Net CO2 assimilation rate | 0.92 | 5 | 15.91 | 79 | 3.35 | 17 | 1.49 | 25 | 0.53 | 0 | 4.54 | 75 | |
| Stomatal conductance | 0.00007 | 8 | 0.00043 | 49 | 0.00031 | 43 | 0.00006 | 15 | 0.00015 | 34 | 0.00021 | 51 | |
| Transpiration | 0.018 | 4 | 0.37 | 72 | 0.13 | 25 | 0.03 | 23 | 0.03 | 8 | 0.09 | 69 | |
| Cumulative plant transpiration | 5.15 | 34 | 10.2 | 66 | −1.37 | 0 | 0.72 | 6 | 11.25 | 92 | 0.32 | 3 | |
| Root traits | RWDa | 958.3 | 11 | 6866.58 | 76 | 1247.78 | 14 | 13.12 | 1 | 1699.72 | 93 | 107.94 | 6 |
| RWDc | 212.97 | 12 | 1122.25 | 55 | 498.44 | 34 | 5.64 | 1 | 341.05 | 90 | 31.79 | 8 | |
| RWDe | 68.67 | 16 | 264.84 | 62 | 95.08 | 22 | 2.5 | 1 | 192.23 | 95 | 7.76 | 4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viana, F.N.; Chaves, M.M.; Kamphorst, S.H.; do Amaral Junior, A.T.; Lima, V.J.d.; Leite, J.T.; Schmidt, K.F.M.; Oliveira, U.A.d.; Lamego, D.L.; Pereira, J.L.; et al. Heritability of Morphophysiological Traits in Popcorn for Drought Tolerance and Their Use as Breeding Indicators of Superior Genotypes. Agronomy 2022, 12, 1517. https://doi.org/10.3390/agronomy12071517
Viana FN, Chaves MM, Kamphorst SH, do Amaral Junior AT, Lima VJd, Leite JT, Schmidt KFM, Oliveira UAd, Lamego DL, Pereira JL, et al. Heritability of Morphophysiological Traits in Popcorn for Drought Tolerance and Their Use as Breeding Indicators of Superior Genotypes. Agronomy. 2022; 12(7):1517. https://doi.org/10.3390/agronomy12071517
Chicago/Turabian StyleViana, Flávia Nicácio, Marcelo Moura Chaves, Samuel Henrique Kamphorst, Antônio Teixeira do Amaral Junior, Valter Jário de Lima, Jhean Torres Leite, Katia Fabiane Medeiros Schmidt, Uéliton Alves de Oliveira, Danielle Leal Lamego, Jacymara Lopes Pereira, and et al. 2022. "Heritability of Morphophysiological Traits in Popcorn for Drought Tolerance and Their Use as Breeding Indicators of Superior Genotypes" Agronomy 12, no. 7: 1517. https://doi.org/10.3390/agronomy12071517
APA StyleViana, F. N., Chaves, M. M., Kamphorst, S. H., do Amaral Junior, A. T., Lima, V. J. d., Leite, J. T., Schmidt, K. F. M., Oliveira, U. A. d., Lamego, D. L., Pereira, J. L., Pena, G. F., Vieira, H. D., Oliveira, J. G. d., Daher, R. F., Campostrini, E., & Bressan-Smith, R. (2022). Heritability of Morphophysiological Traits in Popcorn for Drought Tolerance and Their Use as Breeding Indicators of Superior Genotypes. Agronomy, 12(7), 1517. https://doi.org/10.3390/agronomy12071517











