Alkaline Salt Tolerance of the Biomass Plant Arundo donax
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Characteristics
2.2. Plant Material and Alkaline Salt Treatments
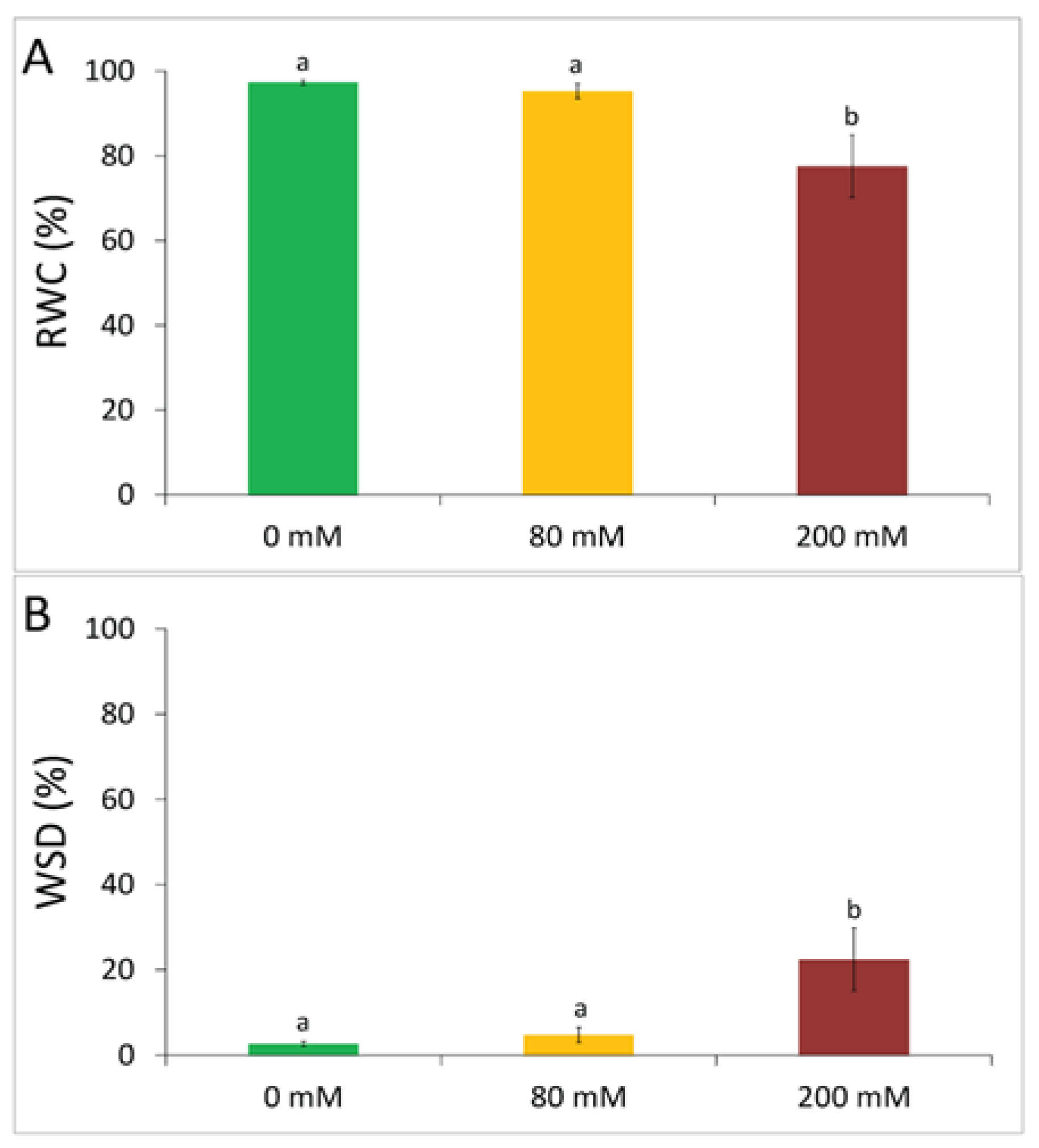
2.3. Determination of Relative Water Content and Water Saturation Deficit
2.4. Determination of Element Concentration
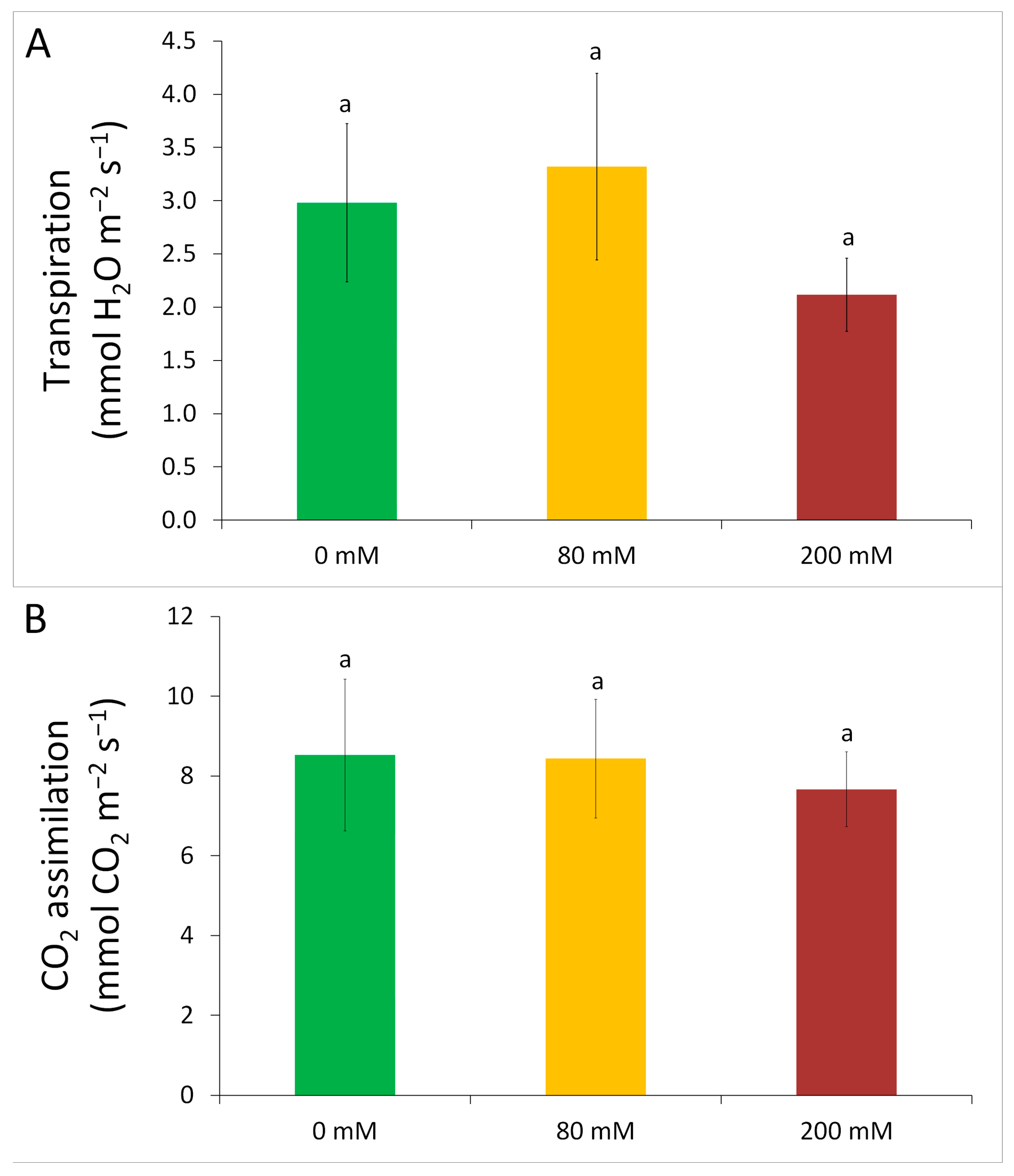
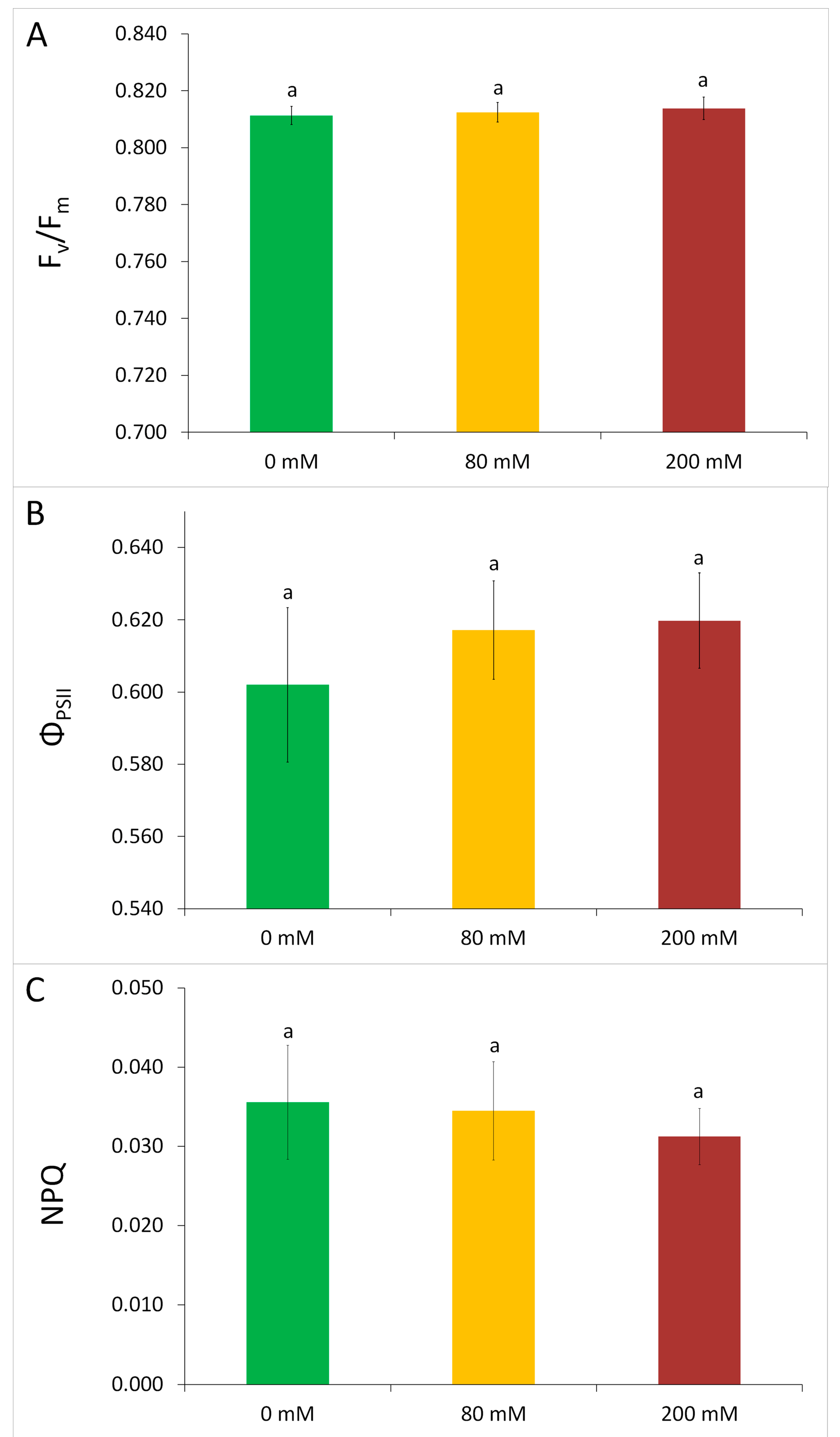
2.5. Measurements of Transpiration and Photosynthetic Performance
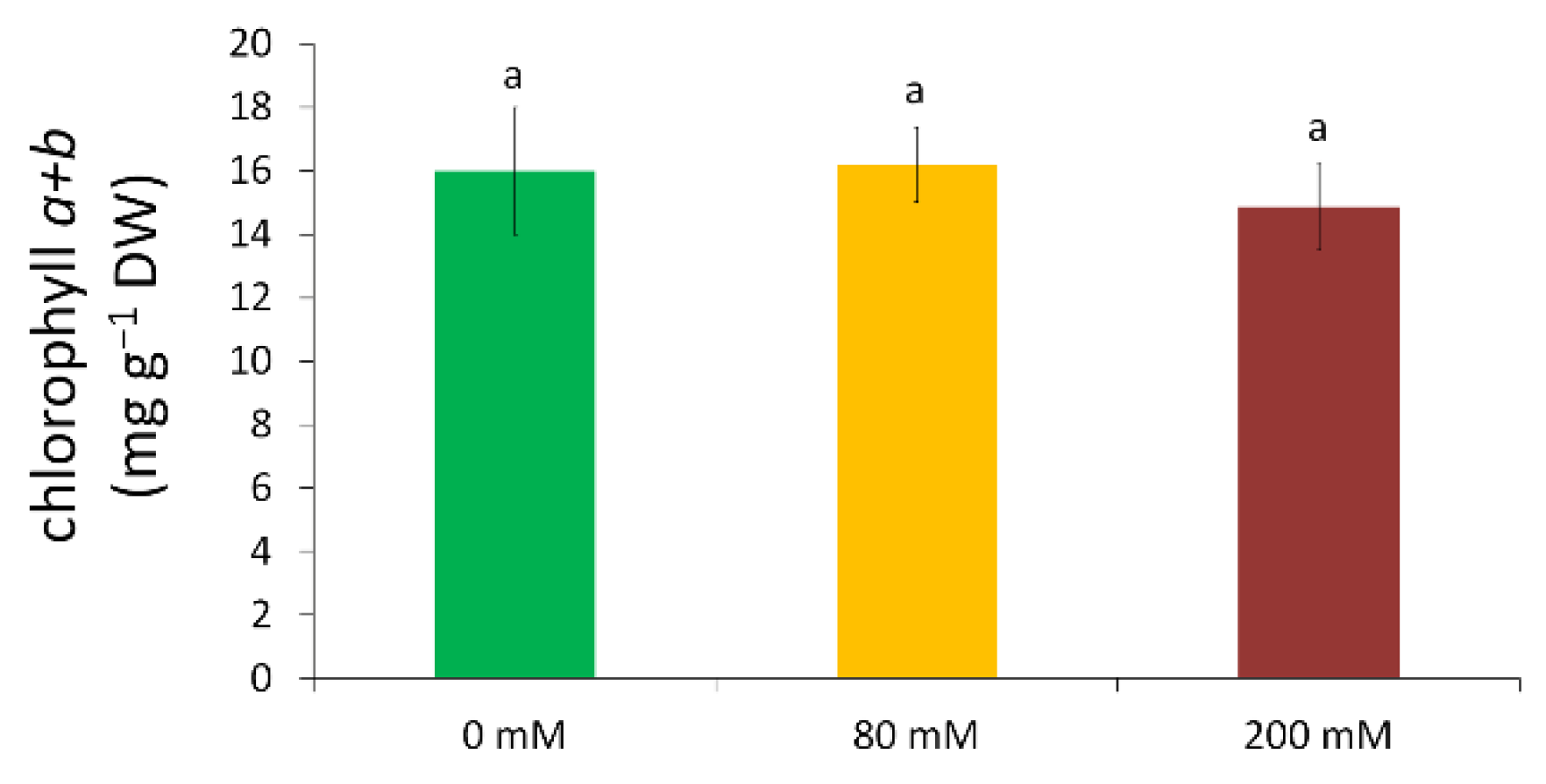
2.6. Determination of Chlorophyll Content
2.7. Malondialdehyde Content
2.8. Determination of Superoxide Dismutase, Class III and Ascorbate Peroxidase Activity
2.9. Glutathione Reductase and Catalase Activity
2.10. Ascorbate Oxidase Activity
2.11. Statistical Analysis
3. Results
3.1. Physiological Tolerance to Alkaline Salt Treatment in Arundo donax
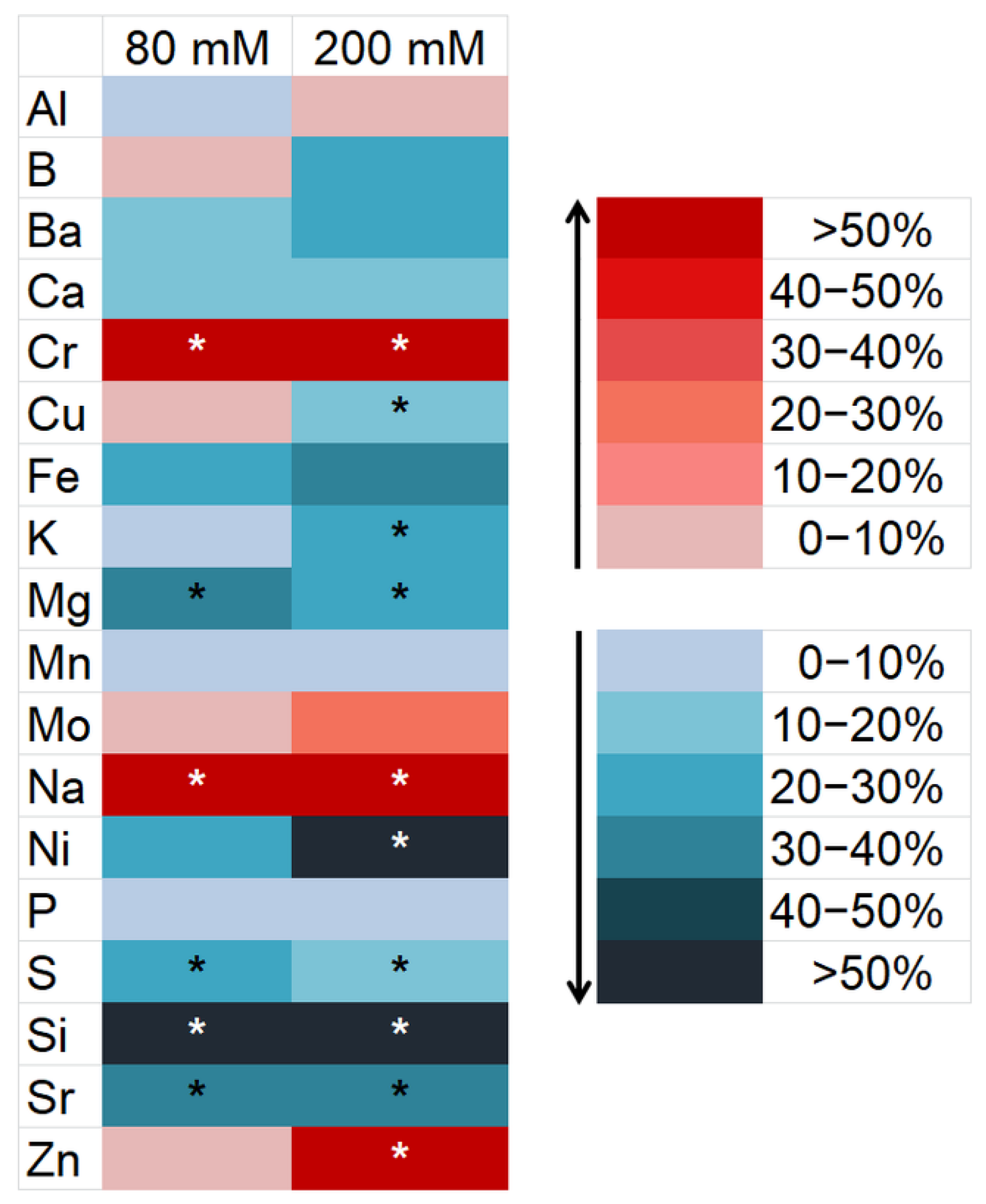
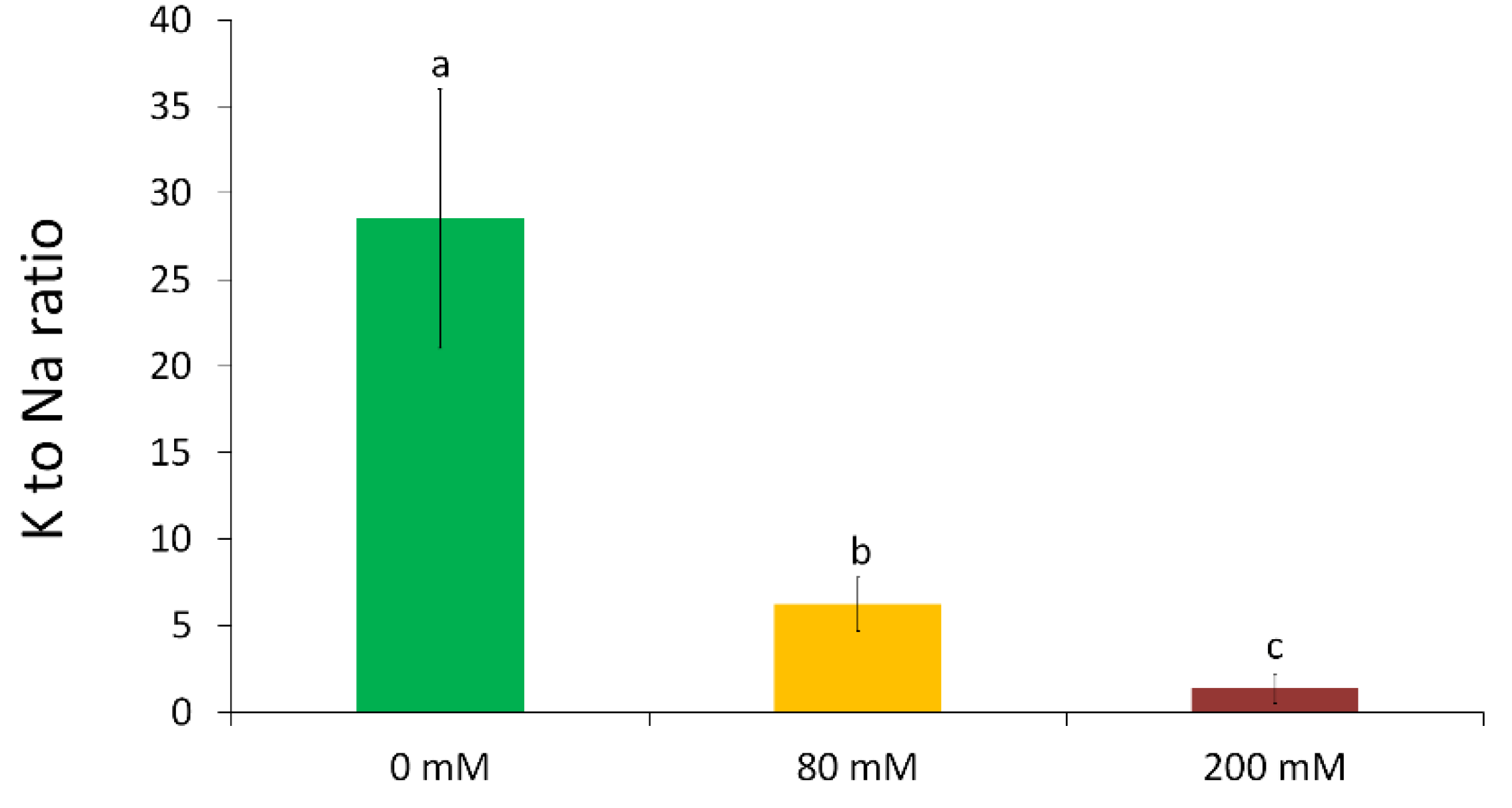
3.2. Changes in the Element Composition
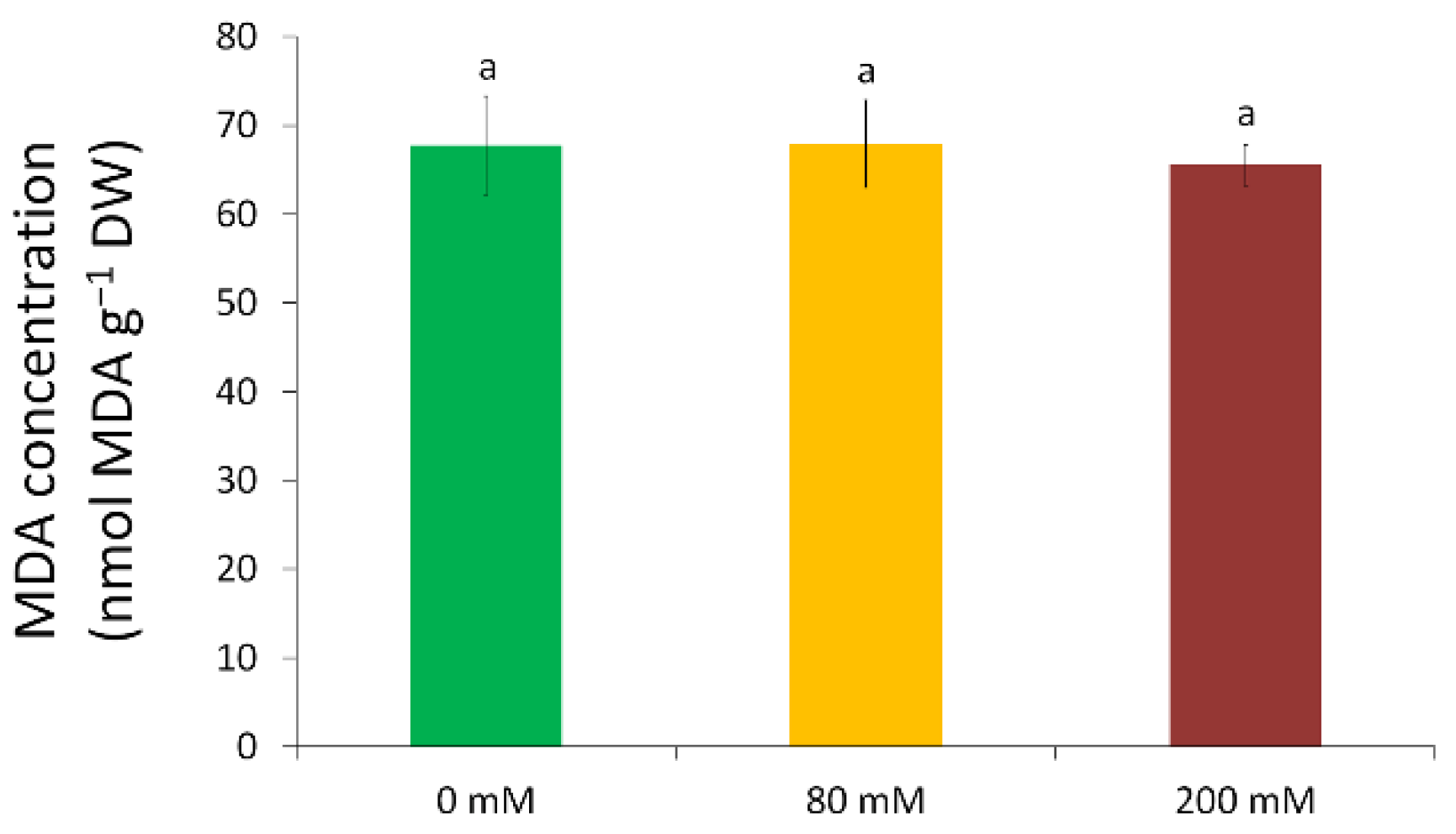
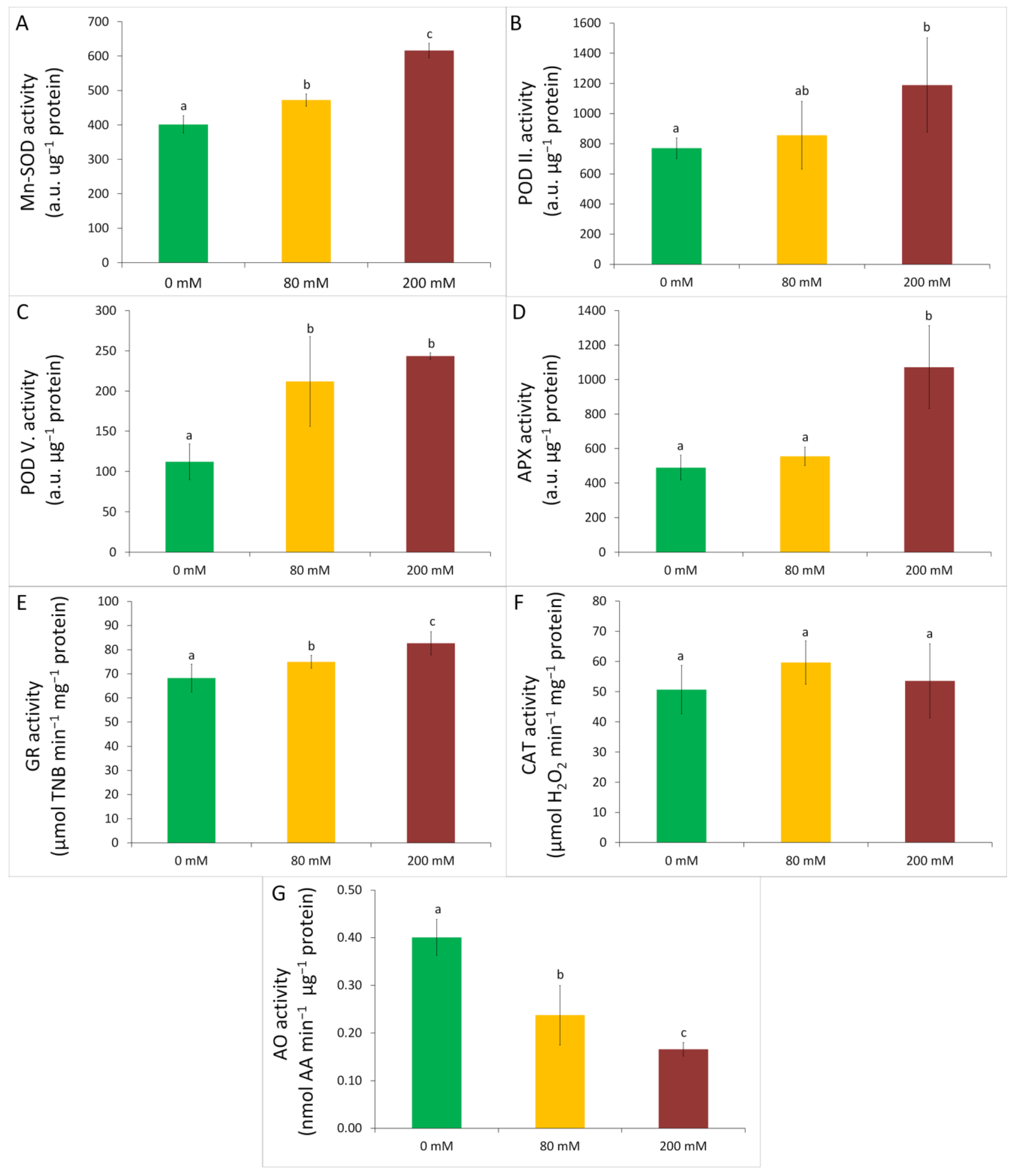
3.3. Oxidative Stress and Antioxidative Defence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srivastava, P.; Wu, Q.S.; Giri, B. Salinity: An overview. In Soil Biology, Volume 56: Microorganisms in Saline Environments: Strategies and Functions; Giri, B., Varma, A., Eds.; Springer: Cham, Switzerland, 2019; Volume 56, pp. 3–18. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. Int. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Negrão, S.; Schmöckel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Velmurugan, A.; Swarnam, P.; Subramani, T.; Meena, B.; Kaledhonkar, M.J. Water demand and salinity. In Desalination—Challenges and Opportunities; Farahani, M.H.D.A., Vatanpour, V., Taheri, A.H., Eds.; IntechOpen: Vienna, Austria, 2020; pp. 45–53. [Google Scholar]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef]
- Calone, R.; Sanoubar, R.; Lambertini, C.; Speranza, M.; Vittori Antisari, L.; Vianello, G.; Barbanti, L. Salt tolerance and Na allocation in Sorghum bicolor under variable soil and water salinity. Plants 2020, 9, 561. [Google Scholar] [CrossRef] [PubMed]
- Isayenkov, S.V.; Maathuis, F.J. Plant salinity stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Farid, I.B.; Marghany, M.R.; Rowezek, M.M.; Sheded, M.G. Effect of salinity stress on growth and metabolomic profiling of Cucumis sativus and Solanum lycopersicum. Plants 2020, 9, 1626. [Google Scholar] [CrossRef]
- Song, T.; Xu, H.; Sun, N.; Jiang, L.; Tian, P.; Yong, Y.; Yang, W.; Cai, H.; Cui, G. Metabolomic analysis of alfalfa (Medicago sativa L.) root-symbiotic rhizobia responses under alkali stress. Front. Plant Sci. 2017, 8, 1208. [Google Scholar] [CrossRef]
- Guo, R.; Shi, L.; Yang, Y. Germination, growth, osmotic adjustment and ionic balance of wheat in response to saline and alkaline stresses. Soil Sci. Plant Nutr. 2009, 55, 667–679. [Google Scholar] [CrossRef]
- Guo, R.; Yang, Z.; Li, F.; Yan, C.; Zhong, X.; Liu, Q.; Xia, X.; Li, H.; Zhao, L. Comparative metabolic responses and adaptive strategies of wheat (Triticum aestivum) to salt and alkali stress. BMC Plant Biol. 2015, 15, 170. [Google Scholar] [CrossRef] [Green Version]
- El Ghazali, G.E. Suaeda vermiculata Forssk. ex JF Gmel.: Structural characteristics and adaptations to salinity and drought: A review. Int. J. Sci. 2020, 9, 28–33. [Google Scholar]
- Percey, W.J.; McMinn, A.; Bose, J.; Breadmore, M.C.; Guijt, R.M.; Shabala, S. Salinity effects on chloroplast PSII performance in glycophytes and halophytes. Funct. Plant Biol. 2016, 43, 1003–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pottosin, I.; Shabala, S. Transport across chloroplast membranes: Optimizing photosynthesis for adverse environmental conditions. Mol. Plant 2016, 9, 356–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamane, K.; Taniguchi, M.; Miyake, H. Salinity-induced subcellular accumulation of H2O2 in leaves of rice. Protoplasma 2012, 249, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, X.; Giraldo, J.P.; Shabala, S. It is not all about sodium: Revealing tissue specificity and signalling roles of potassium in plant responses to salt stress. Plant Soil 2018, 431, 1–17. [Google Scholar] [CrossRef]
- Pan, T.; Liu, M.; Kreslavski, V.D.; Zharmukhamedov, S.K.; Nie, C.; Yu, M.; Kuznetsov, V.V.; Allakhverdiev, S.I.; Shabala, S. Non-stomatal limitation of photosynthesis by soil salinity. Crit. Rev. Environ. Sci. Technol. 2021, 51, 791–825. [Google Scholar] [CrossRef]
- Bai, J.; Qin, Y.; Liu, J.; Wang, Y.; Sa, R.; Zhang, N.; Jia, R. Proteomic response of oat leaves to long-term salinity stress. Environ. Sci. Pollut. Res. 2017, 24, 3387–3399. [Google Scholar] [CrossRef]
- Fooladvand, Z.; Fazelinasab, B. Evaluate the potential halophyte plants to produce biofuels. Eur. J. Biotechnol. Biosci. 2014, 2, 1–3. [Google Scholar]
- Joshi, A.; Kanthaliya, B.; Arora, J. Halophytes: The nonconventional crops as source of biofuel production. In Handbook of Halophytes: From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.-N., Ed.; Springer: Cham, Switzerland, 2020; pp. 2451–2477. [Google Scholar]
- Pilu, R.; Badone, F.C.; Michela, L. Giant reed (Arundo donax L.): A weed plant or a promising energy crop? Afr. J. Biotechnol. 2012, 11, 9163–9174. [Google Scholar]
- Cristaldi, A.; Conti, G.O.; Cosentino, S.L.; Mauromicale, G.; Copat, C.; Grasso, A.; Zuccarello, P.; Fiore, M.; Restuccia, C.; Ferrante, M. Phytoremediation potential of Arundo donax (Giant Reed) in contaminated soil by heavy metals. Environ. Res. 2020, 185, 109427. [Google Scholar] [CrossRef]
- Danelli, T.; Laura, M.; Savona, M.; Landoni, M.; Adani, F.; Pilu, R. Genetic improvement of Arundo donax L.: Opportunities and challenges. Plants 2020, 9, 1584. [Google Scholar] [CrossRef] [PubMed]
- Rossa, B.; Tüffers, A.V.; Naidoo, G.; Von Willert, D.J. Arundo donax L. (Poaceae)—A C3 species with unusually high photosynthetic capacity. Bot. Acta 1998, 111, 216–221. [Google Scholar] [CrossRef]
- Triana, F.; Nassi o Di Nasso, N.; Ragaglini, G.; Roncucci, N.; Bonari, E. Evapotranspiration, crop coefficient and water use efficiency of giant reed (Arundo donax L.) and miscanthus (Miscanthus × giganteus Greef et Deu.) in a Mediterranean environment. GCB Bioenergy 2015, 7, 811–819. [Google Scholar] [CrossRef]
- Sánchez, E.; Scordia, D.; Lino, G.; Arias, C.; Cosentino, S.L.; Nogués, S. Salinity and water stress effects on biomass production in different Arundo donax L. clones. Bioenergy Res. 2015, 8, 1461–1479. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.; Wyman, C.E. Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuel Bioprod. Biorefin. 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta—Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Solórzano, E.; Corpas, F.J.; González-Gordo, S.; Palma, J.M. Reactive oxygen species (ROS) metabolism and nitric oxide (NO) content in roots and shoots of rice (Oryza sativa L.) plants under arsenic-induced stress. Agronomy 2020, 10, 1014. [Google Scholar] [CrossRef]
- Mittler, R.; Zilinskas, B.A. Detection of ascorbate peroxidase activity in native gels by inhibition of the ascorbate-dependent reduction of nitroblue tetrazolium. Anal. Biochem. 1993, 212, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Smith, I.K.; Vierheller, T.L.; Thorne, C.A. Assay of glutathione reductase in crude tissue homogenates using 5,5′-dithiobis(2-nitrobenzoic acid). Anal. Biochem. 1988, 175, 408–413. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Meth. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Diallinas, G.; Pateraki, I.; Sanmartin, M.; Scossa, A.; Stilianou, E.; Panopoulos, N.J.; Kanellis, A.K. Melon ascorbate oxidase: Cloning of a multigene family, induction during fruit development and repression by wounding. Plant Mol. Biol. 1997, 34, 759–770. [Google Scholar] [CrossRef]
- Mosery, O.; Kanellis, A.K. Ascorbate oxidase of Cucumis melo L. var. reticulatus: Purification, characterization and antibody production. J. Exp. Bot. 1994, 45, 717–724. [Google Scholar] [CrossRef] [Green Version]
- Greenway, H.; Munns, R. Mechanism of salt tolerance in non-halophytes. Annu. Rev. Plant Physiol. 1980, 31, 139–168. [Google Scholar] [CrossRef]
- Orcutt, D.M.; Nilsen, E.T. The Physiology of Plants under Stress; John Wiley and Sons, Inc.: New York, NY, USA, 2000. [Google Scholar]
- Basel, S. Water status and protein pattern changes towards salt stress in cotton. J. Stress Physiol. Biochem. 2013, 9, 113–123. [Google Scholar]
- Singh, P.; Singh, N.; Sharma, K.D.; Kuhad, M.S. Plant water relations and osmotic adjustment in Brassica species under salinity stress. J. Am. Sci. 2010, 6, 1–4. [Google Scholar]
- Ali, A.; Raddatz, N.; Pardo, J.M.; Yun, D.J. HKT sodium and potassium transporters in Arabidopsis thaliana and related halophyte species. Physiol. Plant. 2021, 171, 546–558. [Google Scholar] [CrossRef]
- Riedelsberger, J.; Vergara-Jaque, A.; Piñeros, M.; Dreyer, I.; González, W. An extracellular cation coordination site influences ion conduction of OsHKT2;2. BMC Plant Biol. 2019, 19, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, D.F.; de Sousa Lopes, L.; Gomes-Filho, E. Metabolic changes associated with differential salt tolerance in sorghum genotypes. Planta 2020, 252, 34. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Shen, Q.; Cai, S.; Chen, Z.H.; Dai, F.; Zhang, G. Ionomic responses and correlations between elements and metabolites under salt stress in wild and cultivated barley. Plant Cell Physiol. 2013, 54, 1976–1988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seemann, J.R.; Critchley, C. Effects of salt stress on the growth, ion content, stomatal behaviour and photosynthetic capacity of a salt-sensitive species, Phaseolus vulgaris L. Planta 1985, 164, 151–162. [Google Scholar] [CrossRef]
- Wang, R.; Chen, S.; Deng, L.; Fritz, E.; Huttermann, A.; Polle, A. Leaf photosynthesis, fluorescence response to salinity and the relevance to chloroplast salt compartmentation and anti-oxidative stress in two poplars. Trees 2007, 21, 581–591. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2008, 133, 481–489. [Google Scholar] [CrossRef]
- Sofo, A.; Scopa, A.; Nuzzaci, M.; Vitti, A. Ascorbate peroxidase and catalase activities and their genetic regulation in plants subjected to drought and salinity stresses. Int. J. Mol. Sci. 2015, 16, 13561–13578. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Tanveer, M.; Shabala, S. Targeting redox regulatory mechanisms for salinity stress tolerance in crops. In Salinity Responses and Tolerance in Plants, Volume 1; Kumar, V., Wani, S.H., Suprasanna, P., Tran, L.-S.P., Eds.; Springer: Cham, Switzerland, 2018; pp. 213–234. [Google Scholar]
- Bannister, J.V.; Bannister, W.H.; Rotilio, G. Aspects of the structure, function and applications of superoxide dismutase. CRC Crit. Rev. Biochem. 1987, 22, 111–180. [Google Scholar] [CrossRef] [PubMed]
- Zeeshan, M.; Lu, M.; Sehar, S.; Holford, P.; Wu, F. Comparison of biochemical, anatomical, morphological, and physiological responses to salinity stress in wheat and barley genotypes deferring in salinity tolerance. Agronomy 2020, 10, 127. [Google Scholar] [CrossRef] [Green Version]
- Marjamaa, K.; Kukkola, E.M.; Fagerstedt, K.V. The role of xylem class III peroxidases in lignification. J. Exp. Bot. 2009, 60, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Xin, S.; Tao, C.; Jin, X.; Li, H. Cotton ascorbate oxidase promotes cell growth in cultured tobacco bright yellow-2 cells through generation of apoplast oxidation. Int. J. Mol. Sci. 2017, 18, 1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawada, H.; Shim, I.S.; Usui, K. Induction of benzoic acid 2- hydroxylase and salicylic acid biosynthesis Modulation by salt stress in rice seedlings. Plant Sci. 2006, 171, 263–270. [Google Scholar] [CrossRef]
- Pignocchi, C.; Fletcher, J.M.; Wilkinson, J.E.; Barnes, J.D.; Foyer, C.H. The function of ascorbate oxidase in tobacco. Plant Physiol. 2003, 132, 1631–1641. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, B.; Arcoverde Cerveira Sterner, V.; Papp, L.; May, Z.; Orlóci, L.; Gyuricza, C.; Sági, L.; Solti, Á.; Fodor, F. Alkaline Salt Tolerance of the Biomass Plant Arundo donax. Agronomy 2022, 12, 1589. https://doi.org/10.3390/agronomy12071589
Müller B, Arcoverde Cerveira Sterner V, Papp L, May Z, Orlóci L, Gyuricza C, Sági L, Solti Á, Fodor F. Alkaline Salt Tolerance of the Biomass Plant Arundo donax. Agronomy. 2022; 12(7):1589. https://doi.org/10.3390/agronomy12071589
Chicago/Turabian StyleMüller, Brigitta, Vitor Arcoverde Cerveira Sterner, László Papp, Zoltán May, László Orlóci, Csaba Gyuricza, László Sági, Ádám Solti, and Ferenc Fodor. 2022. "Alkaline Salt Tolerance of the Biomass Plant Arundo donax" Agronomy 12, no. 7: 1589. https://doi.org/10.3390/agronomy12071589
APA StyleMüller, B., Arcoverde Cerveira Sterner, V., Papp, L., May, Z., Orlóci, L., Gyuricza, C., Sági, L., Solti, Á., & Fodor, F. (2022). Alkaline Salt Tolerance of the Biomass Plant Arundo donax. Agronomy, 12(7), 1589. https://doi.org/10.3390/agronomy12071589







