Sublethal Effects of Three Insecticides on Development and Reproduction of Spodoptera frugiperda (Lepidoptera: Noctuidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Spodoptera frugiperda Rearing
2.2. Bioassays for LC50
2.3. Effects of Sublethal Concentration on Life Table Parameters
2.4. Data Analyzed
3. Results
3.1. Determination of Sublethal Concentrations
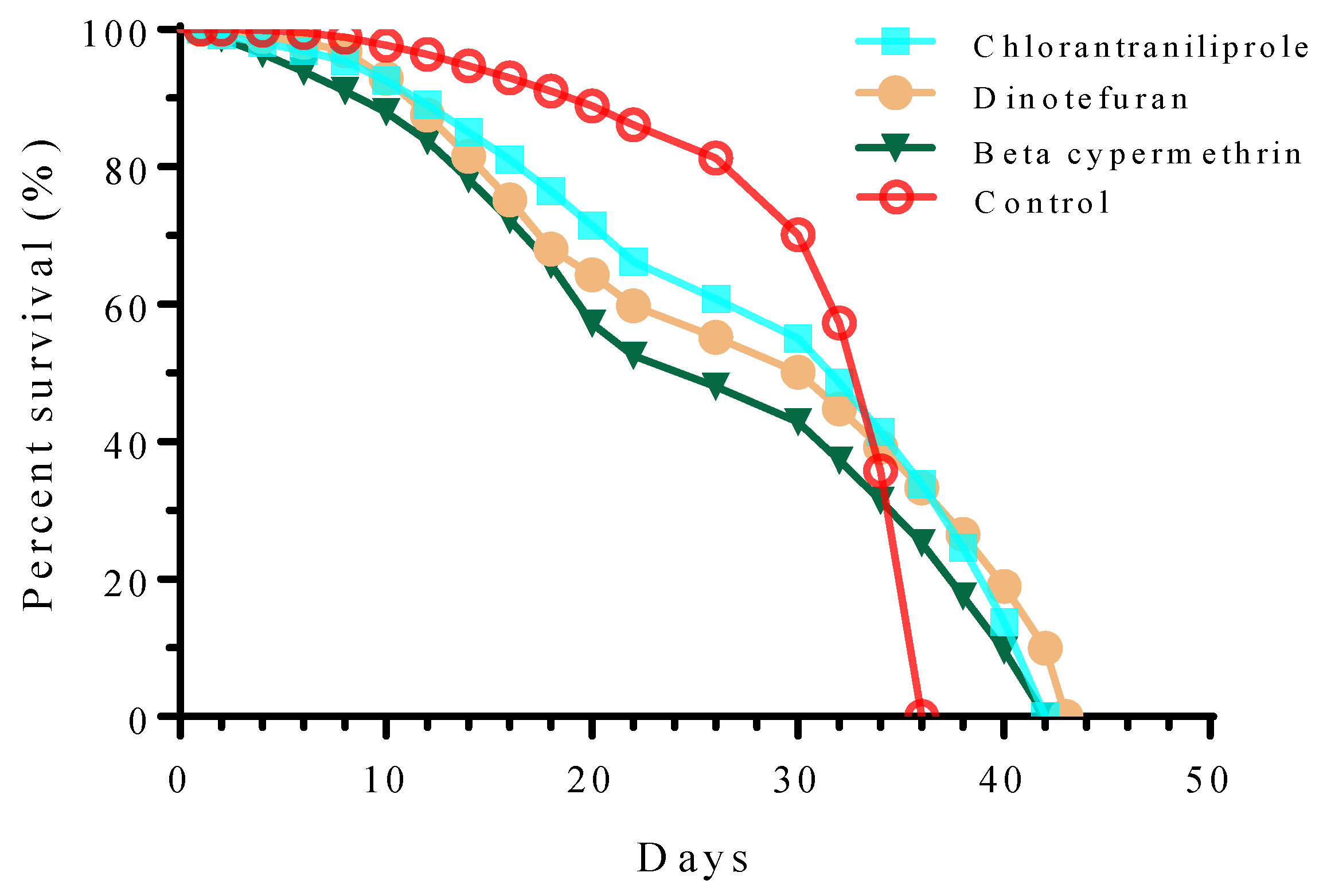
3.2. Effects of Sublethal Concentrations of Three Insecticides on the Mortality of S. frugiperda
3.3. Effects of Sublethal Concentrations of Three Insecticides on Population Growth and Development Duration of Male and Female Pupae Weight of S. frugiperda
3.4. Effects of Sublethal Concentrations of Three Insecticides on Population, Survival, and Fecundity of S. frugiperda
3.5. Effects of Sublethal Concentrations of Three Insecticides on Life Parameters of Adult Population of S. frugiperda
3.6. Sublethal Effect on F1 Generation of S. frugiperda
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamò, M. First Report of Outbreaks of the Fall Armyworm Spodoptera frugiperda (J. E. Smith) (Lepidoptera, Noctuidae), a New Alien Invasive Pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganiger, P.C.; Yeshwanth, H.M.; Muralimohan, K.; Vinay, N.; Kumar, A.R.V.; Chandrashekara, K. Occurrence of the New Invasive Pest, Fall Armyworm, Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae), in the Maize Fields of Karnataka, India. Curr. Sci. 2018, 115, 621. [Google Scholar] [CrossRef]
- Early, R.; González-Moreno, P.; Murphy, S.T.; Day, R. Forecasting the global extent of invasion of the cereal pest Spodoptera frugiperda, the fall armyworm. NeoBiota 2018, 40, 25–50. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Qi, G.; Chen, H.; Ma, J.; Liu, J.; Jiang, Y.; Lee, G.; Otuka, A.; Hu, G. Overseas immigration of fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), invading Korea and Japan in 2019. Insect Sci. 2021, 29, 505–520. [Google Scholar] [CrossRef]
- Kuate, A.F.; Hanna, R.; Fotio, A.R.P.D.; Abang, A.F.; Nanga, S.N.; Ngatat, S.; Tindo, M.; Masso, C.; Ndemah, R.; Suh, C.; et al. Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) in Cameroon: Case study on its distribution, damage, pesticide use, genetic differentiation and host plants. PLoS ONE 2019, 14, e0215749. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Jin, M.H.; Zhang, D.D.; Jiang, Y.Y.; Liu, J.; Wu, K.M.; Xiao, Y.T. Molecular identification of invasive fall armyworm Spodoptera frugiperda in Yunnan Province. Plant Prot. 2019, 45, 19–24+56. (In Chinese) [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, Y.; Liu, J.; Hu, G.; Wu, K. Trajectory modeling revealed a southwest-northeast migration corridor for fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae) emerging from the North China Plain. Insect Sci. 2021, 28, 649–661. [Google Scholar] [CrossRef]
- Wu, Q.-L.; He, L.-M.; Shen, X.-J.; Jiang, Y.-Y.; Liu, J.; Hu, G.; Wu, K.-M. Estimation of the Potential Infestation Area of Newly-invaded Fall Armyworm Spodoptera Frugiperda in the Yangtze River Valley of China. Insects 2019, 10, 298. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Dong, Y.; Huang, W.; Ren, B.; Deng, Q.; Shi, Y.; Bai, J.; Ren, Y.; Geng, Y.; Ma, H. Overwintering Distribution of Fall Armyworm (Spodoptera frugiperda) in Yunnan, China, and Influencing Environmental Factors. Insects 2020, 11, 805. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The Sublethal Effects of Pesticides on Beneficial Arthropods. Annu. Rev. Èntomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Guo, L.; Desneux, N.; Sonoda, S.; Liang, P.; Han, P.; Gao, X.-W. Sublethal and transgenerational effects of chlorantraniliprole on biological traits of the diamondback moth, Plutella xylostella L. Crop Prot. 2013, 48, 29–34. [Google Scholar] [CrossRef]
- Yusoff, N.; Ghani, I.A.; Othman, N.; Aizat, W.; Hassan, M. Toxicity and Sublethal Effect of Farnesyl Acetate on Diamondback Moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae). Insects 2021, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Xia, X. Sublethal effects of methylthio-diafenthiuron on the life table parameters and enzymatic properties of the diamondback moth, Plutella xylostella (L.) (Lepidoptera: Plutellidae). Pestic. Biochem. Physiol. 2020, 162, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.; Su, J. Effects of chlorantraniliprole on development and reproduction of beet armyworm, Spodoptera exigua (Hübner). J. Pest Sci. 2011, 84, 381–386. [Google Scholar] [CrossRef]
- Yu, H.L.; Xiang, X.; Yuan, G.X.; Chen, Y.Q.; Wang, X.G. Effects of sublethal doses of cyantraniliprole on the growth and development and the activities of detoxifying enzymes in Spodoptera exigua (Lepidoptera: Noctuidae). Acta Entomol. Sin. 2015, 58, 634–641. (In Chinese) [Google Scholar] [CrossRef]
- Han, W.; Zhang, S.; Shen, F.; Liu, M.; Ren, C.; Gao, X. Residual toxicity and sublethal effects of chlorantraniliprole on Plutella xylostella (Lepidoptera: Plutellidae). Pest Manag. Sci. 2012, 68, 1184–1190. [Google Scholar] [CrossRef]
- Zhang, R.-M.; Dong, J.-F.; Chen, J.-H.; Ji, Q.-E.; Cui, J.-J. The Sublethal Effects of Chlorantraniliprole on Helicoverpa armigera (Lepidoptera: Noctuidae). J. Integr. Agric. 2013, 12, 457–466. [Google Scholar] [CrossRef]
- Batuxi; Zhang, Z.; Kou, S.; Li, X.R.; Zhang, A.H.; Zhang, Y.H. Impacts of seed coating of thiamethoxam on life parameters of fall armyworm Spodoptera frugiperda (Lepidoptera: Noctuidae). Plant Prot. 2020, 47, 891–899. (In Chinese) [Google Scholar] [CrossRef]
- Gao, Z.; Chen, Y.; He, K.; Guo, J.; Wang, Z. Sublethal Effects of the Microbial-Derived Insecticide Spinetoram on the Growth and Fecundity of the Fall Armyworm (Lepidoptera: Noctuidae). J. Econ. Èntomol. 2021, 114, 1582–1587. [Google Scholar] [CrossRef]
- Saber, M.; Parsaeyan, E.; Vojoudi, S.; Bagheri, M.; Mehrvar, A.; Kamita, S.G. Acute toxicity and sublethal effects of methoxyfenozide and thiodicarb on survival, development and reproduction of Helicoverpa armigera (Lepidoptera: Noctuidae). Crop Prot. 2013, 43, 14–17. [Google Scholar] [CrossRef]
- Zhou, C.; Yang, X.-B.; Yang, H.; Long, G.-Y.; Jin, D.-C. Effects of Sublethal Concentrations of Insecticides on the Fecundity of Sogatella furcifera (Hemiptera: Delphacidae) via the Regulation of Vitellogenin and Its Receptor. J. Insect Sci. 2020, 20, 14. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Potter, M.F.; Haynes, K.F. Behavioral Responses of the Bed Bug to Insecticide Residues. J. Med Èntomol. 2009, 46, 51–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, T.; Heckel, D.; Wu, Y.; Downes, S.; Gordon, K.; Oakeshott, J. Determinants of Insecticide Resistance Evolution: Comparative Analysis Among Heliothines. Annu. Rev. Èntomol. 2022, 67, 387–406. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yang, H.; Li, J.; Wang, C.; Li, C.; Gao, Y. Sublethal Effects of Imidacloprid on the Population Development of Western Flower Thrips Frankliniella occidentalis (Thysanoptera: Thripidae). Insects 2019, 10, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, X.; He, Y.; Wu, J.; Tang, Y.; Gu, J.; Ding, W.; Zhang, Y. Sublethal Effects of Cyantraniliprole and Imidacloprid on Feeding Behavior and Life Table Parameters of Myzus persicae (Hemiptera: Aphididae). J. Econ. Èntomol. 2016, 109, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Cutler, G.C.; Ramanaidu, K.; Astatkie, T.; Isman, M.B. Green peach aphid, Myzus persicae (Hemiptera: Aphididae), reproduction during exposure to sublethal concentrations of imidacloprid and azadirachtin. Pest Manag. Sci. 2009, 65, 205–209. [Google Scholar] [CrossRef]
- Qu, Y.; Xiao, D.; Liu, J.; Chen, Z.; Song, L.; Desneux, N.; Benelli, G.; Gao, X.; Song, D. Sublethal and hormesis effects of beta-cypermethrin on the biology, life table parameters and reproductive potential of soybean aphid Aphis glycines. Ecotoxicology 2017, 26, 1002–1009. [Google Scholar] [CrossRef]
- Tang, Q.; Xiang, M.; Hu, H.; An, C.; Gao, X. Evaluation of Sublethal Effects of Sulfoxaflor on the Green Peach Aphid (Hemiptera: Aphididae) Using Life Table Parameters. J. Econ. Èntomol. 2015, 108, 2720–2728. [Google Scholar] [CrossRef]
- Wang, L.-P.; Shen, J.; Ge, L.-Q.; Wu, J.-C.; Yang, G.-Q.; Jahn, G.C. Insecticide-induced increase in the protein content of male accessory glands and its effect on the fecundity of females in the brown planthopper Nilaparvata lugens Stål (Hemiptera: Delphacidae). Crop Prot. 2010, 29, 1280–1285. [Google Scholar] [CrossRef]
- Wakita, T. Molecular Design of Dinotefuran with Unique Insecticidal Properties. J. Agric. Food Chem. 2010, 59, 2938–2942. [Google Scholar] [CrossRef]
- Troczka, B.; Zimmer, C.T.; Elias, J.; Schorn, C.; Bass, C.; Davies, T.G.E.; Field, L.; Williamson, M.S.; Slater, R.; Nauen, R. Resistance to diamide insecticides in diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae) is associated with a mutation in the membrane-spanning domain of the ryanodine receptor. Insect Biochem. Mol. Biol. 2012, 42, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Cordova, D.; Benner, E.; Sacher, M.; Rauh, J.; Sopa, J.; Lahm, G.; Selby, T.; Stevenson, T.; Flexner, L.; Gutteridge, S.; et al. Anthranilic diamides: A new class of insecticides with a novel mode of action, ryanodine receptor activation. Pestic. Biochem. Physiol. 2006, 84, 196–214. [Google Scholar] [CrossRef]
- Bantz, A.; Camon, J.; Froger, J.-A.; Goven, D.; Raymond, V. Exposure to sublethal doses of insecticide and their effects on insects at cellular and physiological levels. Curr. Opin. Insect Sci. 2018, 30, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Guedes, R.N.C.; Walse, S.S.; Throne, J.E. Sublethal exposure, insecticide resistance, and community stress. Curr. Opin. Insect Sci. 2017, 21, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Lutz, A.L.; Bertolaccini, I.; Scotta, R.R.; Curis, M.C.; Favaro, M.A.; Fernandez, L.N.; Sánchez, D.E. Lethal and sublethal effects of chlorantraniliprole on Spodoptera cosmioides (Lepidoptera: Noctuidae). Pest Manag. Sci. 2018, 74, 2817–2821. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, Z.; Cui, K.; Zhao, Y.; Han, J.; Liu, F.; Mu, W. Effects of Sublethal Concentrations of Cyantraniliprole on the Development, Fecundity and Nutritional Physiology of the Black Cutworm Agrotis ipsilon (Lepidoptera: Noctuidae). PLoS ONE 2016, 11, e0156555. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Lu, Z.; Li, M.; Mao, T.; Wang, H.; Li, F.; Sun, H.; Dai, M.; Ye, W.; Li, B. The mechanism of sublethal chlorantraniliprole exposure causing silkworm pupation metamorphosis defects. Pest Manag. Sci. 2020, 76, 2838–2845. [Google Scholar] [CrossRef]
- Huang, L.; Lu, M.; Han, G.; Du, Y.; Wang, J. Sublethal effects of chlorantraniliprole on development, reproduction and vitellogenin gene (CsVg) expression in the rice stem borer, Chilo suppressalis. Pest Manag. Sci. 2016, 72, 2280–2286. [Google Scholar] [CrossRef]
- Shang, J.; Yao, Y.-S.; Chen, L.-L.; Zhu, X.-Z.; Niu, L.; Gao, X.-K.; Luo, J.-Y.; Ji, J.-C.; Cui, J.-J. Sublethal Exposure to Deltamethrin Stimulates Reproduction and Alters Symbiotic Bacteria in Aphis gossypii. J. Agric. Food Chem. 2021, 69, 15097–15107. [Google Scholar] [CrossRef]
- Deng, D.; Duan, W.; Wang, H.; Zhang, K.; Guo, J.; Yuan, L.; Wang, L.; Wu, S. Assessment of the effects of lethal and sublethal exposure to dinotefuran on the wheat aphid Rhopalosiphum padi (Linnaeus). Ecotoxicology 2019, 28, 825–833. [Google Scholar] [CrossRef]
- Bao, H.; Liu, S.; Gu, J.; Wang, X.; Liang, X.; Liu, Z. Sublethal effects of four insecticides on the reproduction and wing formation of brown planthopper, Nilaparvata lugens. Pest Manag. Sci. 2009, 65, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Dong, S.; Li, C.; Li, L.; Yu, Y.; Men, X.; Yin, S. Sublethal and transgenerational effects of dinotefuran on biological parameters and behavioural traits of the mirid bug Apolygus lucorum. Sci. Rep. 2020, 10, 226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qu, Y.; Ullah, F.; Luo, C.; Monticelli, L.S.; Lavoir, A.-V.; Gao, X.; Song, D.; Desneux, N. Sublethal effects of beta-cypermethrin modulate interspecific interactions between specialist and generalist aphid species on soybean. Ecotoxicol. Environ. Saf. 2020, 206, 111302. [Google Scholar] [CrossRef]
- Quan, L.-F.; Qiu, G.-S.; Zhang, H.-J.; Sun, L.-N.; Li, Y.-Y.; Yan, W.-T. Sublethal Concentration of Beta-Cypermethrin Influences Fecundity and Mating Behavior of Carposina sasakii (Lepidoptera: Carposinidae) Adults. J. Econ. Èntomol. 2016, 109, 2196–2204. [Google Scholar] [CrossRef] [PubMed]
| Treatments | N | Regression Equation | LC30 (μg/g) | 95% Confidence Interval | χ2 |
|---|---|---|---|---|---|
| chlorantraniliprole | 270 | y = 0.5 + 0.91x | 0.456 | 0.162–0.970 | 18.95 |
| dinotefuran | 270 | y = 0.25 + 0.43x | 0.250 | 0.084–0.525 | 4.67 |
| beta-cypermethrin | 270 | y = 0.29 + 1.32x | 0.433 | 0.131–0.848 | 20.12 |
| Treatments | Larval Calendar/d | Pupae Calendar/d | Adults Longevity/d | Pupa Weight (mg) | |||
|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | ||
| control | 19.47 ± 0.45 c | 10.00 ± 0.17 ab | 10.44 ± 0.18 ab | 10.30 ± 0.38 a | 9.11 ± 0.43 ab | 181.38 ± 7.70 a | 197.06 ± 7.41 a |
| chlorantraniliprole | 24.38 ± 0.43 a | 10.11 ± 0.35 ab | 10.14 ± 0.21 b | 10.22 ± 0.39 a | 10.71 ± 0.37 a | 201.35 ± 11.02 a | 205.40 ± 15.91 a |
| dinotefuran | 23.80 ± 0.42 ab | 9.25 ± 0.16 b | 10.33 ± 0.17 ab | 10.22 ± 0.44 a | 9.87 ± 0.57 a | 192.44 ± 13.49 a | 167.25 ± 12.51 a |
| beta-cypermethrin | 21.68 ± 0.31 b | 10.29 ± 0.52 a | 11.00 ± 0.34 a | 9.70 ± 0.40 a | 8.13 ± 0.41 b | 181.11 ± 16.42 a | 185.00 ± 23.20 a |
| Treatments | Pupation Rate (%) | Emergence Rate (%) | Female Ratio (%) | No. of Eggs per Female | Hatching Rate (%) |
|---|---|---|---|---|---|
| control | 71.33 ± 5.21 a | 88.67 ± 0.84 a | 58.64 ± 0.72 a | 506.18 ± 62.55 a | 96.33 ± 3.67 a |
| chlorantraniliprole | 34.67 ± 2.67 b | 79.58 ± 7.23 a | 56.36 ± 4.68 a | 165.36 ± 30.41 b | 81.33 ± 7.31 b |
| dinotefuran | 28.00 ± 3.06 b | 78.09 ± 2.20 a | 54.85 ± 2.89 a | 579.67 ± 71.72 a | 98.67 ± 1.33 a |
| beta-cypermethrin | 36.67 ± 2.91 b | 75.46 ± 6.23 a | 60.66 ± 1.29 a | 288.22 ± 46.55 b | 98.00 ± 2.00 a |
| Treatments | Intrinsic Growth Rate (rm) | Net Reproductive Rate (R0) | Average Generation Period (T) | Finite Rate of Increase (λ) | Population Doubling Time (Dt) |
|---|---|---|---|---|---|
| control | 1.26 ± 0.10 a | 209.17 ± 12.55 a | 4.30 ± 0.29 b | 3.55 ± 0.38 a | 0.54 ± 0.04 b |
| chlorantraniliprole | 0.65 ± 0.09 c | 77.65 ± 21.94 b | 6.61 ± 0.42 a | 1.94 ± 0.17 c | 1.14 ± 0.15 a |
| dinotefuran | 1.04 ± 0.04 ab | 210.48 ± 25.46 a | 5.15 ± 0.30 b | 2.84 ± 0.13 ab | 0.66 ± 0.04 b |
| beta-cypermethrin | 0.89 ± 0.08 bc | 73.17 ± 14.36 b | 4.82 ± 0.21 b | 2.45 ± 0.18 bc | 0.82 ± 0.09 b |
| Treatments | Larval Calendar/d | Pupae Calendar/d | Adults Longevity/d | Pupa Weight (mg) | |||
|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | ||
| control | 17.08 ± 0.08 c | 10.14 ± 0.10 b | 10.64 ± 0.15 ab | 9.53 ± 0.62 a | 8.94 ± 0.42 a | 195.41 ± 5.59 a | 179.60 ± 5.89 b |
| chlorantraniliprole | 18.12 ± 0.092 b | 10.60 ± 0.11 ab | 10.61 ± 0.15 ab | 10.00 ± 0.51 a | 8.35 ± 0.52 a | 209.18 ± 5.91 a | 211.48 ± 8.10 a |
| dinotefuran | 17.92 ± 0.073 b | 10.22 ± 0.13 b | 10.17 ± 0.17 b | 9.29 ± 0.54 a | 8.71 ± 0.54 a | 179.86 ± 7.97 a | 190.32 ± 4.57 ab |
| beta-cypermethrin | 18.55 ± 0.098 a | 10.46 ± 0.14 a | 10.94 ± 0.16 a | 9.41 ± 0.41 a | 8.47 ± 0.69 a | 130.60 ± 4.58 b | 140.00 ± 12.48 c |
| Treatments | Pupation Rate (%) | Emergence Rate (%) | Female Ratio (%) | No. of Eggs per Female | Hatching Rate (%) |
|---|---|---|---|---|---|
| control | 76.7 ± 5.36 a | 92.11 ± 1.80 a | 51.62 ± 4.07 a | 465.33 ± 59.54 ab | 97.00 ± 3.00 a |
| chlorantraniliprole | 67.22 ± 6.41 a | 93.08 ± 2.91 a | 55.73 ± 11.4 a | 335.08 ± 28.32 b | 79.33 ± 13.12 a |
| dinotefuran | 79.45 ± 4.01 a | 89.87 ± 3.57 a | 46.67 ± 3.33 a | 572.43 ± 71.17 a | 96.00 ± 6.93 a |
| beta-cypermethrin | 66.11 ± 6.26 a | 85.49 ± 1.94 a | 53.58 ± 3.69 a | 364.36 ± 26.47 b | 97.33 ± 1.45 a |
| Treatments | Intrinsic Growth Rate (rm) | Net Reproductive Rate (R0) | Average Generation Period (T) | Finite Rate of Increase (λ) | Population-Doubling Time (Dt) |
|---|---|---|---|---|---|
| control | 1.01 ± 0.10 a | 164.76 ± 13.87 a | 5.11 ± 0.44 a | 2.79 ± 0.29 a | 0.70 ± 0.07 a |
| chlorantraniliprole | 1.06 ± 0.05 a | 113.00 ± 15.80 a | 4.46 ± 0.15 ab | 2.89 ± 0.15 a | 0.64 ± 0.05 a |
| dinotefuran | 0.95 ± 0.14 a | 158.55 ± 39.78 a | 4.59 ± 0.17 ab | 2.63 ± 0.33 a | 0.77 ± 0.16 a |
| beta-cypermethrin | 1.23 ± 0.05 a | 141.73 ± 8.90 a | 4.05 ± 0.18 b | 3.42 ± 0.17 a | 0.49 ± 0.04 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.-M.; Feng, H.-L.; Wang, G.-D.; Zhang, L.-L.; Zulu, L.; Liu, Y.-H.; Zheng, Y.-L.; Rao, Q. Sublethal Effects of Three Insecticides on Development and Reproduction of Spodoptera frugiperda (Lepidoptera: Noctuidae). Agronomy 2022, 12, 1334. https://doi.org/10.3390/agronomy12061334
Wu H-M, Feng H-L, Wang G-D, Zhang L-L, Zulu L, Liu Y-H, Zheng Y-L, Rao Q. Sublethal Effects of Three Insecticides on Development and Reproduction of Spodoptera frugiperda (Lepidoptera: Noctuidae). Agronomy. 2022; 12(6):1334. https://doi.org/10.3390/agronomy12061334
Chicago/Turabian StyleWu, Hui-Ming, Hang-Li Feng, Guo-Di Wang, Li-Li Zhang, Lovemore Zulu, Ya-Hui Liu, Yong-Li Zheng, and Qiong Rao. 2022. "Sublethal Effects of Three Insecticides on Development and Reproduction of Spodoptera frugiperda (Lepidoptera: Noctuidae)" Agronomy 12, no. 6: 1334. https://doi.org/10.3390/agronomy12061334
APA StyleWu, H.-M., Feng, H.-L., Wang, G.-D., Zhang, L.-L., Zulu, L., Liu, Y.-H., Zheng, Y.-L., & Rao, Q. (2022). Sublethal Effects of Three Insecticides on Development and Reproduction of Spodoptera frugiperda (Lepidoptera: Noctuidae). Agronomy, 12(6), 1334. https://doi.org/10.3390/agronomy12061334







