Effects of Root Temperature and Cluster Position on Fruit Quality of Two Cocktail Tomato Cultivars
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Experimental Design and Root-Temperature Management
2.3. Harvest and Sample Preparation
2.4. Sugar Quantification
2.5. Carotenoids Determination
2.6. Analysis of Organic Acid
2.7. Determination of Carbon, Nitrogen, Sulfur, and Other Elements
2.8. Statistical Analysis
3. Results
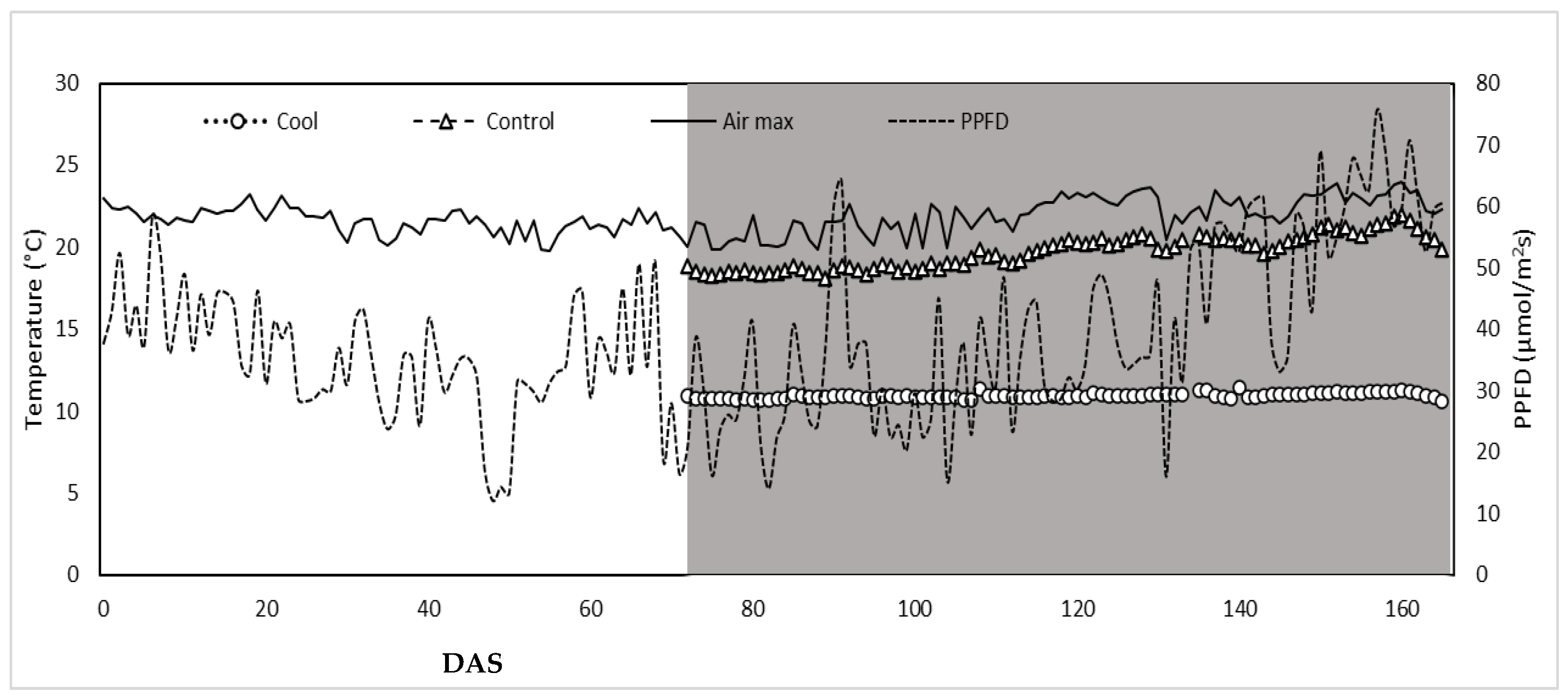
3.1. Climatic Parameters within Greenhouse
3.2. Plant Growth and Fruit Yield
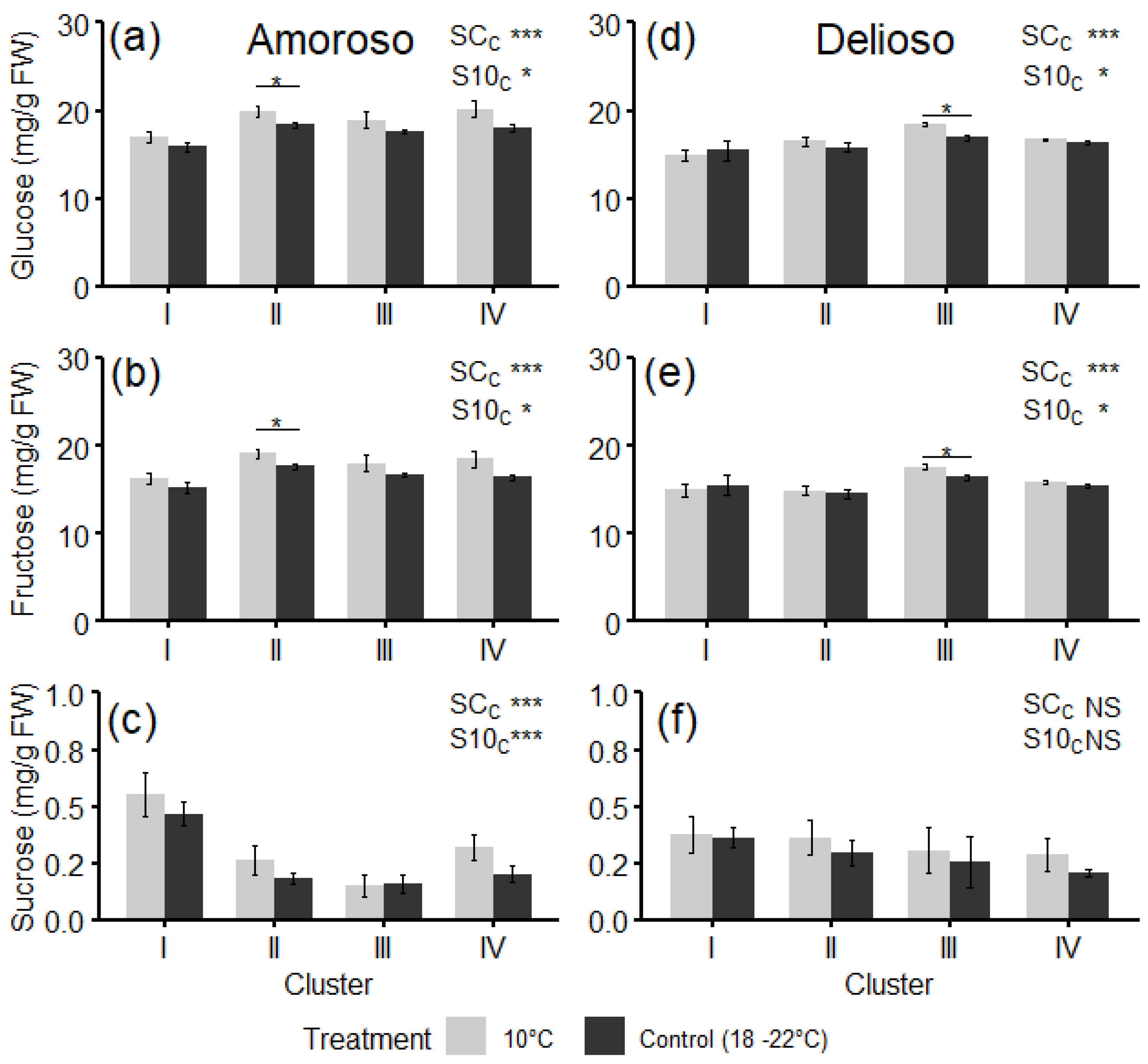
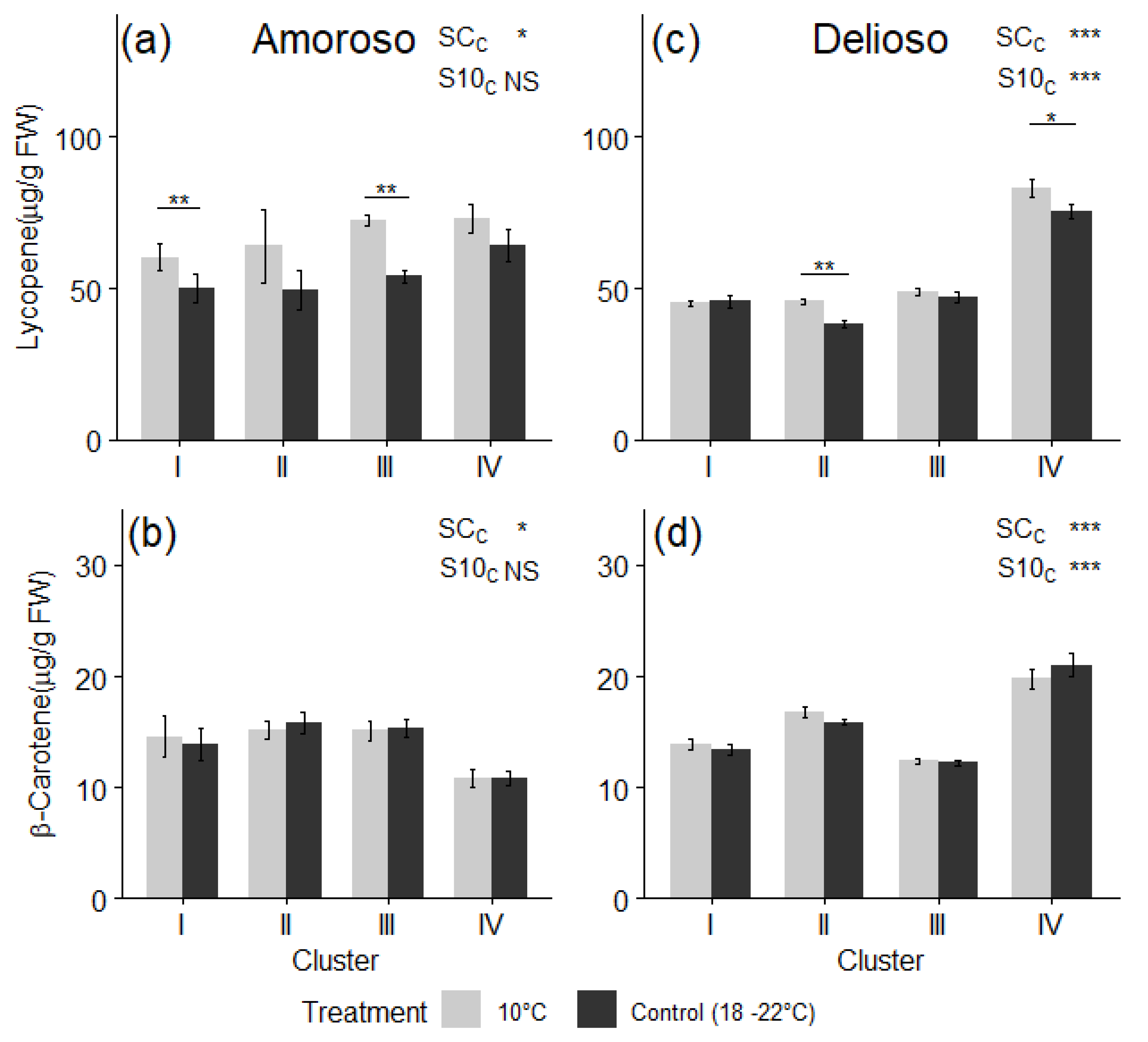
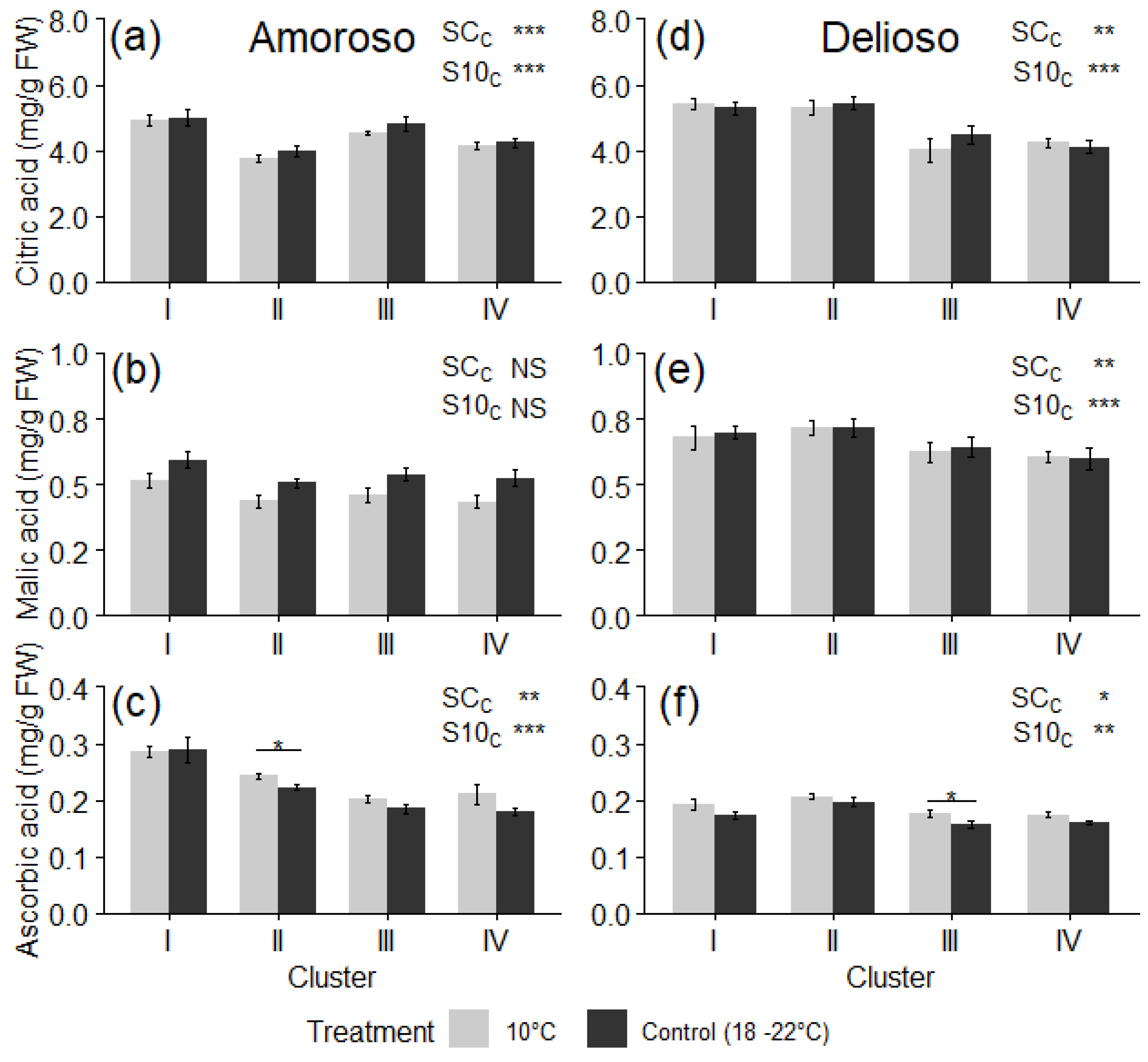
3.3. Bioactive Compounds
3.4. Minerals
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Perveen, R.; Suleria, H.A.R.; Anjum, F.M.; Butt, M.S.; Pasha, I.; Ahmad, S. Tomato (Solanum lycopersicum) Carotenoids and Lycopenes Chemistry; Metabolism, Absorption, Nutrition, and Allied Health claims—A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 919–929. [Google Scholar] [CrossRef]
- Anlar, H.G.; Bacanli, M. Lycopene as an antioxidant in human health and diseases. In Pathology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 247–254. [Google Scholar]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M.; et al. Tomato and Lycopene and Multiple Health Outcomes: Umbrella Review. Food Chem. 2021, 343, 128396. [Google Scholar] [CrossRef] [PubMed]
- Zushi, K.; Suehara, C.; Shirai, M. Effect of Light Intensity and Wavelengths on Ascorbic Acid Content and the Antioxidant System in Tomato Fruit Grown in Vitro. Sci. Hortic. 2020, 274, 109673. [Google Scholar] [CrossRef]
- Sainju, U.M.; Dris, R.; Singh, B. Mineral Nutrition of Tomato. Int. J. Food Agric. Environ. 2003, 1, 176–183. [Google Scholar]
- Islam, M.Z.; Lee, Y.-T.; Mele, M.A.; Choi, I.-L.; Kang, H.-M. The Effect of Phosphorus and Root Zone Temperature on Anthocyanin of Red Romaine Lettuce. Agronomy 2019, 9, 47. [Google Scholar] [CrossRef]
- Abreu, P.; Meneses, J.F.; Gary, C.; Tompousse, A. Model of Yield Prediction for Tomato Crops: Calibration Study for Unheated Plastic Greenhouses. Acta Hortic. 2000, 519, 141–150. [Google Scholar] [CrossRef]
- De Koning, A.N.M. Quantifying the Responses to Temperature of Different Plant Processes Involved in Growth and Development of Glasshouse Tomato. In Proceedings of the II IFAC/ISHS Workshop: Mathematical & Control Applications in Agriculture & Horticulture 406, Matsuyama, Japan, 30 September–3 October 1994; pp. 99–104. [Google Scholar] [CrossRef]
- He, F.; Thiele, B.; Kraus, D.; Bouteyine, S.; Watt, M.; Kraska, T.; Schurr, U.; Kuhn, A.J. Effects of Short-Term Root Cooling before Harvest on Yield and Food Quality of Chinese Broccoli (Brassica Oleracea Var. Alboglabra Bailey). Agronomy 2021, 11, 577. [Google Scholar] [CrossRef]
- Variyar, P.S.; Banerjee, A.; Akkarakaran, J.J.; Suprasanna, P. Chapter 12-Role of Glucosinolates in Plant Stress Tolerance. In Emerging Technologies and Management of Crop Stress Tolerance; Ahmad, P., Rasool, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 271–291. ISBN 9780128008768. [Google Scholar]
- Lam, V.P.; Kim, S.J.; Bok, G.J.; Lee, J.W.; Park, J.S. The Effects of Root Temperature on Growth, Physiology, and Accumulation of Bioactive Compounds of Agastache Rugosa. Agriculture 2020, 10, 162. [Google Scholar] [CrossRef]
- Chourak, Y.; Belarbi, E.H.; Martínez-Rivera, E.Y.; Loeiro da Cunha-Chiamolera, T.P.; Peña-Fernández, A.A.; Guil-Guerrero, J.L.; Urrestarazu, M. Fertigation Temperature Adjustment Enhances the Yield and Quality of Saffron Grown in a Soilless Culture System. HortScience 2021, 56, 1191–1194. [Google Scholar] [CrossRef]
- Sachs, R.M.; Sisto, I.; Jenkins, B.M.; Forister, G.W. Plant Response and Energy Savings for Bench-Top-Heated Greenhouses. Sci. Hortic. 1992, 49, 135–146. [Google Scholar] [CrossRef]
- Bertin, N. Competition for Assimilates and Fruit Position Affect Fruit Set in Indeterminate Greenhouse Tomato. Ann. Bot. 1995, 75, 55–65. [Google Scholar] [CrossRef]
- Caruso, G.; Gucci, R.; Sifola, M.I.; Selvaggini, R.; Urbani, S.; Esposto, S.; Taticchi, A.; Servili, M. Irrigation and Fruit Canopy Position Modify Oil Quality of Olive Trees (cv. Frantoio). J. Sci. Food Agric. 2017, 97, 3530–3539. [Google Scholar] [CrossRef] [PubMed]
- Alcobendas, R.; Mirás-Avalos, J.M.; Alarcón, J.J.; Pedrero, F.; Nicolás, E. Combined Effects of Irrigation, Crop Load and Fruit Position on Size, Color and Firmness of Fruits in an Extra-Early Cultivar of Peach. Sci. Hortic. 2012, 142, 128–135. [Google Scholar] [CrossRef]
- Tsormpatsidis, E.; Ordidge, M.; Henbest, R.G.C.; Wagstaffe, A.; Battey, N.H.; Hadley, P. Harvesting Fruit of Equivalent Chronological Age and Fruit Position Shows Individual Effects of UV Radiation on Aspects of the Strawberry Ripening Process. Environ. Exp. Bot. 2011, 74, 178–185. [Google Scholar] [CrossRef]
- Pék, Z.; Szuvandzsiev, P.; Nemenyi, A.; Helyes, L.; Lugasi, A. The Effect of Natural Light on Changes in Antioxidant Content and Color Parameters of Vine-Ripened Tomato (Solanum lycopersicum L.) Fruits. HortScience 2011, 46, 583–585. [Google Scholar] [CrossRef]
- Coyago-Cruz, E.; Corell, M.; Moriana, A.; Hernanz, D.; Stinco, C.M.; Meléndez-Martínez, A.J. Effect of the Fruit Position on the Cluster on Fruit Quality, Carotenoids, Phenolics and Sugars in Cherry Tomatoes (Solanum lycopersicum L.). Food Res. Int. 2017, 100, 804–813. [Google Scholar] [CrossRef]
- Trad, M.; Gaaliche, B.; Renard, C.M.G.C.; Mars, M. Inter- and Intra-Tree Variability in Quality of Figs. Influence of Altitude, Leaf Area and Fruit Position in the Canopy. Sci. Hortic. 2013, 162, 49–54. [Google Scholar] [CrossRef]
- Pék, Z.; Helyes, L. The Effect of Daily Temperature on Truss Flowering Rate of Tomato. J. Sci. Food Agric. 2004, 84, 1671–1674. [Google Scholar] [CrossRef]
- He, F.; Thiele, B.; Watt, M.; Kraska, T.; Ulbrich, A.; Kuhn, A.J. Effects of Root Cooling on Plant Growth and Fruit Quality of Cocktail Tomato during Two Consecutive Seasons. J. Food Qual. 2019, 2019, 3598172. [Google Scholar] [CrossRef]
- Wu, M.; Kubota, C. Effects of High Electrical Conductivity of Nutrient Solution and Its Application Timing on Lycopene, Chlorophyll and Sugar Concentrations of Hydroponic Tomatoes during Ripening. Sci. Hortic. 2008, 116, 122–129. [Google Scholar] [CrossRef]
- Viola, R.; Davies, H.V. A Microplate Reader Assay for Rapid Enzymatic Quantification of Sugars in Potato Tubers. Potato Res. 1992, 35, 55–58. [Google Scholar] [CrossRef]
- Murai-Hatano, M.; Kuwagata, T.; Sakurai, J.; Nonami, H.; Ahamed, A.; Nagasuga, K.; Matsunami, T.; Fukushi, K.; Maeshima, M.; Okada, M. Effect of Low Root Temperature on Hydraulic Conductivity of Rice Plants and the Possible Role of Aquaporins. Plant Cell Physiol. 2008, 49, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Landhäusser, S.M.; Zwiazek, J.J.; Lieffers, V.J. Root Water Flow and Growth of Aspen (Populus tremuloides) at Low Root Temperatures. Tree Physiol. 1999, 19, 879–884. [Google Scholar] [CrossRef]
- Ntatsi, G.; Savvas, D.; Huntenburg, K.; Druege, U.; Hincha, D.K.; Zuther, E.; Schwarz, D. A Study on ABA Involvement in the Response of Tomato to Suboptimal Root Temperature Using Reciprocal Grafts with Notabilis, a Null Mutant in the ABA-Biosynthesis Gene LeNCED1. Environ. Exp. Bot. 2014, 97, 11–21. [Google Scholar] [CrossRef]
- Hofmann, R.W.; Jahufer, M.Z.Z. Tradeoff between Biomass and Flavonoid Accumulation in White Clover Reflects Contrasting Plant Strategies. PLoS ONE 2011, 6, e18949. [Google Scholar] [CrossRef] [PubMed]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth–Defense Tradeoffs in Plants: A Balancing Act to Optimize Fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef]
- Fujimura, S.; Suzuki, K.; Nagao, M.; Okada, M. Acclimation to Root Chilling Increases Sugar Concentrations in Tomato (Solanum lycopersicum L.) Fruits. Sci. Hortic. 2012, 147, 34–41. [Google Scholar] [CrossRef]
- Marcelis, L.F.M. Fruit Shape in Cucumber as Influenced by Position within the Plant, Fruit Load and Temperature. Sci. Hortic. 1994, 56, 299–308. [Google Scholar] [CrossRef]
- Pannico, A.; Cirillo, C.; Giaccone, M.; Scognamiglio, P.; Romano, R.; Caporaso, N.; Sacchi, R.; Basile, B. Fruit Position within the Canopy Affects Kernel Lipid Composition of Hazelnuts. J. Sci. Food Agric. 2017, 97, 4790–4799. [Google Scholar] [CrossRef]
- Basile, B.; Solari, L.I.; Dejong, T.M. Intra-Canopy Variability of Fruit Growth Rate in Peach Trees Grafted on Rootstocks with Different Vigour-Control Capacity. J. Hortic. Sci. Biotechnol. 2007, 82, 243–256. [Google Scholar] [CrossRef]
- Farina, V.; Lo Bianco, R.; Inglese, P. Vertical Distribution of Crop Load and Fruit Quality within Vase- and Y-Shaped Canopies of ‘Elegant Lady’ Peach. HortScience 2005, 40, 587–591. [Google Scholar] [CrossRef]
- Akhtar, S.; Hazra, P. Others Sampling Technique for Optimum Worth of the Fruit Characters in Tomato (Solanum lycopersicum). Indian J. Agric. Sci. 2013, 83, 1179–1183. [Google Scholar]
- Gautier, H.; Rocci, A.; Buret, M.; Grasselly, D.; Causse, M. Fruit Load or Fruit Position Alters Response to Temperature and Subsequently Cherry Tomato Quality. J. Sci. Food Agric. 2005, 85, 1009–1016. [Google Scholar] [CrossRef]
- Klee, H.J.; Tieman, D.M. The Genetics of Fruit Flavour Preferences. Nat. Rev. Genet. 2018, 19, 347–356. [Google Scholar] [CrossRef]
- Patrick, J.W.; Botha, F.C.; Birch, R.G. Metabolic Engineering of Sugars and Simple Sugar Derivatives in Plants. Plant Biotechnol. J. 2013, 11, 142–156. [Google Scholar] [CrossRef]
- He, J.; Tan, L.P.; Lee, S.K. Root-Zone Temperature Effects on Photosynthesis, 14C-Photoassimilate Partitioning and Growth of Temperate Lettuce (Lactuca sativa Cv. “Panama”) in the Tropics. Photosynthetica 2009, 47, 95–103. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Effect of Root-Zone Temperature on Growth and Quality of Hydroponically Grown Red Leaf Lettuce (Lactuca sativa L. Cv. Red Wave). Am. J. Plant Sci. 2015, 6, 2350–2360. [Google Scholar] [CrossRef]
- Chadirin, Y.; Hidaka, K.; Takahashi, T.; Sago, Y.; Wajima, T.; Kitano, M. Application of Temperature Stress to Roots of Spinach I. Effect of the Low Temperature Stress on Quality. Environ. Control. Biol. 2011, 49, 133–139. [Google Scholar] [CrossRef][Green Version]
- He, F.; Thiele, B.; Santhiraraja-Abresch, S.; Watt, M.; Kraska, T.; Ulbrich, A.; Kuhn, A.J. Effects of Root Temperature on the Plant Growth and Food Quality of Chinese Broccoli (Brassica oleracea Var. Alboglabra bailey). Agronomy 2020, 10, 702. [Google Scholar] [CrossRef]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of Sugars under Abiotic Stress. Plant Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Yan, Q.-Y.; Duan, Z.-Q.; Mao, J.-D.; Li, X.; Dong, F. Low Root Zone Temperature Limits Nutrient Effects on Cucumber Seedling Growth and Induces Adversity Physiological Response. J. Integr. Agric. 2013, 12, 1450–1460. [Google Scholar] [CrossRef]
- Marconi, O.; Floridi, S.; Montanari, L. Organic Acids Profile in Tomato Juice by HPLC with UV Detection. J. Food Qual. 2007, 30, 43–56. [Google Scholar] [CrossRef]
- Anthon, G.E.; LeStrange, M.; Barrett, D.M. Changes in pH, Acids, Sugars and Other Quality Parameters during Extended Vine Holding of Ripe Processing Tomatoes. J. Sci. Food Agric. 2011, 91, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.V. Response to Selection and Associated Changes in Genetic Variance for Soluble Solids and Titratable Acids Contents in Strawberries. J. Am. Soc. Hortic. Sci. 1990, 115, 839–843. [Google Scholar] [CrossRef]
- Hidaka, K.; Yasutake, D.; Kitano, M.; Takahashi, T.; Sago, Y.; Ishikawa, K.; Kawano, T. Production of High Quality Vegetable by Applying Low Temperature Stress to Roots. In Proceedings of the International Symposium on High Technology for Greenhouse System Management: Greensys, Naples, Italy, 4 October 2007; pp. 1431–1436. [Google Scholar]
- Langi, P.; Kiokias, S.; Varzakas, T.; Proestos, C. Carotenoids: From Plants to Food and Feed Industries. Methods Mol. Biol. 2018, 1852, 57–71. [Google Scholar] [CrossRef]
- Martínez-Valverde, I.; Periago, M.J.; Provan, G.; Chesson, A. Phenolic Compounds, Lycopene and Antioxidant Activity in Commercial Varieties of Tomato (Lycopersicum esculentum): Phenolics, Lycopene and Antioxidant Activity in Tomatoes. J. Sci. Food Agric. 2002, 82, 323–330. [Google Scholar] [CrossRef]
- Thompson, K.A.; Marshall, M.R.; Sims, C.A.; Wei, C.I.; Sargent, S.A.; Scott, J.W. Cultivar, Maturity, and Heat Treatment on Lycopene Content in Tomatoes. J. Food Sci. 2000, 65, 791–795. [Google Scholar] [CrossRef]
- Zanfini, A.; Franchi, G.G.; Massarelli, P.; Corbini, G.; Dreassi, E. Phenolic Compounds, Carotenoids and Antioxidant Activity in Five Tomato (Lycopesicon esculentum Mill.) Cultivars. Ital. J. Food Sci. 2017, 29, 90–100. [Google Scholar] [CrossRef]
- Brandt, S.; Lugasi, A.; Barna, É.; Hóvári, J.; Pék, Z.; Helyes, L. Effects of the Growing Methods and Conditions on the Lycopene Content of Tomato Fruits. Acta Aliment. 2003, 32, 269–278. [Google Scholar] [CrossRef]
- Roselló, S.; Adalid, A.M.; Cebolla-Cornejo, J.; Nuez, F. Evaluation of the Genotype, Environment and Their Interaction on Carotenoid and Ascorbic Acid Accumulation in Tomato Germplasm. J. Sci. Food Agric. 2011, 91, 1014–1021. [Google Scholar] [CrossRef]
- Ensminger, I.; Busch, F.; Huner, N.P.A. Photostasis and Cold Acclimation: Sensing Low Temperature through Photosynthesis. Physiol. Plant. 2006, 126, 28–44. [Google Scholar] [CrossRef]
- He, Y.; Yang, J.; Zhu, B.; Zhu, Z.-J. Low Root Zone Temperature Exacerbates the Ion Imbalance and Photosynthesis Inhibition and Induces Antioxidant Responses in Tomato Plants under Salinity. J. Integr. Agric. 2014, 13, 89–99. [Google Scholar] [CrossRef]
- Sakamoto, M.; Suzuki, T. Elevated Root-Zone Temperature Modulates Growth and Quality of Hydroponically Grown Carrots. Agric. Sci. China 2015, 6, 749. [Google Scholar] [CrossRef]
- Malik, S.; Andrade, S.A.L.; Sawaya, A.C.H.F.; Bottcher, A.; Mazzafera, P. Root-Zone Temperature Alters Alkaloid Synthesis and Accumulation in Catharanthus Roseus and Nicotiana Tabacum. Ind. Crops Prod. 2013, 49, 318–325. [Google Scholar] [CrossRef]
- Lewallen, K.S.; Marini, R.P. Relationship between Flesh Firmness and Ground Color in Peach as Influenced by Light and Canopy Position. J. Am. Soc. Hortic. Sci. 2003, 128, 163–170. [Google Scholar] [CrossRef]
- Forlani, M.; Basile, B.; Cirillo, C.; Iannini, C. Effects of Harvest Date and Fruit Position along with Tree Canopy on Peach Fruit Quality. Acta Hortic. 2002, 592, 459–466. [Google Scholar] [CrossRef]
- Fanasca, S.; Martino, A.; Heuvelink, E.; Stanghellini, C. Effect of Electrical Conductivity, Fruit Pruning, and Truss Position on Quality in Greenhouse Tomato Fruit. J. Hortic. Sci. Biotechnol. 2007, 82, 488–494. [Google Scholar] [CrossRef]
- Balliu, A.; Ibro, V. Influence of Different Levels of Potassium Fertilizers on Growth, Yield and Ascorbic Acid Content of Tomato Fruit Grown in Non-Heated Greenhouse. Acta Hortic. 2000, 579, 385–388. [Google Scholar] [CrossRef]
- Borghesi, E.; González-Miret, M.L.; Escudero-Gilete, M.L.; Malorgio, F.; Heredia, F.J.; Meléndez-Martínez, A.J. Effects of Salinity Stress on Carotenoids, Anthocyanins, and Color of Diverse Tomato Genotypes. J. Agric. Food Chem. 2011, 59, 11676–11682. [Google Scholar] [CrossRef]
- Poiroux-Gonord, F.; Bidel, L.P.R.; Fanciullino, A.-L.; Gautier, H.; Lauri-Lopez, F.; Urban, L. Health Benefits of Vitamins and Secondary Metabolites of Fruits and Vegetables and Prospects to Increase Their Concentrations by Agronomic Approaches. J. Agric. Food Chem. 2010, 58, 12065–12082. [Google Scholar] [CrossRef]
- Osorio, S.; Ruan, Y.-L.; Fernie, A.R. An Update on Source-to-Sink Carbon Partitioning in Tomato. Front. Plant Sci. 2014, 5, 516. [Google Scholar] [CrossRef] [PubMed]
- Pollock, C.J.; Eagles, C.F. Low Temperature and the Growth of Plants. Symp. Soc. Exp. Biol. 1988, 42, 157–180. [Google Scholar] [PubMed]
- Nagel, K.A.; Kastenholz, B.; Jahnke, S.; van Dusschoten, D.; Aach, T.; Mühlich, M.; Truhn, D.; Scharr, H.; Terjung, S.; Walter, A.; et al. Temperature Responses of Roots: Impact on Growth, Root System Architecture and Implications for Phenotyping. Funct. Plant Biol. 2009, 36, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Poiré, R.; Schneider, H.; Thorpe, M.R.; Kuhn, A.J.; Schurr, U.; Walter, A. Root Cooling Strongly Affects Diel Leaf Growth Dynamics, Water and Carbohydrate Relations in Ricinus Communis. Plant Cell Environ. 2010, 33, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Quadir, Q.F.; Watanabe, T.; Chen, Z.; Osaki, M.; Shinano, T. Ionomic Response of Lotus Japonicus to Different Root-Zone Temperatures. Soil Sci. Plant Nutr. 2011, 57, 221–232. [Google Scholar] [CrossRef]
- Clarke, S.J.; Lamont, K.J.; Pan, H.Y.; Barry, L.A.; Hall, A.; Rogiers, S.Y. Spring Root-Zone Temperature Regulates Root Growth, Nutrient Uptake and Shoot Growth Dynamics in Grapevines. Aust. J. Grape Wine Res. 2015, 21, 479–489. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, J.; Qu, Z.; Zou, D.; Sha, H.; Liu, H.; Sun, J.; Zheng, H.; Wang, J.; Yang, L.; et al. Effects of Low Water Temperature during Reproductive Growth on Photosynthetic Production and Nitrogen Accumulation in Rice. Field Crops Res. 2019, 242, 107587. [Google Scholar] [CrossRef]
- Füllner, K.; Temperton, V.M.; Rascher, U.; Jahnke, S.; Rist, R.; Schurr, U.; Kuhn, A.J. Vertical Gradient in Soil Temperature Stimulates Development and Increases Biomass Accumulation in Barley. Plant Cell Environ. 2012, 35, 884–892. [Google Scholar] [CrossRef]
- Ahn, S.-J.; Im, Y.-J.; Chung, G.-C.; Seong, K.-Y.; Cho, B.-H. Sensitivity of Plasma Membrane H+-ATPase of Cucumber Root System in Response to Low Root Temperature. Plant Cell Rep. 2000, 19, 831–835. [Google Scholar] [CrossRef]
- Feng, H.; Yan, M.; Fan, X.; Li, B.; Shen, Q.; Miller, A.J.; Xu, G. Spatial Expression and Regulation of Rice High-Affinity Nitrate Transporters by Nitrogen and Carbon Status. J. Exp. Bot. 2011, 62, 2319–2332. [Google Scholar] [CrossRef]
- George, H.L.; Davies, F.S.; Crane, J.H.; Schaffer, B. Root Temperature Effects on “Arkin” Carambola (Averrhoa carambola L.) Trees: I. Leaf Gas Exchange and Water Relations. Sci. Hortic. 2002, 96, 53–65. [Google Scholar] [CrossRef]
- Pettersson, S. Low Root Zone Temperature Effects on Net Mineral Nutrient Uptake and Distribution in Barley (Hordeum vulgare). J. Plant Physiol. 1995, 145, 459–464. [Google Scholar] [CrossRef]
- Aidoo, M.K.; Sherman, T.; Lazarovitch, N.; Fait, A.; Rachmilevitch, S. Physiology and Metabolism of Grafted Bell Pepper in Response to Low Root-Zone Temperature. Funct. Plant Biol. 2019, 46, 339–349. [Google Scholar] [CrossRef]
- Janská, A.; Marsík, P.; Zelenková, S.; Ovesná, J. Cold Stress and Acclimation-What Is Important for Metabolic Adjustment? Plant Biol. 2010, 12, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Thorp, T.G.; Ferguson, I.B.; Boyd, L.M.; Bornett, A.M. Fruiting Position, Mineral Concentration and Incidence of Physiological Pitting in “Hayward” Kiwifruit. J. Hortic. Sci. Biotechnol. 2003, 78, 512–517. [Google Scholar] [CrossRef]
- Cronje, P.J.R.; Barry, G.H.; Huysamer, M. Fruiting Position during Development of “Nules Clementine” Mandarin Affects the Concentration of K, Mg and Ca in the Flavedo. Sci. Hortic. 2011, 130, 829–837. [Google Scholar] [CrossRef]
| Treatment | Yield per Plant (g) | Marketable Yield per Plant (g) | Shoot Diameter (mm) | Shoot Height (cm) | |
|---|---|---|---|---|---|
| ‘Amoroso’ | Control | 1580 ± 153 | 1570 ± 152 | 17.7 ± 0.7 | 206.5 ± 13.0 |
| 10 °C | 1557 ± 169 | 1551 ± 173 | 17.4 ± 0.5 | 198.7 ± 15.6 | |
| ST b | NS | NS | NS | NS | |
| ‘Delioso’ | Control | 1705 ± 211 | 1672 ± 207 | 15.7 ± 0.4 | 213.1 ± 15.8 |
| 10 °C | 1699 ± 140 | 1669 ± 147 | 15.2 ± 0.5 | 220.6 ± 19.2 | |
| ST b | NS | NS | NS | NS |
| Cluster | FW (g) | DW (g) | WC (%) | Equ (mm) | Long (mm) | SSC (°Brix) | ||
|---|---|---|---|---|---|---|---|---|
| ‘Amoroso’ | I | Control | 354 ± 73.3 | 23.8 ± 9.01 | 93.4 ± 1.13 | 39.6 ± 1.85 | 33.6 ± 1.57 | 6.63 ± 0.58 |
| 10 °C | 345 ± 71.1 | 22.4 ± 6.66 | 93.6 ± 1.35 | 38.5 ± 2.09 | 32.8 ± 1.69 | 6.93 ± 0.32 | ||
| ST a | NS | NS | NS | NS | NS | NS | ||
| II | Control | 403 ± 66.0 | 27.8 ± 6.20 | 93.1 ± 0.82 | 40.9 ± 1.13 | 34.3 ± 1.21 | 6.53 ± 0.24 | |
| 10 °C | 415 ± 35.0 | 23.0 ± 2.27 | 94.5 ± 0.49 | 40.3 ± 0.88 | 34.2 ± 0.46 | 7.07 ± 0.50 | ||
| ST a | NS | NS | * | NS | NS | * | ||
| III | Control | 412 ± 55.2 | 27.8 ± 3.23 | 93.4 ± 0.31 | 41.3 ± 1.60 | 34.3 ± 1.22 | 6.44 ± 0.21 | |
| 10 °C | 410 ± 58.7 | 24.7 ± 5.38 | 93.8 ± 0.97 | 40.5 ± 2.10 | 34.0 ± 1.73 | 6.99 ± 0.76 | ||
| ST a | NS | NS | NS | NS | NS | NS | ||
| IV | Control | 400 ± 25.4 | 26.2 ± 2.94 | 93.5 ± 0.47 | 41.9 ± 1.16 | 35.1 ± 0.83 | 6.31 ± 0.42 | |
| 10 °C | 399 ± 78.9 | 25.1 ± 7.63 | 93.8 ± 1.00 | 41.1 ± 2.23 | 34.6 ± 1.84 | 6.96 ± 0.83 | ||
| ST a | NS | NS | NS | NS | NS | NS | ||
| SCC a | NS | NS | NS | * | NS | NS | ||
| S10C a | NS | NS | NS | * | NS | NS | ||
| ‘Delioso’ | I | Control | 353 ± 63.6 | 20.1 ± 3.72 | 94.3 ± 0.58 | 39.7 ± 1.2 | 32.3 ± 0.89 | 6.36 ± 0.32 |
| 10 °C | 350 ± 57.0 | 18.4 ± 1.93 | 94.6 ± 1.03 | 39.3 ± 1.51 | 32.2 ± 1.03 | 6.64 ± 0.29 | ||
| ST a | NS | NS | NS | NS | NS | * | ||
| II | Control | 428 ± 50.8 | 26.4 ± 2.59 | 93.8 ± 0.38 | 41.5 ± 0.92 | 33.1 ± 0.92 | 6.69 ± 0.25 | |
| 10 °C | 419 ± 42.2 | 25.1 ± 2.68 | 94.0 ± 0.38 | 41.9 ± 1.1 | 33.8 ± 1.09 | 6.83 ± 0.18 | ||
| ST a | NS | NS | NS | NS | NS | NS | ||
| III | Control | 434 ± 63.3 | 27.3 ± 4.39 | 93.7 ± 0.12 | 42.9 ± 1.59 | 34.0 ± 1.12 | 6.66 ± 0.32 | |
| 10 °C | 417 ± 41.7 | 25.6 ± 3.62 | 93.9 ± 0.57 | 41.9 ± 1.45 | 33.6 ± 1.41 | 6.79 ± 0.46 | ||
| ST a | NS | NS | NS | NS | NS | NS | ||
| IV | Control | 489 ± 64.1 | 28.5 ± 4.10 | 94.1 ± 0.69 | 43.3 ± 1.23 | 34.7 ± 1.05 | 6.40 ± 0.42 | |
| 10 °C | 513 ± 56.6 | 30.9 ± 4.38 | 94.0 ± 0.58 | 43.3 ± 1.65 | 34.5 ± 1.18 | 6.64 ± 0.47 | ||
| ST a | NS | NS | NS | NS | NS | NS | ||
| SCC a | *** | *** | NS | *** | *** | NS | ||
| S10C a | *** | *** | NS | *** | *** | NS |
| Cluster | C (%) | N (%) | P (%) | Na (mg/kg) | Mg (%) | K (%) | Ca (%) | Zn (mg/kg) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| ‘Amoroso’ | I | Control | 42.5 ± 0.58 | 2.05 ± 0.21 | 0.42 ± 0.05 | 127 ± 30.5 | 0.09 ± 0.02 | 3.49 ± 0.47 | 0.04 ± 0.01 | 17.5 ± 2.57 |
| 10 °C | 42.5 ± 0.55 | 2.05 ± 0.10 | 0.44 ± 0.04 | 135 ± 28.8 | 0.09 ± 0.01 | 3.60 ± 0.29 | 0.04 ± 0.01 | 17.7 ± 2.17 | ||
| ST a | NS | NS | NS | NS | NS | NS | NS | NS | ||
| II | Control | 42.3 ± 0.48 | 1.88 ± 0.15 | 0.38 ± 0.04 | 107 ± 16.4 | 0.07 ± 0.01 | 3.21 ± 0.27 | 0.03 ± 0.01 | 15.8 ± 2.21 | |
| 10 °C | 43.1 ± 0.29 | 1.99 ± 0.21 | 0.40 ± 0.06 | 114 ± 19.2 | 0.08 ± 0.01 | 3.33 ± 0.39 | 0.03 ± 0.01 | 15.3 ± 2.16 | ||
| ST a | * | NS | NS | NS | NS | NS | NS | NS | ||
| III | Control | 42.9 ± 0.32 | 1.93 ± 0.09 | 0.40 ± 0.03 | 123 ± 19.4 | 0.08 ± 0.01 | 3.56 ± 0.24 | 0.04 ± 0.01 | 18.4 ± 2.83 | |
| 10 °C | 42.6 ± 0.38 | 1.79 ± 0.30 | 0.38 ± 0.04 | 120 ± 10.6 | 0.08 ± 0.01 | 3.40 ± 0.37 | 0.04 ± 0.01 | 16.4 ± 3.82 | ||
| ST a | NS | NS | NS | NS | NS | NS | NS | NS | ||
| IV | Control | 42.3 ± 0.97 | 1.70 ± 0.27 | 0.36 ± 0.05 | 117 ± 17.7 | 0.08 ± 0.02 | 3.13 ± 0.43 | 0.05 ± 0.01 | 17.2 ± 2.78 | |
| 10 °C | 42.1 ± 0.97 | 1.68 ± 0.18 | 0.37 ± 0.05 | 109 ± 15.9 | 0.07 ± 0.01 | 3.02 ± 0.42 | 0.04 ± 0.01 | 16.1 ± 2.26 | ||
| ST a | NS | NS | NS | NS | NS | NS | NS | NS | ||
| SCC a | * | *** | NS | NS | NS | NS | * | NS | ||
| S10C a | NS | * | NS | NS | NS | NS | NS | NS | ||
| ‘Delioso’ | I | Control | 41.9 ± 0.51 | 1.82 ± 0.08 | 0.43 ± 0.02 | 152 ± 27.6 | 0.10 ± 0.01 | 4.06 ± 0.13 | 0.04 ± 0.01 | 17.1 ± 1.65 |
| 10 °C | 41.9 ± 0.53 | 1.91 ± 0.25 | 0.43 ± 0.03 | 150 ± 19.9 | 0.10 ± 0.01 | 4.05 ± 0.30 | 0.04 ± 0.01 | 14.0 ± 2.03 | ||
| ST a | NS | NS | NS | NS | NS | NS | NS | ** | ||
| II | Control | 42.2 ± 0.60 | 1.74 ± 0.11 | 0.35 ± 0.03 | 135 ± 22.9 | 0.08 ± 0.01 | 3.68 ± 0.24 | 0.03 ± 0.01 | 14.9 ± 2.23 | |
| 10 °C | 42.5 ± 0.54 | 1.80 ± 0.14 | 0.38 ± 0.03 | 134 ± 20.4 | 0.09 ± 0.01 | 3.31 ± 0.15 | 0.03 ± 0.01 | 14.0 ± 1.55 | ||
| ST a | NS | NS | NS | NS | NS | * | NS | NS | ||
| III | Control | 42.2 ± 0.69 | 1.66 ± 0.07 | 0.35 ± 0.02 | 134 ± 10.2 | 0.09 ± 0.01 | 3.47 ± 0.16 | 0.04 ± 0.01 | 16.3 ± 1.43 | |
| 10 °C | 42.1 ± 0.52 | 1.62 ± 0.15 | 0.35 ± 0.02 | 143 ± 13.0 | 0.09 ± 0.00 | 3.48 ± 0.10 | 0.04 ± 0.01 | 15.7 ± 1.68 | ||
| ST a | NS | NS | NS | NS | NS | NS | NS | NS | ||
| IV | Control | 42.3 ± 0.38 | 1.62 ± 0.12 | 0.34 ± 0.02 | 123 ± 12.9 | 0.08 ± 0.00 | 3.47 ± 0.24 | 0.04 ± 0.01 | 15.0 ± 2.35 | |
| 10 °C | 42.4 ± 0.21 | 1.57 ± 0.08 | 0.34 ± 0.01 | 133 ± 8.0 | 0.08 ± 0.00 | 3.15 ± 0.25 | 0.04 ± 0.01 | 15.6 ± 2.71 | ||
| ST a | NS | NS | NS | NS | NS | NS | NS | NS | ||
| SCC a | NS | ** | *** | NS | * | *** | NS | NS | ||
| S10C a | NS | *** | *** | NS | ** | *** | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, F.; Thiele, B.; Kraska, T.; Schurr, U.; Kuhn, A.J. Effects of Root Temperature and Cluster Position on Fruit Quality of Two Cocktail Tomato Cultivars. Agronomy 2022, 12, 1275. https://doi.org/10.3390/agronomy12061275
He F, Thiele B, Kraska T, Schurr U, Kuhn AJ. Effects of Root Temperature and Cluster Position on Fruit Quality of Two Cocktail Tomato Cultivars. Agronomy. 2022; 12(6):1275. https://doi.org/10.3390/agronomy12061275
Chicago/Turabian StyleHe, Fang, Björn Thiele, Thorsten Kraska, Ulrich Schurr, and Arnd Jürgen Kuhn. 2022. "Effects of Root Temperature and Cluster Position on Fruit Quality of Two Cocktail Tomato Cultivars" Agronomy 12, no. 6: 1275. https://doi.org/10.3390/agronomy12061275
APA StyleHe, F., Thiele, B., Kraska, T., Schurr, U., & Kuhn, A. J. (2022). Effects of Root Temperature and Cluster Position on Fruit Quality of Two Cocktail Tomato Cultivars. Agronomy, 12(6), 1275. https://doi.org/10.3390/agronomy12061275








