1. Introduction
Minerals play an essential role in the growth and development of plants and humans alike. In recent years, the correlation between well-nourished crops and balanced human nutrition is becoming more meaningful as we come to realize the effects of nutrient management in intensified agriculture on food composition. Increasing population and the need to ensure food security necessitate agriculture to become more efficient and sustainable [
1,
2]. Progress is achieved through improving agricultural practices, e.g., by explicitly addressing plant needs for nutrients by accounting for physiological and environmental conditions. In the process, the nutritional quality of the end product, namely food, should also be studied and taken into consideration to sustain human requirements and maintain fruit and vegetables as a rich source of minerals for population diet and health [
3,
4].
Minerals in the body possess a wide range of crucial roles in necessary functions, e.g., in bone health, central nervous system function, hormonal regulation, and cardiovascular performance. Many micro-elements (Cu, Fe, Mn, Mg, Se, and Zn) are cofactors of enzymes, in addition to having other vital functions in the formation of erythrocytes (Co, I, and Fe), glucose level regulation (Cr), and cell protection via the activation of antioxidant enzymes (Mo) [
5]. Macro elements participate in nerve cell function and in controlling blood pressure (Na, Ca, and K) [
5]. Minerals also have structural roles in bones and teeth (Ca, Mg, P, Mn, B, and F) and are involved in immune (Ca, Mg, Cu, Se, and Zn) and brain (Cr and Mn) functions [
5].
A large number of works have shown the effect of plant nutrition on crop mineral contents, reporting a wide array of responses to fertilization. Such responses include either no change in food mineral levels with increasing mineral nutrition, an increase or a decrease in minerals, or alternatively an optimum curve response [
6,
7]. The existence of various patterns implies the possibility to allocate more nutrients into edible plant parts, hence controlling food mineral levels and concomitantly its nutritional quality. Such biofortification can be achieved by manipulating fertilization regimes and designing plant nutrition protocols to optimize food composition [
5,
8]. Examples of the effect of plant nutrition on foodstuffs are available for essentially all elements and include field crops, fruit trees, grains, and oil crops, to name a few. At the same time, some reports might present contradictory data, potentially due to differences in experimental parameters, e.g., genetic background, cultivation conditions (including the interplay with other elements), and environmental effects, also impacting final outcomes.
Sulfur (
S) is the seventh most abundant element in the human body, supplied mainly by the essential amino acid methionine (Met), originating in plant and animal proteins. Met is required for protein synthesis, and deficiency will lead to malnutrition [
9]. Additionally,
S is a cofactor of some antioxidant enzymes, e.g., glutathione peroxidase, regulating oxidative stress. Sulfur deprivation might thus also lead to undesirable metabolic outcomes, e.g., inflammation. As animal products are a significant source of
S, maintaining the recommended
S consumption is critically vital in increasingly popular plant-based diets. In addition to Met,
S in plants is also involved in glucosinolate biosynthesis, a nutritionally significant plant secondary metabolite group in
Brassicaceae, possessing health-beneficial properties [
10]. Hence,
S has a significant impact on the nutritional quality of crops.
In recent years, crop
S deficiency has become a widespread phenomenon in agricultural land due to the rapid depletion of soil
S in many countries [
11,
12], resulting from reduced input combined with increased output of
S. The reduced input is caused by the efficient reduction in
S emissions into the atmosphere, leading to reduced deposition into agricultural fields [
13], as well as the use of highly concentrated fertilizers that do not contain
S, i.e., urea and triple superphosphate instead of ammonium-sulfate and superphosphate. The increased S outputs are the result of higher crop yields to meet the growing demand for food accompanied by higher removal of soil sulfur [
11]. Intensive greenhouse agriculture often employs substrates and sandy soils poor in S, which is thus mainly supplied by irrigation water. However, global water shortage, especially in arid and semi-arid climates, leads to rapid growth in using desalinated water for irrigating crops, where
S concentration is rather low, which might cause sulfur deficiencies in the crops.
S constitutes about 0.2%–0.5% of the total plant dry matter, therefore defined as a macro-nutrient [
14,
15,
16].
S is found in a number of amino acids (cysteine and methionine) and is essential for the synthesis of proteins [
10,
14,
15], thus in
S deficiencies, protein synthesis is disrupted [
14]. In addition,
S deficiencies also typically result in decreased leaf chlorophyll content [
14,
17], as well as reduced activities of some important plant enzymes, e.g., PEP-carboxylase, malate dehydrogenase, or glutamate synthase [
14,
18]. Furthermore,
S-deficient plants had increased concentrations of soluble inorganic nitrate with
S-free amino acid accumulation. Tomato
S deficiencies develop rapidly [
19], appearing as purple dots on the leaf blade and necrosis at the margins of mature leaves [
20]. The young leaves turn yellow and shrink upwards and later develop necrosis in all leaf parts [
21], and photosynthesis is impaired [
22].
In
S-sufficient plants,
S concentration increased when supplied with
S [
17]. Fruit-bearing tomato plants had decreased yield when sulfur concentration dropped from 5.2 mM to 0, but an increase in concentration from 5.2 to 20 mM had no effect on yield and quality, despite a decrease in magnesium, calcium, and phosphorus uptake [
23]. No difference in yield and development of tomato plants was observed when sulfur increased from 1.0 to 3.8 mM [
24]. Courbet et al. (2019) stated that
S deficiency mainly acts by reducing plant growth, which in turn restricts the uptake of
N, K, and Mg by the roots [
25]. Conversely, deficiencies in
N, K, or Mg reduce
S uptake. Interestingly, Batool et al. (2018) reported that the requirement for cysteine for the biosynthesis of abscisic acid and subsequent stomatal closure implicates sulfur nutrition and metabolism as crucial for plant water relations [
26]. Sulfate (SO
42−) is the only form of
S ion plants absorb through the root system [
14,
27]. The adsorbed Sulfate moves mainly in the xylem, whereas in most plants,
S translocation from the mature leaves to other organs through the phloem is very slow. Consequently, signs of sulfur deficiency are first observed in young leaves [
27,
28].
The negative effect of high nitrate supply on the uptake of other anions, mainly chloride, is well documented [
29,
30,
31,
32]. Additionally, sulfate uptake is greatly affected by plant
N status [
25], therefore we hypothesized that
N concentration in the solution should impact
S uptake by tomato plants and vice versa. However, information is lacking regarding sulfate/nitrate interaction in the
Solanum genus generally and specifically the tomato species.
Although
S deficiency effects on crop yield are well-reported, information about fruit quality implications is scarce. Some works reported that low
S levels resulted in low levels of
S-containing amino acids and subsequently reduced quality, e.g., in wheat grain [
15]. Sulfate potentially plays a significant role in oilseed crops, increasing seed yield and oil content in rapeseed, groundnut, sunflower, and soybean [
15]. In potato,
S fertilization affected free amino acid and sugar levels [
33], and in broccoli sprouts, a sulfate treatment enhanced glucosinolate levels and antioxidant capacity [
34].
Given the limited knowledge regarding the effect of S concentration and the ratio between S and nitrate on tomato fruit yield and quality, and even more so in Mediterranean climatic conditions, the primary purpose of the current work is to determine the level of S needed in desalinated irrigation water and its ratio to nitrate for optimal fruit yield and mineral composition of tomato grown in arid areas.
2. Materials and Methods
Experiment I—a field experiment was set up in a large semi-commercial net house situated in the Ramat Negev research station in the south of Israel (30°59′ N, 34°43′ E). Tomato plants (Solanum lycopersicum L., Var. Ikram; Zeraim Gedera, Israel) were received as seedlings (Hishtil Nurseries, Israel) and were transplanted into polystyrene containers (100 L × 50 W × 20 H cm; Polybid, Israel), filled with Perlite (Perlite #2; Agrekal Habonim Ind., Israel). Each plot included 3 containers and each container was populated with 5 plants in two rows with each row lined with a drip line (Netafim, Israel). The distance between the centers of two adjacent plots was 2.0 m.
Five treatments of sulfate (C
S) varied from 0.19 to 3.55 mmol/L with fixed
N concentration (C
N) at 3.6 mmol/L and three treatments of C
S varied from 0.19 to 3.55 mmol/L with fixed
N concentration (at 10.8 mmol/L) (
Table 1) were introduced via laterals with integral drippers of 1.6 L/h, 20 cm between drippers, one lateral for each row, 2 laterals for each container. The irrigation system of each treatment was fed from a separate 5000 l tank containing complete solution. Solutions were prepared by filling the tanks with desalinized water from a small scale on-site reverse osmosis plant (Argad, Israel) and later adding concentrated fertilizer solutions of NH
4NO
3, H
3PO
4, B(OH)
3 (Fertilizers & Chemicals LTD, Israel) and salts of KH
2PO
4, NaNO
3, KNO
3, Ca(NO
3)
2, CaSO
4, Mg(NO
3)
2, MgSO
4, K
2SO
4, CaCl
2, MgCl
2, KCl, NaCl from various suppliers to fulfill treatment specifications. The micro nutrients Fe, Mn, Zn, Cu and Mo were supplied as chelates of EDTA (Fertilizers & Chemicals LTD, Israel). All fertilizers were added one by one into the solution tank where the solution was mixed by a pump that circulated the water from the bottom to the top of the tank. The basic fertigation solution delivered the following elemental concentrations: 1.09 mmol/L P, 3.4 mmol/L K, 2.0 mmol/L Ca, 0.94 mmol/L Mg, 8.7 mmol/L Na, 17.9 µmol/L Fe, 9.1 µmol/L Mn, 3.8 µmol/L Zn, 0.57 µmol/L Cu, 0.28 µmol/L Mo and 27.7 µmol/L B. NH
4-
N was between 10% to 20% of the total
N in all treatments. All nutrients’ concentrations were the same in all treatments except
N,
S and Cl that were varied according to the treatments with fixed sum of the concentrations of nitrate, sulfate and chloride, 18.0 mmol/L (
Table 1). Complete solution pH was adjusted between 5.5 and 6.0 with 1 M NaHCO
3 solution.
Treatments were distributed according to a statistical design of randomized blocks and were repeated 6 times (blocks). Irrigation started on day of transplanting on 10 September 2009 and was applied 4 to 6 times daily depending on actual water consumption. Fertigation with the differential treatments initiated gradually five weeks after transplanting of the seedlings on 15 October 2009, the target treatments were set on 23 November 2009 and lasted throughout the tomato plant growth until termination of the experiment on 13 April 2010. To avoid salt accumulation within the container, the irrigation solution dose was adjusted to allow an excess of up to 40% of the water consumed by evapotranspiration.
Experiment II—a second experiment following the same methodology and the same treatments as the 1st experiment. The experiment was carried out starting with transplanting on 24 August 2011 and initiating the differential treatments on 11 September 2011 and the experiment lasted until 10 April 2012.
Measurements—Diagnostic leaves, defined as the fully developed youngest leaf, and fruits were sampled several times throughout plants’ growth, and the concentrations of nutrients in these leaves and fruits samples were determined. Sampling of whole plants was conducted in experiment II on 10 October 2011, with one plant per plot sampled. In each sampling date leaves were separated from the main stem and then split into two age groups according to location along the main stem, mature leaves and diagnostic leaves. All plant parts were weighed immediately for fresh matter, washed with distilled water, dried in a ventilated oven at 60 °C, weighed again for dry matter, and stored pending chemical analysis. Yield measurements took place throughout the season with ripe fruits that had reached 80% red color selectively harvested weekly and weighed. Mineral concentrations, and N, P, K, S, Ca, Mg, Na, Fe, Mn, Zn, Cu, Mo and B in the different plant parts was analyzed in the sampled leaves and in red ripe fruits. The dry tissue (DW) of plant organs was ground to pass a 20-mesh sieve.
To extract
N, P and K, a subsample (100 mg) of the powder was digested using sulfuric acid and peroxide, as previously described [
35].
N and P concentrations in water-diluted extractions were determined using a Quickchem 8000 Autoanalyzer (Lachat Instruments, Loveland, CO, USA) and the K concentration was determined by flame photometry (Sherwood M410, Sherwood Scientific Ltd., Cambridge, UK). To extract
S, Ca, Mg, Na, Fe, Mn, Zn, Cu, Mo and B a subsample (100 mg) of the powder was wet-digested with 2 mL of concentrated HNO
3 and oxidized with few drops of HClO
4, and their concentrations were determined in the diluted acid using inductively coupled plasma (ICP-ICAP 6500 DUO Thermo, Salford, UK).
Statistical analysis was done with JMP12 Software (SAS Institute Inc., Cary, NC, USA). Following the randomized complete block design, one-way analysis of variance (ANOVA) was carried out. The measured values of plant parts’ weight, their nutrient concentrations and the various yield parameters were analyzed by treatment and block with means comparison done for all pairs using the Tukey-Kramer HSD test. Differences with a probability larger than 95% were deemed significant. For leaf and fruit mineral concentrations a one-way ANOVA was carried out, comparing all eight treatment levels. As one-way ANOVA showed no interactions between the two main variables, a two-way ANOVA was completed using three S levels (0.1, 1 and 4 mM) and two N levels (4 and 11 mM), to determine the specific effects of each variable, differences with a probability larger than 95% were deemed significant.
3. Results
The current work aimed at evaluating the effect of S on tomato plant growth and yield, and tomato fruit nutritional quality, under low and high N levels using desalinized water. We intended to mimic commercial tomato cultivation in the south of Israel in real-life conditions. We focused on the nutritional value of tomato fruits, and specifically the mineral composition perspective, to determine whether, under such commercial conditions, S-fertilization will result in fruit S biofortification while retaining fruit nutritional quality, in addition to maintaining plant growth and yield.
3.1. Effects of S Fertilization on Plant Growth and Yield Parameters
S deficiency was evident in tomato plant leaves, as shown in
Figure 1. Tomato plant
S deficiency symptoms appeared on leaves of treatments 1 and 6 where
S concentrations were low (
Table 1), starting with mature leaf yellowing and followed by leaf blade purple dots and necrosis at the margins of mature leaves, as previously reported [
19,
20].
Plant growth parameters for season 2 are presented in
Table 2 and include biomass of dry leaves (51.8–66.0 g/plant), dry stem (19.0–21.7 g/plant), dry shoot (72.0–87.1 g/plant), dry fruits (66.1–83.2 g/plant) and total dry biomass (112.9–134.7 g/plant). These did not significantly change among treatments, indicating that under the current experimental conditions, plant growth was not affected by
S levels, under either low or high
N levels.
Table 2 also presents data regarding the total fresh fruit yield for seasons 1 and 2. In season 1, the yield was not affected by
S fertilization, under either low (4 mM) or high (11 mM)
N conditions and ranged from 9.1 to 10.2 kg/m
2. At the same time, in season 2, fresh fruit yield was not affected by
S treatments under low
N levels (20.7–22.9 kg/m
2), whereas under high
N conditions, 1 mM
S treatment had higher fruit yield compared to 4 mM
S, with 0.1 mM
S not significantly different from other treatments (25.4, 24.4 and 22.0 kg/m
2, respectively). Overall, yield in all treatments was high, and in spite of the 10.7% decrease in yield under low
N levels, these plants are considered high-yielding by commercial standards.
3.2. Diagnostic Leaf Mineral Concentrations
Table 3 presents our results for mineral concentrations in young (diagnostic) leaves in seasons 1 and 2. Our bivariate analysis (
Table 4) showed that leaf
N and
S levels were exclusively affected by their respective fertilization concentrations. This way, leaf
S levels were solely affected by
S fertilization, with no effect of the
N level, and significantly increased by 4.31-fold from 2.1 to 10.9 mg/g
S in low (0.1 mM) compared to high (4 mM)
S, respectively. Similarly, leaf
N levels were only affected by
N fertilization levels, with no effects observed by levels of
S fertilization, and increased in 14.33% from 30.5 to 34.8 mg/g in 4 and 11 mM
N, respectively.
We then used the bivariate model (
Table 4) to assess the
S fertilization effect on other leaf mineral concentrations when the model indicated that the
S fertilization effect was statistically significant.
S fertilization levels increased K (14.2 and 19.5 mg/g at 0.1 and 4 mM
S, respectively), Na (4.2 and 7 mg/g), Cl (11.4 and 16.9 mg/g), and Cu (6.20 and 8.10 mg/kg) leaf levels, while negatively affecting B (58.30 and 51.35 mg/kg), Ca (26.0 and 25.7 mg/g), and Mo (4 to 1.4 mg/kg) concentrations in leaves (
Table 3; mineral concentration values result from the bivariate model, and thus are the mean of the values under 0.1 or 4 mM
S under both 4 and 11 mM
N).
N treatment increased leaf Na (4.4 to 7.0 mg/g on 4 and 11 mM
N, respectively) and decreased Cl (20.6 to 7.3 mg/g) leaf levels.
In season 1, other elements were not affected by neither
S nor
N fertilization: P (6.9–7.4 mg/g, mean 7.2), Mg (3.5–6.0 mg/g, mean 5.1), Fe (175.70–232.50, mean 208.08 mg/kg), Zn (36.0–54.3, mean 44.12 mg/kg) and Mn (279.7–330.8, mean 308.93 mg/kg) (
Table 3). As specified for season 1, in season 2 leaf
S and
N levels increased with corresponding fertilization, from 2.6 to 5.9 and 6.5 mg/g in 0.1, 1 and 4 mM
S, respectively, and from 42.4 to 46.8 mg/g
N at 4 and 11 mM
N, respectively. Increased
S fertilization in season 2 resulted in enhanced leaf Na (1.6 and 2.3 mg/g at 0.1 and 4 Mm
S, respectively), B (41.3 and 45.4 mg/kg), Cu (13.4 and 15.5 mg/kg), and Mo (8.3 to 3.9 mg/kg), while Ca levels were again negatively affected (15.0 to 11.7 mg/g, respectively).
N fertilization in season 2 positively affected P (0.77 to 0.83 at 4 and 11 mM N, respectively) leaf levels, as well as Ca (13.3–15.9 mg/g), S (4.7 to 5.4 mg/g), Zn (27.7 to 33.4 mg/kg), and Cu (13.9 to 15.2 mg/kg). However, leaf levels of other minerals were negatively affected, i.e., Cl (17.8 to 7.3 mg/g) and Mo (6.0 to 5.0 mg/kg). At the same time, levels of other element, K (4.12–4.83, mean 4.46), Mg (4.6–5.9 mg/kg, mean 5.3), Fe (142.0–165.1, mean 153.7 mg/kg) and Mn (81.8–92.8, mean 86.7 mg/kg) were not significantly altered by treatments.
3.3. Fruit Mineral Composition
Table 5 presents results for fruit mineral concentrations during seasons 1 and 2. Fruit mineral contents generally followed the same trend recorded in diagnostic leaves during seasons 1 and 2. Bivariate analysis revealed that, like the results observed in leaves, fruit
S and
N levels were exclusively affected by their respective fertilization levels. Fruit
S increased from 1.21 mg/g in 0.1 mM
S to 2.0 mg/g in 1 and 4 mM
S, and fruit
N was positively affected by fertilization
N, increasing by 10.7% from 15.6 to 17.3 mg/g under 4 and 11 mM
N, respectively (
Table 5). For both fruit
S and
N levels, fruit concentrations were not affected by levels of other minerals.
In season 1, S fertilization levels affected fruit by decreasing Ca from 1.79 to 1.48 mg/g in 0.1 and 4 mM S, respectively. Fruit Cl was also negatively affected by S, from 6.17 to 5.15 mg/g at increasing S from 0.1 to 4 mM, respectively. Fruit Mo levels were affected by S alone, decreasing from 2.27 to 0.77 mg/kg with increasing S from 0.1 to 4 mM, respectively. Fruit Ca concentrations were negatively affected by fertilization N, decreasing from 1.94 to 1.56 mg/g in 4 and 11 mM N, respectively. Cl was also negatively affected by N fertilization, decreasing from 7.68 to 3.69 mg/g in 4 and 11 mM N, respectively, probably owing to the lower irrigation-Cl levels, while fruit Na increased from 0.183 to 0.28, respectively.
All other fruit minerals remained unaffected by fertilization, including P (5.0–5.4 mg/g, mean 5.2), K (31.7–34.3 mg/g, mean 33.3), Mg (1.6–1.8 mg/g, mean 1.7), Fe (32.2–36.2, mean 34.57 mg/kg), Zn (12.50–14.30, mean 13.30 mg/kg), B (7.40–9.20, mean 8.48 mg/kg), Mn (17.70–20.50, mean 19.22 mg/kg) and Cu (4.10 to 4.90, mean 4.50 mg/kg) (
Table 5).
In season 2, the previously described trend of S and N fertilization affecting S and N fruit accumulation in respect was also apparent, as fruit S raised from 1.32 to 1.69 and 1.83 mg/g in 0.1, 1, and 4 mM fertilization S respectively, and fruit N increased from 26.6 to 28.1 mg/g in 4 and 11 mM N, respectively.
S treatments elevated fruit N (25.8 to 28.9 mg/g in 0.1 and 4 mM S, respectively), and Na (0.68 to 0.91 mg/g), while decreasing fruit Ca (1.39 to 1.15 mg/g) and Mo (2.36 to 1.40 mg/kg). N treatments increased fruit P (6.4 to 6.9 mg/g in 4 and 11 mM N, respectively), K (48.0 to 51.2 mg/g), Ca (1.17 to 1.43 mg/g), Mg (2.0 to 2.4 mg/g), and S (1.55 to 1.68 mg/g), while fruit Cl (9.08–4.23 mg/g) and Mo (1.90 to 1.50 mg/kg) decreased. Fruit Fe (44.30–5.60, mean 50.82 mg/kg), Zn (24.8–28.6, mean 26.78 mg/kg), B (18.50–21.60, mean 19.65 mg/kg), Mn (16.50–19.70, mean 17.27 mg/kg) and Cu (9.90–11.30, mean 10.48 mg/kg) were not affected by experimental treatments.
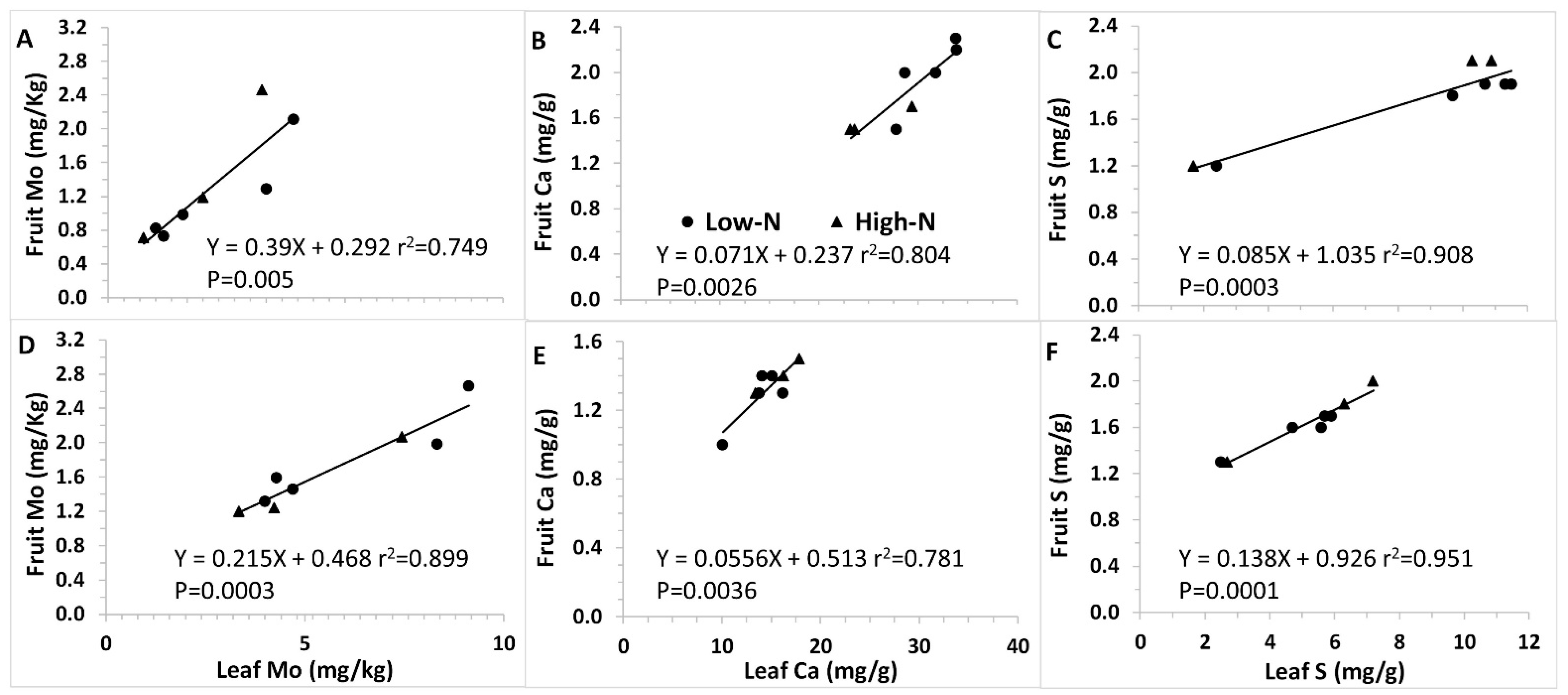
To evaluate the association between leaf and fruit concentrations of the affected elements, the two organs’ Mo, Ca, and
S levels of were correlated. Results show that leaf accumulation positively corresponded to fruit levels for all three minerals, with linear correlation as the best fit. Linear regression equation and correlation coefficient for Mo (Y = 0.2153x + 0.4678; R
2 = 0.899), Ca (Y = 0.0556x + 0.0513; R
2 = 0.781) and
S (Y = 0.1378x + 0.0926; R
2 = 0.951) are presented in
Figure 2.
4. Discussion
Current decrease in available global resources necessitates adjustments in agricultural practices, including using poor soils and desalinated water. Such applications should be carefully examined to assure that food’s nutritional quality is not impaired, to sustain population healthy nutrition and food security. In the current work, we conducted a whole-season experiment in semi-commercial conditions to assess S fertilization effects on tomato fruit sulfur levels and mineral composition, under low and high N fertilization, in addition to evaluating the effects on plant mineral status (as reflected by diagnostic leaves), growth and total yield.
To study S and N impact on fruit mineral status and composition, we designed conditions where yield is sufficient and is not negatively affected, and fruit quality is also maintained. We did not evaluate a broader range of physiological conditions where S is highly deficient since yield would be adversely affected. Hence, the data possess a practical range of values on agriculture, supplying information regarding high-yield and high-quality tomato production with varying S levels, demonstrating actual cultivation conditions.
It was also vital to study two different N levels since S and N are both macronutrients present as high-concentration anions, thus competing in uptake. On the other hand, previous studies showed that N deficiency is associated with reduced S in plants (24). Thus, it was essential to evaluate N background when fertilizing with different S levels to elucidate any N interference. Accepted N fertigation in Israel ranges between 60 and 100 mg/L. Hence, 50 and 110 mg/L in the irrigation were chosen for the current experiment as low and high N levels, respectively. These reflect realistic N fertilization levels, as we intended to conduct a real-life experiment using realistic conditions. Moreover, high N levels were also of interest to account for frequent excessive N fertilization by growers (yet under no effect on yield). Under the current experimental conditions, in the first season, fruit S was not affected by the higher N level, whereas in season 2, even a slight significant increase was observed in fruit S by high N treatments. Furthermore, low N levels did not result in undesirable plant growth or yield consequences, reflecting N sufficiency. We thus concluded that for tomato fruit, high N levels could be effective in improving fruit S concentrations.
Both fruit and leaf S and N levels demonstrated a correlation between fertilization S and N levels and their corresponding fruit and leaf levels, with enhanced S and N concentrations at higher S and N treatments, respectively. This expected interrelationship indicates that the experiment was well performed and technically sound, with fertilizer application performed as planned, resulting in these elements accumulating in fruit and leaves. In this respect, it should be mentioned that the fully developed youngest leaf, defined as diagnostic leaves, is a diagnostic plant organ, providing an indication for plant status and changes taking place due to treatments. This is especially true for low mobility nutrients with limited translocation from old to young leaves like S, Ca, Fe, Mn, Zn, Cu, B, and Mo. Therefore, we analyzed the diagnostic leaves as an additional plant organ which indicates physiological outcomes of the experimental interventions. Although some differences were recorded between fruit and leaf data, as some effects observed in leaf mineral concentrations were not apparent in fruit, our primary focus was fruit composition, while diagnostic leaf data supplied supportive information to establish the trends observed in fruit, the edible organ. The high positive correlation described between leaf and fruit concentrations of the three most significantly affected minerals corroborated the merit of diagnostic leaves as valuable fruit mineral alternation indicators.
Several tendencies are consistently repeated throughout the experiment, defining a well-established pattern. First, increasing
S fertilization resulted in decreased fruit Mo and Ca levels. These results repeated for both years, forming a clear trend. Furthermore, for all other minerals, no
S effect was evident on their fruit levels, with the exception of Na, which increased in leaves in response to
S during both years but in fruit only during season 2. The observed decrease in Mo in the leaves and fruits with the increase in
S supply is in agreement with previous studies in other plants [
25] and in tomato [
36]. The mechanism for this effect is competition in uptake and transport through transporters that serve both nutrients. The observed decrease in fruit Ca levels with increasing
S is conceivably due to the formation of calcium sulfate salt in the root zone, resulting in a decrease in free Ca levels, owing to the low solubility of the calcium sulfate formed. This chemical reaction results in calcium sulfate accumulation in the root zone, subsequently leading to hindered Ca uptake. In addition, even prior to CaSO
4 precipitation, sulfate and Ca ions form ion pairs in the solution, which also interferes with Ca uptake. These processes result in lower levels of available Ca, manifested in low fruit Ca concentrations. Although significantly changing, fruit Ca levels are rather low and within a narrow range (1–2.5 mg/g), an order of magnitude lower than these of leaf (10.1–33.9 mg/g). The practical meaning of these changes is thus limited, and might only play a role in fruit sensitivity to blossom-end rot, as disorder incidence is associated with Ca deficiency [
37].
Tomato is a staple food in many world regions, with 16MT produced in 2020 [
38], and is thus cultivated under a wide range of conditions, some of which may be disadvantageous and impair its mineral composition [
39]. At the same time, tomatoes provide an important source of minerals in many diets, and their nutritional quality should be carefully retained. In the current work, fertilization levels were adequate, potentially optimizing tomato fruit mineral contents. Fruit Zn was in the range of 12.5–28.6 mg/kg, equal to 10%–24% of recommended daily intake (RDI) in 100 g tomato, and likewise, Fe was in the range of 32.2–58.6 mg/kg, providing 18%–33% of women’s RDI in 100 g. Mg (1.6–2.5 mg/g; 50% of women’s RDI), Mn (16–20 mg/kg; 91%–110%), K (32–52 mg/g; 120%–190%), P (5.0–7.5 mg/g; 71%–105%), and Cu (4–11.8 mg/kg; 44%–129%) provided levels even higher than RDI, presenting a significant mineral source in the diet.
Biofortification is a rapidly growing concept in agriculture, related to food security, suggesting sustainably improving food’s nutritional value through fertilization and breeding strategies [
40]. It has been suggested for enriching fruit and vegetables, cereals, and pulse crops, among others, with a wide variety of nutrients, delivering high levels of selected elements to fight both malnutrition in developing countries and hidden hunger in developed areas [
41,
42]. In the current work, we successfully biofortified tomato fruit with
S through
S fertilization treatments. Using varying levels of
S, we were able to alter fruit mineral composition, manipulating fruit mineral concentrations to enhance its nutritional quality. A similar approach was previously suggested to improve quality in other crops [
43].
Taken together, the nutritional significance of the results lies in several aspects. Firstly, most fruit mineral levels were not affected by
S treatments, which indicates that fruit quality was preserved under experimental conditions. In addition,
S levels increased from 1.2 to 2.1 mg/g, indicating a biofortification process crucial for enhancing crop
S levels and preventing
S deficiency, even under poor-
S soil and irrigation water. Furthermore, although fruit Ca levels decreased from 2.3 to 1.5 mg/g, these levels were initially already low; thus, from a nutritional perspective, these changes are insignificant. As for the observed decrease in Mo from 2.29 to 0.77 mg/kg, the primary dietary sources of Mo are legumes, grains, and nuts, rather than fresh fruit and vegetables [
41]. In addition, Mo deficiency is rare, possibly thanks to the human body’s ability to adapt to a wide range of molybdenum intake levels.