Changes in Photosynthetic Characteristics of Paeonia suffruticosa under High Temperature Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Determination Items and Methods
2.2.1. Measurement of Photosynthetic Gas Exchange Parameters
2.2.2. Determination of the Kinetic Curve of Rapid Chlorophyll Fluorescence Induction
2.2.3. Statistical Analysis
3. Results
3.1. Effects of High-Temperature Stress on Leaf Photosynthetic Characteristics of Peony
3.2. Effects of High-Temperature Stress on the Rapid Chlorophyll Fluorescence Characteristics of the Peony Leaves
3.2.1. Changes in Chlorophyll Fluorescence Parameters
3.2.2. Effects of High-Temperature Stress on VJ and Quantum Yield
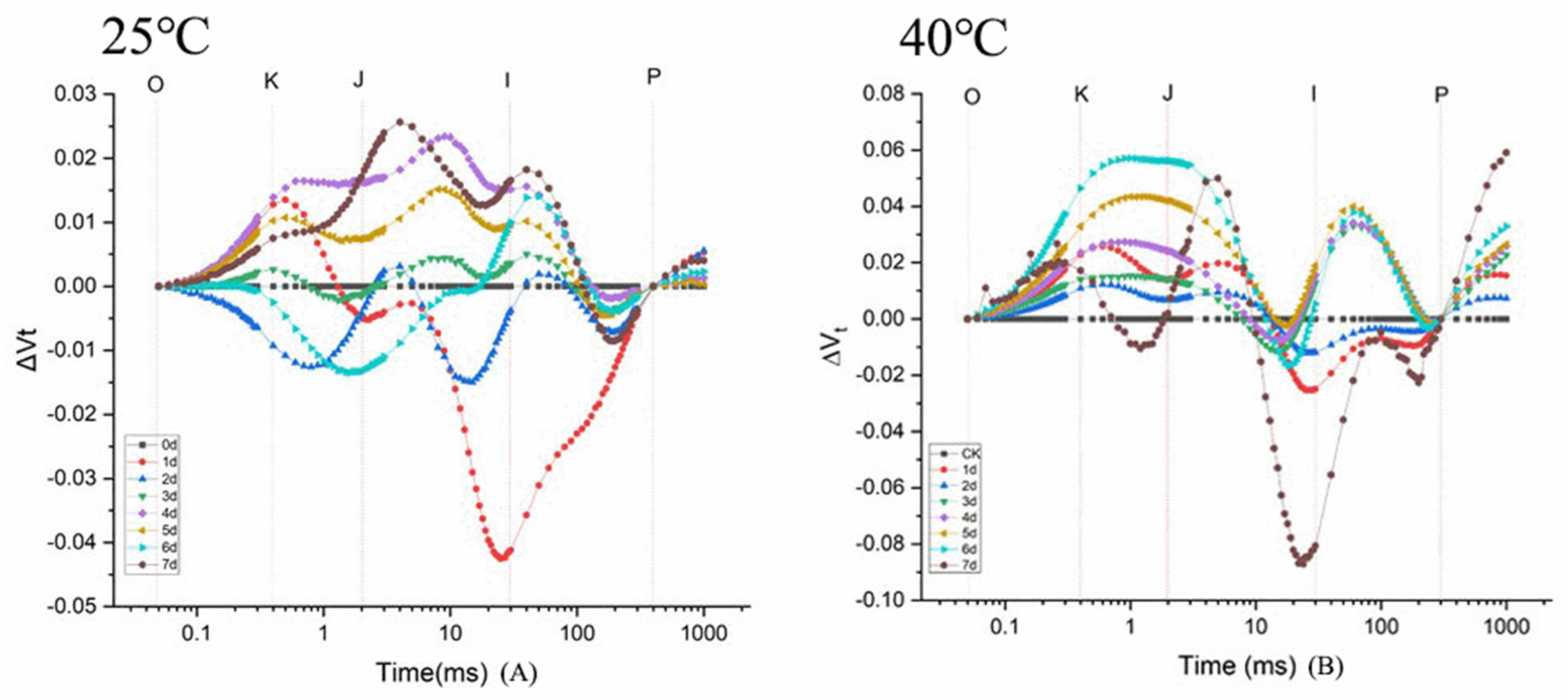
3.2.3. Kinetic Curve of Rapid Chlorophyll Fluorescence Induction
4. Discussion
4.1. Effect of High Temperature on Photosynthetic Characteristics of the Peony Leaves
4.2. The Effect of High Temperature on the Rapid Chlorophyll Fluorescence Characteristics of Peony Leaves
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, Y.Q.; Wu, Q.Z.; Yu, M.Y.; Huang, Y.; Lin, X.; Li, Z.Z. Adaptability Analysis of Paeonia ostii ‘FengDan’ in Different Regions of Fujian. Chin. J. Trop. Crops 2021, 42, 592–598. [Google Scholar]
- Zhang, Y.Z.; Cheng, Y.W.; Ya, H.Y.; Han, J.M.; Zheng, L. Identification of heat shock proteins via transcriptome profiling of tree peony leaf exposed to high temperature. Genet. Mol. Res. 2015, 14, 8431–8442. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.A. The Impact of Climate Change on Crop Production: An Empirical Study in Zhejiang, China. Ph.D. Thesis, Zhejiang University, Zhejiang, China, 2019. [Google Scholar]
- Knutti, R.; Rogelj, J.; Sedláček, J.; Fischer, E.M. A scientific critique of the two-degree climate change target. Nat. Geosci. 2016, 9, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Luo, S.Q.; Ma, Y.L.; Li, L.Y.; Xie, Y.F.; Zhang, W.X. Chlorophyll a Fluorescence Transient and 2-Dimensional Electrophoresis Analyses Reveal Response Characteristics of Photosynthesis to Heat Stress in Malus. ‘Prairifire’. Plants 2020, 9, 1040. [Google Scholar] [CrossRef]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 67, 313–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, L.Q.; Zhang, Y.L.; Guo, F.; Wan, S.B.; Meng, W.; Li, X.G. Damaging mechanisms of peanut (Arachis hypogaea L.) photosystems caused by high-temperature and drought under high irradiance. Acta Ecol. Sin. 2011, 31, 1835–1843. [Google Scholar]
- Yan, K.; Chen, P.; Shao, H.; Shao, C.; Zhao, S.; Brestic, M. Dissection of photosynthetic electron transport process in Sweet sorghum under heat stress. PLoS ONE 2013, 8, e62100. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, D.; Jajoo, A. Study of high temperature stress induced damage and recovery in photosystem II (PSII) and photosystem I (PSI) in Spinach leaves (Spinacia oleracia). J. Plant Biochem. 2021, 30, 532–544. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. BBA-Bioenergetics 2007, 1767, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.X.; Chang, Q.S.; Hou, X.G.; Wang, J.Z.; Chen, S.D.; Zhang, Q.M.; Wang, Z.; Yin, Y.; Liu, J.K. The Effect of high-temperature stress on the physiological indexes, chloroplast ultrastructure, and photosystems of two herbaceous peony cultivars. J. Plant Growth Regul. 2022, 1–16. [Google Scholar] [CrossRef]
- Li, P.M.; Gao, H.Y.; Strasser, R.J. Application of the Fast ChlorophyII Fluorescence Induction Dynamics Analysis in Photosynthesis Study. Acta Photophysiol. Sin. 2005, 31, 559–566. [Google Scholar]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Micheal, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. Probing Photosynth. Mech. 2000, 445–483. [Google Scholar]
- Stefanov, D.; Petkova, V.; Denev, I.D. Screening for heat tolerance in common bean (Phaseolus vulgaris L.) lines and cultivars using JIP-test. Sci. Hortic. 2011, 128, 1–6. [Google Scholar] [CrossRef]
- Giorio, P.; Sellami, M.H. Polyphasic OKJIP Chlorophyll a Fluorescence Transient in a Landrace and a Commercial Cultivar of Sweet Pepper (Capsicum annuum L.) under Long-Term Salt Stress. Plants 2021, 10, 887. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, S.; Paunov, M.; Pavlova, B.; Dankov, K.; Kouzmanova, M.; Velikova, V.; Tsonev, T.; Kalaji, H.M.; Goltsev, V. Photosynthetic efficiency of two Platanus orientalis L. ecotypes exposed to moderately high temperature–JIP-test analysis. Photosynthetica 2020, 58, 657–675. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.X.; Zhao, S.; Liu, C.M.; Li, Y.L. Effects of Shading Treatments on Chlorophyll Fluorescence Characteristics of Sabina vulgaris Seedlings Grown in Iron Tailings Media. Sci. Silvae Sin. 2015, 51, 17–26. [Google Scholar]
- Kong, F.L.; Xiao, Y.; Wang, G.Z.; Zhang, K. Effects of Fertilizer Dosage on Photosynthesis and Fast Chlorophyll Fluorescence Characteristics of Juglans regia in Mountainous Region. Sci. Silvae Sin. 2016, 29, 764–769. [Google Scholar]
- Chen, S.; Yang, J.; Zhang, M.; Strasser, R.J.; Qiang, S. Classification and characteristics of heat tolerance in Ageratina adenophora populations using fast chlorophyll a fluorescence rise OJIP. Environ. Exp. Bot. 2016, 122, 126–140. [Google Scholar] [CrossRef]
- Liu, J.J. Effect of High Temperature and Drought Stress on PSII Function and Light Distribution in Peony Leaves with Different Resistance. North Hortic. 2019, 11, 72–79. [Google Scholar]
- Liu, C.Y.; Chen, D.Y.; Gai, S.P.; Zhang, Y.X.; Zheng, G.S. Effects of high-and low temperature stress on the leaf PSⅡ functions and physiological characteristics of tree peony. Chin. J. Appl. Ecol. 2012, 23, 133–139. [Google Scholar]
- Zhu, S.H.; Ma, J.; Hao, L.H.; Zhang, L.L. Identification and Analysis of Heat-resistant Differential Protein in Leaves of Paeonia suffruticosa. MPB 2021, 19, 419–431. [Google Scholar]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Govindjee; Sarin, N.B. Overexpression of γ-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: Physiological and chlorophyll a fluorescence measurements. BBA 2010, 1797, 1428–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahid, A.; Gelani, S.; Ashraf, M. Heat tolerance in plants: An overview. Environ. Exp Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.J.; Wang, M.H.; Shah, L.; Lu, S.Q.; Wang, X.B.; Ma, C.X. Unraveling Field Crops Sensitivity to Heat Stress: Mechanisms, Approaches, and Future Prospects. Agronomy 2018, 8, 128. [Google Scholar] [CrossRef] [Green Version]
- Gao, G.L.; Feng, Q.; Zhang, X.Y.; Si, J.H.; Yu, T.F. An Overview of stomatal and non-stomatal limitations to photosynthesis of plants. Arid Zone Res. 2018, 35, 929–937. [Google Scholar]
- Zhang, Y.D.; Yang, Z.Q.; Lu, S.Y.; Yang, L.; Zheng, H. Response of photosynthetic characteristics of leaves of protected chrysanthemum variety ‘Jinbeidahong’ to high temperature stress. North Hortic. 2021, 72–80. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, Z.H.; Yao, B.Q.; Ma, Z.; Huang, X.T.; Zhou, B.R.; Xu, M.H.; Guo, J.; Zhou, H.K. Effects of drought and heat on the productivity and photosynthetic characteristics of alpine meadow plants on the Qinghai-Tibetan Plateau. J Mt. Sci.-Engl. 2021, 18, 2079–2093. [Google Scholar] [CrossRef]
- Su, C.J.; Jin, Y.Z.; Wu, X.; Fan, B.W.; Song, Y.N.; Yang, F.J. Research status on the influence of high temperature stress on photosynthetic system of tomato and mitigating mechanism. J. Heilongjiang Bayi Agric. Univ. 2021, 33, 13–20. [Google Scholar]
- Cai, J.G.; Wei, M.Q.; Zhang, Y.; Wei, Y.L. Effects of shading on photosynthetic characteristics and chlorophyll fluorescence parameters in leaves of Hydrangea macrophylla. Chin. J. Plant Ecol. 2017, 41, 570–576. [Google Scholar]
- Xu, D.Q. Photosynthesis; Science Press: Beijing, China, 2013. [Google Scholar]
- Shahsavandi, F.; Eshghi, S.; Gharaghani, A.; Ghasemi-Fasaei, R.; Jafarinia, M. Effects of bicarbonate induced iron chlorosis on photosynthesis apparatus in grapevine. Sci. Hortic. 2020, 270, 109427. [Google Scholar] [CrossRef]
- Wang, Y.Q. Response Mechanism of Different Wheat Cultivars to High Temperature, Drought and Their Combined Stress. Master’s Thesis, Shandong Agricultural University, Shandong, China, 2019. [Google Scholar]
- Han, Y.Q.; NLiu, X.; Hu, W.P.; Zhang, P.J.; Deng, J.C.; Cheng, Z.L. Effects of CO2 Enrichment on Chlorophyll Fluorescence Characteristics of Vallisneria natans. Bull. Bot. Res. 2017, 37, 45–51. [Google Scholar]
- Liu, Q.Q.; Ma, S.B.; Feng, X.H.; Sun, Y.; Yi, Y.J.; Liu, W.X. Effects of Grafting on the Fast Chlorophyll Fluorescence Induction Dynamics of Pepper Seedlings Under Temperature Stress. Acta Hortic. Sin. 2016, 43, 885–896. [Google Scholar]
- Tian, G. Study on the Selection of Heat-Resistant Varieties of Ornamental Chrysanthemum and Its Heat-Resistant Mechanism. Master’s Thesis, Henan Agricultural University, Henan, China, 2020. [Google Scholar]
- Guo, Y.Y.; Li, H.J.; Liu, J.; Bai, Y.W.; Xue, J.Q.; Zhang, R.H. Melatonin alleviates drought-Induced damage of photosynthetic apparatus in maize seedlings. Russ. J. Plant Physiol. 2020, 67, 312–322. [Google Scholar] [CrossRef]
- Liu, K.Y. Study on Drought Resistance of Clematis acrophylla and Schizonepeta chinensis. Master’s Thesis, Henan Agricultural University, Henan, China, 2018. [Google Scholar]
- Hu, D.Y.; Liu, X.F.; Wang, K.J.; He, S.L.; Xie, R.J.; Yi, S.L.; Deng, L. Effects of tree pruning in a closed citrus orchard on some parameters of photochemical reactions and fruit quality. J. Friut Sci. 2017, 34, 552–566. [Google Scholar]
- Zhang, D.; Chen, C.S.; Li, P.M.; Ma, F.W. Effects of Drought on the Photosynthetic Apparatus in Malus hupehensis Leaves Explored by Simultaneous Measurement of Prompt Fluorescence, Delayed Fluorescence and Modulated Light Refl ection at 820 nm. Plant Physiol. J. 2013, 49, 551–560. [Google Scholar]
- Wei, X.D.; Chen, G.X.; Shi, D.W.; Liu, D.; Tang, J.H.; Li, X. Effects of drought on fluorescence characteristics of photosystem II in leaves of Ginkgo biloba. Acta Ecol. Sin. 2012, 32, 7492–7500. [Google Scholar]
- Fatma, M.; Iqbal, N.; Sehar, Z.; Alyemeni, M.N.; Kaushik, P.; Khan, N.A.; Ahmad, P. Methyl Jasmonate protects the PS II system by maintaining the stability of chloroplast D1 protein and accelerating enzymatic antioxidants in heat-stressed wheat plants. Antioxidants 2021, 10, 1216. [Google Scholar] [CrossRef]
- Fei, L.W.; Chu, J.P.; Zhang, X.; Dong, S.X.; Dai, X.L.; He, M.R. Physiological and proteomic analyses indicate delayed sowing improves photosynthetic capacity in wheat flag leaves under heat stress. Front. Plant Sci. 2022, 13, 848464. [Google Scholar] [CrossRef]
- Yuan, J.L.; Ma, C.; Feng, Y.L.; Zhang, J.; Yang, F.Q.; Li, Y.J. Response of chlorophyll fluorescence transient in leaves of wheats with different drought resistances to drought stresses and rehydration. Plant Physiol. J. 2018, 54, 1119–1129. [Google Scholar]
- Yang, X.; Zhang, Q.C.; Sun, S.Y.; Chen, K.N. Effects of water depth on the growth of Vallisneria natans and photosynthetic system Ⅱ photochemical characteristics of the leaves. Chin. J. Appl. Ecol. 2014, 25, 1623–1631. [Google Scholar]
- Xiao, F. Effects of High Temperature Stress on Physiological and Gene Expression Characteristics of Grapevine (Vitis viniferu L. Hongti) during Seedling Stage. Ph.D. Thesis, Nanjing University of Information Science & Technology, Nanjing, China, 2018. [Google Scholar]
- Essemine, J.; Govindachary, S.; Ammar, S.; Bouzid, S.; Carpentier, R. Enhanced sensitivity of the photosynthetic apparatus to heat stress in digalactosyl-diacylglycerol deficient Arabidopsis. Environ. Exp. Bot. 2012, 80, 16–26. [Google Scholar] [CrossRef]
- Huang, W.; Yang, Y.J.; Hu, H.; Zhang, S.B. Responses of photosystem I compared with photosystem II to fluctuating light in the shade-establishing tropical tree species Psychotria henryi. Front. Plant Sci. 2016, 7, 1549. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Yang, Y.J.; Zhang, J.L.; Hu, H.; Zhang, S.B. Superoxide generated in the chloroplast stroma causes photoinhibition of photosystem I in the shade-establishing tree species Psychotria henryi. Photosynth. Res. 2017, 132, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Xu, C. Study on the Mechanism and Simulation of the Effect of High Temperature in the Seedling Stage on the Growth and Fruit Quality of Strawberry. Ph.D. Thesis, Nanjing University of Information Science & Technology, Nanjing, China, 2021. [Google Scholar]
- Xu, C.; Wang, M.T.; Yang, Z.Q.; Han, W.; Zheng, S.H. Effects of high temperature on photosynthetic physiological characteristics of strawberry seedlings in greenhouse and construction of stress level. Chin. J. Appl. Ecol. 2021, 32, 231–240. [Google Scholar]
- Guo, Y.J.; Lu, Y.P.; Goltsev, V.; Strasser, R.J.; Kalaji, H.M.; Wang, H.; Wang, X.X.; Chen, S.G.; Qiang, S. Comparative effect of tenuazonic acid, diuron, bentazone, dibromothymoquinone and methyl viologen on the kinetics of Chl a fluorescence rise OJIP and the MR820 signal. Plant Physiol. Biochem. 2020, 156, 39–48. [Google Scholar] [CrossRef] [PubMed]



| Formula and Terms | Illustrations |
|---|---|
| Data extracted from the recorded fluorescence transient OJIP | |
| Ft | Fluorescence at time t after onset of actinic illumination |
| F50 µs or F20 µs | Minimal reliable recorded fluorescence, at 50 μs or 20 μs |
| F300 µs | Fluorescence intensity at 300 μs |
| FJ ≡ F2ms | Fluorescence intensity at the J-step (2 ms) of OJIP |
| FJ ≡ F30ms | Fluorescence intensity at the I-step (30 ms) of OJIP |
| FP | Maximal recorded fluorescence intensity, at the peak P of OJIP |
| tFM | Time (in ms) to reach the maximal fluorescence intensity FM |
| Fluorescence parameters derived from the extracted data | |
F0  F50µs or F50µs or  F20µs F20µs | Minimal fluorescence (all PSII RCs are assumed to be open) |
| FM (=FP) | Maximal fluorescence, when all PSII RCs are closed (equal to FP when the actinic light intensity is above 500 μmol photons m−2 s−1 and provided that all RCs are active as QA reducing) |
| Fυ ≡ Ft − F0 | Variable fluorescence at time t |
| FV ≡ FM − F0 | Maximal variable fluorescence |
| FV/FM | Maximal quantum efficiency of PSII |
| VJ | J point is relatively variable fluorescence intensity |
| Vt ≡ Fυ/FV ≡ (Ft − F0)/(FM − F0) | Relative variable fluorescence at time t |
| ΔVt = (Ft − F0)/(FM − F0) − Vt (control) | Relative variable fluorescence |
| M0 ≡ [(ΔF/Δt)0]/(FM − F50μs)≡4(F300μs − F50μs)/(FM − F50μs) | Approximated initial slope (in ms−1) of the fluorescence transient normalized on the maximal variable fluorescence FV |
| Specific energy fluxes [per QA-reducing PSII reaction center (RC)] | |
| ABS/RC = MO (1/VJ)(1/φPo) | Absorption flux (of antenna Chls) per RC |
| TRO/RC = MO (1/VJ) | Trapped energy flux per RC (at t = 0) |
| ETO/RC = MO (1/VJ)ψEo | Electron transport flux (further than QA−) per RC (at t = 0) |
| DIO/RC = (ABS/RC) − (TRO/RC) | Dissipated energy flux per RC (at t = 0) |
| Yields or flux rations | |
| φEo ≡ ET0/ABS = [1 − (F0/FM)]ψEo | Quantum yield for electron transport (ET)(at t = 0) |
| Ψo = ETO/TRO = (1 − VJ) | Probability that a trapped exciton moves an electron into the electron transport chain beyond QA− (at t = 0) |
| φDo = 1 − φPO = (FO/Fm) | Quantum ratio used for heat dissipation |
| Performance indexes (products of terms expressing partial potentials at steps of energy bifurcations) | |
| PIABS ≡ (RC/ABS)[φPo/(1 − φPo)][Ψo/(1 − Ψo)] | Performance index on absorption basis |
| PItotal ≡ PIABS[ δRo/(1 − δRo)] | Performance index (potential) for energy conservation from exciton to the reduction of PSI end acceptors |
| Time (d) | Temperature | Pn (µmol·m−2·s−1) | Gs (mmol·m−2·s−1) | Ci (µmol/mol) | Tr (g·m−2·h−1) |
|---|---|---|---|---|---|
| 0 | 25 °C | 5.8203 ± 0.3775 a | 0.0582 ± 0.0042 a | 248.4497 ± 1.9420 b | 0.5802 ± 0.0188 a |
| 40 °C | 5.2338 ± 0.3429 a | 0.0596 ± 0.0025 a | 367.6925 ± 22.0826 ab | 0.5540 ± 0.0380 a | |
| 1 | 25 °C | 5.6017 ± 0.1824 a | 0.0628 ± 0.0059 a | 269.3729 ± 8.4733 ab | 0.5490 ± 0.0536 a |
| 40 °C | 3.5051 ± 0.0621 b | 0.0369 ± 0.0013 c | 333.0420 ± 46.4777 ab | 0.3848 ± 0.0240 b | |
| 2 | 25 °C | 2.5803 ± 0.2868 d | 0.0205 ± 0.0016 c | 209.1131 ± 36.8020 b | 0.3635 ± 0.0168 d |
| 40 °C | 1.2469 ± 0.3513 b | 0.0197 ± 0.0007 d | 325.2725 ± 23.8841 ab | 0.1829 ± 0.0169 c | |
| 3 | 25 °C | 5.0258 ± 0.1830 b | 0.0400 ± 0.0028 b | 223.9212 ± 21.8206 b | 0.5054 ± 0.0197 b |
| 40 °C | 0.6174 ± 0.0740 c | 0.0073 ± 0.0010 de | 220.6303 ± 30.8073 b | 0.1056 ± 0.0205 d | |
| 4 | 25 °C | 4.7060 ± 0.1808 b | 0.0373 ± 0.0013 b | 244.7754 ± 1.4064 b | 0.3919 ± 0.0063 d |
| 40 °C | 0.4703 ± 0.0967 c | 0.0046 ± 0.0011 e | 286.5971 ± 64.4177 b | 0.0616 ± 0.0171 d | |
| 5 | 25 °C | 3.1041 ± 0.3254 c | 0.0339 ± 0.0032 b | 292.7151 ± 16.2667 a | 0.4493 ± 0.03893 c |
| 40 °C | 0.4253 ± 0.0912 c | 0.0096 ± 0.0034 d | 410.0798 ± 123.7733 a | 0.1596 ± 0.0594 cd | |
| 6 | 25 °C | 1.4205 ± 0.1654 e | 0.0156 ± 0.0008 cd | 267.4682 ± 9.7356 ab | 0.2411 ± 0.0118 e |
| 40 °C | 0.3069 ± 0.0630 c | 0.0227 ± 0.0037 b | 266.7287 ± 25.6768 b | 0.4007 ± 0.0793 b | |
| 7 | 25 °C | 1.0134 ± 0.1410 e | 0.0127 ± 0.0014 d | 292.9464 ± 27.4262 a | 0.1962 ± 0.0238 f |
| 40 °C | −0.1586 ± 0.0276 d | 0.0086 ± 0.0011 d | 302.4088 ± 120.2667 ab | 0.1429 ± 0.0154 cd |
| Time (d) | Temperature | VJ | Ψo | φEo | φDo |
|---|---|---|---|---|---|
| 0 | 25 °C | 0.4992 ± 0.0564 a | 0.5008 ± 0.0564 a | 0.4097 ± 0.0505 a | 0.1831 ± 0.0110 a |
| 40 °C | 0.4406 ± 0.0348 b | 0.5594 ± 0.0348 a | 0.4580 ± 0.0312 a | 0.1815 ± 0.0056 e | |
| 1 | 25 °C | 0.4959 ± 0.0521 a | 0.4959 ± 0.0521 a | 0.4113 ± 0.0478 a | 0.1852 ± 0.0105 a |
| 40 °C | 0.4519 ± 0.0136 b | 0.5481 ± 0.0136 ab | 0.4404 ± 0.0103 ab | 0.1964 ± 0.0036 ce | |
| 2 | 25 °C | 0.4908 ± 0.0483 a | 0.5092 ± 0.0483 a | 0.4145 ± 0.0446 a | 0.1870 ± 0.0114 a |
| 40 °C | 0.4716 ± 0.0483 ab | 0.5284 ± 0.0483 ab | 0.4203 ± 0.0438 bc | 0.2055 ± 0.0132 ce | |
| 3 | 25 °C | 0.5038 ± 0.0673 a | 0.4962 ± 0.0673 a | 0.4066 ± 0.0599 a | 0.1820 ± 0.0109 a |
| 40 °C | 0.4509 ± 0.0497 b | 0.5491 ± 0.0497 ab | 0.4354 ± 0.0400 ab | 0.2071 ± 0.0096 c | |
| 4 | 25 °C | 0.5159 ± 0.0575 a | 0.4842 ± 0.0757 a | 0.3953 ± 0.0671 a | 0.1854 ± 0.0133 a |
| 40 °C | 0.4636 ± 0.0373 ab | 0.5364 ± 0.0373 ab | 0.4230 ± 0.0376 b | 0.2124 ± 0.0197 c | |
| 5 | 25 °C | 0.5082 ± 0.0715 a | 0.4918 ± 0.0715 a | 0.4007 ± 0.0648 a | 0.1873 ± 0.0153 a |
| 40 °C | 0.4776 ± 0.0271 ab | 0.5224 ± 0.0271 b | 0.4111 ± 0.0207 bc | 0.2130 ± 0.0097 bc | |
| 6 | 25 °C | 0.4858 ± 0.0473 a | 0.5142 ± 0.0473 a | 0.4201 ± 0.0389 a | 0.1830 ± 0.0092 a |
| 40 °C | 0.4916 ± 0.0251 a | 0.5084 ± 0.0251 b | 0.3889 ± 0.0295 c | 0.2358 ± 0.0265 b | |
| 7 | 25 °C | 0.5154 ± 0.0665 a | 0.4846 ± 0.0665 a | 0.3939 ± 0.0597 a | 0.1890 ± 0.0140 a |
| 40 °C | 0.4586 ± 0.0296 ab | 0.5414 ± 0.0296 ab | 0.3924 ± 0.0439 bc | 0.2737 ± 0.0875 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, W.; Luo, H.; Song, Y.; Hong, E.; Li, Z.; Lin, B.; Fan, C.; Wang, H.; Song, X.; Jin, S.; et al. Changes in Photosynthetic Characteristics of Paeonia suffruticosa under High Temperature Stress. Agronomy 2022, 12, 1203. https://doi.org/10.3390/agronomy12051203
Ji W, Luo H, Song Y, Hong E, Li Z, Lin B, Fan C, Wang H, Song X, Jin S, et al. Changes in Photosynthetic Characteristics of Paeonia suffruticosa under High Temperature Stress. Agronomy. 2022; 12(5):1203. https://doi.org/10.3390/agronomy12051203
Chicago/Turabian StyleJi, Wen, Haiyan Luo, Yuqin Song, Erman Hong, Zhijun Li, Bangyu Lin, Chenwei Fan, Huasen Wang, Xinzhang Song, Songheng Jin, and et al. 2022. "Changes in Photosynthetic Characteristics of Paeonia suffruticosa under High Temperature Stress" Agronomy 12, no. 5: 1203. https://doi.org/10.3390/agronomy12051203
APA StyleJi, W., Luo, H., Song, Y., Hong, E., Li, Z., Lin, B., Fan, C., Wang, H., Song, X., Jin, S., Chen, X., & Zhu, X. (2022). Changes in Photosynthetic Characteristics of Paeonia suffruticosa under High Temperature Stress. Agronomy, 12(5), 1203. https://doi.org/10.3390/agronomy12051203








