Hydrochemical Assessment of Water Used for Agricultural Soil Irrigation in the Water Area of the Three Morava Rivers in the Republic of Serbia
Abstract
:1. Introduction
2. Materials and Methods
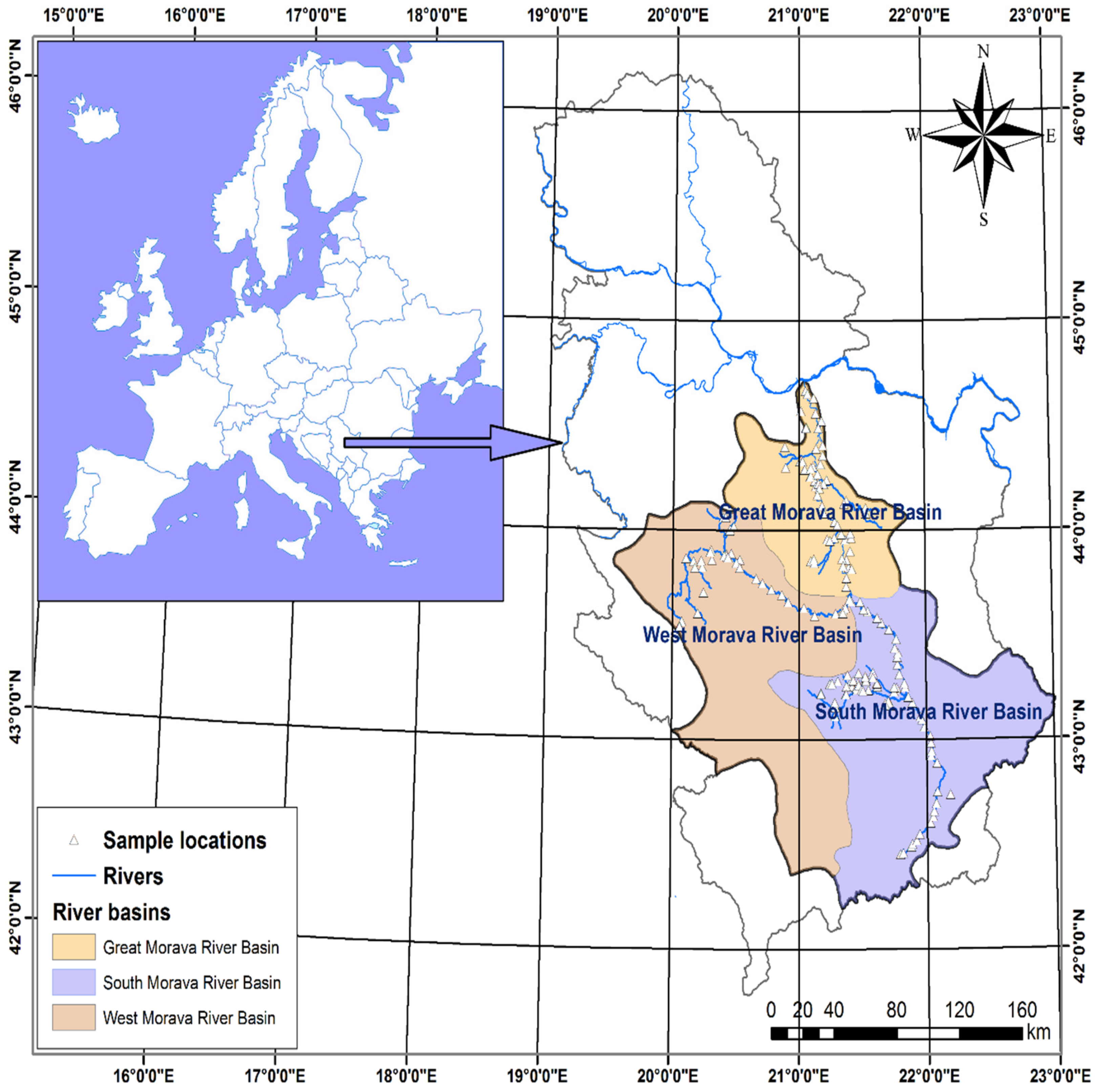
2.1. Description of the Study Area
2.1.1. Location, Hydrological Setting, and Climate
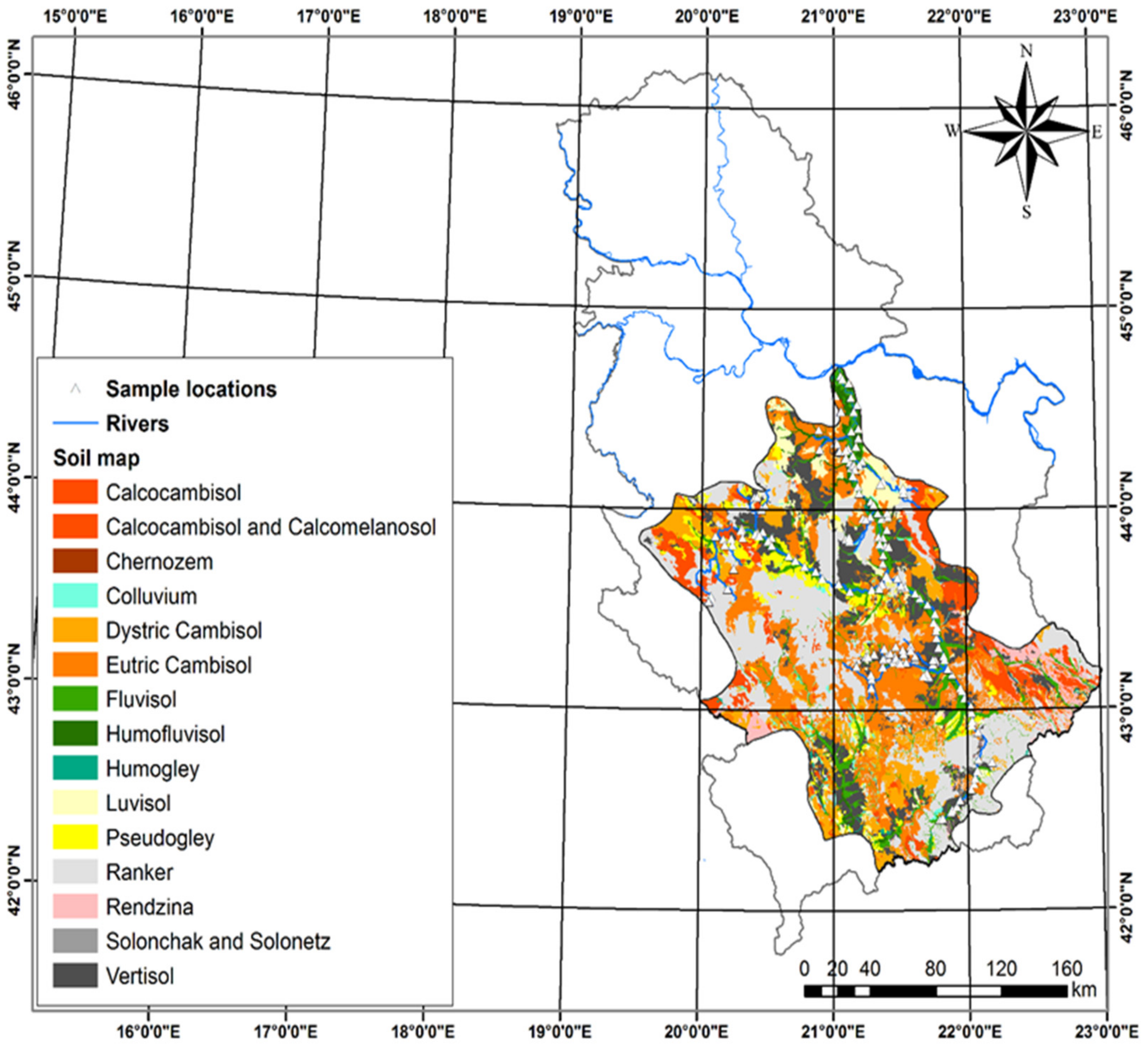
2.1.2. Geological and Hydrogeological Setting
2.1.3. Soil Use Type
2.2. Sampling and Collection of Water Samples
2.3. Laboratory Analysis
2.4. Data Analysis
2.5. Spatial Distribution
2.6. Irrigation Water Quality Evaluation
3. Results and Discussion
3.1. Irrigation Water Suitability Indicators
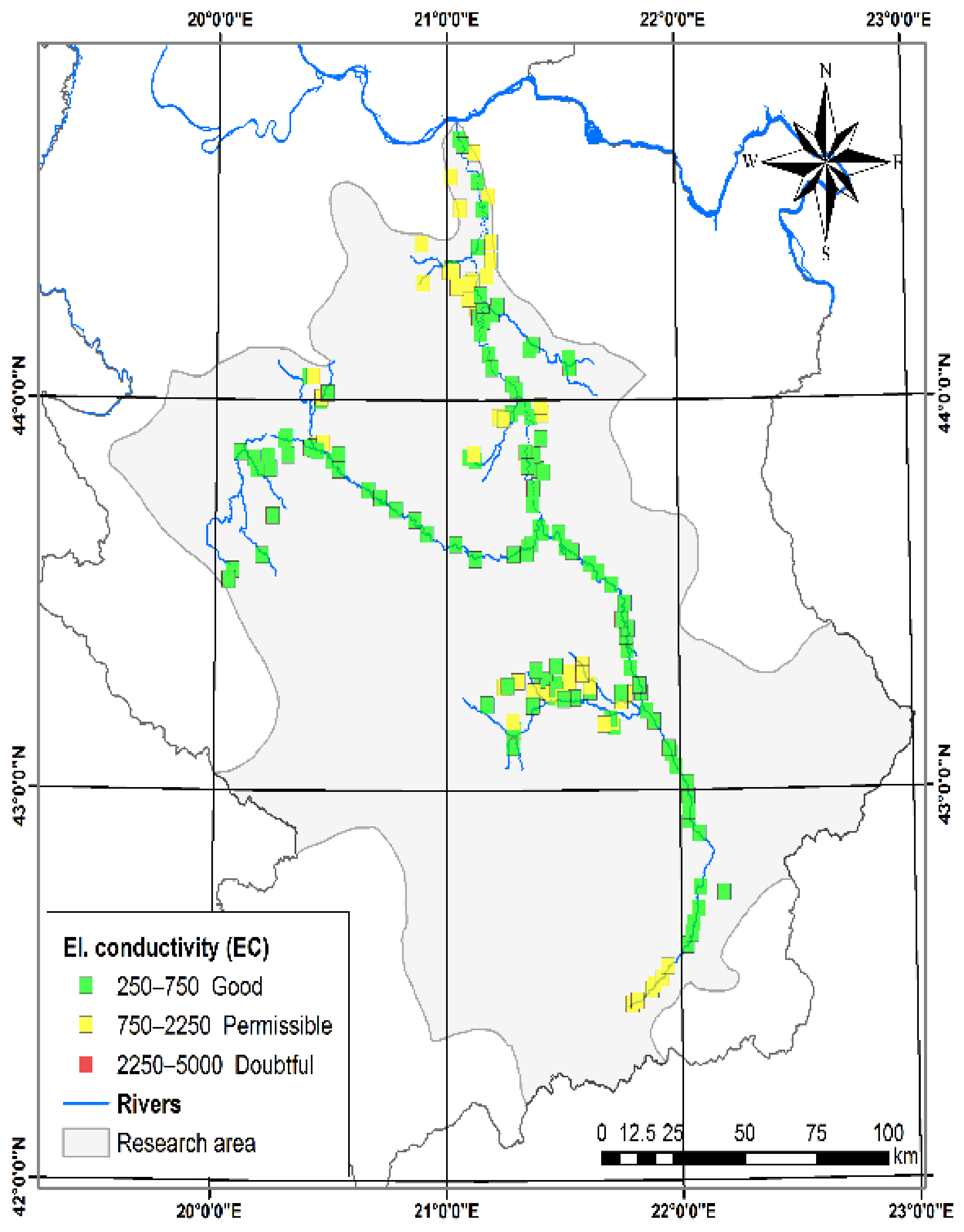
3.1.1. Electrical Conductivity (EC)
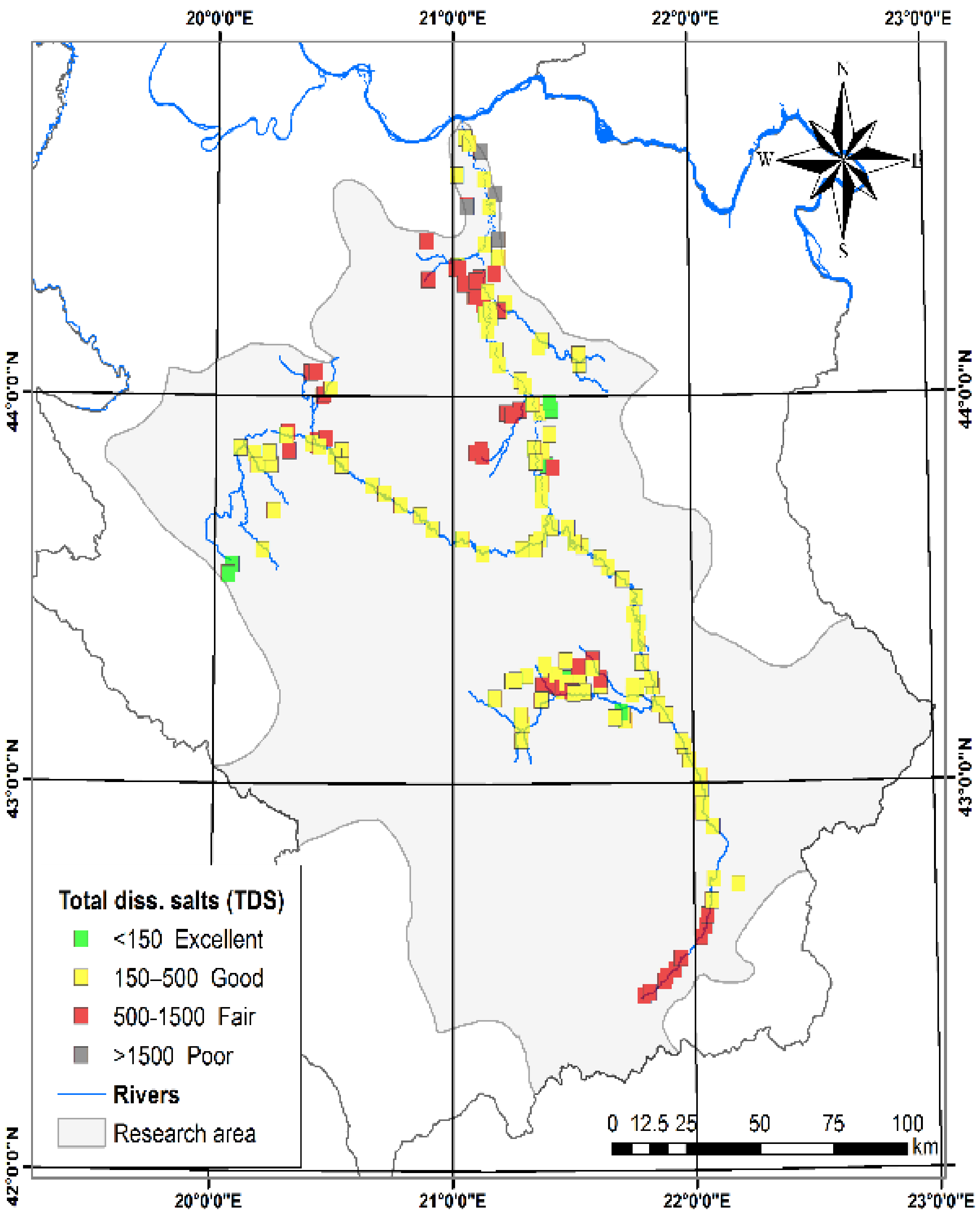
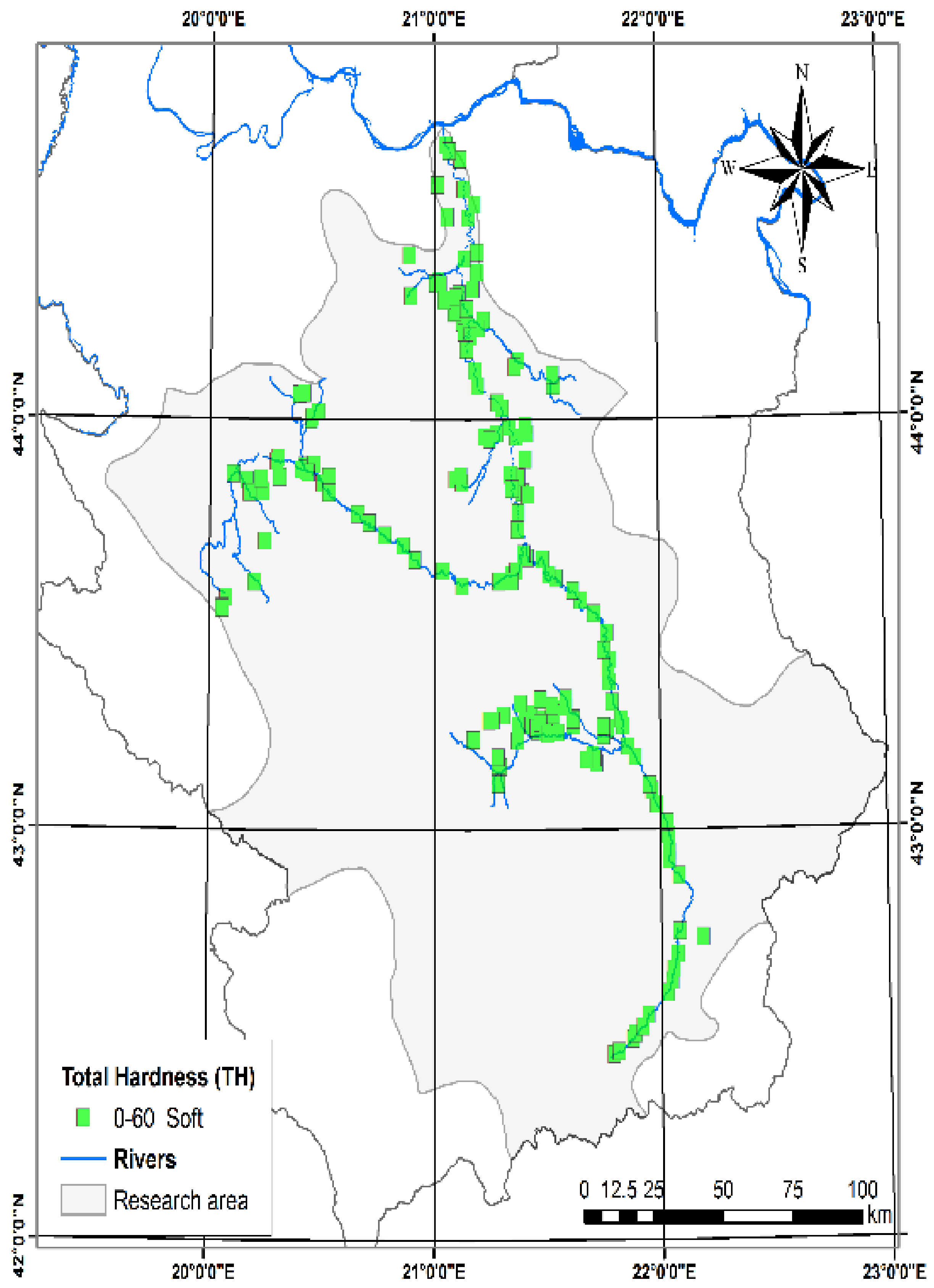
3.1.2. Total Dissolved Solids (TDS) and Total Hardness (TH)
3.1.3. Sodium Absorption Ratio (SAR)
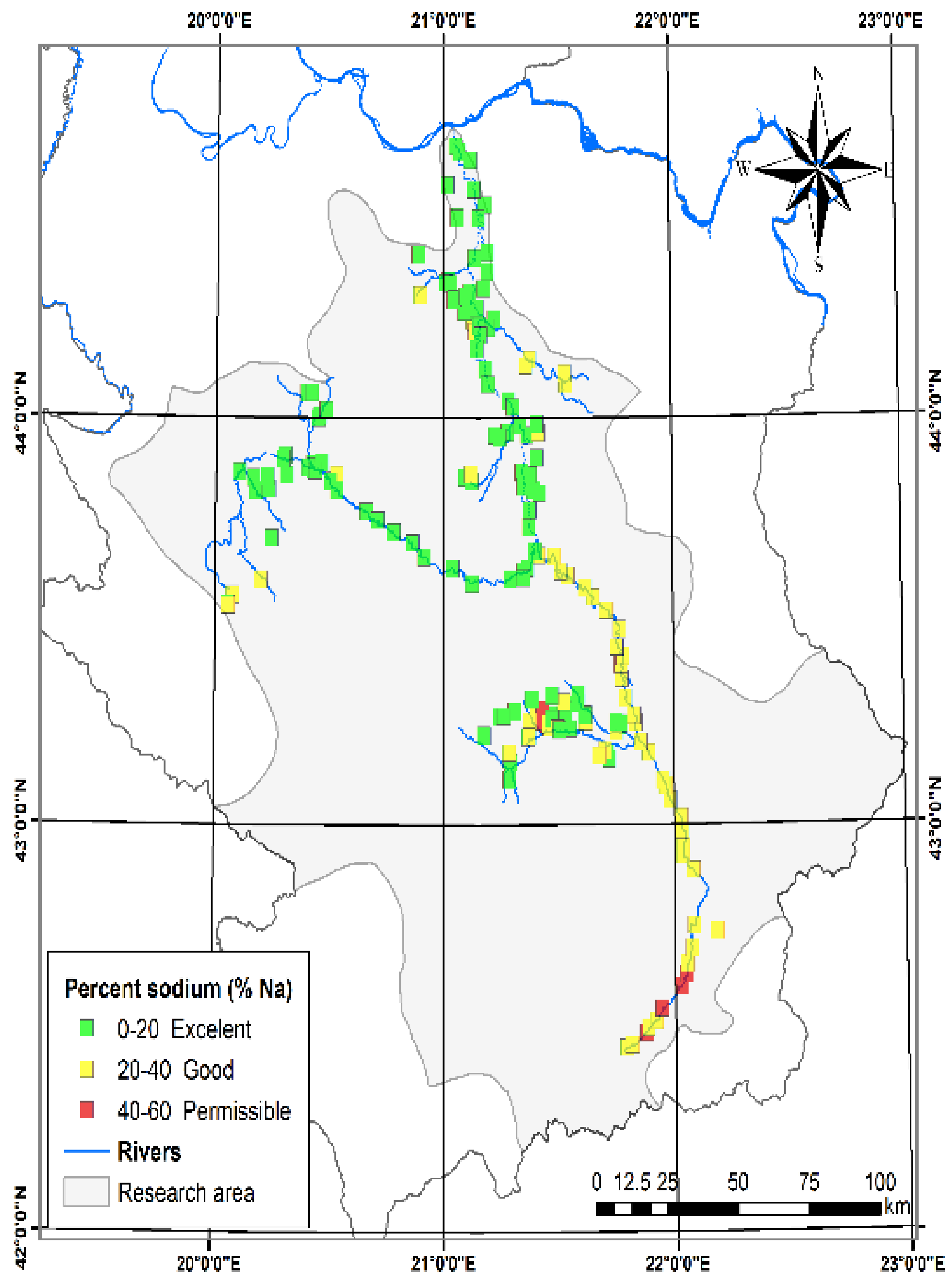
3.1.4. Sodium Hazard (Na %)
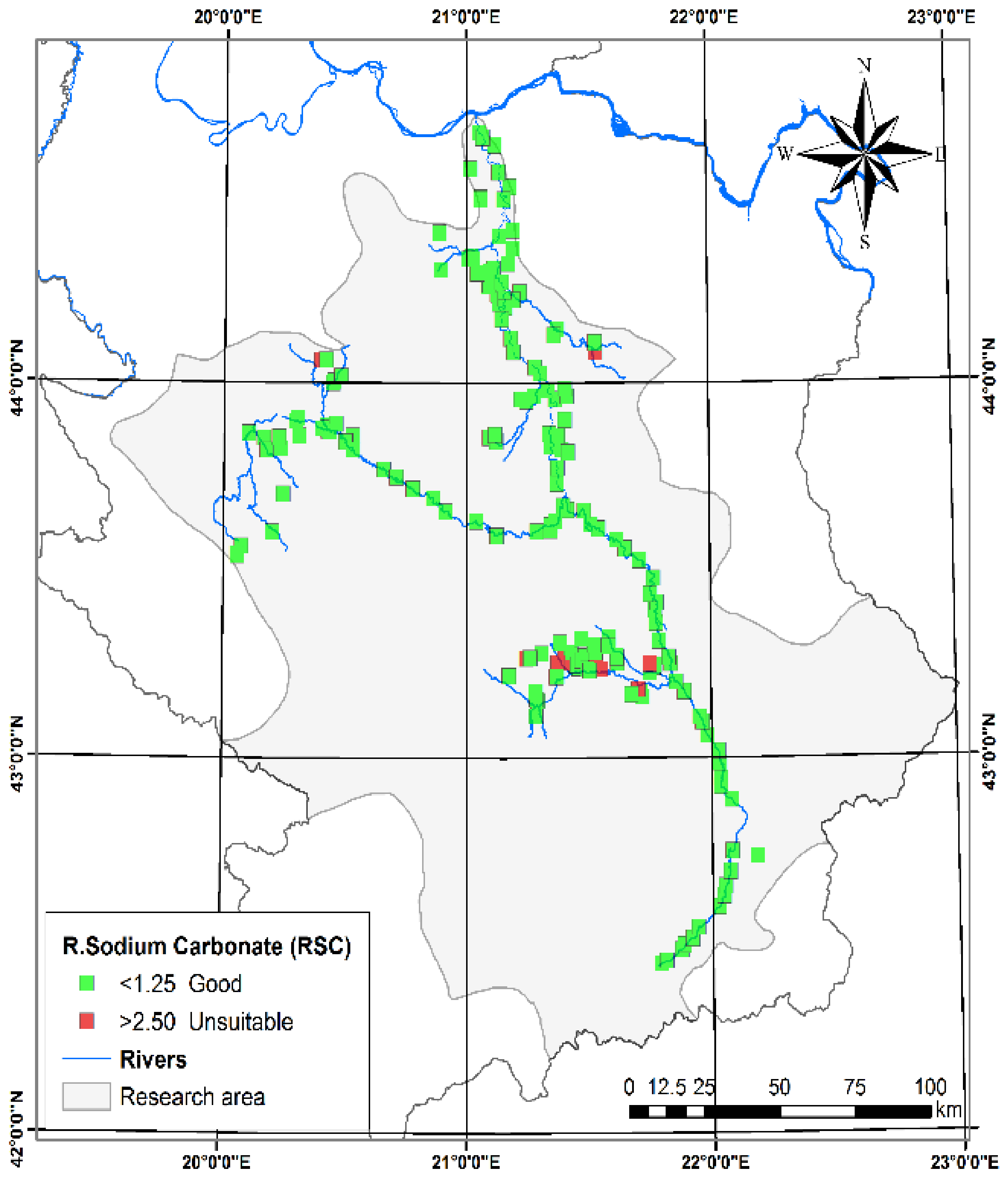
3.1.5. Residual Sodium Carbonate (RSC)
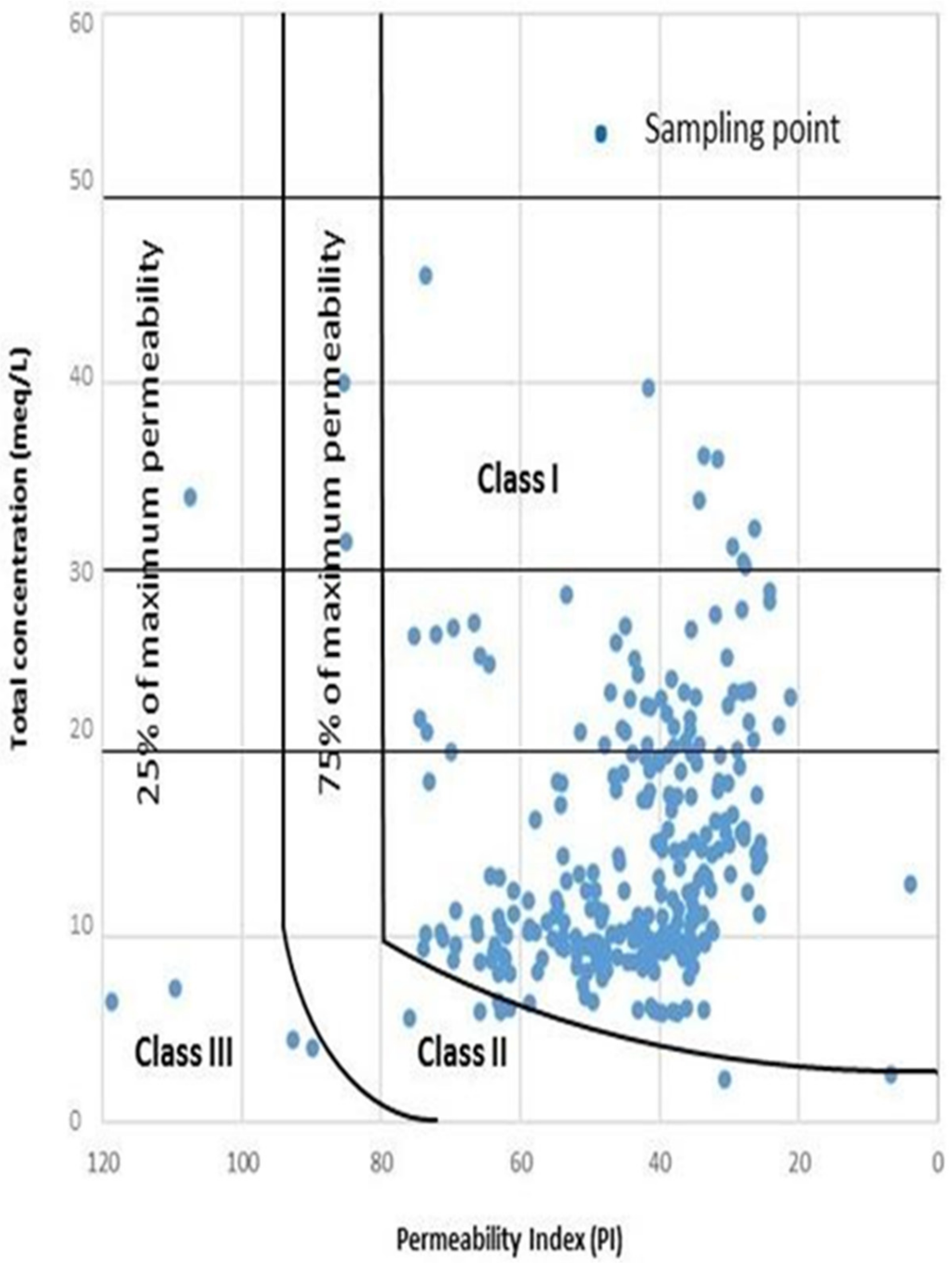
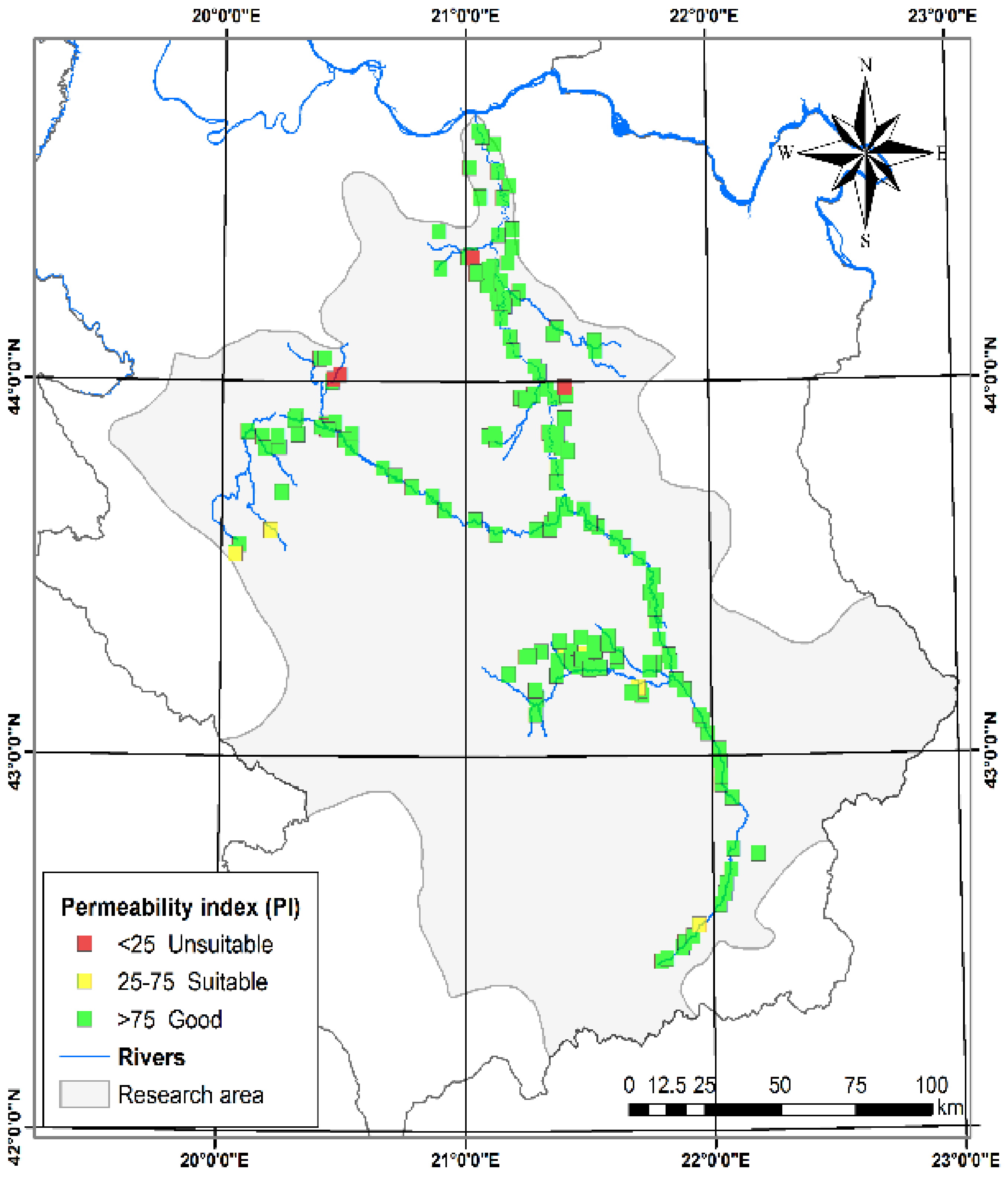
3.1.6. Permeability Index (PI) and Doneen Diagram
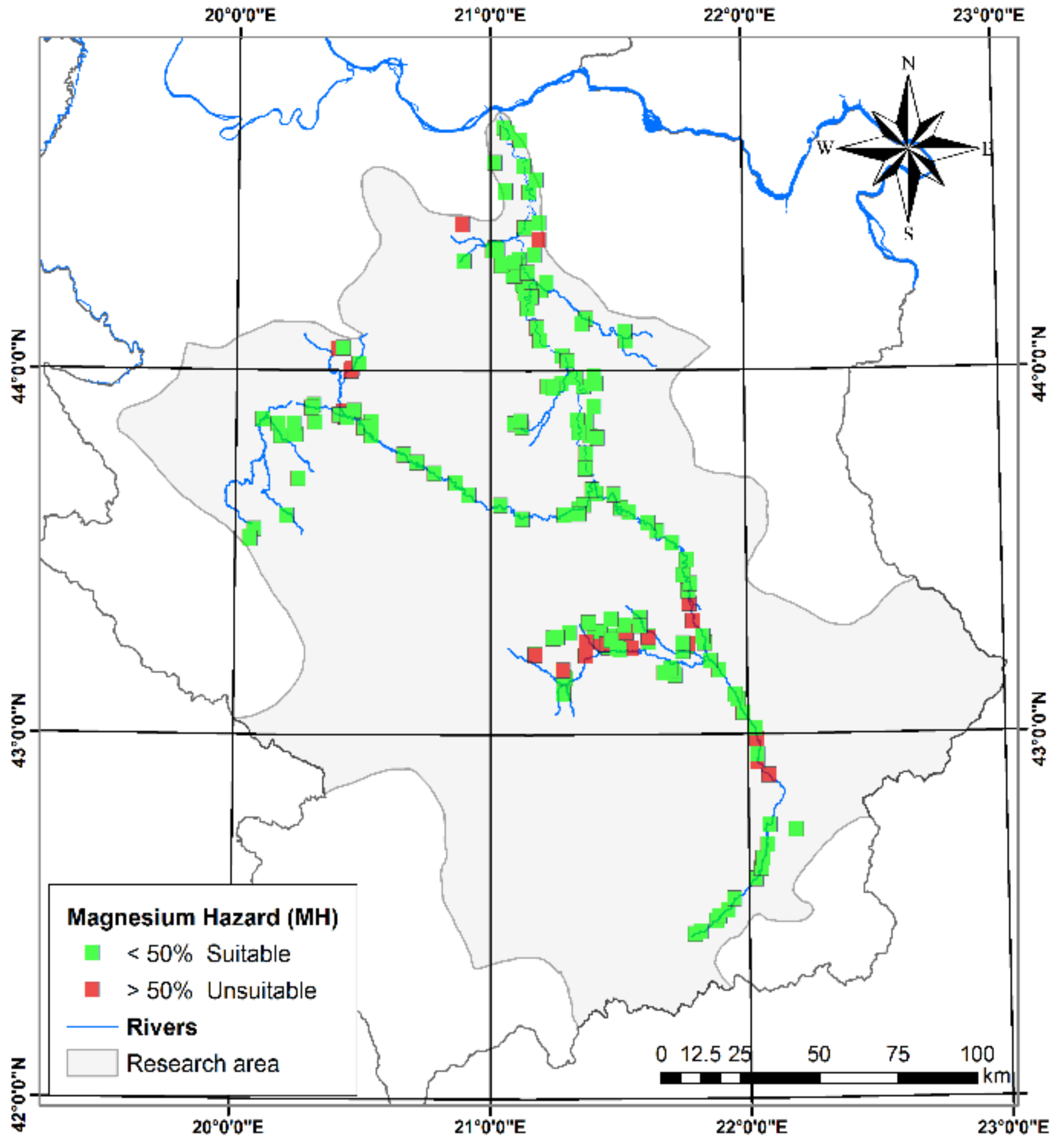
3.1.7. Magnesium Hazard (MH)
3.1.8. Kelley Index (KI)
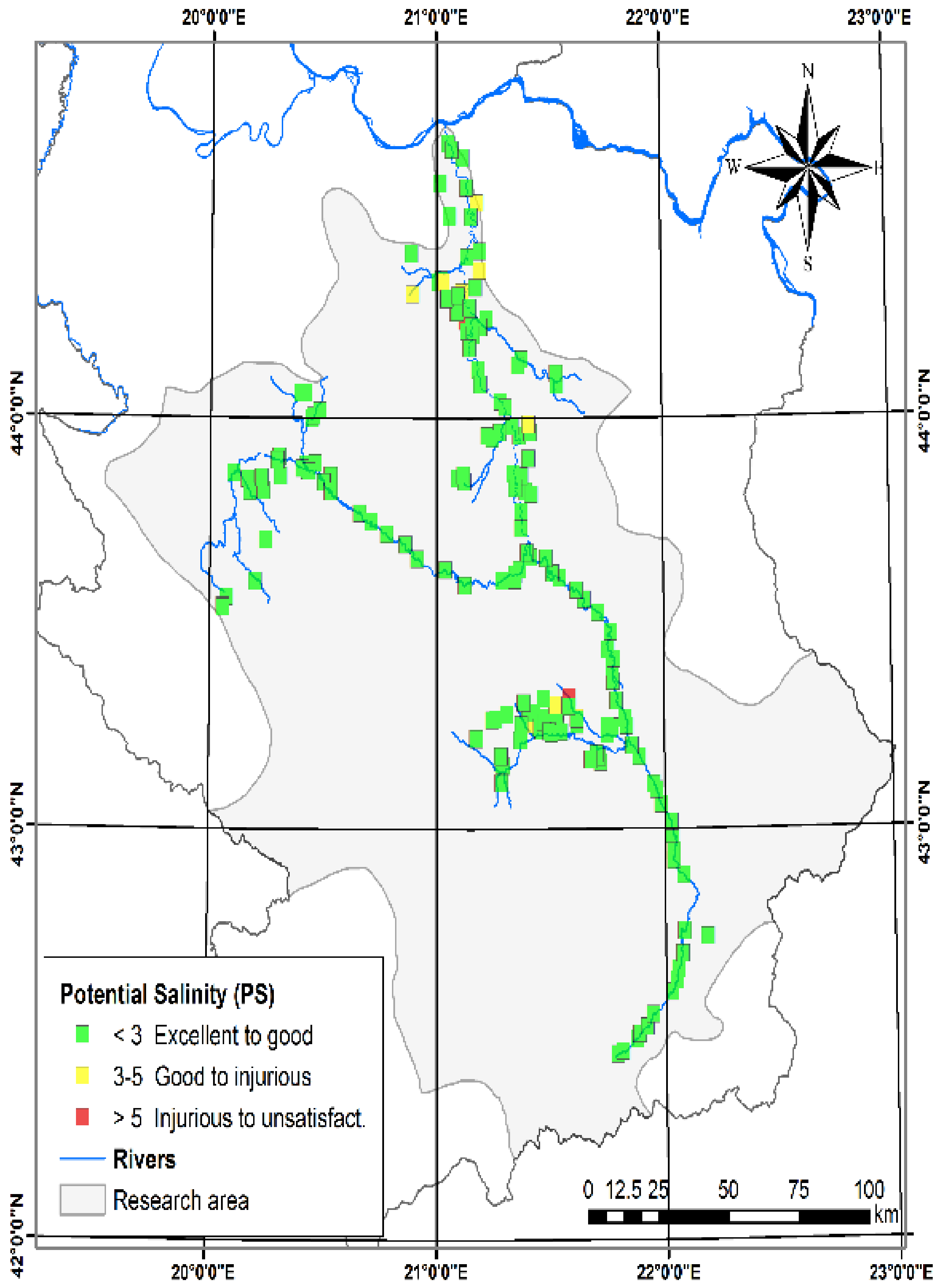
3.1.9. Potential Salinity (PS)
3.1.10. USSL Salinity Diagram
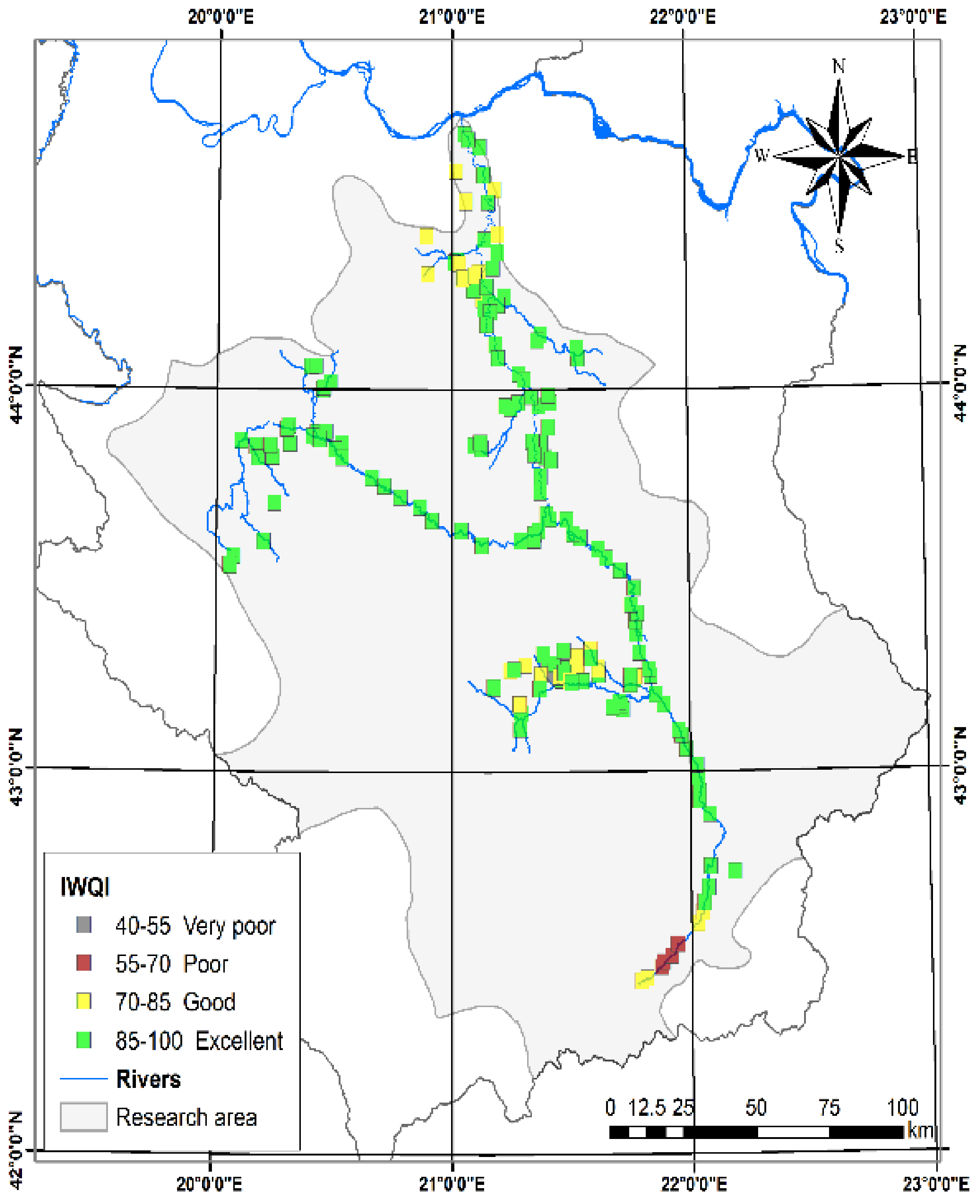
3.1.11. Irrigation Water Quality Index (IWQI)
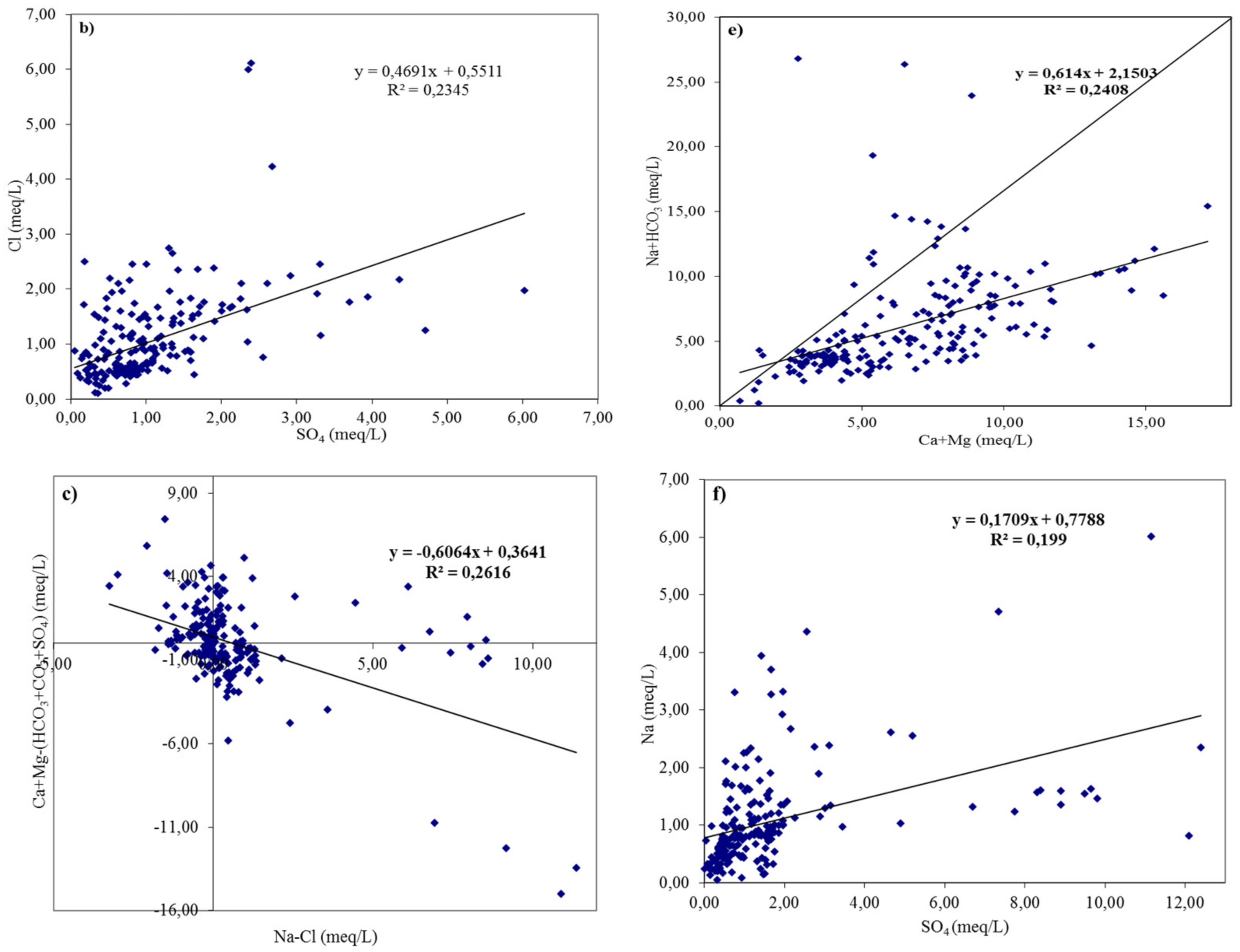
3.2. Geochemical Facies and Controlling Mechanisms
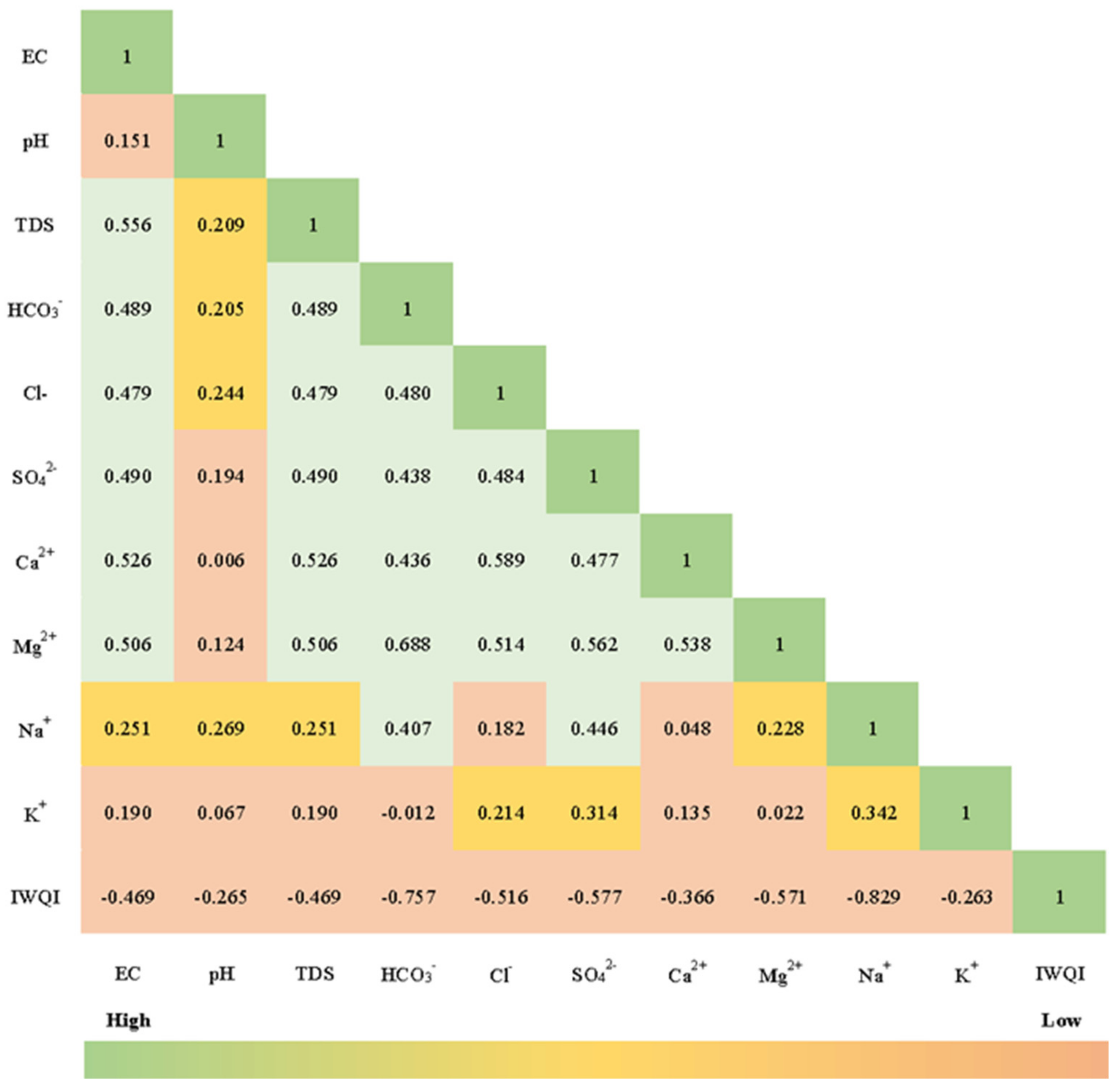
3.3. Multivariate Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pivić, R.; Dinić, Z.; Stanojković Sebić, A. Irrigation Water Quality of Drina River in Republic of Serbia; LAP Lambert Academic Publishing, OmniScriptum GmbH & Co KG: Sunnyvale, CA, USA; Saarbrücken, Germany, 2017; p. 84. [Google Scholar]
- Pivić, R.; Stanojković Sebić, A.; Maksimović, J.; Dinić, D. Soils of central Serbia areas and quality of available water for irrigation. Serbian Academy of Sciences and Arts. In Rational Use of Land and Water in Serbia, Proceedings of the III Scientific Meeting of the Department of Chemical and Biological Sciences, Banja Koviljaca, Serbia, 25 September 2020; Serbian Academy of Sciences and Arts: Belgrade, Serbia, 2020; pp. 71–87. (In Serbian) [Google Scholar]
- Statistical Office of the Republic of Serbia. Report of Irrigation in 2021; Statistical Office Republic of Serbia: Belgrade, Serbia, No. 002-year LXXII; 2021. (In Serbian) [Google Scholar]
- Ramakrishnaiah, C.R.; Adashiv, C.; Ranganna, G. Assessment of water quality index for the groundwater in Tumkur Taluk, Karnataka State, India. E-J. Chem. 2009, 6, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; FAO Irrigation and Drainage Paper: Rome, Italy, 1985; p. 29. [Google Scholar]
- Mirabbasi, R.; Mazloumzadeh, S.M.; Rahnama, M.B. Evaluation of irrigation water quality using fuzzy logic. Res. J. Environ. Sci. 2008, 2, 340–352. [Google Scholar] [CrossRef] [Green Version]
- Yıldız, S.; Karakuş, C.B. Estimation of irrigation water quality index with development of an optimum model: A case study. Environ. Dev. Sustain. 2020, 22, 4771–4786. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Jarvie, H.P.; Whitton, B.A.; Neal, C. Nitrogen and phosphorus in east coast British rivers: Speciation, sources and biological significance. Sci. Total Environ. 1998, 210–211, 79–109. [Google Scholar] [CrossRef]
- Simeonova, V.; Stratisb, J.A.; Samarac, C.; Zachariadisb, G.; Voutsac, D.; Anthemidis, A.; Sofonioub, M.; Kouimtzisc, T. Assessment of the surface water quality in Northern Greece. Water Res. 2003, 37, 4119–4124. [Google Scholar] [CrossRef]
- Ghazaryan, K.; Chen, Y. Hydrochemical assessment of surface water for irrigation purposes and its influence on soil salinity in Tikanlik oasis, China. Environ. Earth Sci. 2016, 75, 383. [Google Scholar] [CrossRef]
- Amezketa, E. An integrated methodology for assessing soil salinization, a pre-condition for land desertification. J. Arid. Environ. 2006, 67, 594–606. [Google Scholar] [CrossRef]
- Singh, S.; Ghosh, N.C.; Gurjar, S.; Krishan, G.; Kumar, S.; Berwal, P. Index-based assessment of suitability of water quality for irrigation purpose under Indian conditions. Environ. Monit. Assess. 2018, 190, 29. [Google Scholar] [CrossRef]
- Singh, A.K.; Mondal, G.C.; Kumar, S.; Singh, T.B.; Tewary, B.K.; Sinha, A. Major ion chemistry, weathering processes and water quality assessment in upper catchment of Damodar River basin, India. Environ Geol. 2008, 54, 745–758. [Google Scholar] [CrossRef]
- Etteieb, S.; Cherif, S.; Tarhouni, J. Hydrochemical assessment of water quality for irrigation: A case study of the Medjerda River in Tunisia. Appl. Water Sci. 2017, 7, 469–480. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Ma, L.; Abuduwaili, J.; Ge, Y.; Issanova, G.; Saparov, G. Hydrochemical characteristics and irrigation suitability of surface water in the Syr Darya River, Kazakhstan. Environ. Monit. Assess. 2019, 191, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gad, M.; El-Hattab, M. Integration of water pollution indices and DRASTIC model for assessment of groundwater quality in El Fayoum Depression, Western Desert, Egypt. J. Afr. Earth Sci. 2019, 158, 103554. [Google Scholar] [CrossRef]
- Hem, J.D. Study and Interpretation of the Chemical Characteristics of Natural Water; Department of the Interior, US Geological: Alexandria, VA, USA, 1985; Volume 2254, pp. 117–120.
- Cieszynska, M.; Wesolowski, M.; Bartoszewicz, M.; Michalska, M.; Nowacki, J. Application of physicochemical data for water-quality assessment of watercourses in the Gdansk Municipality (South Baltic coast). Environ. Monit. Assess. 2012, 184, 2017–2029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Wu, J.; Qian, H. Assessment of groundwater quality for irrigation purposes and identification of hydrogeochemical evolution mechanisms in Pengyang County, China. Environ. Earth Sci. 2013, 69, 2211–2225. [Google Scholar] [CrossRef]
- Fakhre, A. Evaluation of hydrogeochemical parameters of groundwater for suitability of domestic and irrigational purposes: A case study from central Ganga Plain, India. Arab. J. Geosci. 2014, 7, 4121–4131. [Google Scholar] [CrossRef]
- Saeedi, M.; Abessi, O.; Sharifi, F.; Meraji, H. Development of groundwater quality index. Environ. Monit. Assess. 2010, 163, 327–335. [Google Scholar] [CrossRef]
- Meireles, A.; Andrade, E.M.; Chaves, L.; Frischkorn, H.; Crisostomo, L.A. A new proposal of the classification of irrigation water. Rev. Cienc. Agron. 2010, 413, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Abbasnia, A.; Radfard, M.; Mahvi, A.H.; Nabizadeh, R.; Yousefi, M.; Soleimani, H.; Alimohammadi, M. Groundwater quality assessment for irrigation purposes based on irrigation water quality index and its zoning with GIS in the villages of Chabahar, Sistan and Baluchistan, Iran. Data Brief 2018, 19, 623–631. [Google Scholar] [CrossRef]
- Batarseh, M.; Imreizeeq, E.; Tilev, S.; Al Alaween, M.; Wael Suleiman, W.; Al Remeithi, A.M.; Al Tamimi, M.K.; Al Alawneh, M. Assessment of groundwater quality for irrigation in the arid regions using irrigation water quality index (IWQI) and GIS-Zoning maps: Case study from Abu Dhabi Emirate, UAE. Groundw. Sustain. Dev. 2021, 14, 100611. [Google Scholar] [CrossRef]
- Hussein, H.A.; Ricka, A.; Kuchovsky, T.; El Osta, M.M. Groundwater hydrochemistry and origin in the south-eastern part of Wadi El Natrun, Egypt. Arab. J. Geosci. 2017, 10, 170–184. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and Improvement of Saline Alkali Soils. In Agriculture Handbook; US Department of Agriculture: Washington, DC, USA, 1954; Volume 60, p. 160. [Google Scholar]
- Wilcox, L.V. Classification and Use of Irrigation Waters; U.S. Department of Agriculture: Washington, DC, USA, 1955; p. 367.
- Doneen, L.D. Notes on Water Quality in Agriculture. Published as a Water Science and Engineering; Department of Water Sciences and Engineering, University of California: Oakland, CA, USA, 1964; Volume 4001. [Google Scholar]
- Gibbs, R.J. Mechanisms controlling world water chemistry. Science 1970, 170, 10881. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.; El Osta, M. Geochemical controlling mechanisms and quality of the groundwater resources in El Fayoum Depression, Egypt. Arab. J. Geosci. 2020, 13, 861. [Google Scholar] [CrossRef]
- Kamtchueng, B.T.; Fantong, W.Y.; Wirmvem, M.J. Hydrogeochemistry and quality of surface water and groundwater in the vicinity of Lake Monoun, West Cameroon: Approach from multivariate statistical analysis and stable isotopic characterization. Environ. Monit. Assess. 2016, 188, 524. [Google Scholar] [CrossRef]
- Rakotondrabe, F.; Ngoupayou, J.R.; Mfonka, Z. Water quality assessment in the Betare-Oya gold mining area (East-Cameroon): Multivariate statistical analysis approach. Sci. Total Environ. 2018, 610, 831–844. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Tian, R.; Liu, R. Solute geochemistry and multivariate analysis of water quality in the Guohua Phosphorite Mine, Guizhou Province, China. Expo. Health 2019, 11, 81–94. [Google Scholar] [CrossRef]
- Tanasijević, Đ.; Antonović, G.; Aleksić, Ž.; Pavićević, N.; Filipović, Đ.; Spasojević, M. The Soils of the West and Northwestern Serbia; Institute of Soil Science in Topčider: Belgrade, Serbia, 1966. (In Serbian) [Google Scholar]
- Tanasijević, Đ.; Antonović, G.; Kovačević, R.; Aleksić, Ž.; Pavićević, N.; Filipović, Đ.; Jeremić, M.; Vojinović, Ž.; Spasojević, M. The Soils of the Velika Morava and Mlava basin; Institute of Soil Science in Topčider: Belgrade, Serbia, 1965. (In Serbian) [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014. International Soil Classification System for Naming soils and Creating Legends for Soil Maps. Update 2015; World Soil Resources Reports; FAO: Rome, Italy, 2015; Volume 106. [Google Scholar]
- Z1.111:1987; Measurement of pH—Potentiometric Method. Institute for Standardization of Serbia SRPS: Belgrade, Serbia, 1987.
- SRPS EN 27888:1993; Determination of Electrical Conductivity. Institute for Standardization of Serbia SRPS: Belgrade, Serbia, 1993.
- APHA. Standard Methods for the Examination of Water and Wastewater, 18th ed.; Greenberg, A.E., Clesceri, L.S., Eaton, A.D., Eds.; American Public Health Association: Washington, DC, USA, 1992. [Google Scholar]
- Rhoades, J.D.; Kandiah, A.; Mashali, M. The Use of Saline Waters for Crop Production—FAO Irrigation & Drainage Paper; FAO: Rome, Italy, 1992; Volume 48. [Google Scholar]
- ISO 11885:2007; Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). ISO: Geneva, Switzerland, 2007.
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2005.
- Li, P.; Zhang, Y.; Yang, N.; Jing, L.; Yu, P. Major ion chemistry and quality assessment of groundwater in and around a mountainous tourist town of China. Expo. Health 2016, 8, 239–252. [Google Scholar] [CrossRef]
- Deutsch, W.J. Groundwater Geochemistry Fundamentals and Application to Contamination; Lewis Publishers: Boca Raton, FL, USA, 1997. [Google Scholar]
- Raghunath, H.M. Groundwater, 2nd ed.; Wiley Eastern Ltd.: New Delhi, India, 1987; p. 563. [Google Scholar]
- Kelley, W.P. Use of saline irrigation water. Soil Sci. 1963, 95, 385–391. [Google Scholar] [CrossRef]
- Durfor, C.N.; Becker, E. Public Water Supplies of the 100 Largest Cities in the United States. Water Supply Paper; Government Publishing Office: Washington, DC, USA, 1964; Volume 1812, pp. 343–346. [CrossRef]
- Begum, A.; Harikrishna, R. Study on the Quality of Water in Some Streams of Cauvery River. J. Chem. 2008, 2, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Narsimha, A.; Sudarshan, V. Contamination of fluoride in groundwater and its effect on human health: A case study in hard rock aquifers of Siddipet, Telangana State, India. Appl. Water Sci. 2017, 7, 2501–2512. [Google Scholar] [CrossRef] [Green Version]
- Adimalla, N.; Venkatayogi, S. Geochemical characterization and evaluation of groundwater suitability for domestic and agricultural utility in semi-arid region of Basara, Telangana State, South India. Appl. Water Sci. 2018, 8, 44. [Google Scholar] [CrossRef] [Green Version]
- Sharma, D.A.; Rishi, M.S.; Keesari, T. Evaluation of groundwater quality and suitability for irrigation and drinking purposes in southwest Punjab, India using hydrochemical approach. Appl. Water Sci. 2017, 7, 3137–3150. [Google Scholar] [CrossRef] [Green Version]
- Almeida, C.; Quintar, S.; González, P.; Mallea, M. Assessment of irrigation water quality. A proposal of a quality profile. Environ. Monit. Assess. 2008, 142, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Todd, D.K. Groundwater Hydrology, 1st ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 1960; p. 336. [Google Scholar]
- González-Acevedo, Z.I.; Padilla-Reyes, D.A.; Ramos-Leal, J.A. Quality assessment of irrigation water related to soil salinization in Tierra Nueva, San Luis Potosí, Mexico. Rev. Mex. De Cienc. Geológicas 2016, 33, 271–285. [Google Scholar]
- Rao, N.K.; Latha, P.S. Groundwater quality assessment using water quality index with a special focus on vulnerable tribal region of Eastern Ghats hard rock terrain, Southern India. Arab. J. Geosci. 2019, 12, 267. [Google Scholar] [CrossRef]
- Sutradhar, S.; Mondal, P. Groundwater suitability assessment based on water quality index and hydrochemical characterization of Suri Sadar Sub-division, West Bengal. Ecol. Inform. 2021, 64, 101335. [Google Scholar] [CrossRef]
- Vasanthavigar, M.; Srinivasamoorthy, K.; Prasanna, M.V. Evaluation of groundwater suitability for domestic, irrigational, and industrial purposes: A case study from Thirumanimuttar river basin, Tamilnadu, India. Environ Monit Assess. 2012, 184, 405–420. [Google Scholar] [CrossRef]
- Taloor, A.K.; Pir, R.A.; Adimalla, N.; Ali, S.; Manhas, D.S.; Roy, S.; Singh, A.K. Spring water quality and discharge assessment in the Basantar watershed of Jammu Himalaya using geographic information system (GIS) and water quality Index (WQI). Groundw. Sustain. Dev. 2020, 10, 100364. [Google Scholar] [CrossRef]
- Salifu, M.; Aidoo, F.; Hayford, M.S.; Adomako, D.; Asare, E. Evaluating the suitability of groundwater for irrigational purposes in some selected districts of the Upper West region of Ghana. Appl. Water Sci. 2017, 7, 653–662. [Google Scholar] [CrossRef]
- Eaton, F.M. Significance of carbonates in irrigation waters. Soil Sci. 1950, 69, 123–134. [Google Scholar] [CrossRef]
- Singaraja, C. Relevance of water quality index for groundwater quality evaluation: Thoothukudi District, Tamil Nadu, India. Appl. Water Sci. 2017, 7, 2157–2173. [Google Scholar] [CrossRef]
- Ravikumar, P.; Mehmood, M.A.; Somasheka, R.K. Water quality index to determine the surface water quality of Sankey tank and Mallathahalli lake, Bangalore urban district, Karnataka, India. Appl. Water Sci. 2013, 3, 247–261. [Google Scholar] [CrossRef] [Green Version]
- Rawat, K.S.; Singh, S.K.; Gautam, S.K. Assessment of groundwater quality for irrigation use: A peninsular case study. Appl. Water Sci. 2018, 8, 233. [Google Scholar] [CrossRef] [Green Version]
- Singh, K.K.; Tewari, G.; Kumar, S. Evaluation of groundwater quality for suitability of irrigation purposes: A Case Study in the Udham Singh Nagar, Uttarakhand. J. Chem. 2020, 2020, 6924026. [Google Scholar] [CrossRef]
- Li, P.; Wu, J.; Qian, H.; Zhang, Y.; Yang, N.; Jing, L.; Yu, P. Hydrogeochemical characterization of groundwater in and around a wastewater irrigated forest in the southeastern edge of the Tengger Desert, Northwest China. Expo. Health 2016, 8, 331–348. [Google Scholar] [CrossRef]
- El-Amier, Y.A.; Kotb, W.K.; Bonanomi, G.; Fakhry, H.; Marraiki, N.A.; Abd-ElGawad, A.M. Hydrochemical Assessment of the Irrigation Water Quality of the El-Salam Canal, Egypt. Water 2021, 13, 2428. [Google Scholar] [CrossRef]
- Szabolcs, I.; Darab, C. The Influence of Irrigation Water of High Sodium Carbonate Content of Soils. In Proceedings of the 8th International Congress of ISSS, Bucharest, Romania, 31 August–9 September 1964; Volume II, pp. 803–812. [Google Scholar]
- Keesari, T.; Kulkarni, U.P.; Deodhar, A.; Ramanjaneyulu, P.S.; Sanjukta, A.K.; Kumar, U.S. Geochemical characterization of groundwater from an arid region in India. Environ. Earth Sci. 2014, 71, 4869–4888. [Google Scholar] [CrossRef]
- Kelley, W.P. Permissible Composition and Concentration of Irrigated Waters. Proc. Am. Soc. Civ. Eng. 1940, 66, 607–613. [Google Scholar]
- Dhembare, A.J. Assessment of water quality indices for irrigation of Dynaneshwar Dam Water, Ahmednagar, Maharashtra, India. Arch. Appl. Sci. Res. 2012, 4, 348–352. [Google Scholar]
- Sundaray, S.K.; Nayak, B.B.; Bhatta, D. Environmental studies on river water quality with reference to suitability for agricultural purposes: Mahanadi river estuarine system, India—A case study. Environ. Monit. Assess. 2009, 155, 227–243. [Google Scholar] [CrossRef]
- He, S.; Li, P. A MATLAB based graphical user interface (GUI) for quickly producing widely used hydrogeochemical diagrams. Geochemistry 2019, 80, 125550. [Google Scholar] [CrossRef]
- Sadashivaiah, C.; Ramakrishnaiah, C.; Ranganna, G. Hydrochemical analysis and evaluation of groundwater quality in tumkur taluk, Karnataka state, India. Int. J. Environ. Res. Publ. Health 2008, 5, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Adimalla, N.; Taloor, A.K. Hydrogeochemical investigation of groundwater quality in the hard rock terrain of South India using Geographic Information System (GIS) and groundwater quality index (GWQI) techniques. Groundw. Sustain. Dev. 2020, 10, 100288. [Google Scholar] [CrossRef]
- Stoner, J.D. Water-Quality Indices for Specific Water Uses; Department of the Interior, Geological Survey: Arlington, VA, USA, 1978; p. 12. [CrossRef] [Green Version]
- Bortolini, L.; Maucieri, C.; Borin, M. A tool for the evaluation of irrigation water quality in the arid and semi-arid regions. Agronomy 2018, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Spandana, M.P.; Suresh, K.R.; Prathima, B. Developing an irrigation water quality index for vrishabavathi command area. Int. J. Eng. Res. Technol. 2013, 2, 821–830. [Google Scholar]
- Zaman, M.; Shahid, S.A.; Heng, L. Irrigation Water Quality. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer International Publishing: Cham, Switzerland, 2018; p. 113. [Google Scholar] [CrossRef] [Green Version]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water-analyses. EOS Trans. Am. Geophys. Union. 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Appelo, C.A.J.; Postma, D.; Balkema, A.A. Geochemistry, Groundwater and Pollution; Balkema, A.A., Ed.; Cambridge University Press: Rotterdam, The Netherlands, 1993; p. 536. [Google Scholar]
- El Osta, M.; Milad Masoud, M.; Alqarawy, A.; Elsayed, S.; Gad, M. Groundwater Suitability for Drinking and Irrigation Using Water Quality Indices and Multivariate Modeling in Makkah Al-Mukarramah Province, Saudi Arabia. Water 2022, 14, 483. [Google Scholar] [CrossRef]
- Chadha, D.K. A proposed new diagram for geochemical classification of natural waters and interpretation of chemical data. Hydrogeol. J. 1999, 7, 431–439. [Google Scholar] [CrossRef]
- Varol, S.; Davraz, A. Assessment of geochemistry and hydrogeochemical processes in groundwater of the Tefenni plain (Burdur/Turkey). Environ. Earth Sci. 2014, 71, 4657–4673. [Google Scholar] [CrossRef]
- Roy, A.; Keesari, T.; Mohokar, H.; Sinha, U.K.; Bitra, S. Assessment of groundwater quality in hard rock aquifer of central Telangana state for drinking and agriculture purposes. Appl. Water Sci. 2018, 8, 124. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Huang, Q.; Lin, Y.; Fang, Y.; Qian, H.; Liu, R.; Ma, H. Hydrogeochemical Characteristics and Quality Assessment of Groundwater in an Irrigated Region, Northwest China. Water 2019, 11, 96. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Feng, W.; Qian, H.; Zhang, Q. Hydrogeochemical Characterization and Irrigation Quality Assessment of Shallow Groundwater in the Central-Western Guanzhong Basin, China. Int. J. Environ. Res. Public Health 2019, 16, 1492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Li, P.; Qian, H. Hydrochemical characterization of drinking groundwater with special reference to fluoride in an arid area of China and the control of aquifer leakage on its concentrations. Environ. Earth Sci. 2015, 73, 8575–8588. [Google Scholar] [CrossRef]
- Nurlan, S.S. Current hydroecological state of the lower watercourse of Syrdarya and use of her resources of the drain. Astrakhan Bull. Ecol. Educ. 2017, 2, 50–55. [Google Scholar]
- Issanova, G.; Jilili, R.; Abuduwaili, J.; Kaldybayev, A.; Saparov, G.; Yong Xiao, G. Water availability and state of water resources within water-economic basins in Kazakhstan. Paddy Water Environ. 2018, 16, 183–191. [Google Scholar] [CrossRef]
- Karlykhanov, O.K.; Toktaganova, G.B. The assessment of irrigated land salinization in the Aral Sea Region. Int. J. Environ. Sci. Educ. 2016, 11, 7946–7960. [Google Scholar]
- Pearson, K. Mathematical Contributions to the Theory of Evolution. III. Regression, Heredity, and Panmixia. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 1896, 187, 253–318. [Google Scholar] [CrossRef] [Green Version]
- Islam, A.R.M.T.; Mamun, A.A.; Rahman, M.M.; Zahid, A. Simultaneous comparison of modified-integrated water quality and entropy weighted indices: Implication for safe drinking water in the coastal region of Bangladesh. Ecol. Indic. 2020, 113, 106229. [Google Scholar] [CrossRef]
- Danielsson, Å.; Cato, I.; Carman, R.; Rahm, L. Spatial clustering of metals in the sediments of the Skagerrak/Kattegat. Appl. Geochem. 1999, 14, 689–706. [Google Scholar] [CrossRef]
- Lattin, J.M.; Carroll, J.D.; Green, P.E.; Green, P.E. Analyzing Multivariate Data; Thomson Brooks/Cole: Pacific Grove, CA, USA, 2003. [Google Scholar]





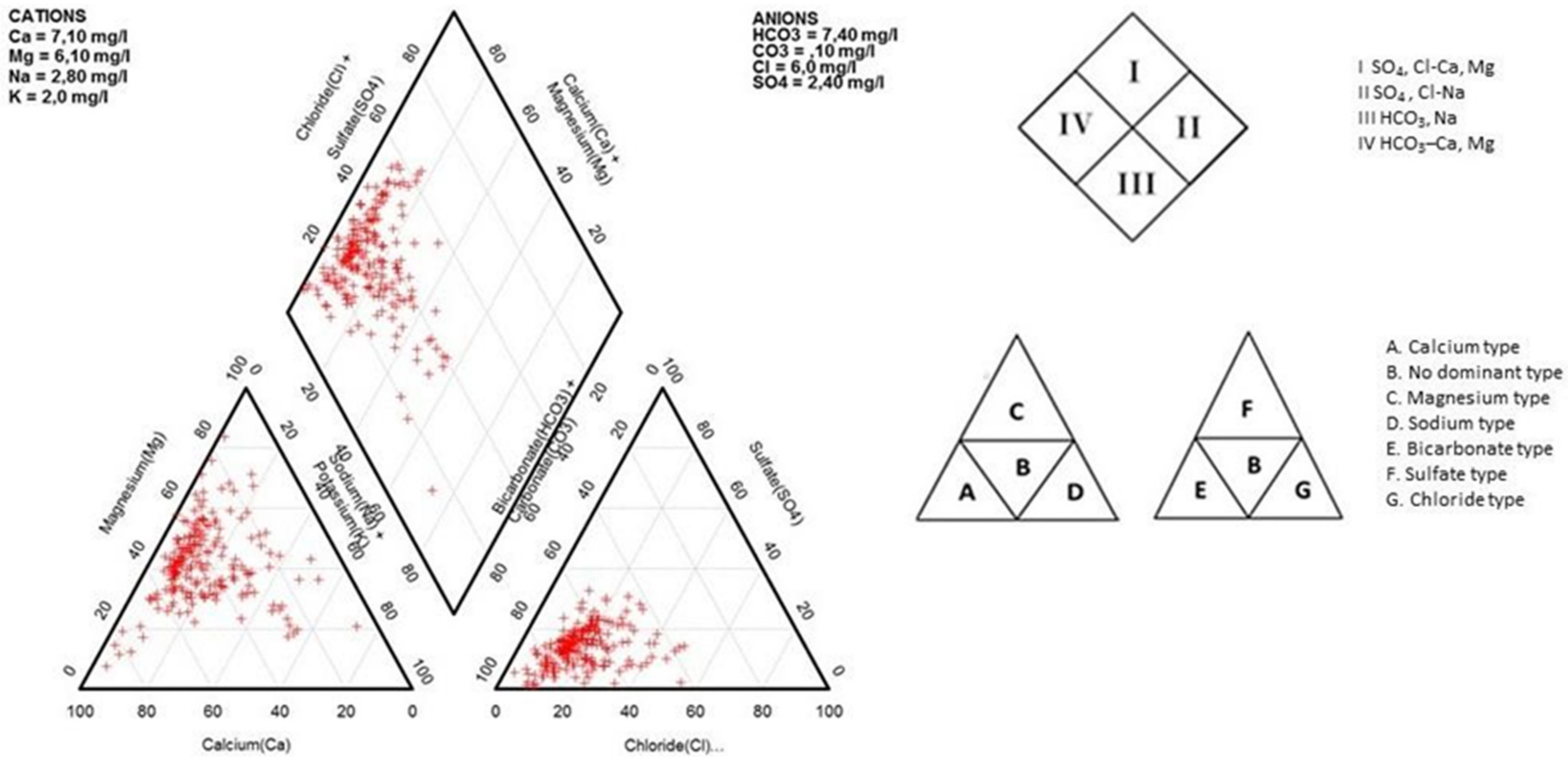
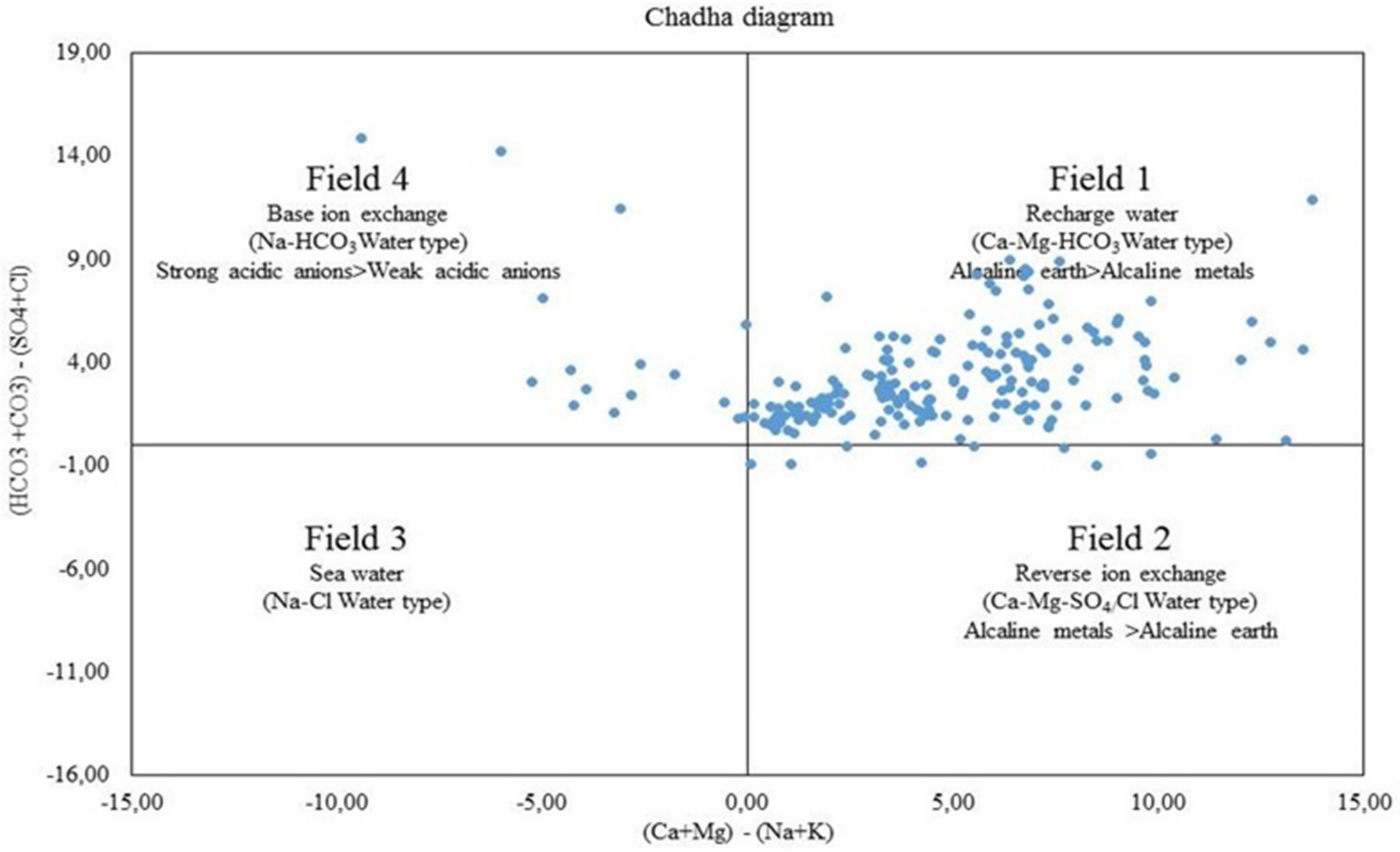
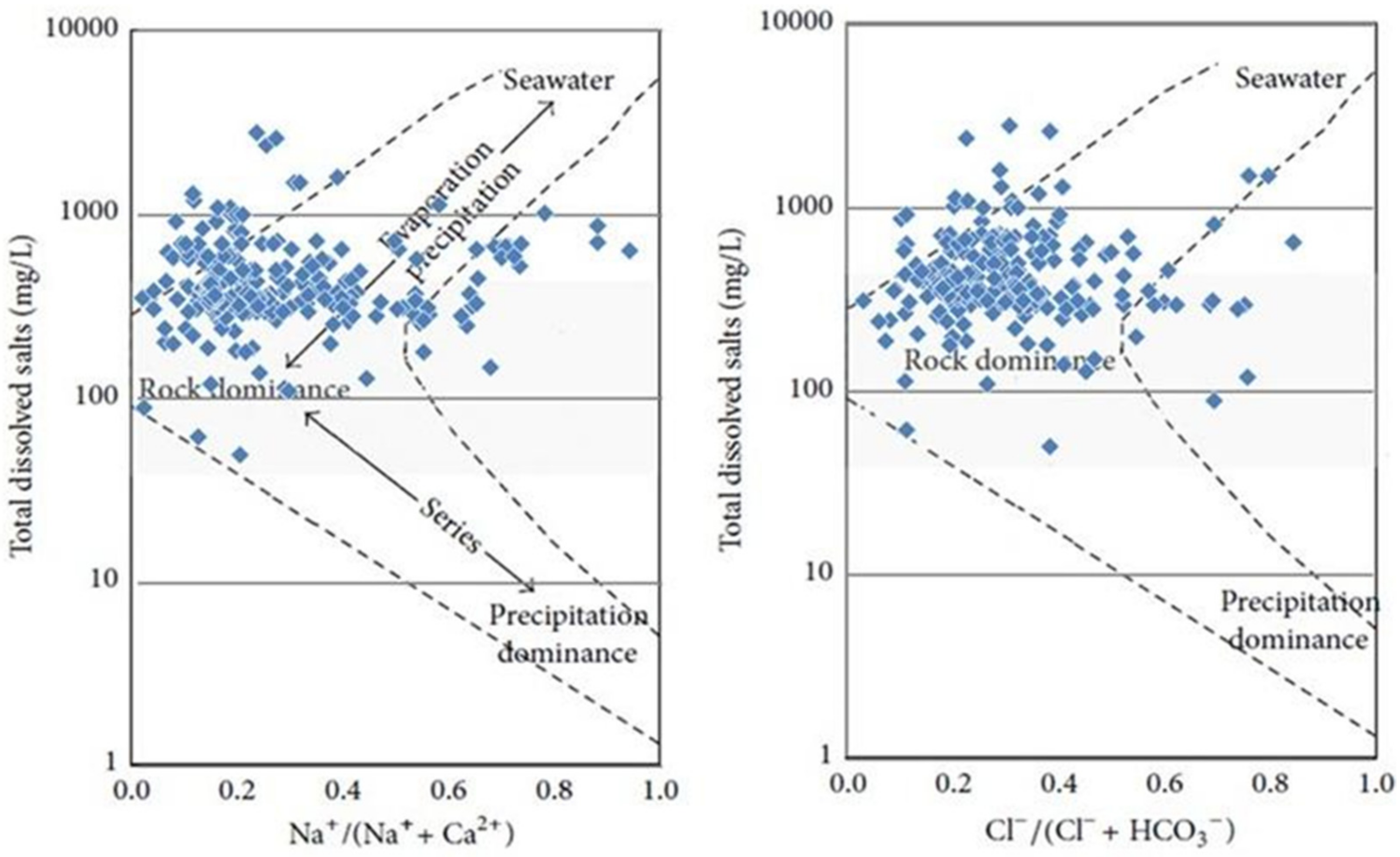
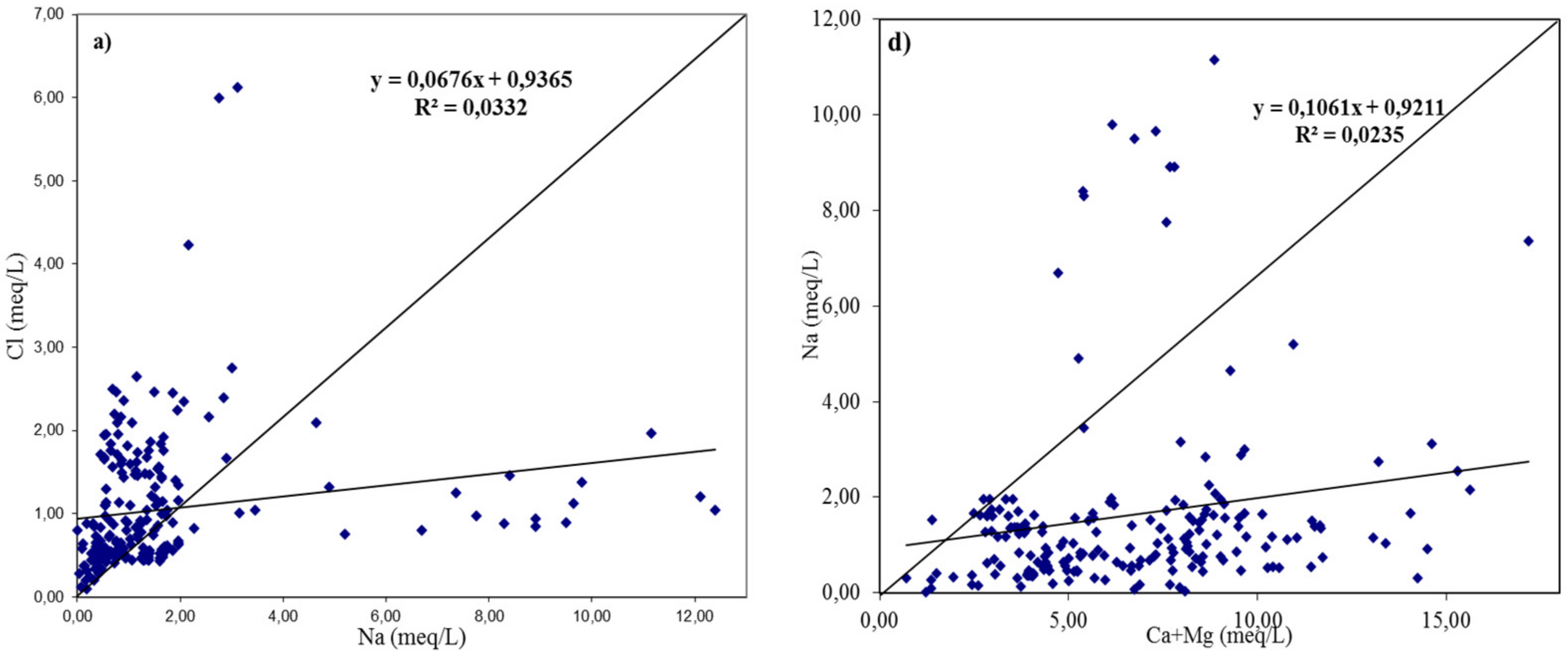

| qi | EC | SAR | Na | Cl | HCO3 |
|---|---|---|---|---|---|
| (μS m−1) | ((mmol L−1)0.5) | (meq L−1) | (meq L−1) | (meq L−1) | |
| 85–100 | [200–750) | [2–3) | [2–3) | [1–4) | [1–1.5) |
| 60–85 | [750–1500) | [3–6) | [3–6) | [4–7) | [1.5–4.5) |
| 35–60 | [1500–3000) | [6–12) | [6–9) | [7–10) | [4.5–8.5) |
| 0–35 | EC < 200 or | SAR < 2 or | Na < 2 or | Cl < 1 or | HCO3 < 1 or |
| EC ≥ 3000 | SAR ≥ 12 | Na ≥ 9 | Cl ≥ 10 | HCO3 ≥ 8.5 |
| Parameters | Wi |
|---|---|
| [EC] | 0.211 |
| [Na] | 0.204 |
| [HCO3] | 0.202 |
| [Cl] | 0.194 |
| [SAR] | 0.189 |
| Total | 1 |
| Parameters | Unit | Range | Mean | STDEV | |
|---|---|---|---|---|---|
| pH | / | 6.20–8.90 | 7.73 | ± | 0.47 |
| EC | µSm−1 | 20–2260 | 650.91 | ± | 370.02 |
| TDS | mg L−1 | 50–2800 | 497.42 | ± | 359.76 |
| CO32− | meq L−1 | 0.00–14.70 | 4.47 | ± | 2.49 |
| HCO3− | meq L−1 | 0.00–3.60 | 0.83 | ± | 0.56 |
| Cl− | meq L−1 | 0.10–6.12 | 1.04 | ± | 0.80 |
| SO42− | meq L−1 | 0.05–6.02 | 1.05 | ± | 0.82 |
| Ca2+ | meq L−1 | 0.46–11.03 | 3.85 | ± | 1.84 |
| Mg2+ | meq L−1 | 0.16–10.91 | 2.54 | ± | 1.71 |
| K+ | meq L−1 | 0.01–12.4 | 1.60 | ± | 2.15 |
| Na+ | meq L−1 | 0.01–4.30 | 0.48 | ± | 0.53 |
| Classification Pattern | Sample Range | Categories | Ranges | Description | Number of Samples | Sample (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| Min. | Max | Mean | STDEV | ||||||
| Sodium absorption ratio (SAR) [27] | 0.01 | 10.34 | 0.93 | 1.27 | Excellent | 0–10 | Don’t have sodium hazard | 214 | 99.53 |
| Good | 10–18 | Low sodium hazard | 1 | 0.47 | |||||
| Fair | 18–26 | Harmful for almost all types of soils | |||||||
| Poor | >26 | Unsuitable for irrigation | |||||||
| Percent sodium (% Na) [28] | 0.49 | 51.89 | 18.80 | 9.86 | Excellent | 0–20 | Excellent for irrigation | 136 | 63.26 |
| Good | 20–40 | Good for irrigation | 72 | 33.49 | |||||
| Permissible | 40–60 | Permissible for irrigation | 7 | 3,26 | |||||
| Doubtful | 60–80 | Doubtful for irrigation | |||||||
| Unsuitable | >80 | Unsuitable for irrigation | |||||||
| Residual sodium carbonate (RSC) [27] | −8.79 | 14.16 | −1.08 | 2.62 | Good | <1.25 | Generally safe for irrigation | 197 | 91.63 |
| Medium | 1.25–2.5 | Marginal as an irrigation source | |||||||
| Bad | >2.5 | Generally not suitable for irrigation without improvement | 18 | 8.37 | |||||
| Permeability index (PI) [29] | 0.07 | 1.19 | 0.48 | 0.17 | Class-I | >75 | Good for irrigation | 201 | 93.49 |
| Class-II | 25–75 | Suitable for irrigation | 9 | 4.19 | |||||
| Class-III | <25 | Unsuitable for irrigation | 5 | 2.33 | |||||
| Electrical conductivity (EC, µS cm−1) [28] | 20.00 | 2260.00 | 650.91 | 370.02 | Good | 250–750 | Medium salinity water | 135 | 62.79 |
| Permissible | 750–2250 | High-salinity water | 79 | 36.74 | |||||
| Doubtful | 2250–5000 | Doubtful for irrigation | 1 | 0.47 | |||||
| Unsuitable | >5000 | Unsuitable for irrigation | |||||||
| Total dissolved salts (TDS, mg L−1) [27] | 50.00 | 280000 | 497.42 | 359.76 | Excellent | <150 | Low salinity hazard | 8 | 3.72 |
| Good | 150–500 | Permissible for irrigation | 134 | 62.33 | |||||
| Fair | 500–1500 | Doubtful for irrigation | 69 | 32.09 | |||||
| Poor | >1500 | Unsuitable for irrigation | 4 | 1.86 | |||||
| Magnesium Hazard (MH) [46] | 4.94 | 77.68 | 38.35 | 12.55 | MH | <50% | Suitable | 173 | 85.12 |
| MH | >50% | Unsuitable | 32 | 14.88 | |||||
| Kelly’s Index (KI) [47] | 0.00 | 4.42 | 0.29 | 0.42 | KI | <1 | Suitable | 202 | 93.95 |
| KI | >1 | Unsuitable | 13 | 6.05 | |||||
| Potential Salinity (PS) (meq L−1) [29] | 0.28 | 7.32 | 1.57 | 1.06 | PS | <3.0 | Excellent to good | 197 | 91.63 |
| PS | 3.0–5.0 | Good to injurious | 15 | 6.98 | |||||
| PS | >5.0 | Injurious to unsatisfactory | 3 | 1.4 | |||||
| Total Hardness (TH) (meq L−1) [48] | 0.70 | 17.17 | 6.38 | 3.11 | TH | 0–60 | Soft | 215 | 100 |
| TH | 61–120 | Moderate | |||||||
| TH | 121–180 | Hard | |||||||
| TH | >181 | Very hard | |||||||
| IWQI [23] | 52.96 | 99.42 | 89.55 | 9.19 | ClassI | 85–100 | Excellent | 164 | 76.27 |
| ClassII | 70–85 | Good | 41 | 19.07 | |||||
| ClassIII | 55–70 | Poor | 7 | 3.26 | |||||
| ClassIV | 40–55 | Very poor | 3 | 1.4 | |||||
| ClassV | 0–40 | Unsuitable | |||||||
| IWQI | Exploitation Restrictions | Recommendation | |
|---|---|---|---|
| Soil | Crops | ||
| (85–100) | No restriction (NR) | Water can be used for almost all types of soil. Soil is exposed to lower risks of salinity/sodicity problems | No toxicity risk for most plants |
| (70–85) | Low restriction (LR) | Irrigated soils with a light texture or moderate permeability can be adapted to this range. To avoid soil sodicity in heavy textures, soil leaching is recommended. | Elevated risks for salt sensitive plants |
| (55–70) | Moderate restriction (MR) | The water in this range would be better used for soils with moderate to high permeability values. Moderate leaching of salts is highly recommended to avoid soil degradation. | Plants with moderate tolerance to salts may be grow |
| (40–55) | High restriction (HR) | This range of water can be used in soils with high permeability without compact layers. High frquency irrigation schedule | Suitable for irrigation of plants with moderate to high tolerance to salts with special salinity control practices, except water with low Na, Cl and HCO3 values |
| (0–40) | Severe restriction | Using this range of water for irrigaion under normal conditions should be avoided. | Only plants with high salt tolerance, except for waters with extremely low values of Na, Cl and HCO3−. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pivić, R.; Maksimović, J.; Dinić, Z.; Jaramaz, D.; Majstorović, H.; Vidojević, D.; Stanojković-Sebić, A. Hydrochemical Assessment of Water Used for Agricultural Soil Irrigation in the Water Area of the Three Morava Rivers in the Republic of Serbia. Agronomy 2022, 12, 1177. https://doi.org/10.3390/agronomy12051177
Pivić R, Maksimović J, Dinić Z, Jaramaz D, Majstorović H, Vidojević D, Stanojković-Sebić A. Hydrochemical Assessment of Water Used for Agricultural Soil Irrigation in the Water Area of the Three Morava Rivers in the Republic of Serbia. Agronomy. 2022; 12(5):1177. https://doi.org/10.3390/agronomy12051177
Chicago/Turabian StylePivić, Radmila, Jelena Maksimović, Zoran Dinić, Darko Jaramaz, Helena Majstorović, Dragana Vidojević, and Aleksandra Stanojković-Sebić. 2022. "Hydrochemical Assessment of Water Used for Agricultural Soil Irrigation in the Water Area of the Three Morava Rivers in the Republic of Serbia" Agronomy 12, no. 5: 1177. https://doi.org/10.3390/agronomy12051177
APA StylePivić, R., Maksimović, J., Dinić, Z., Jaramaz, D., Majstorović, H., Vidojević, D., & Stanojković-Sebić, A. (2022). Hydrochemical Assessment of Water Used for Agricultural Soil Irrigation in the Water Area of the Three Morava Rivers in the Republic of Serbia. Agronomy, 12(5), 1177. https://doi.org/10.3390/agronomy12051177






