Morphological and Biochemical Factors Associated with Constitutive Defense to Thrips in Alfalfa
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth and Treatments
2.2. Thrips Resistance Evaluation
| Severity Level | Severity Level (%) |
|---|---|
| 0 | 0 |
| 1 | 0~4 |
| 2 | 5~9 |
| 3 | 10~19 |
| 4 | 20~29 |
| 5 | 30~49 |
| 6 | 50~100 |
2.3. Morphological Measurements
3. Results
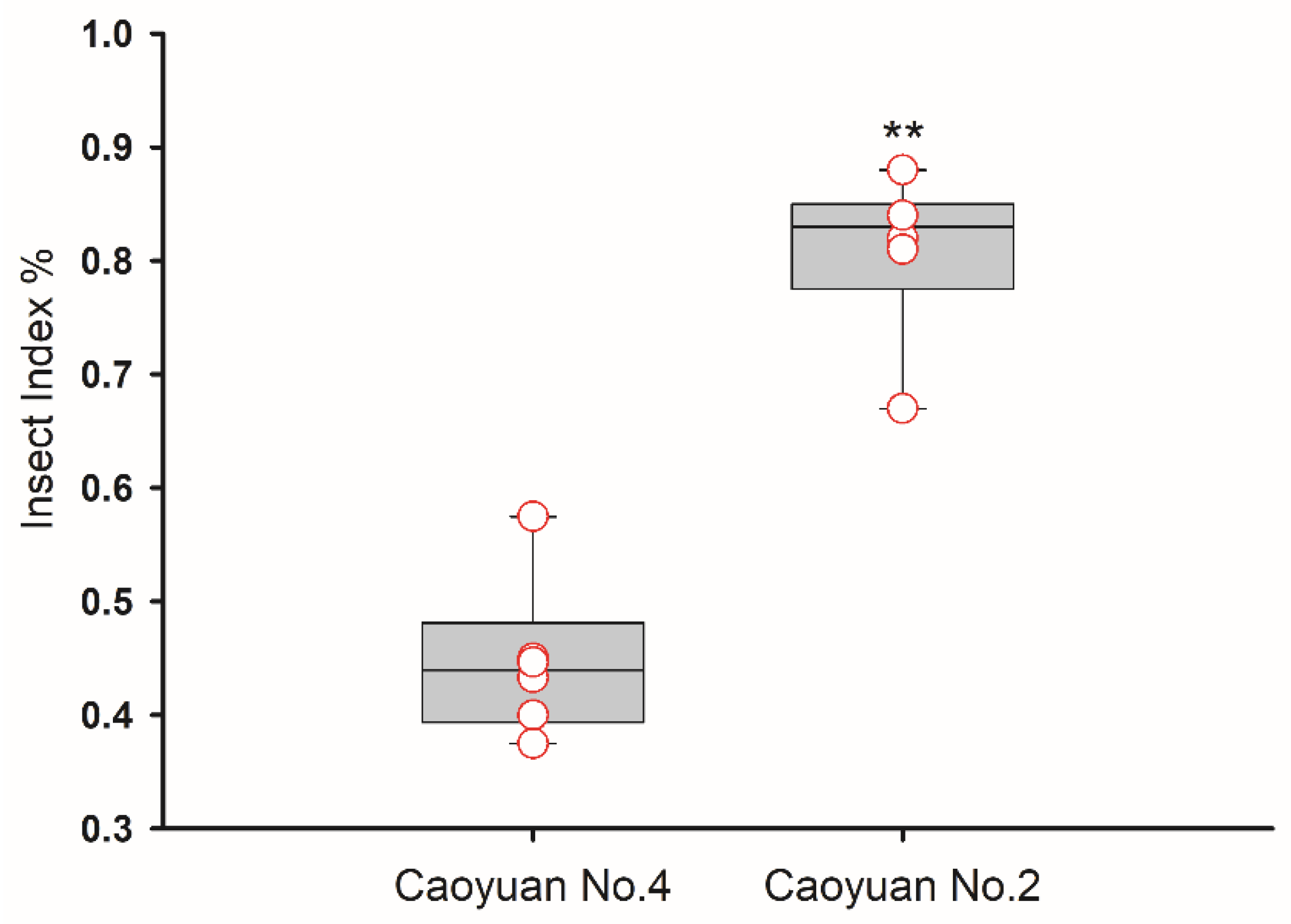
3.1. Thrips Resistance Identification of Caoyuan No.4 and Caoyuan No.2
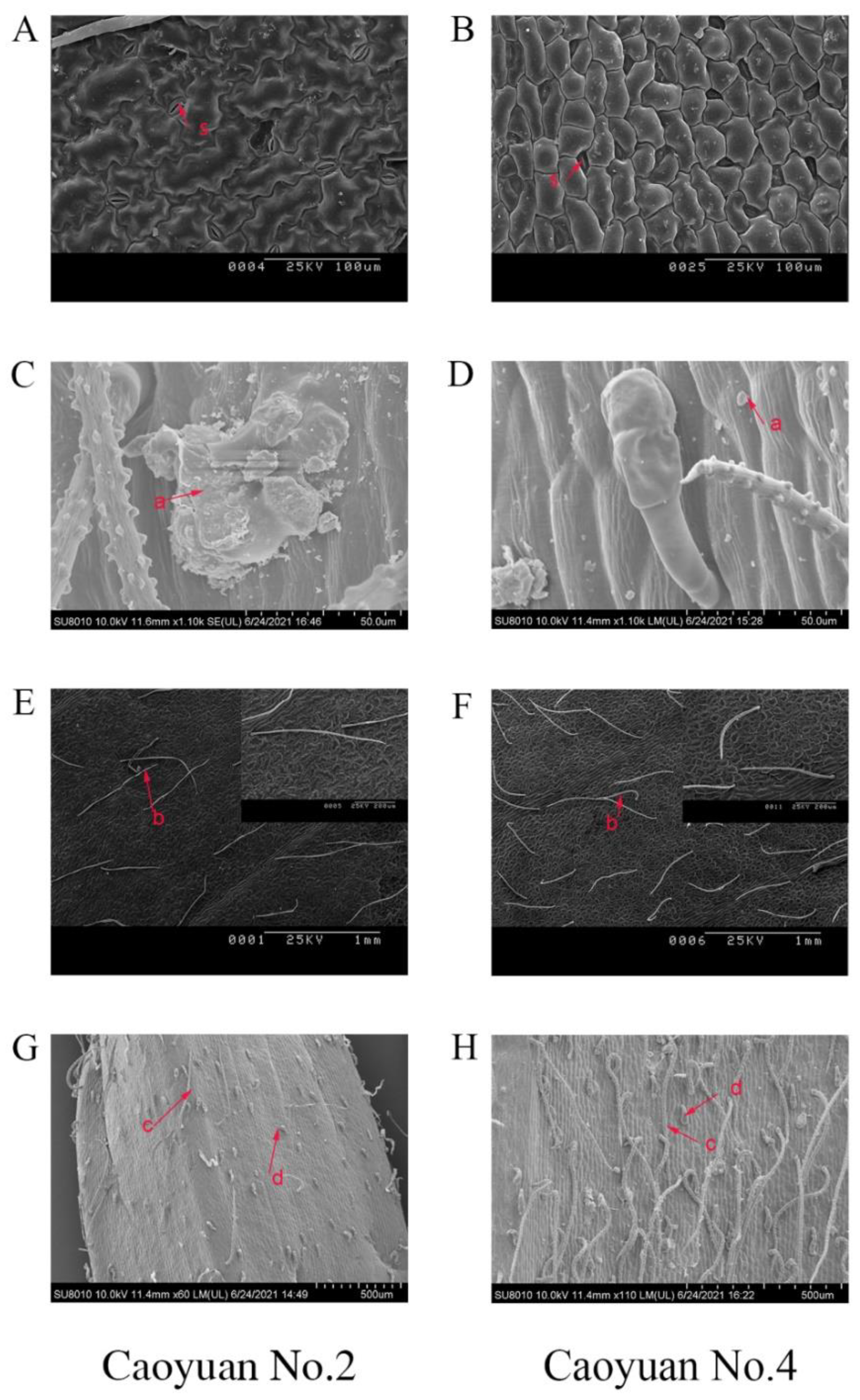
3.2. Morphological Factors
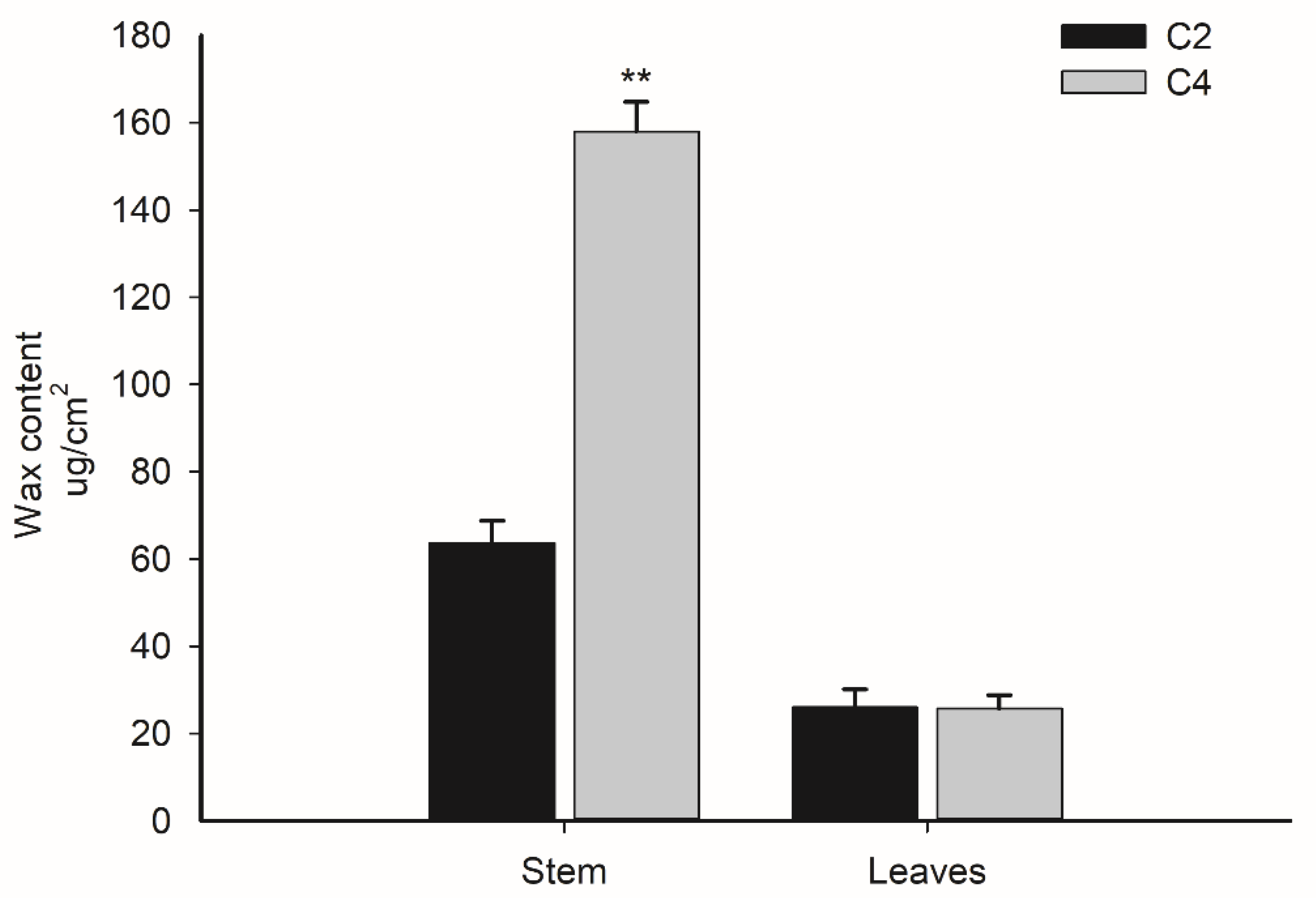
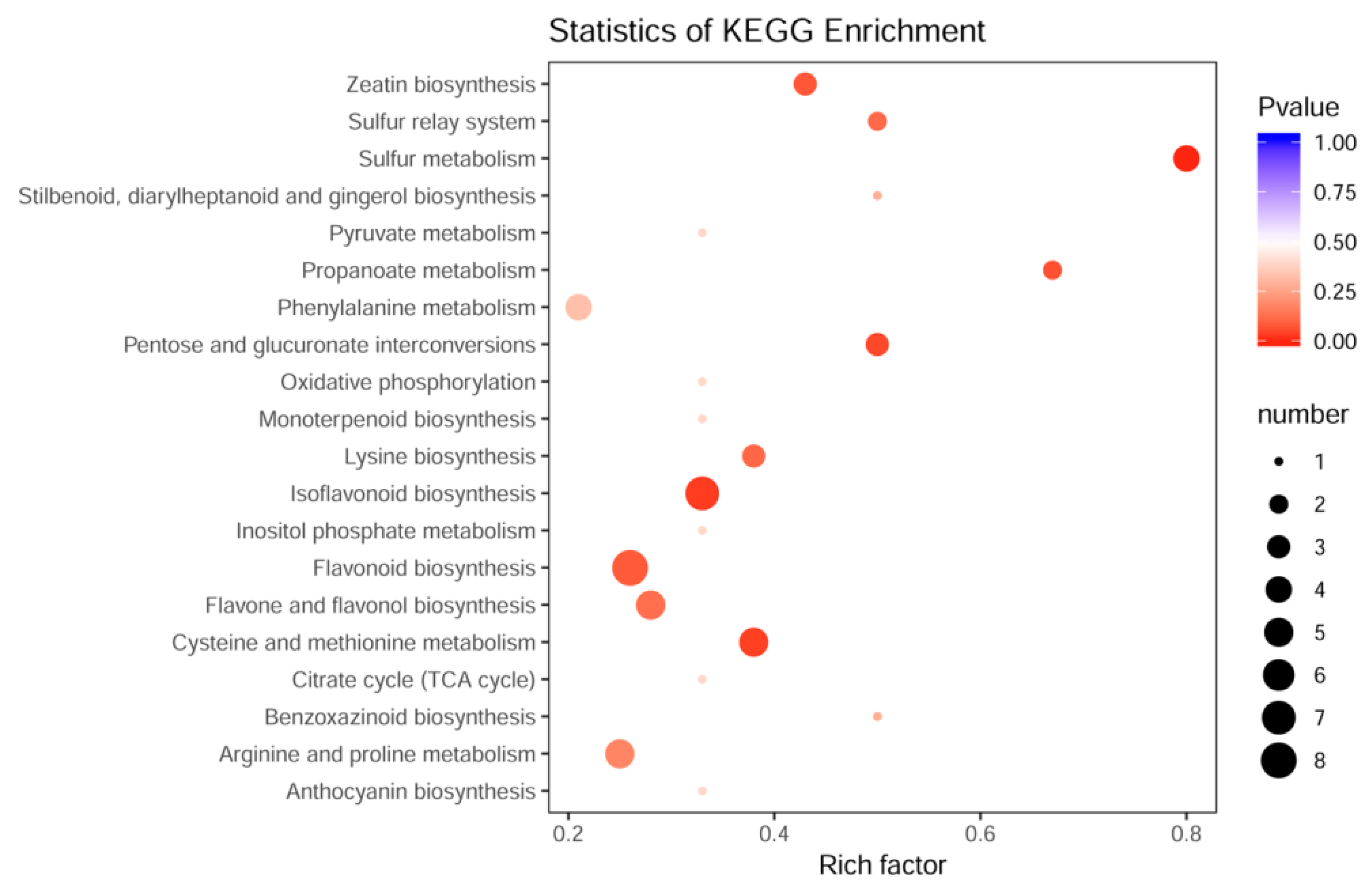
3.3. Biochemical Factors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, B.; Zhou, M.Q.; Wang, J.; Pu, Y.; Zhang, L.; Yuan, M.L. Species checklist and research status of alfalfa insect pests reported in China. Pratacultural Sci. 2016, 33, 785–812. (In Chinese) [Google Scholar]
- Wu, S.L.; Tang, L.D.; Zhang, X.R.; Xing, Z.L.; Lei, Z.R.; Gao, Y.L. A decade of a thrips invasion in China: Lessons learned. Ecotoxicology 2018, 27, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Steenbergen, M.; Broekgaarden, C.; Pieterse, C.M.J.; Van Wees, S.C.M. Bioassays to Evaluate the Resistance of Whole Plants to the Herbivorous Insect Thrips. Methods Mol. Biol. 2020, 2085, 93–108. [Google Scholar] [PubMed]
- Tu, X.; Liu, Z.; Zhang, Z. Comparative transcriptomic analysis of resistant and susceptible alfalfa cultivars (Medicago sativa L.) after thrips infestation. BMC Genom. 2018, 19, 116. [Google Scholar] [CrossRef]
- Ma, J.; Chen, H.; Wang, Y. Thrips Species investigation and identification of Alfalfa in Ningxia. Jinagsu Agric. Sci. 2017, 45, 88–91. (In Chinese) [Google Scholar] [CrossRef]
- Wu, Y.F.; Wei, L.; Zhao, X.; Temuer, B. Screening test of resistance source of alfalfa to thrips. Chin. Grassl. (In Chinese). 1990, 5, 61–63+65. [Google Scholar]
- Temuer, B.H.; Xiao, Y. Breeding of new variety of Medicago sativa cv.Caoyuan No.4. Pratacultural Sci. (In Chinese). 2017, 34, 855–860. [Google Scholar]
- Zhang, R.; Yang, F.; Xian, C.Z.; Ma, J.H.; Zhang, S.H. A study on the yield loss and economic threshold of alfalfa damaged by thrip, Odentothrips loti. Plant Prot. 2005, 31, 47–49. [Google Scholar]
- Yang, Q. Guide to Alfalfa Production and Management; China Forestry Press: Beijing, China, 2003. (In Chinese) [Google Scholar]
- Steenbergen, M.; Abd-El-Haliem, A.; Bleeker, P.; Dicke, M.; Escobar-Bravo, R.; Cheng, G.; Haring, M.A.; Kant, M.R. Thrips advisor: Exploiting thrips-induced defences to combat pests on crops. J. Exp. Bot. 2018, 69, 1837–1848. [Google Scholar] [CrossRef]
- Gao, Y.L.; Lei, Z.R.; Reitz, S.R. Western flower thrips resistance to insecticides: Detection, mechanisms and management strategies. Pest Manag. Sci. 2012, 68, 1111–1121. [Google Scholar] [CrossRef]
- Chen, J.J.; Zhen, Y.; Sun, X.H.; Yang, D.X.; Yi, C.Q.; Liu, X.X.; Yue, Y.; Liu, T.H. Preliminary study on the effect of 5 kinds of pesticides on the control of thrips. China Plant Prot. 2021, 41, 5. (In Chinese) [Google Scholar]
- Maria, V.A.; Loana, G.; Ramona, S.; Alin, C.; Levente, M.; Veaceslav, M. Biological control of Odontothrips loti (Hal.) with anthocorid predators, Orius minutus (L.) and Orius niger (Wolf.). J. Biotechnol. 2016, 231, S88. [Google Scholar] [CrossRef]
- Mujuka, E.A.; Affognon, H.; Muriithi, B.W.; Subramanian, S.; Irungu, P.; Mburu, J. Returns to research and outreach for integrated pest management of western flower thrips infesting French bean and tomato in Kenya. Int. J. Trop. Insect Sci. 2017, 37, 114–124. [Google Scholar] [CrossRef]
- Dalir, S.; Hajiqanbar, H.; Fathipour, Y.; Khanamani, M. A comprehensive picture of foraging strategies of Neoseiulus cucumeris and Amblyseius swirskii on western flower thrips. Pest Manag. Sci. 2021, 77, 5418–5429. [Google Scholar] [CrossRef]
- Haperen, P.V.; Voorrips, R.E.; Lucatti, A.F.; Schellart, W.; Van Loon, J.J.A.; Vosman, B. The effect of a thrips resistance QTL in different Capsicum backgrounds. Euphytica 2020, 216, 187. [Google Scholar] [CrossRef]
- Wu, F.; Shi, S.; Li, Y.; Miao, J.; Kang, W.; Zhang, J.; Yun, A.; Liu, C. Physiological and biochemical response of different resistant alfalfa cultivars against thrips damage. Physiol. Mol. Biol. Plants 2021, 27, 649–663. [Google Scholar] [CrossRef]
- Züst, T.; Agrawal, A.A. Trade-Offs Between Plant Growth and Defense Against Insect Herbivory: An Emerging Mechanistic Synthesis. Annu. Rev. Plant Biol. 2017, 68, 513–534. [Google Scholar] [CrossRef]
- Mouden, S.; Leiss, K.A. Host plant resistance to thrips (Thysanoptera: Thripidae)—Current state of art and future research avenues. Curr. Opin. Insect Sci. 2021, 45, 28–34. [Google Scholar] [CrossRef]
- Tu, X.B.; Fan, Y.L.; Ji, M.S.; Liu, Z.K.; Xie, N.; Liu, Z.Y.; Zhang, Z.H. Improving a method for evaluating alfalfa cultivar resistance to thrips. J. Integr. Agric. 2016, 15, 600–607. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Chen, Q.; Tan, Y.; Shuang, S.; Dai, R.; Jiang, X.H.; Temuer, B.H. Combined Transcriptome and Metabolome Analysis of Alfalfa Response to Thrips Infection. Genes 2021, 12, 1967. [Google Scholar] [CrossRef]
- Scott-Brown, A.S.; Arnold, S.; Kite, G.C.; Farrell, I.W.; Stevenson, P.C. Mechanisms in mutualisms: A chemically mediated thrips pollination strategy in common elder. Planta 2019, 250, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Wahyuni, D.S.C.; Choi, Y.H.; Leiss, K.A.; Klinkhamer, P.G.L. Morphological and Chemical Factors Related to Western Flower Thrips Resistance in the Ornamental Gladiolus. Plants 2021, 10, 1384. [Google Scholar] [CrossRef] [PubMed]
- Kariyat, R.R.; Smith, J.D.; Stephenson, A.G.; De Moraes, C.M.; Mescher, M.C. Non- glandular trichomes of Solanum carolinense deter feeding by Manduca sexta caterpillars and cause damage to the gut peritrophic matrix. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162323. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.W. Glandular hairs providing resistance to aphids in certain wild potato species. Ann. Appl. Biol. 2010, 68, 113–119. [Google Scholar] [CrossRef]
- Tayal, M.; Somavat, P.; Rodriguez, I.; Thomas, T.; Christoffersen, B.; Kariyat, R. Polyphenol-Rich Purple Corn Pericarp Extract Adversely Impacts Herbivore Growth and Development. Insects 2020, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kariyat, R.R. Exposure to polyphenol-rich purple corn pericarp extract restricts fall armyworm (Spodoptera frugiperda) growth. Plant Signal. Behav. 2020, 15, 1784545. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Li, J.; Ban, L.P. Morphology and Distribution of Antennal Sensilla in Three Species of Thripidae (Thysanoptera) Infesting Alfalfa Medicago sativa. Insects 2021, 12, 81. [Google Scholar] [CrossRef]
- Li, J.; Gu, H.; Liu, Y.; Wei, S.; Hu, G.; Wang, X.; Mcneill, M.R.; Ban, L. RNA-seq reveals plant virus composition and diversity in alfalfa, thrips, and aphids in Beijing, China. Arch. Virol. 2021, 166, 1711–1722. [Google Scholar] [CrossRef]
- Liu, Y.L.; Mi, F.G.; Temuer, B.H.; Wang, P.C.; Ma, X.T. Relationship between Alfalfa Salicylic Acid Content and Its Thrips Resistance. Acta Bot. Boreali-Occident. 2011, 31, 588–594. (In Chinese) [Google Scholar]
- Temuer, B.H.; Si, Q. Anti-thrips alfalfa form features and fanti-insect sex research. J. Inn. Mong. Agric. Univ. 2014, 35, 51–58. (In Chinese) [Google Scholar]
- Jia, X.L.; Wang, G.L.; Xiong, F.; Yu, X.R.; Xu, Z.S.; Wang, F.; Xiong, A.S. De novo assembly, transcriptome characterization, lignin accumulation, and anatomic characteristics: Novel insights into lignin biosynthesis during celery leaf development. Sci. Rep. 2015, 5, 8259. [Google Scholar] [CrossRef] [PubMed]
- Macel, M.; Visschers, I.G.S.; Peters, J.L.; Van Dam, N.M.; De Graaf, R.M. High Concentrations of Very Long Chain Leaf Wax Alkanes of Thrips Susceptible Pepper Accessions (Capsicum spp.). J. Chem. Ecol. 2020, 46, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gong, L.; Guo, Z.; Wang, W.S.; Zhang, H.Y.; Liu, X.Q.; Yu, S.B.; Xiong, L.Z. A Novel Integrated Method for Large-Scale Detection, Identification, and Quantification of Widely Targeted Metabolites: Application in the Study of Rice Metabolomics. Mol. Plant 2013, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Visschers, I.G.S.; Peters, J.L.; Vondervoort, J.a.H.V.D.; Hoogveld, R.H.M.; Dam, N.M.V. Thrips Resistance Screening Is Coming of Age: Leaf Position and Ontogeny Are Important Determinants of Leaf-Based Resistance in Pepper. Front. Plant Sci. 2019, 10, 518. [Google Scholar] [CrossRef]
- Zhou, J.; Johnson, D.T.; Tzanetakis, I.E. Assessing soybean genotypes for feeding damage by Neohydatothrips variabilis (Thysanoptera: Thripidae). Crop Prot. 2020, 128, 104983. [Google Scholar] [CrossRef]
- Njau, G.M.; Nyomora, A.M.S.; Dinssa, F.F.; Chang, J.-C.; Malini, P.; Subramanian, S.; Srinivasan, R. Evaluation of onion (Allium cepa) germplasm entries for resistance to onion thrips, Thrips tabaci (Lindeman) in Tanzania. Int. J. Trop. Insect Sci. 2017, 37, 98–113. [Google Scholar] [CrossRef]
- Shakunthala, N.; Kristine, B.S.; Knauft, D.A. Resistance mechanisms in Pieris Taxa (Ericaceae) to Stephanitis takeyai (Hemiptera: Tingidae). Environ. Entomol. 2012, 41, 1153–1162. [Google Scholar]
- Jacob, T.K.; Kumar, C.; Devasahayam, S.; D’silva, S.; Ankegowda, S. Plant morphological traits associated with field resistance to cardamom thrips (Sciothrips cardamomi) in cardamom (Elettaria cardamomum). Ann. Appl. Biol. 2020, 177, 143–151. [Google Scholar] [CrossRef]
- Scott-Brown, A.S.; Gregory, T.; Farrell, I.W.; Stevenson, P.C. Leaf trichomes and foliar chemistry mediate defence against glasshouse thrips; Heliothrips haemorrhoidalis (Bouche) in Rhododendron simsii. Funct. Plant Biol. 2016, 43, 1170–1182. [Google Scholar] [CrossRef]
- Bac-Molenaar, J.A.; Mol, S.; Verlaan, M.G.; Van Elven, J.; Kim, H.K.; Klinkhamer, P.G.L.; Leiss, K.A.; Vrieling, K. Trichome independent resistance against Western Flower Thrips in tomato. Plant Cell Physiol. 2019, 60, 1011–1024. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Klinkhamer, P.G.L.; Escobar-Bravo, R. Constitutive and inducible resistance to thrips do not correlate with differences in trichome density or enzymatic-related defenses in Chrysanthemum. J. Chem. Ecol. 2020, 46, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Khosa, J.; Hunsaker, D.; Havey, M.J. Identities of and phenotypic variation for epicuticular waxes among Leaves and plants from inbred nion populations. Hortscience 2020, 55, 2008–2010. [Google Scholar] [CrossRef]
- Voorrips, R.E.; Steenhuis-Broers, G.; Tiemens-Hulscher, M.; Bueren, E.T.L.V. Plant traits associated with resistance to Thrips tabaci in cabbage (Brassica oleracea var capitata). Euphytica 2008, 163, 409–415. [Google Scholar] [CrossRef][Green Version]
- Damon, S.J.; Groves, R.L.; Havey, M.J. Variation for epicuticular waxes on onion foliage and impacts on numbers of onion thrips. J. Am. Soc. Hortic. Sci. 2014, 139, 495–501. [Google Scholar] [CrossRef]
- Eigenbrode, S.D.; Pillai, S.K. Neonate Plutella xylostella responses to surface wax components of a resistant cabbage (Brassica oleracea). J. Chem. Ecol. 1998, 24, 1611–1627. [Google Scholar] [CrossRef]
- Braccini, C.L.; Vega, A.S.; Araoz, M.V.C.; Teal, P.E.; Cerrillo, T.; Zavala, J.A.; Fernandez, P.C. Both Volatiles and Cuticular Plant Compounds Determine Oviposition of the Willow Sawfly Nematus oligospilus on Leaves of Salix spp. (Salicaceae). J. Chem. Ecol. 2015, 41, 985–996. [Google Scholar] [CrossRef]
- Mitra, S.; Sarkar, N.; Barik, A. Long-chain alkanes and fatty acids from Ludwigia octovalvis weed leaf surface waxes as short-range attractant and ovipositional stimulant to Altica cyanea (Weber) (Coleoptera: Chrysomelidae). Bull. Entomol. Res. 2017, 107, 391–400. [Google Scholar] [CrossRef]
- Rutherford, R.S.; Van Staden, J. Towards a rapid near-infrared technique for prediction of resistance to sugarcane borer Eldana saccharina walker (Lepidoptera: Pyralidae) using stalk surface wax. J. Chem. Ecol. 1996, 22, 681–694. [Google Scholar] [CrossRef]
- Abdelmaksoud, E.M.; El-Refai, S.A.; Mahmoud, K.W.; Ragab, M.E. Susceptibility of some new strawberry genotypes to infestation by western flower thrips, Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) in the nursery. Ann. Agric. Sci. 2020, 65, 144–148. [Google Scholar] [CrossRef]
- Macel, M.; Visschers, I.G.S.; Peters, J.L.; Kappers, I.F.; De Vos, R.C.H.; Van Dam, N.M. Metabolomics of thrips resistance in pepper (Capsicum spp.) reveals monomer and dimer acyclic diterpene glycosides as potential chemical defenses. J. Chem. Ecol. 2019, 45, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lou, Y.-R.; Tzin, V.; Jander, G. Alteration of plant primary metabolism in response to insect herbivory. Plant Physiol. 2015, 169, 1488–1498. [Google Scholar] [CrossRef] [PubMed]
- Goldar, X.L.; Villari, C.; Bonello, P.; Borg-Karlson, A.K.; Grivet, D.; Zas, R.; Sampedro, L. Inducibility of plant secondary metabolites in the stem predicts genetic variation in resistance against a key insect herbivore in Maritime Pine. Front. Plant Sci. 2018, 9, 1651. [Google Scholar] [CrossRef] [PubMed]
- Michael, W. Plant secondary metabolites modulate insect behavior-steps toward addiction? Front. Physiol. 2018, 9, 364. [Google Scholar]
- Yang, M.; Yang, J.; Su, L.; Sun, K.; Li, D.; Liu, Y.; Wang, H.; Chen, Z. Metabolic profile analysis and identification of key metabolites during rice seed germination under low-temperature stress. Plant Sci. 2019, 289, 110282. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, L.; Huang, X.; Wang, X.; Yang, R.; Mao, J.; Wang, X.; Wang, X. Identification of Nutritional Components in Black Sesame Determined by Widely Targeted Metabolomics and Traditional Chinese Medicines. Molecules 2018, 23, 1180. [Google Scholar] [CrossRef]
- Roumani, M.; Besseau, S.; Gagneul, D.; Robin, C.; Larbat, R. Phenolamides in plants: An update on their function, regulation, and origin of their biosynthetic enzymes. J. Exp. Bot. 2021, 72, 2334–2355. [Google Scholar] [CrossRef]
- Alamgir, K.M.; Hojo, Y.; Christeller, J.T.; Fukumoto, K.; Isshiki, R.; Shinya, T.; Baldwin, I.T.; Galis, I. Systematic analysis of rice (Oryza sativa) metabolic responses to herbivory. Plant Cell Environ. 2016, 39, 453–466. [Google Scholar] [CrossRef]
- Xiao, J.; Gu, C.; He, S.; Zhu, D.; Zhou, Q. Widely targeted metabolomics analysis reveals new biomarkers and mechanistic insights on chestnut (Castanea mollissima Bl.) calcification process. Food Res. Int. 2021, 141, 110128. [Google Scholar] [CrossRef]
- Wang, X.S.; Yang, C.L.; Wang, S.S.; Hu, G.X. Changes of phenols and lignin contents in alfalfa leaf damaged by Odontothrips loti. Ying Yong Sheng Tai Xue Bao = J. Appl. Ecol. 2014, 25, 1688–1692. [Google Scholar]
- Liu, X.J.; Klinkhamer, P.G.; Vrieling, K. The effect of structurally related metabolites on insect herbivores: A case study on pyrrolizidine alkaloids and western flower thrips. Phytochemistry 2017, 138, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Nuringtyas, T.R.; Choi, Y.H.; Verpoorte, R.; Klinkhamer, P.G.L.; Leiss, K.A. Differential tissue distribution of metabolites in jacobaea vulgaris, Jacobaea aquatica and their crosses. Phytochemistry 2012, 78, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Agbahoungba, S.; Karungi, J.; Odong, T.L.; Badji, A.; Kumi, F.; Mwila, N.; Rubaihayo, P.R. Biochemical constituents influencing the reistance to flower bud thrips in cowpea Vigna unguiculata (L.) Walp. Germplasm. J. Anim. Plant Sci. 2018, 28, 128–137. [Google Scholar]
- Cheng, D.; Kirk, H.; Vrieling, K.; Mulder, P.P.J.; Klinkhamer, P.G.L. The relationship between structurally different pyrrolizidine alkaloids and Western Flower Thrips resistance in F-2 hybrids of Jacobaea vulgaris and Jacobaea aquatica. J. Chem. Ecol. 2011, 37, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Leiss, K.A.; Cristofori, G.; Van Steenis, R.; Verpoorte, R.; Klinkhamer, P.G.L. An eco-metabolomic study of host plant resistance to Western flower thrips in cultivated, biofortified and wild carrots. Phytochemistry 2013, 93, 63–70. [Google Scholar] [CrossRef]
- Kandakoor, S.B.; Khan, H.K.; Chakravarthy, A.K.; Kumar, C.T.A.; Venkataravana, P. Biochemical constituents influencing thrips resistance in groundnut germplasm. J. Environ. Biol. 2014, 35, 675–681. [Google Scholar]



| Type | Number | Percentage |
|---|---|---|
| Organic acids and derivatives | 102 | 13.01% |
| Flavone | 97 | 12.37% |
| Amino acid and derivatives | 93 | 11.86% |
| Lipids | 72 | 9.18% |
| Phenylpropanoids | 64 | 8.16% |
| Nucleotide and derivates | 54 | 6.89% |
| Alkaloids | 36 | 4.59% |
| Flavonol | 30 | 3.83% |
| Flavonoid | 27 | 3.44% |
| Phenolamides | 26 | 3.32% |
| Flavanone | 26 | 3.32% |
| Terpene | 23 | 2.93% |
| Vitamins and derivatives | 21 | 2.68% |
| Alcohols | 19 | 2.42% |
| Carbohydrates | 17 | 2.17% |
| Isoflavone | 14 | 1.79% |
| Anthocyanins | 11 | 1.40% |
| Polyphenol | 6 | 0.77% |
| Indole derivatives | 6 | 0.77% |
| Sterides | 5 | 0.64% |
| Quinones | 3 | 0.38% |
| Proanthocyanidins | 1 | 0.13% |
| Others | 31 | 3.95% |
| Total | 776 | 100% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Chen, Q.; Sa, R.; Dai, R.; Shuang, S.; Jiang, X.; Liu, H.; Tan, Y.; Tang, F.; Temuer, B. Morphological and Biochemical Factors Associated with Constitutive Defense to Thrips in Alfalfa. Agronomy 2022, 12, 1175. https://doi.org/10.3390/agronomy12051175
Zhang Z, Chen Q, Sa R, Dai R, Shuang S, Jiang X, Liu H, Tan Y, Tang F, Temuer B. Morphological and Biochemical Factors Associated with Constitutive Defense to Thrips in Alfalfa. Agronomy. 2022; 12(5):1175. https://doi.org/10.3390/agronomy12051175
Chicago/Turabian StyleZhang, Zhiqiang, Qi Chen, Rula Sa, Rui Dai, Shuang Shuang, Xiaohong Jiang, Huijie Liu, Yao Tan, Fang Tang, and Buhe Temuer. 2022. "Morphological and Biochemical Factors Associated with Constitutive Defense to Thrips in Alfalfa" Agronomy 12, no. 5: 1175. https://doi.org/10.3390/agronomy12051175
APA StyleZhang, Z., Chen, Q., Sa, R., Dai, R., Shuang, S., Jiang, X., Liu, H., Tan, Y., Tang, F., & Temuer, B. (2022). Morphological and Biochemical Factors Associated with Constitutive Defense to Thrips in Alfalfa. Agronomy, 12(5), 1175. https://doi.org/10.3390/agronomy12051175





