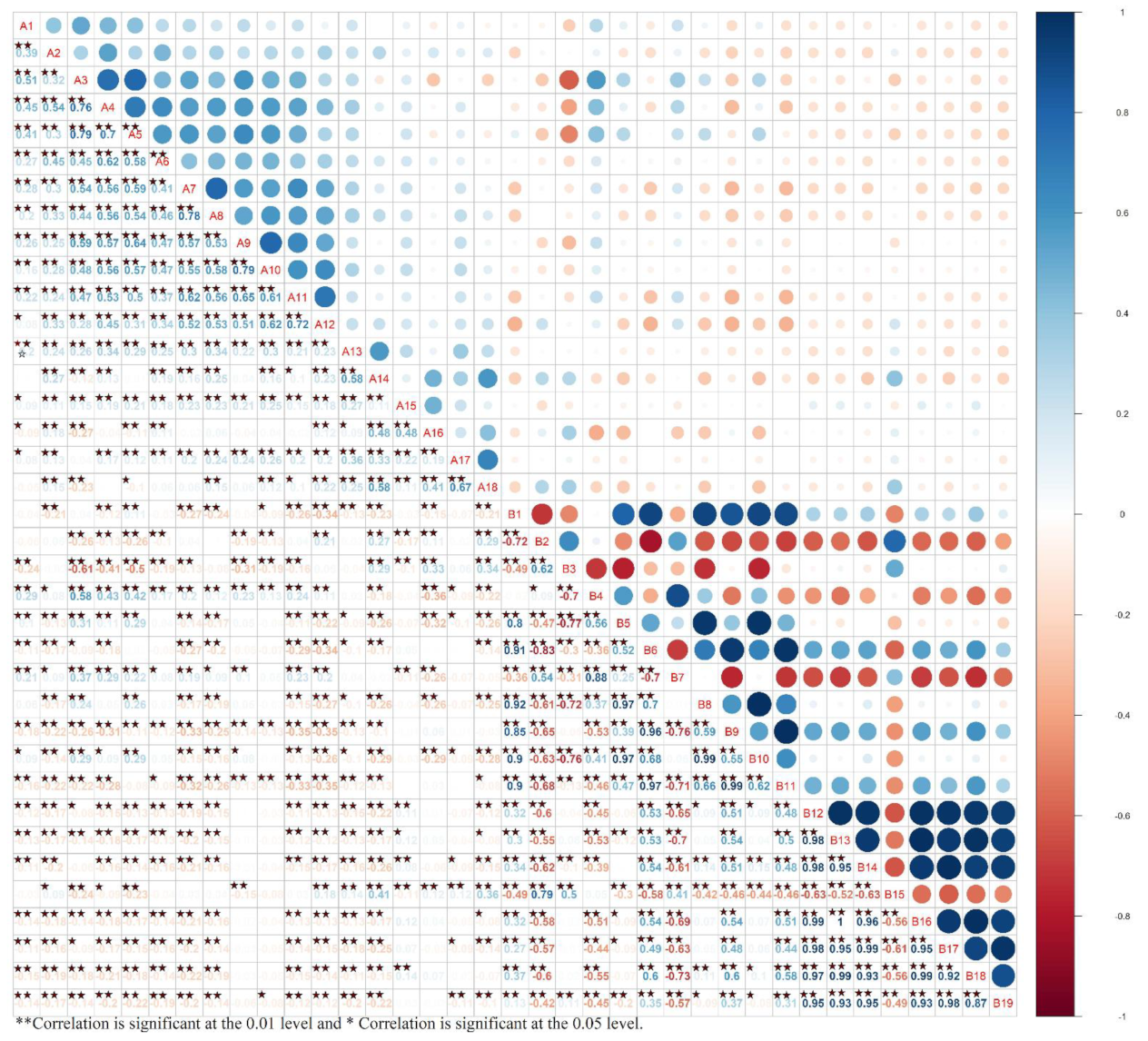
Appendix A
Qualitative and quantitative morphological description of the
Rosa sericea complex based on
Table S1.
Prickles; terete, prickles present, sometimes very broad in pairs below the leaves, dense or scattered, robust to fine, abruptly flaring, and the base is broad, sometimes bristles are very dense. Phyllotactic arrangement; not distichous. Vein on adaxial surface; mostly sunken and sometimes flat. Leaf margin; single serrate. leaf margins 2/3 of apex with serrate, ½ with serrate margin were noted. Serrate tooth edge of leaf margin with gland absent. Thorns; thorns of petiole and rachis sometimes absent, rare, and sparse were examined. Thorns of midrib sometimes absent, and rare were noted. Indumentum of leaf adaxially; pubescent and glabrous. Gland of leaf adaxially absent. Indumentum of leaf abaxially; pubescent and glabrous. Gland of the leaf is abaxially mostly absent sometimes rare, sparse, and medium. Indumentum of stipule abaxially; sometimes absent, rarely pubescent, pubescent, sericeous, and densely hairy. Gland of stipule, sometimes absent, only on leaf margins, rarely on leaf margin abaxially, sparsely except leaf margin, and densely except leaf margin. Indumentum of petiole and rachis; sometimes absent, rarely pubescent, pubescent, sericeous, and densely hairy. Glands of petiole and rachis are sometimes absent, rare, sparse, medium, and dense. Indumentum of the pedicel is sometimes absent, rarely pubescent. Indumentum of pedicel; sometimes absent, rarely pubescent, pubescent, sericeous, and densely hairy. The gland of the pedicel is sometimes absent, rare, sparse, medium, and dense. Indumentum of the receptacle; sometimes absent, rarely pubescent, pubescent, sericeous, and densely hairy. Gland of receptacle sometimes absent, rare, sparse, medium, and dense. Sepal morphology; ovate-lanceolate, abaxially sparsely pubescent or subglabrous, adaxially villous, margin entire, apex acuminate, or acute. Indumentum of sepal; sometimes absent, rarely pubescent, pubescent, sericeous, and densely hairy. The gland of the sepal is sometimes absent, rare, sparse, medium, and dense. The number of petals was four mostly few have five. Stalk; not inflated. Hip color; red or bright to reddish, orange to red or red-purple, immature green or greenish.
Prickles; terete, prickles in pairs mostly in pairs below leaves, or intermixed sparse, bristles with dense slender. Phyllotactic arrangement; not distichous. The vein on adaxial surface; is mostly sunken and sometimes flat. Leaf margin; double serrate, ½ with a serrate margin. Serrate tooth edge of leaf margin with gland present. Thorns; thorns of petiole and rachis rare, sparse, medium, and dense were examined. Thorns of midrib were medium and dense were noted. Indumentum of leaf adaxially; sericeous. Gland of leaf adaxially absent. Indumentum of leaf abaxially; sericeous. Gland of leaf abaxially mostly sparse, medium, and dense. Indumentum of stipule abaxially; pubescent, sericeous, and densely hairy. Gland of stipule, sometimes absent, only on leaf margins, rarely on leaf margin abaxially, sparsely except leaf margin, and densely except leaf margin. Indumentum of petiole and rachis; sometimes absent, rarely pubescent, pubescent, sericeous, and densely hairy. Glands of petiole sometimes absent, rare, sparse, medium, and dense. Indumentum of pedicel; sometimes absent, rarely pubescent, pubescent, sericeous, and densely hairy. Gland of the pedicel is sometimes absent, rare, sparse, medium, and dense. These specimens have more hairs on the leaf abaxial surface as well as on the petiole, and fruits.
Phyllotactic arrangement; not distichous. The vein on adaxial surface; is mostly sunken and sometimes flat. Leaf margin; single serrate. leaf margins all serrate, 2/3 of apex with serrate, ½ with serrate margin were observed. Serrate tooth edge of leaf margin with gland absent. Thorns; thorns of petiole and rachis sometimes absent, rare, and sparse were examined. Thorns of midrib sometimes absent, rare, sparse, medium and dense were noted. Indumentum of leaf adaxially; glabrous. Gland of leaf adaxially absent. Indumentum of leaf abaxially; glabrous. Gland of leaf abaxially mostly rarely and sometimes absent. Indumentum of stipule abaxially; sometimes absent, rarely pubescent, pubescent, sericeous, and densely hairy. Gland of stipule, sometimes absent, only on leaf margins, and rarely on leaf margin abaxially. Indumentum of petiole and rachis; sometimes absent, rarely pubescent, pubescent, sericeous, and densely hairy. Glands of the petiole are sometimes absent, rare, sparse, medium, and dense. Indumentum of pedicel; mostly absent, rarely pubescent, and pubescent. Gland of the pedicel is sometimes absent, rare, and sparse. Indumentum of the receptacle; mostly absent, rarely pubescent, and pubescent. Gland of receptacle sometimes absent, rare, and sparse. Sepal morphology; lanceolate, abaxially subglabrous, adaxially sparsely pubescent, margin entire, apex acuminate or long caudate. Indumentum of sepal; rarely pubescent, pubescent, sericeous, and densely hairy. Gland of sepal sometimes absent, rare and sparse. Petals; number of petals were four mostly few have five. Color of petals mostly white, yellowish and pink. Stalk; half inflated. Hip color; red or bright to reddish, orange to red or red purple, immature green or greenish. Prickles; wing like, prickles present in paired below the leaves with broad base, dense or scattered, abruptly flaring, bristles when present very dense, apex tapering or abruptly.
Prickles; stout, prickles in pairs mostly in pairs below leaves, mostly dense sometimes scattered, bristles dense slender. Phyllotactic arrangement; not distichous. Vein on adaxial surface; mostly flat. Leaf margin; double serrate. Serrate tooth edge of leaf margin with gland present. Leaf margin tooth single tooth. Thorns; thorns of petiole and rachis not present. Thorns of midrib were absent. Indumentum of leaf adaxially; absent. Gland of leaf adaxially rarely. Indumentum of leaf abaxially; sericeous. Gland of leaf abaxially sparse. Indumentum of stipule abaxially; absent. Gland of stipule mostly absent, sometimes in only on leaf margin. Indumentum of petiole and rachis; sometimes absent, and rarely pubescent. Glands of petiole sparse, and medium. Indumentum of pedicel; sometimes absent, rarely pubescent, and pubescent. Gland of pedicel sometimes absent, and rare. Indumentum of receptacle; absent. Gland of receptacle sometimes absent, and rare. Sepal morphology; lanceolate, abaxially glabrous, sometimes sparsely glandular, adaxially densely pubescent, margin entire, apex long acuminate. Indumentum of sepal; sometimes absent mostly rarely pubescent. Gland of sepal sometimes absent, sparse and medium. Gland of receptacle sometimes absent, and rare. Petals; number of petals were four. Color of petals mostly white. Stalk; all inflated. Hip color; orange to red or red purple.
Prickles; terete, and winglike, prickles present, sometimes very broad in pairs below the leaves, dense or scattered, robust to fine, apex tapering, and the base is broad, sometimes bristles are mostly scattered and sometimes dense. Phyllotactic arrangement; not distichous. Vein on adaxial surface; mostly sunken and sometimes flat. Leaf margin; single serrate. leaf margins 2/3 of apex with serrate, ½ with serrate margin were noted. Serrate tooth edge of leaf margin with gland present. Thorns; thorns of petiole and rachis absent. Thorns of midrib mostly absent, and sometimes dense present. Indumentum of leaf adaxially; pubescent. Gland of leaf adaxially present and dense. Indumentum of leaf abaxially; absent. Gland of leaf abaxially dense. Indumentum of stipule abaxially; sometimes absent, pubescent, and sometimes sericeous. Gland of stipule, rarely on leaf margin abaxially, and densely except from leaf margin. Indumentum of petiole and rachis; absent. Glands of petiole and rachis sometimes medium, and dense. Indumentum of pedicel; sometimes absent, rarely pubescent, and pubescent. Gland of pedicel absent. Indumentum of receptacle; absent. Gland of receptacle absent. Sepal morphology; ovate or lanceolate, abaxially sparsely pubescent, adaxially densely pubescent, margin entire, apex acuminate. Indumentum of sepal; pubescent, sericeous, and densely hairy. Gland of sepal sometimes absent, rare, sparse, and medium. Number of petals were four. White color. Stalk; not inflated and all inflated. Hip color; red or bright to reddish.
Prickles; flat, prickles present, sometimes very broad in pairs below the leaves, dense or scattered, robust to fine, tapering, and the base is broad, sometimes bristles are very dense. Phyllotactic arrangement; not distichous. Vein on adaxial surface; sunken on adaxial side. Leaf margin; double serrate. leaf margins double tooth. Serrate tooth edge of leaf margin with gland absent. Thorns; thorns of petiole and rachis sometimes were rare. Thorns of midrib were mostly absent, sometimes rare. Indumentum of leaf adaxially; pubescent. Gland of leaf adaxially absent, rare and sometimes sparse. Indumentum of leaf abaxially; absent. Gland of leaf abaxially mostly rare, and sparse. Indumentum of stipule abaxially; sometimes rarely pubescent, pubescent, sericeous, and densely hairy. Gland of stipule absent. Indumentum of petiole and rachis; sometimes absent, rarely pubescent, pubescent, sericeous, and densely hairy. Glands of petiole and rachis sometimes absent, rare, sparse, and medium. Indumentum of pedicel sometimes rarely pubescent, pubescent, sericeous, and densely hairy. Indumentum of pedicel; sometimes, pubescent, sericeous, and densely hairy. Gland of pedicel absent. Indumentum of receptacle; sometimes absent, and sericeous. Gland of receptacle absent. Sepal morphology; lanceolate, 9–12 mm, both surfaces densely pubescent, margin entire, apex long acuminate, caudate. Indumentum of sepal; sometimes absent, and rarely pubescent. Gland of sepal sometimes absent, rare, and sparse. The number of petals were four. Stalk; not inflated. Hip color; dark red, immature green or greenish.
The length ranged from 7–15 mm, but most of the specimens had an average 11 mm of prickles. The width ranges from 7–12 mm. The prickles diameter ranges from 0.9–2.9 cm. Leaf morphology; the number of leaflets minimum ranges from 5–11, number of leaflets maximum ranges from 9–15. Length of leaf minimum ranges from 12.5–50 mm, length of leaf maximum ranges from 20–80 mm. Length of first leaflet on apex minimum ranges from 5.12–17.4 mm, length of the first leaflet on apex maximum ranges from 6.71–31.21 mm. Width of the first leaflet on the apex minimum 2.56–6.43 mm, the width of first leaflet on apex maximum ranges from 3.49–9.8 mm. Length of first leaflet on base minimum ranges from 2–7 mm, length of first leaflet on base maximum ranges from 4–18 mm. Width of the leaflet on base minimum 1.5–8.23 mm, width of the first leaflet on base maximum 2–9.78 mm. Pedicel size; length of pedicle minimum ranges from 1–27 mm, length of pedicel maximum ranges from 2–41.6 mm. Sepal size; length of sepal minimum ranges from 3–19 mm, length of sepal maximum ranges from 6–29 mm. Width of sepal minimum ranges from 1.2–8.7 mm, width of sepal maximum ranges from 1.3–3.5 mm.
The length of prickles was examined various in different specimens of the same species, variation also have been observed, it was ranging from 3–15 mm, but the average length was between 7–10 mm. The width was ranges from 2–12 mm. Prickle diameter was ranging from 1.5–3 cm. Leaf morphology; number of leaflets minimum ranges from 5–11, number of leaflets maximum ranges from 13–17. Length of leaflet minimum ranges from 20–80 mm, length of leaf maximum ranges from 35–115 mm. Length of first leaflet on apex minimum ranges from 5–20 mm, length of the first leaflet on apex maximum ranges from 9–41.1 mm. Width of the first leaflet on apex minimum 2.54–8 mm, width of first leaflet on apex maximum ranges from 3.98–12 mm. Length of first leaflet on base minimum ranges from 3.46–11.12 mm, length of first leaflet on base maximum ranges from 5–17 mm. Width of the leaflet on base minimum 2–6 mm, width of the first leaflet on base maximum 3–8 mm. Pedicel size; length of pedicle minimum ranges from 1–21.5 mm, length of pedicel maximum ranges from 2–34.13 mm. Sepal size; length of sepal minimum ranges from 3.9–16 mm, length of sepal maximum ranges from 5–23 mm. Width of sepal minimum ranges from 1.89–5 mm, width of sepal maximum ranges from 2–6.4 mm.
The prickles length was from 7–15 mm, mostly was from 7–10 mm. The width ranges from 2–12 mm width noted. The prickles diameter ranges from 0.8–1.3 cm. Leaf morphology; number of leaflets minimum ranges from 7–9, number of leaflets maximum ranges from 9–13. Length of leaflet minimum ranges from 13.41–30.23 mm, length of leaf maximum ranges from 30.12–63.56 mm. The length of first leaflet on apex minimum ranges from 5.12–10.29 mm, length of the first leaflet on apex maximum ranges from 8.11–19.78 mm. Width of the first leaflet on the apex minimum 3.34–5.34 mm, the width of the first leaflet on the apex maximum ranges from 4.51–7.53 mm. The length of the first leaflet on the base minimum ranges from 3.12–6.78 mm, length of the first leaflet on the base maximum ranges from 5.14–11.23 mm. Width of the leaflet on-base minimum 2.34–4.32 mm, the width of the first leaflet on-base maximum of 3.03–6.03 mm. Pedicel size; length of pedicle minimum ranges from 3.4–13 mm, length of pedicel maximum ranges from 9.1–32 mm. Sepal size; length of sepal minimum ranges from 4.9–14 mm, length of sepal maximum ranges from 7.9–22 mm. The width of sepal minimum ranges from 1.7–4 mm, the width of sepal minimum ranges from 2.9–9 mm, the width of sepal maximum ranges from 2.5–9 mm.
The prickles length was from 7–15 mm, mostly was from 7–10 mm. The width ranges from 2–12 mm width noted. The prickle’ diameter ranges from 0.8 to 1.3 cm. Leaf morphology; the number of leaflets minimum were 7, the number of leaflets maximum ranges from 9–11. The length of leaflet minimum ranges from 15–30 mm, length of leaf maximum ranges from 24–45 mm. Length of first leaflet on apex minimum ranges from 5–8 mm, length of the first leaflet on apex maximum ranges from 7–12 mm. The width of the first leaflet on apex minimum 2.5–5 mm, the width of the first leaflet on apex maximum ranges from 3–7 mm. Length of first leaflet on base minimum ranges from 4–6 mm, length of the first leaflet on base maximum ranges from 5–9 mm. The width of the leaflet on base minimum ranges from 2–4 mm, width of the first leaflet on base maximum 3–5.5 mm. Pedicel size; length of pedicle minimum ranges from 4–7 mm, length of pedicel maximum ranges from 5–10 mm. Sepal size; length of sepal minimum ranges from 7–14 mm, length of sepal maximum ranges from 8–14 mm. The width of sepal minimum ranges from 2–3 mm, width of sepal maximum ranges from 2.5–3.5 mm.
The prickles length was from 7–15 mm, mostly was from 7–10 mm. The width ranges from 2–12 mm width noted. The prickles diameter ranges from 0.8–1.3 cm. Leaf morphology; the number of leaflets minimum were 7–9, number of leaflets maximum ranges from 9–11. The length of leaflet minimum ranges from 20–30 mm, length of leaf maximum ranges from 25–40 mm. The length of the first leaflet on the apex minimum ranges from 7–11 mm, length of the first leaflet on the apex maximum ranges from 9–13 mm. The width of the first leaflet on the apex minimum 3–4.5 mm, the width of first leaflet on apex maximum ranges from 3.5–5.5 mm. The length of first leaflet on base minimum ranges from 3–6 mm, length of first leaflet on the base maximum ranges from 5–9 mm. The width of the leaflet on base minimum ranges from 2–3 mm, width of the first leaflet on a base maximum 2.5–4 mm. Pedicel size; length of pedicle minimum ranges from 2–7 mm, length of pedicel maximum ranges from 3–8 mm. Sepal size; length of sepal minimum ranges from 5–10 mm, length of sepal maximum ranges from 6–12 mm. The width of sepal minimum ranges from 2.5–3 mm, the width of sepal maximum ranges from 3–4 mm.
Rosa zhongdianensis
The prickles length was from 6–8 mm. The width ranges from 4–7 mm width noted. Leaf morphology; the number of leaflets minimum were 5–9, number of leaflets maximum ranges from 9–11. The length of leaflet minimum ranges from 19–42 mm, length of leaf maximum ranges from 32–58 mm. The length of the first leaflet on apex minimum ranges from 7–11 mm, length of the first leaflet on apex maximum ranges from 10–14 mm. Width of the first leaflet on the apex minimum 5–7 mm, the width of first leaflet on apex maximum ranges from 7–8.5 mm. Length of first leaflet on base minimum ranges from 5–8 mm, length of first leaflet on base maximum ranges from 7–13 mm. Width of the leaflet on base minimum ranges from 3–4.5 mm, width of the first leaflet on base maximum 4–5.5 mm. Pedicel size; length of pedicle minimum ranges from 7–14 mm, length of pedicel maximum ranges from 10–15 mm. Sepal size; length of sepal minimum ranges from 9–13 mm, length of sepal maximum ranges from 11–17 mm. Width of sepal minimum ranges from 2.5–3.5 mm, width of sepal maximum ranges from 3–3.5 mm.