Achromobacter xylosoxidans and Enteromorpha intestinalis Extract Improve Tomato Growth under Salt Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biological Materials
2.2. Greenhouse Assay
2.3. Tyrosine Nitration Quantification
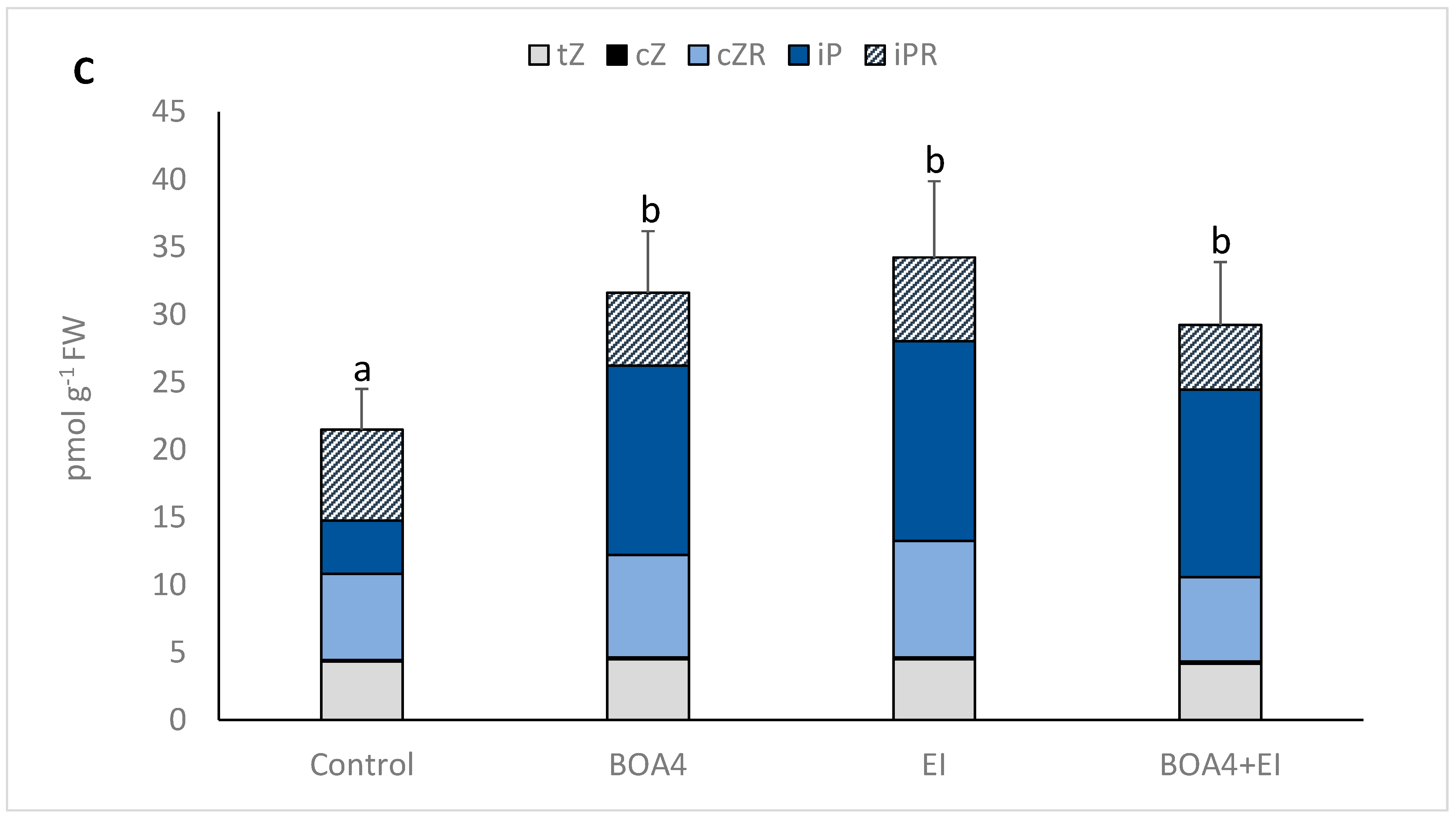
2.4. Cytokinin Quantification
2.5. Statistical Analysis
3. Results and Discussion
3.1. BOA4 Production of Cytokinin and Carbon Source Use
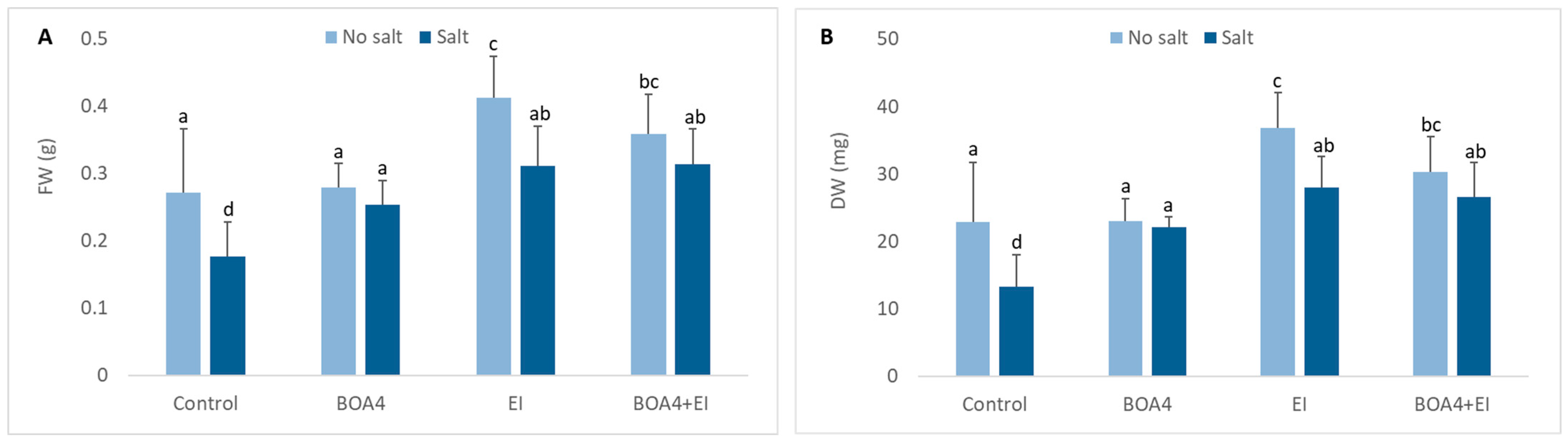
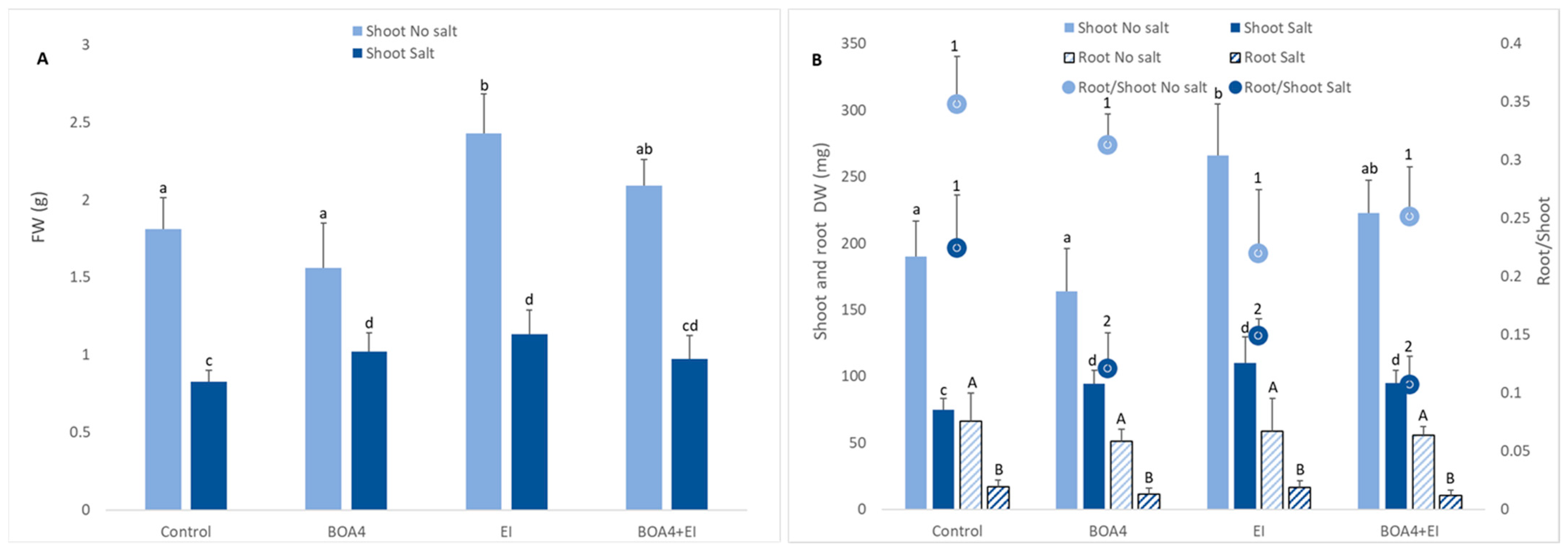
3.2. Effect of BOA4 and EI Extract on Plant Growth
3.3. Effect of BOA4 and EI Extract on Root/Shoot Ratios
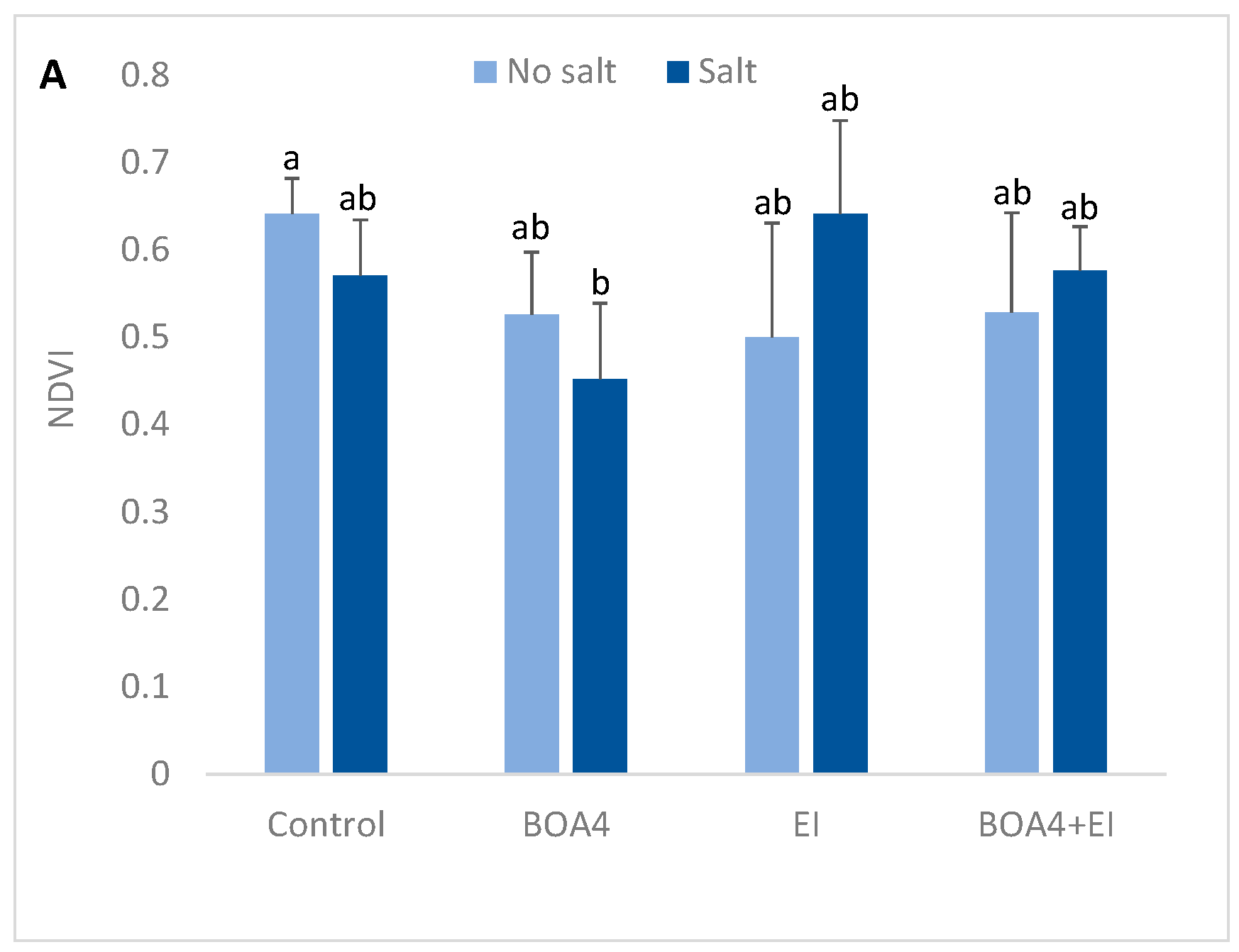
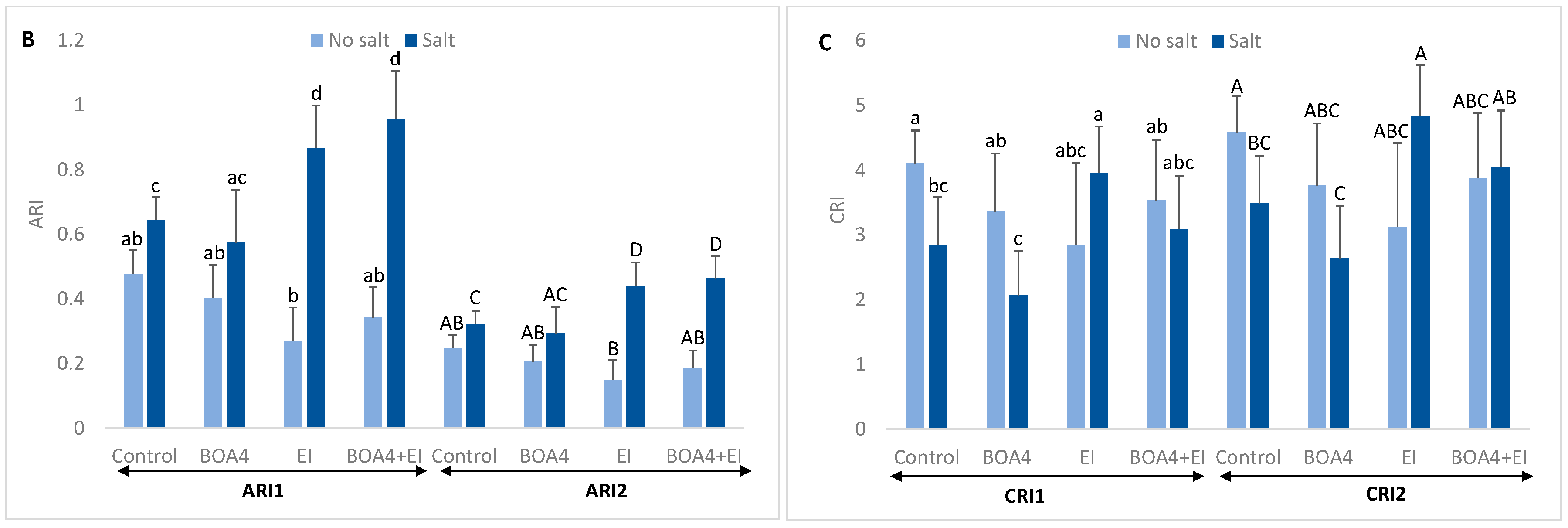
3.4. Leaf Content in Anthocyanins and Carotenoids
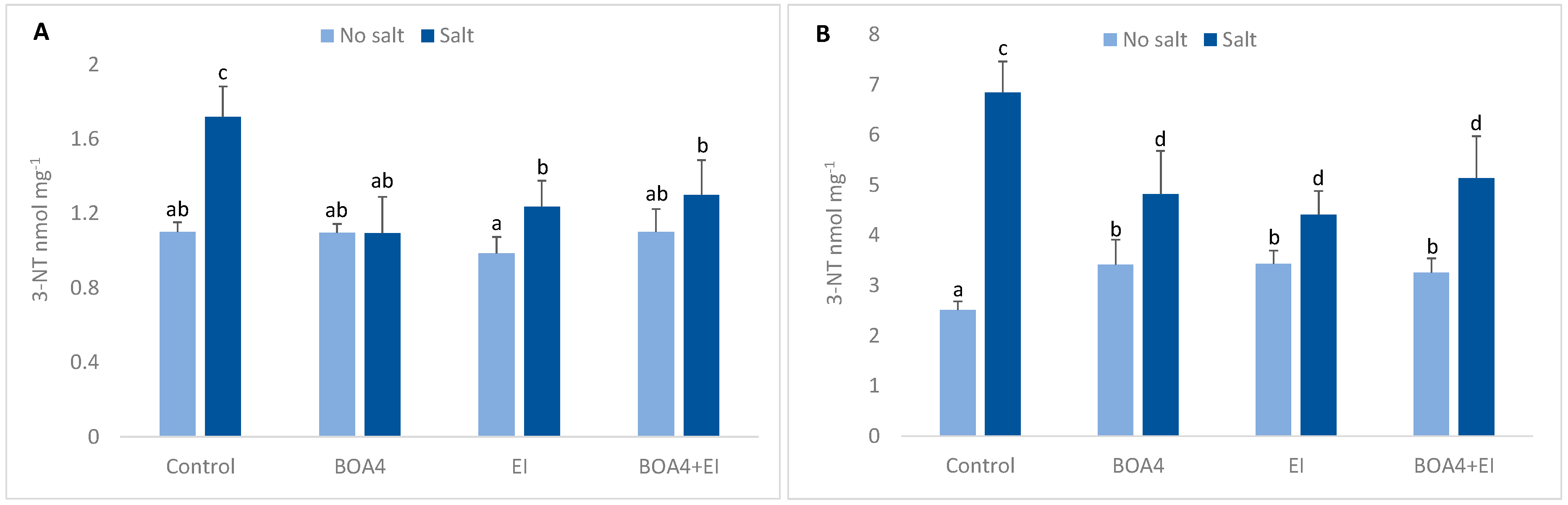
3.5. Effect of BOA4 and EI Extract on Tyrosine Nitration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamil, A.; Riaz, S.; Ashraf, M.; Foolad, M.R. Gene Expression Profiling of Plants Under Salt Stress. Crit. Rev. Plant Sci. 2011, 30, 435–458. [Google Scholar] [CrossRef]
- Ashraf, M.; Ahmad, M.S.A.; Öztürk, M.; Aksoy, A. Crop Improvement Through Different Means: Challenges and Prospects. In Crop Production for Agricultural Improvement; Ashraf, M., Öztürk, M., Ahmad, M.S.A., Aksoy, A., Eds.; Springer Science & Business Media, B.V: Dordrecht, The Netherlands, 2012; pp. 1–15. [Google Scholar]
- Rai, A.; Cherif, A.; Cruz, C.; Nabti, E. Extracts from Marine Macroalgae and Opuntia ficus-indica Cladodes Enhance Halotolerance and Enzymatic Potential of Diazotrophic Rhizobacteria and Their Impact on Wheat Germination Under Salt Stress. Pedosphere 2018, 27, 241–254. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant Growth-Promoting Bacteria Confer Resistance in Tomato Plants to Salt Stress. Plant Physiol. Biochem. 2004, 42, 565–572. [Google Scholar]
- Qurashi, A.W.; Sabri, A.N. Osmoadaptation and Plant Growth Promotion by Salt Tolerant Bacteria Under Salt Stress. Afr. J. Microbiol. Res. 2011, 5, 3546–3554. [Google Scholar]
- Ullah, U.; Ashraf, M.; Shahzad, S.M.; Siddiqui, A.R.; Piracha, M.A.; Suleman, M. Growth Behavior of Tomato (Solanum lycopersicum L.) Under Drought Stress in the Presence of Silicon and Plant Growth Promoting Rhizobacteria. Soil Environ. 2016, 35, 65–75. [Google Scholar]
- Egamberdieva, D.; Wirth, S.; Bellingrath-Kimura, S.D.; Mishra, J.; Arora, N.K. Salt-Tolerant Plant Growth Promoting Rhizobacteria for Enhancing Crop Productivity of Saline Soils. Front. Microbiol. 2019, 10, 2791. [Google Scholar] [CrossRef] [Green Version]
- Baudouin, E.; Hancock, J.T. Nitric Oxide Signaling in Plants. Front. Plant Sci. 2014, 4, 553. [Google Scholar] [CrossRef] [Green Version]
- Gow, A.J.; Farkouh, C.R.; Munson, D.A.; Posencheg, M.A.; Ischiropoulos, H. Biological Significance of Nitric Oxide-Mediated Protein Modifications. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 287, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Mata-Pérez, C.; Begara-Morales, J.C.; Chaki, M.; Sánchez-Calvo, B.; Valderrama, R.; Padilla, M.N.; Corpas, F.J.; Barroso, J.B. Protein Tyrosine Nitration During Development and Abiotic Stress Response in Plants. Front. Plant Sci. 2016, 7, 1699. [Google Scholar] [CrossRef]
- Bartesaghi, S.; Radi, R. Fundamentals on the Biochemistry of Peroxynitrite and Protein Tyrosine Nitration. Redox. Biol. 2018, 14, 618–625. [Google Scholar] [CrossRef]
- Radi, R. Nitric Oxide, Oxidants, and Protein Tyrosine Nitration. Proc. Natl. Acad. Sci. USA 2004, 101, 4003–4008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corpas, F.J.; Hayashi, M.; Mano, S.; Nishimura, M.; Barroso, J.B. Peroxisomes are Required for in Vivo Nitric Oxide Accumulation in the Cytosol Following Salinity Stress of Arabidopsis plants. Plant Physiol. 2009, 15, 2083–2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freschi, L. Nitric Oxide and Phytohormone Interactions: Current Status and Perspectives. Front. Plant Sci. 2013, 4, 398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.-Z.; Kong, D.-D.; Gu, X.-X.; Gao, H.-B.; Wang, J.-Z.; Xia, M.; Gao, Q.; Tian, L.; Xu, Z.; Bao, F.; et al. Cytokinins Can Act as Suppressors of Nitric Oxide in Arabidopsis. Proc. Natl. Acad. Sci. USA 2013, 11, 1548–1553. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, M.; Brütting, C.; Meza-Canales, I.D.; Großkinsky, D.K.; Vankova, R.; Baldwin, I.T.; Meldau, S. The Role of Cis-Zeatin-Type Cytokinins in Plant Growth Regulation and Mediating Responses to Environmental Interactions. J. Exp. Bot. 2015, 66, 4873–4884. [Google Scholar] [CrossRef] [Green Version]
- Santos, B.M.S.; Silva, M.S.R.A.; Chávez, D.W.H.; Rigobelo, E.C. Genetic and Nutritional Diversity of Bacillus subtilis Isolates Demonstrating Different Aspects Related to Plant Growth Promotion. Aust. J. Crop Sci. 2020, 14, 880–888. [Google Scholar] [CrossRef]
- Zhang, T.-T.; Zeng, S.-L.; Gao, Y.; Ouyang, Z.-T.; Li, B.; Fang, C.-M.; Zhao, B. Using Hyperspectral Vegetation Indices as a Proxy to Monitor Soil Salinity. Ecol. Indic. 2011, 11, 1552–1562. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Olaetxea, M.; Mora, V.; Bacaicoa, E.; Baigorri, R.; Garnica, M.; Fuentes, M.; Zamarreño, A.M.; Spíchal, L.; Garcia-Mína, J.M. Root ABA and H+-ATPase are Key Players in the Root and Shoot Growth-Promoting Action of Humic Acids. Plant Direct 2019, 3, e00175. [Google Scholar] [CrossRef] [Green Version]
- Gill, S.S.; Tuteja, N. Reactive Oxygen Species and Antioxidant Machinery in Abiotic Stress Tolerance in Crop Plants. Plant Physiol. Biochem 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Kuzuyama, T.; Seto, H. Two Distinct Pathways for Essential Metabolic Precursors for Isoprenoid Biosynthesis. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2012, 88, 41–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, W.C.; Song, H.; Liu, H.W.; Liu, P. Current Development in Isoprenoid Precursor Biosynthesis and Regulation. Curr. Opin. Chem. Biol. 2013, 17, 571–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahzad, R.; Khan, A.L.; Waqas, M.; Ullah, I.; Bilal, S.; Kim, Y.-H.; Asaf, S.; Kang, S.-M.; Lee, I.-J. Metabolic and Proteomic Alteration in Phytohormone-producing Endophytic Bacillus amyloliquefaciens RWL-1 During Methanol Utilization. Metabolomics 2019, 22, 16. [Google Scholar] [CrossRef]
- Alfonso, F.C.; Vigueras-Ramírez, G.; Rosales-Colunga, L.M.; del Monte-Martínez, A.; Hernández, R.O. Propionate as the Preferred Carbon Source to Produce 3-indoleacetic acid in B. subtilis: Comparative Flux Analysis Using Five Carbon Sources. Mol. Omics 2021, 17, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Permentier, H.P.; de Weger, L.A.; Wijffelman, C.A.; Lugtenberg, B.J.J. Amino Acid Synthesis is Necessary for Tomato Root Colonization by Pseudomonas fluorescens strain WCS365. Mol. Plant Microbe Interact. 1997, 10, 102–106. [Google Scholar] [CrossRef] [Green Version]
- Kravchenko, L.V.; Azarova, T.S.; Leonova-Erko, E.I.; Shaposhnikov, A.I.; Makarova, N.M.; Tikhonovichet, I.S. Root Exudates of Tomato Plants and Their Effect on the Growth and Antifungal Activity of Pseudomonas strains. Microbiology 2003, 72, 37–41. [Google Scholar] [CrossRef]
- Jones, D.L. Organic Acids in the Rhizosphere-a Critical Review. Plant Soil 1998, 205, 25–44. [Google Scholar] [CrossRef]
- Rai, A.; Santana, M.M.; Maia, R.N.; Tavares, J.; Nabti, E.; Cruz, V. Distinct Proline Balance for Tomato Growth Improvement Under Salt Stress Through Bacterial Inoculation and Irrigation with Opuntia Rackets or Marine Algae Extracts; Laboratoire de Gestion et Valorisation des Ressources Naturelles et Assurance Qualité, Faculté SNVST, Université Akli: Bouira, Algeria, 2022; to be submitted. [Google Scholar]
- Edwards, D.M.; Reed, R.H.; Stewart, W.D.P. Osmoacclimation in Enteromorpha intestinalis: Long-term Effects of Osmotic Stress on Organic Solute Accumulation. Mar. Biol. 1988, 98, 467–476. [Google Scholar] [CrossRef]
- Kirst, G.O. Osmotic Adjustment in Phytoplankton and Macroalgae. The Use of Dimethylsulfoniopropionate (DMSP). In Biological and Environmental Chemistry of DMSP and Related Sulfonium Compounds; Kiene, R.P., Visscher, P.T., Keller, M.D., Kirst, G.O., Eds.; Springer: Boston, MA, USA, 1996; pp. 121–129. [Google Scholar]
- Ghoul, M.; Minet, J.; Bernard, T.; Dupray, E.; Cormier, M. Marine Macroalgae as a Source for Osmoprotection for Escherichia Coli. Microb. Ecol. 1995, 30, 171–181. [Google Scholar] [CrossRef]
- Kim, K.; Jang, Y.J.; Lee, S.M.; Oh, B.T.; Chae, J.C.; Lee, K.J. Alleviation of Salt Stress by Enterobacter sp. EJ01 in Tomato and Arabidopsis is Accompanied by Up-Regulation of Conserved Salinity Responsive Factors in Plants. Mol. Cells 2014, 37, 109–117. [Google Scholar] [CrossRef]
- Diagne, N.; Ndour, M.; Djighaly, P.I.; Ngom, D.; Ngom, M.C.M.; Ndong, G.; Svistoonoff, S.; Cherif-Silini, H. Effect of Plant Growth Promoting Rhizobacteria (PGPR) and Arbuscular Mycorrhizal Fungi (AMF) on Salt Stress Tolerance of Casuarina obesa (Miq.). Front. Sustain. Food Syst. 2020, 4, 266. [Google Scholar] [CrossRef]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of Proline under Changing Environments: A Review. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Signorelli, S.; Dans, P.D.; Coitiño, E.L.; Borsani, O.; Monza, J. Connecting Proline and γ-Aminobutyric Acid in Stressed Plants Through Non-Enzymatic Reactions. PLoS ONE 2015, 10, e0115349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hidri, R.; Barea, J.M.; Metoui-Ben Mahmoud, O.; Abdelly, C.; Azcón, R. Impact of Microbial Inoculation on Biomass Accumulation by Sulla carnosa Provenances, and in Regulating Nutrition, Physiological and Antioxidant Activities of this Species under Non-saline and Saline Conditions. J. Plant Physiol. 2016, 201, 28–41. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.-L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant Growth-Promoting Rhizobacteria and Root System Functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef] [Green Version]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk Amongst Phytohormones from Planta and PGPR Under Biotic and Abiotic Stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Prinsen, E.; Veselov, S.U.; Martinenko, E.V.; Melentiev, A.I.; Kudoyarova, G.R. Cytokinin Producing Bacteria Enhance Plant Growth in Drying Soil. Plant Soil 2007, 292, 305–315. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Vysotskaya, L.B.; Cherkozyanova, A.; Dodd, I.C. Effect of Partial Rootzone Drying on the Concentration of Zeatin-Type Cytokinins in Tomato (Solanum lycopersicum L.) Xylem Sap and Leaves. J. Exp. Bot. 2007, 58, 161–168. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van Onckelen, H.; Schmülling, T. Cytokinin-Deficient Transgenic Arabidopsis Plants Show Multiple Developmental Alterations Indicating Opposite Functions of Cytokinins in the Regulation of Shoot and Root Meristem Activity. Plant Cell 2003, 15, 2532–2550. [Google Scholar] [CrossRef] [Green Version]
- Frensch, J. Primary Responses of Root and Leaf Elongation to Water Deficits in the Atmosphere and Soil Solution. J. Exp. Bot. 1997, 48, 985–999. [Google Scholar] [CrossRef]
- Farber, M.; Attia, Z.; Weiss, D. Cytokinin Activity Increases Stomatal Density and Transpiration Rate in Tomato. J. Exp. Bot. 2016, 67, 6351–6362. [Google Scholar] [CrossRef] [PubMed]
- Koenig, R.L.; Morris, R.O.; Polacco, J.C. tRNA is the Source of Low-Level Trans-Zeatin Production in Methylobacterium spp. J. Bact. 2002, 184, 1832–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, J.; Gelvin, S.B.; Meilan, R.; Morris, R.O. Transfer RNA is the Source of Extracellular Isopentenyladenine in a Ti-plasmidless Strain of Agrobacterium tumefaciens. Plant Physiol. 1996, 110, 431–438. [Google Scholar] [CrossRef] [Green Version]
- Silva-Navas, J.; Conesa, C.M.; Saez, A.; Navarro-Neila, S.; Garcia-Mina, J.M.; Zamarreño, A.M.; Baigorri, R.; Swarup, R.; del Pozo, J.C. Role of Cis-Zeatin in Root Responses to Phosphate Starvation. New Phytol. 2019, 224, 242–257. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.S.; Edwards, D.G.; Campbell, L.C. Phosphorus Enhancement of Salt Tolerance of Tomato. Crop Sci. 1990, 30, 123–128. [Google Scholar] [CrossRef]
- Žižková, E.; Dobrev, P.I.; Muhovski, Y.; Hošek, P.; Hoyerová, K.; Haisel, D.; Procházková, D.; Lutts, S.; Motyka, V.; Hichri, I. Tomato (Solanum lycopersicum L.) SlIPT3 and SlIPT4 Isopentenyltransferases Mediate Salt Stress Response in Tomato. BMC Plant Biol. 2015, 15, 85. [Google Scholar] [CrossRef]
- Ghanem, M.E.; Albacete, A.; Smigocki, A.C.; Frébort, I.; Pospísilová, H.; Martínez-Andújar, C.; Acosta, M.; Bravo, J.S.; Lutts, S.; Dodd, I.; et al. Root-Synthesized Cytokinins Improve Shoot Growth and Fruit Yield in Salinized Tomato (Solanum lycopersicum L.) Plants. J. Exp. Bot. 2011, 62, 125–140. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Stirk, W.A.; Novak, M.S.; van Staden, J. Cytokinins in Macroalgae. Plant Growth Regul. 2003, 41, 13–24. [Google Scholar] [CrossRef]
- Hart, D.S.; Keightley, A.; Sappington, D.; Nguyen, P.T.; Chritton, C.; Seckinger, G.R.; Torres, K.C. Stability of Adenine-Based Cytokinins in Aqueous Solution. Vitr. Cell. Dev. Biol. Plant 2016, 52, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Mathur, C.; Rai, S.; Sase, N.; Krish, S.; Jayasri, M.A. Enteromorpha intestinalis Derived Seaweed Liquid Fertilizers as Prospective Biostimulant for Glycine max. Braz. Arch. Biol. Technol. 2015, 58, 813–820. [Google Scholar] [CrossRef] [Green Version]
- Steyn, W.J.; Wand, S.J.; Holcroft, D.M.; Jacobs, G. Anthocyanins in Vegetative Tissues: A Proposed Unified Function in Photoprotection. New Phytol. 2002, 155, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of Photosystem II Under Environmental Stress. Biochim. Biophys. Acta 2007, 1767, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalker-Scott, L. Environmental Significance of Anthocyanins in Plant Stress Responses. Photochem. Photobiol. 1999, 70, 1–9. [Google Scholar] [CrossRef]
- Deikman, J.; Hammer, P.E. Induction of Anthocyanin Accumulation by Cytokinins in Arabidopsis thaliana. Plant Physiol. 1995, 108, 47–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, P.K.; Shin, D.H.; Choi, S.-B.; Yoo, S.-D.; Choi, G.; Park, Y.-I. Cytokinins Enhance Sugar-Induced Anthocyanin Biosynthesis in Arabidopsis. Mol. Cells 2012, 34, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Müller, R.; Acosta-Motos, J.R.; Großkinsky, D.K.; Hernández, J.A.; Lütken, H.; Barba-Espin, G. UV-B Exposure of Black Carrot (Daucus carota ssp. sativus var. atrorubens) Plants Promotes Growth, Accumulation of Anthocyanin, and Phenolic compounds. Agronomy 2019, 9, 323. [Google Scholar] [CrossRef] [Green Version]
- Viña, A.; Gitelson, A.A. Sensitivity to Foliar Anthocyanin Content of Vegetation Indices Using Green Reflectance. IEEE Geosci. Remote Sens. Lett. 2011, 8, 464–468. [Google Scholar] [CrossRef] [Green Version]
- Graßmann, J.; Hippeli, S.; Elstner, E.F. Plant’s Defence and its Benefits for Animal and Medicine: Role of Phenolics and Terpenoids in Avoiding Oxygen Stress. Plant Physiol. Biochem. 2002, 40, 471–478. [Google Scholar] [CrossRef]
- Mibei, E.K.; Ambuko, J.; Giovannoni, J.J.; Onyango, A.N.; Owino, W.O. Carotenoid Profiling of the Leaves of Selected African Eggplant Accessions Subjected to Drought Stress. Food Sci. Nutr. 2017, 5, 113–122. [Google Scholar] [CrossRef]
- Yarsi, G.; Sivaci, A.; Dasgan, H.-Y.; Altuntas, O.; Binzet, R.; Akhoundnejad, Y. Effects of Salinity Stress on Chlorophyll and Carotenoid Contents and Stomata Size of Grafted and Ungrafted Galia C8 Melon Cultivar. Pak. J. Bot. 2017, 49, 421–426. [Google Scholar]
- Reddy, A.R.; Chaitanya, K.V.; Vivekanandan, M. Drought Induced Responses of Photosynthesis and Antioxidant Metabolism in Higher Plants. J. Plant Physiol. 2004, 161, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Ak, I.; Turker, G. Antioxidant Activity of Five Seaweed Extracts. New Knowl. J. Sci. 2018, 7, 149–155. [Google Scholar]
- Corpas, F.J.; Barroso, J.B. Nitro-Oxidative Stress vs Oxidative or Nitrosative Stress in Higher Plants. New Phytol. 2013, 199, 633–635. [Google Scholar] [CrossRef]
- Kolbert, Z.; Feigl, G.; Bordé, A.; Molnár, A.; Erdei, L. Protein Tyrosine Nitration in Plants: Present knowledge, Computational Prediction and Future Perspectives. Plant Physiol. Biochem. 2017, 113, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Jovanović, A.M.; Durst, S.; Nick, P. Plant Cell Division is Specifically Affected by Nitrotyrosine. J. Exp. Bot. 2010, 61, 901–909. [Google Scholar] [CrossRef] [Green Version]
- Correa-Aragunde, N.; Graziano, M.; Lamattina, L. Nitric Oxide Plays a Central Role in Determining Lateral Root Development in Tomato. Planta 2004, 218, 900–905. [Google Scholar] [CrossRef]
- Feng, J.; Wang, C.; Chen, Q.; Chen, H.; Ren, B.; Li, X.; Zuo, J. S-nitrosylation of Phosphotransfer Proteins Represses Cytokinin Signaling. Nat. Commun. 2013, 4, 1529. [Google Scholar] [CrossRef] [Green Version]
- Radi, R. Protein Tyrosine Nitration: Biochemical Mechanisms and Structural Basis of its Functional Effects. Acc. Chem. Res. 2013, 46, 550–559. [Google Scholar] [CrossRef] [Green Version]
- Tsuda, T.; Kato, Y.; Osawa, T. Mechanism for the Peroxynitrite Scavenging Activity by Anthocyanins. FEBS Lett. 2000, 484, 207–210. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Ichiyanagi, T.; Komiyama, T.; Hatano, Y.; Konishi, T. Superoxide Radical-and Peroxynitrite-Scavenging Activity of Anthocyanins; Structure-Activity Relationship and Their Synergism. Free Rad. Res. 2006, 40, 993–1002. [Google Scholar] [CrossRef] [PubMed]

| Treatment | No Salt Stress (%) | Salt Stress (%) |
|---|---|---|
| Control | 72 ± 4.9 a | 64 ± 9.8 a |
| BOA4 | 96 ± 4.0 b | 52 ± 8.0 a |
| EI | 76 ± 9.8 ab | 60 ± 6.3 a |
| BOA4 + EI | 68 ± 10.2 a | 64 ± 7.5 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana, M.M.; Rosa, A.P.; Zamarreño, A.M.; García-Mina, J.M.; Rai, A.; Cruz, C. Achromobacter xylosoxidans and Enteromorpha intestinalis Extract Improve Tomato Growth under Salt Stress. Agronomy 2022, 12, 934. https://doi.org/10.3390/agronomy12040934
Santana MM, Rosa AP, Zamarreño AM, García-Mina JM, Rai A, Cruz C. Achromobacter xylosoxidans and Enteromorpha intestinalis Extract Improve Tomato Growth under Salt Stress. Agronomy. 2022; 12(4):934. https://doi.org/10.3390/agronomy12040934
Chicago/Turabian StyleSantana, Margarida Maria, Ana Paula Rosa, Angel M. Zamarreño, José María García-Mina, Abdelwahab Rai, and Cristina Cruz. 2022. "Achromobacter xylosoxidans and Enteromorpha intestinalis Extract Improve Tomato Growth under Salt Stress" Agronomy 12, no. 4: 934. https://doi.org/10.3390/agronomy12040934
APA StyleSantana, M. M., Rosa, A. P., Zamarreño, A. M., García-Mina, J. M., Rai, A., & Cruz, C. (2022). Achromobacter xylosoxidans and Enteromorpha intestinalis Extract Improve Tomato Growth under Salt Stress. Agronomy, 12(4), 934. https://doi.org/10.3390/agronomy12040934







