Weed Composition in Hungarian Phacelia (Phacelia tanacetifolia Benth.) Seed Production: Could Tine Harrow Take over Chemical Management?
Abstract
1. Introduction
2. Materials and Methods
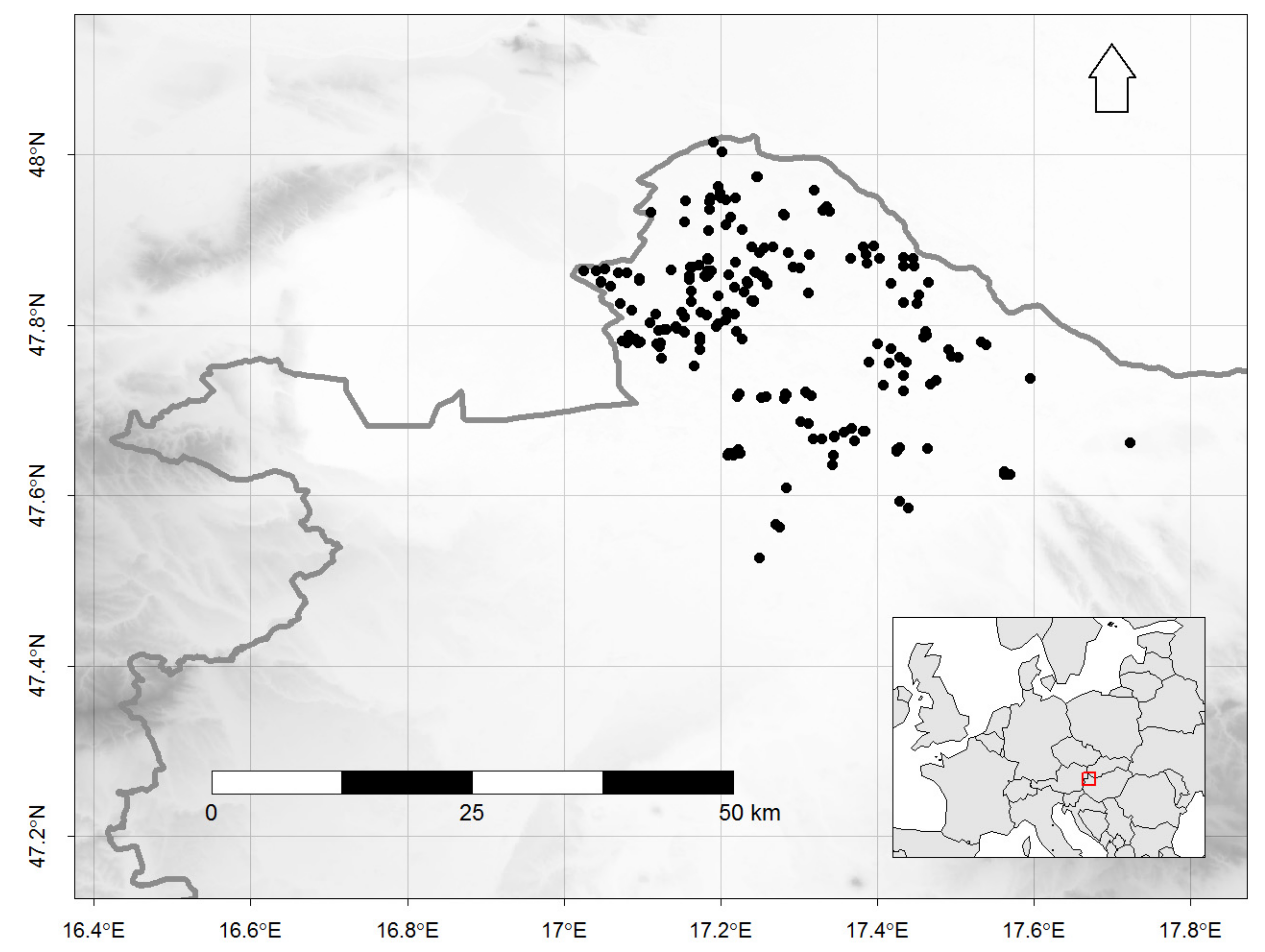
2.1. Data Collection
2.2. Statistical Analysis
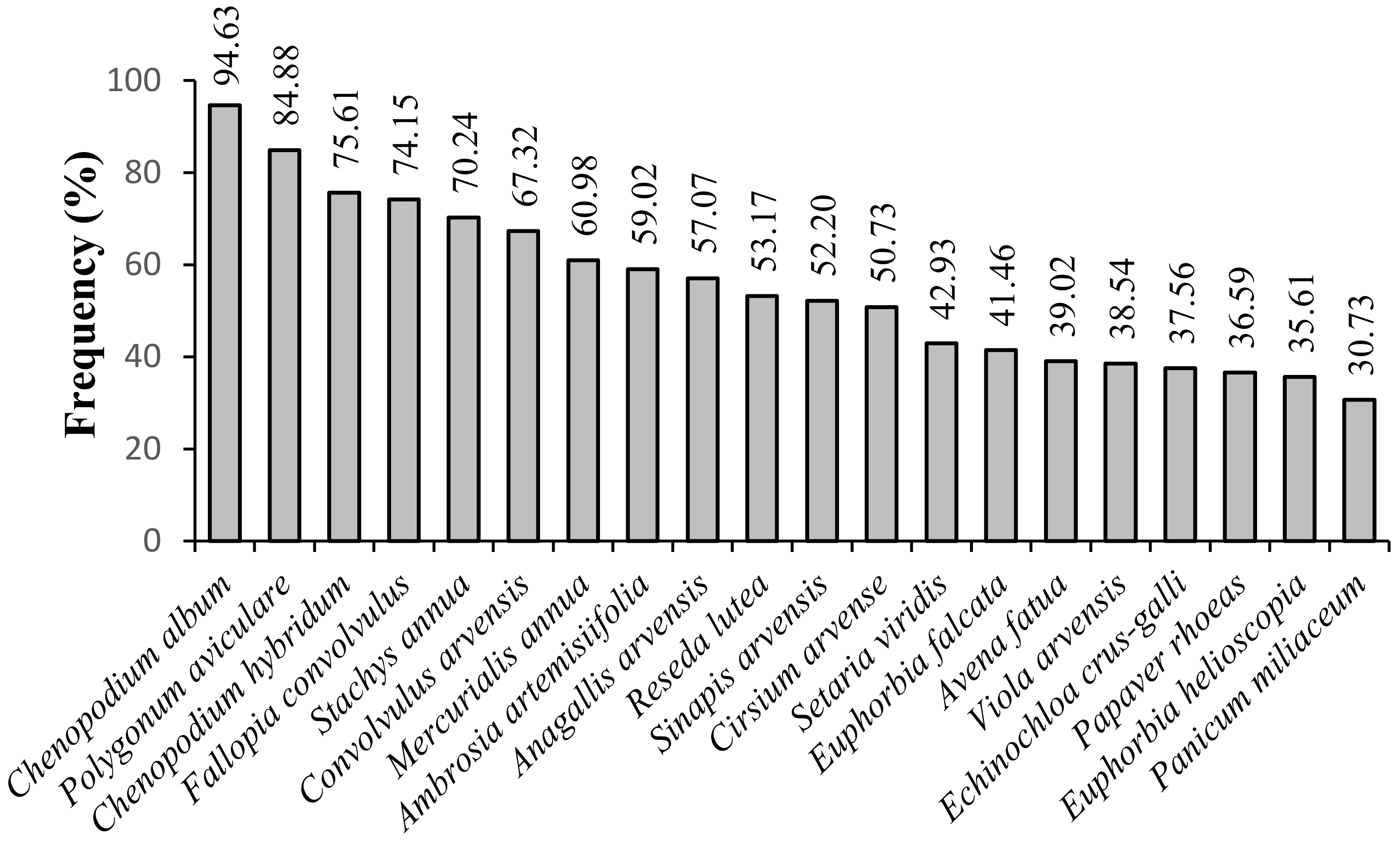
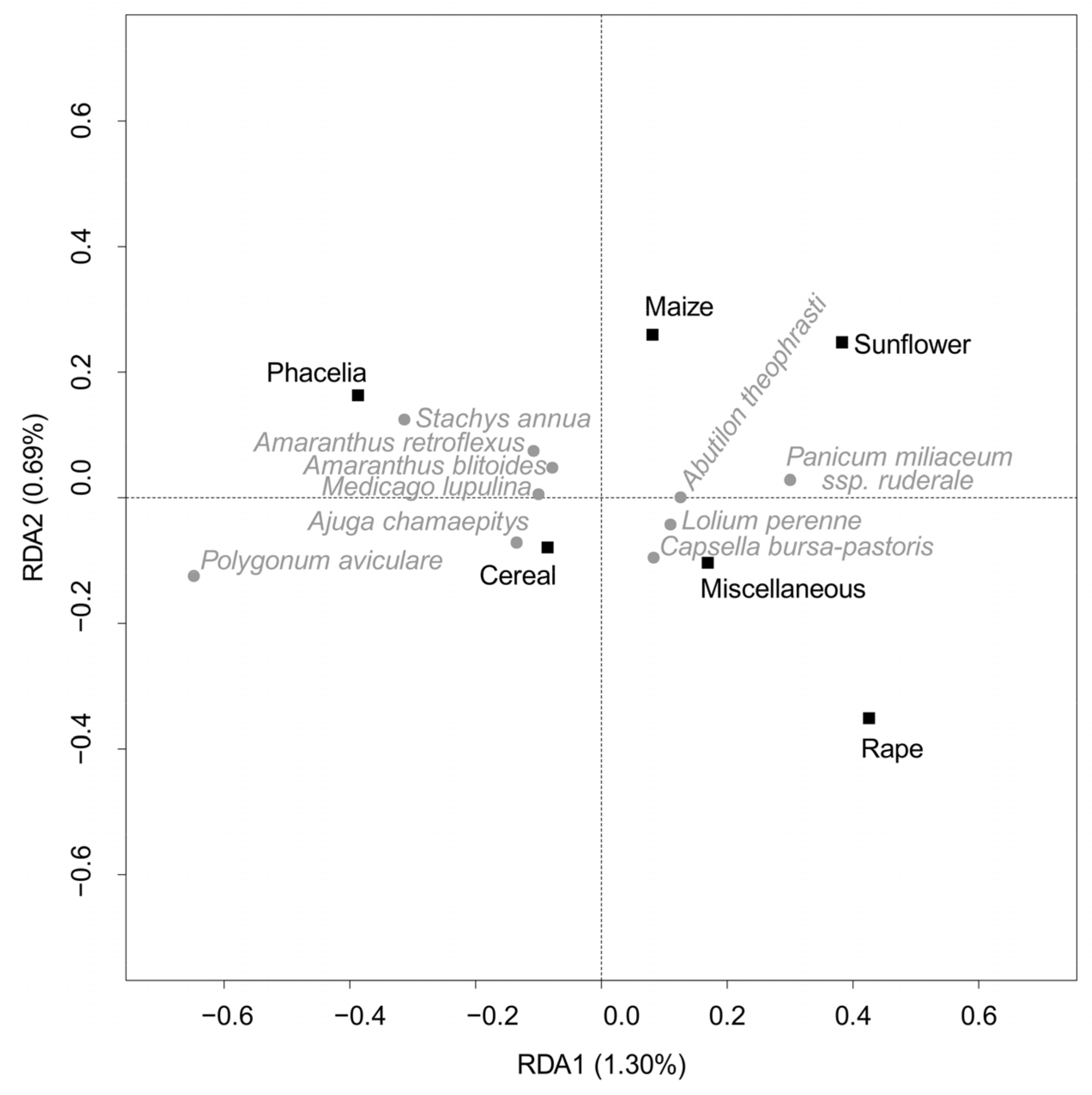
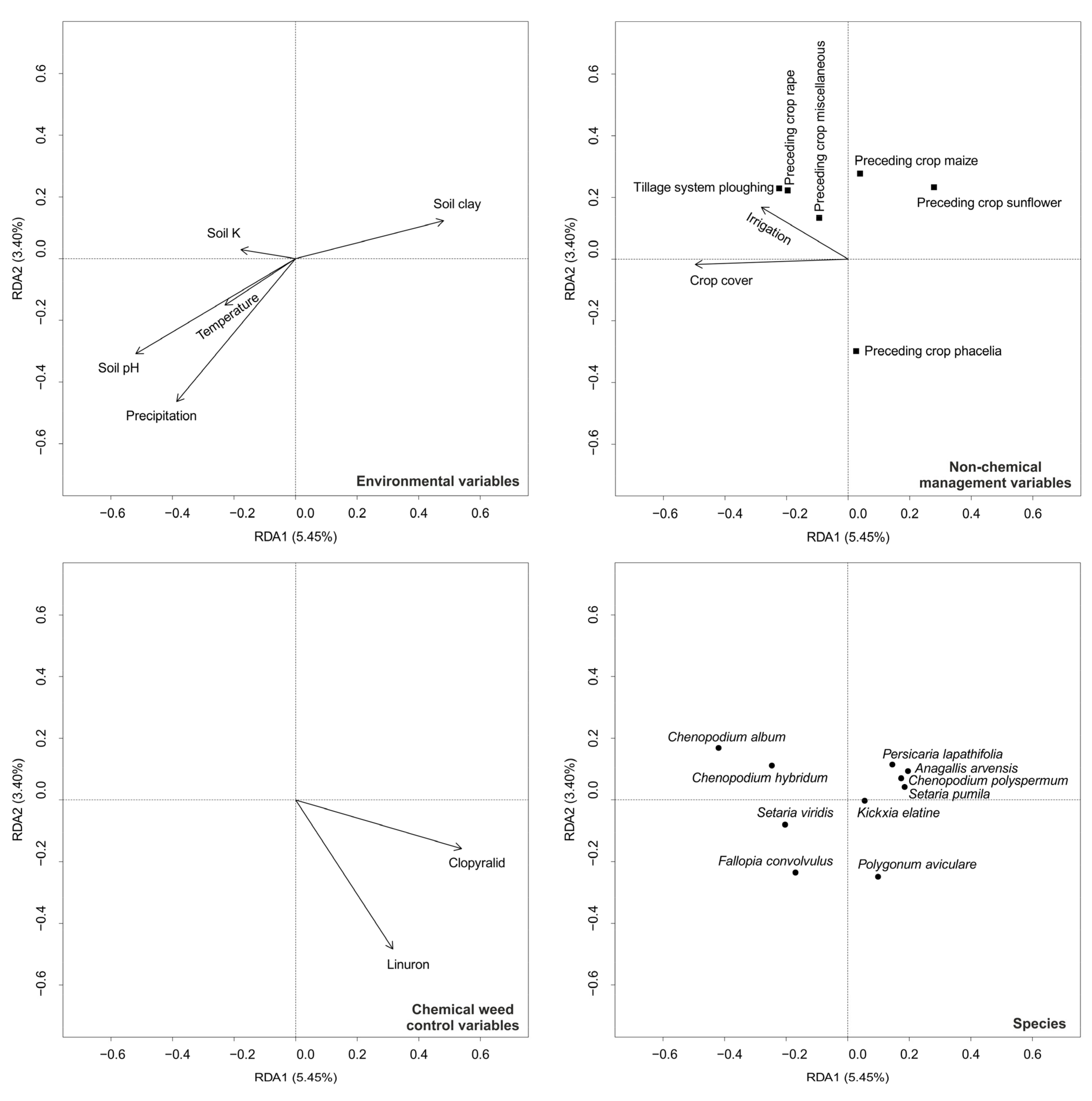
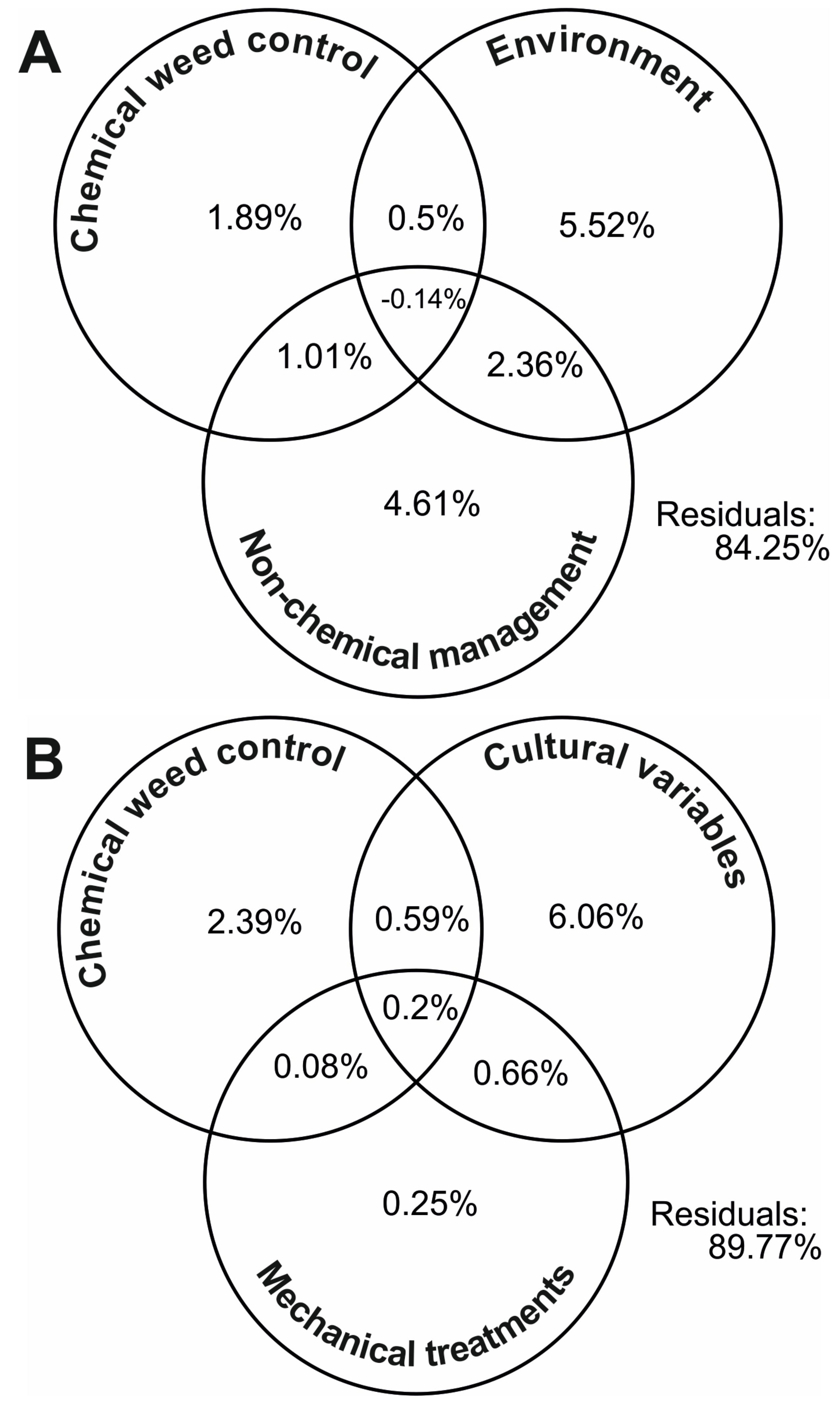
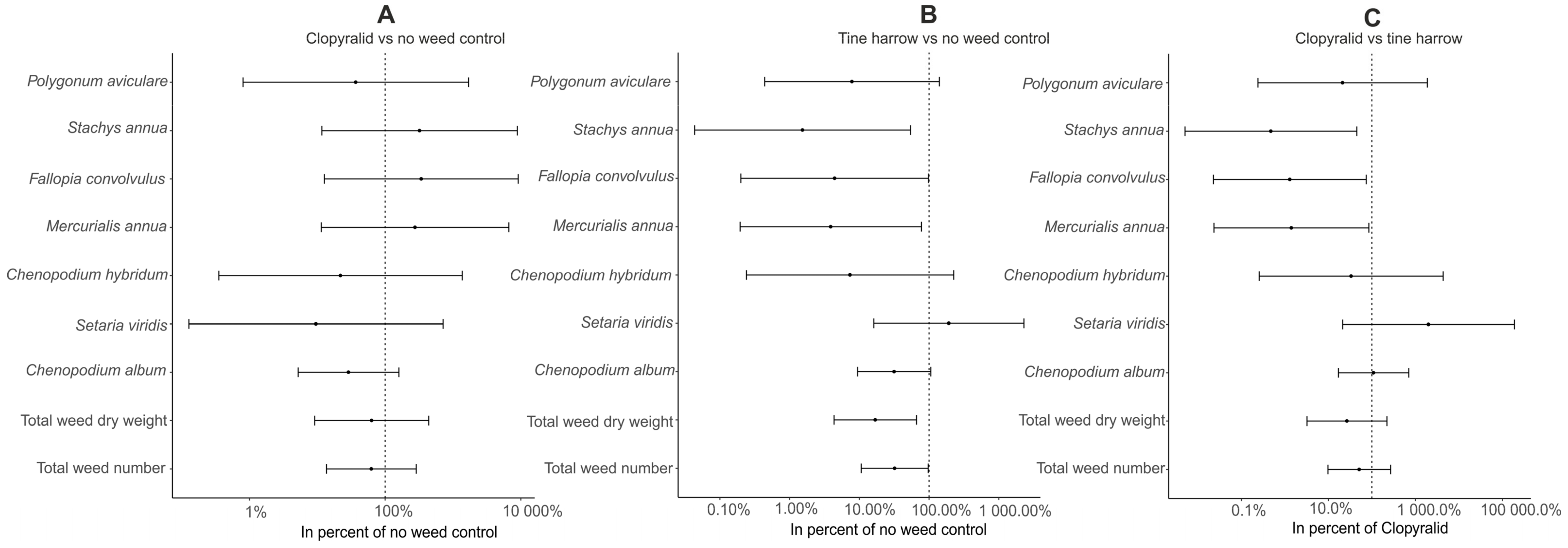
3. Results
4. Discussion
4.1. Environmental Variables
4.2. Non-Chemical Management Variables
4.2.1. Cultural Practices
4.2.2. Mechanical Weed Management
4.3. Herbicides
4.4. Biodiversity Issues
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kirk, W. Phacelia. Bee World 2005, 86, 14–16. [Google Scholar] [CrossRef]
- Farkas, Á.; Zajácz, E. Nectar production for the Hungarian honey industry. Eur. J. Plant Sci. Biotechnol. 2007, 1, 125–151. [Google Scholar]
- Kus, P.; Jerkovic, I.; Marijanovic, Z.; Kranjac, M.; Tuberoso, C. Unlocking Phacelia tanacetifolia benth. honey characterization through melissopalynological analysis, color determination and volatiles chemical profiling. Food Res. Int. 2018, 106, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Stanek, N.; Teper, D.; Kafarski, P.; Jasicka-Misiak, I. Authentication of phacelia honeys (Phacelia tanacetifolia) based on a combination of HPLC and HPTLC analyses as well as spectrophotometric measurements. LWT 2019, 107, 199–207. [Google Scholar] [CrossRef]
- Hickman, J.; Wratten, S. Use of Phacelia tanacetifolia strips to enhance biological control of aphids by hoverfly larvae in cereal fields. J. Econ. Entomol. 1996, 89, 832–840. [Google Scholar] [CrossRef]
- Owayss, A.; Shebl, M.; Iqbal, J.; Awad, A.; Raweh, H.; Alqarni, A. Phacelia tanacetifolia can enhance conservation of honey bees and wild bees in the drastic hot-arid subtropical Central Arabia. J. Apic. Res. 2020, 59, 569–582. [Google Scholar] [CrossRef]
- Petanidou, T. Introducing plants for bee-keeping at any cost? Assessment of Phacelia tanacetifolia as nectar source plant under xeric Mediterranean conditions. Plant Syst. Evol. 2003, 238, 155–168. [Google Scholar] [CrossRef]
- Sprague, R.; Boyer, S.; Stevenson, G.; Wratten, S. Assessing pollinators’ use of floral resource subsidies in agri-environment schemes: An illustration using Phacelia tanacetifolia and honeybees. PeerJ 2016, 4, e2677. [Google Scholar] [CrossRef] [PubMed]
- Williams, I.; Christian, D. Observations on Phacelia tanacetifolia bentham (Hydrophyllaceae) as a food plant for honey bees and bumble bees. J. Apic. Res. 1991, 30, 3–12. [Google Scholar] [CrossRef]
- Bacq-Labreuil, A.; Crawford, J.; Mooney, S.; Neal, A.; Ritz, K. Phacelia (Phacelia tanacetifolia Benth.) affects soil structure differently depending on soil texture. Plant Soil 2019, 441, 543–554. [Google Scholar] [CrossRef]
- Patkowska, E.; Konopinski, M. The role of oats, common vetch and tansy phacelia as cover plants in the formation of microorganisms communities in the soil under the cultivation of root chicory (Cichorium intybus var. sativum Bisch.) and salsify [Tragopogon porrifolius var. sativus (Gaterau) Br.]. Acta Sci. Pol. Hortorum Cultus 2013, 12, 179–191. [Google Scholar]
- Büchi, L.; Wendling, M.; Amosse, C.; Jeangros, B.; Charles, R. Cover crops to secure weed control strategies in a maize crop with reduced tillage. Field Crops Res. 2020, 247, 107583. [Google Scholar] [CrossRef]
- Gaweda, D.; Wesolowski, M.; Kwiatkowski, C. Weed infestation of spring barley (Hordeum vulgare L.) depending on the cover crop and weed control method. Acta Agrobot. 2014, 67, 77–84. [Google Scholar] [CrossRef]
- Kolodziejczyk, M. The effect of living mulches and conventional methods of weed control on weed infestation and potato yield. Sci. Hortic. 2015, 191, 127–133. [Google Scholar] [CrossRef]
- Nagy, I. A magyarországi facélia (mézontófű) vetőmag-előállítás számokban. Agrofórum 2021, 32, 176–178. [Google Scholar]
- Schmidt, R. Facélia. In Növénytermesztéstan; Antal, J., Ed.; Mezőgazda Kiadó: Budapest, Hungary, 2005; pp. 476–481. [Google Scholar]
- Kádár, A. Mézontófű (facélia). In Vegyszeres Gyomirtás és Termésszabályozás; Magánkiadás: Budapest, Hungary, 2019; pp. 293–294. [Google Scholar]
- Hillocks, R. Farming with fewer pesticides: EU pesticide review and resulting challenges for UK agriculture. Crop Prot. 2012, 31, 85–93. [Google Scholar] [CrossRef]
- Bell, C.E.; Boutwell, B.E.; Ogbuchiekwe, E.J.; McGiffen, M.E. Weed control in carrots: The efficacy and economic value of linuron. Hortscience 2000, 35, 1089–1091. [Google Scholar] [CrossRef]
- Soltani, N.; Nurse, R.E.; Shropshire, C.; Sikkema, P.H. Weed management in cranberry bean with linuron. Can. J. Plant Sci. 2011, 91, 881–888. [Google Scholar] [CrossRef][Green Version]
- Doma, C.; Horváth, I.; Horváth, E.; Vass, Z.; Aurbech, A.; Molnár, K.; Boronkai, A. A mézontófű (Phacelia tanacetifolia) vegyszeres gyomirtásának lehetőségei. In Növényvédelmi Tudományos Napok; Horváth, J., Ed.; Magyar Növényvédelmi Társaság: Budapest, Hungary, 2017; Volume 63, p. 79. [Google Scholar]
- Armengot, L.; Jose-Maria, L.; Chamorro, L.; Xavier Sans, F. Weed harrowing in organically grown cereal crops avoids yield losses without reducing weed diversity. Agron. Sustain. Dev. 2013, 33, 405–411. [Google Scholar] [CrossRef]
- Peruzzi, A.; Martelloni, L.; Frasconi, C.; Fontanelli, M.; Pirchio, M.; Raffaelli, M. Machines for non-chemical intra-row weed control in narrow and wide-row crops: A review. J. Agric. Eng. 2017, 48, 57–70. [Google Scholar] [CrossRef]
- Weis, M.; Gutjahr, C.; Rueda Ayala, V.; Gerhards, R.; Ritter, C.; Scholderle, F. Precision farming for weed management: Techniques. Gesunde Pflanz. 2008, 60, 171–181. [Google Scholar] [CrossRef]
- Brandsaeter, L.O.; Mangerud, K.; Rasmussen, J. Interactions between pre- and post-emergence weed harrowing in spring cereals. Weed Res. 2012, 52, 338–347. [Google Scholar] [CrossRef]
- Lundkvist, A. Effects of pre- and post-emergence weed harrowing on annual weeds in peas and spring cereals. Weed Res. 2009, 49, 409–416. [Google Scholar] [CrossRef]
- Johnson, W.; Luo, X. Integrating cultivation using a tine weeder with herbicides in conventional peanut production. Weed Technol. 2019, 33, 374–379. [Google Scholar] [CrossRef]
- Pannacci, E.; Tei, F.; Guiducci, M. Mechanical weed control in organic winter wheat. Ital. J. Agron. 2017, 12, 336–342. [Google Scholar] [CrossRef]
- Rueda-Ayala, V.; Peteinatos, G.; Gerhards, R.; Andujar, D. A non-chemical system for online weed control. Sensors 2015, 15, 7691–7707. [Google Scholar] [CrossRef]
- Zeng, Z.; Martin, A.; Chen, Y.; Ma, X. Weeding performance of a spring-tine harrow as affected by timing and operational parameters. Weed Sci. 2021, 69, 247–256. [Google Scholar] [CrossRef]
- Pinke, G.; Pál, R.W.; Tóth, K.; Karácsony, P.; Czúcz, B.; Botta-Dukát, Z. Weed vegetation of poppy (Papaver somniferum) fields in hungary: Effects of management and environmental factors on species composition. Weed Res. 2011, 51, 621–630. [Google Scholar] [CrossRef]
- Pinke, G.; Blazsek, K.; Magyar, L.; Nagy, K.; Karácsony, P.; Czúcz, B.; Botta-Dukát, Z. Weed species composition of conventional soyabean crops in Hungary is determined by environmental, cultural, weed management and site variables. Weed Res. 2016, 56, 470–481. [Google Scholar] [CrossRef]
- Pinke, G.; Karácsony, P.; Czúcz, B.; Botta-Dukát, Z. When herbicides don’t really matter: Weed species composition of oil pumpkin (Cucurbita pepo L.) fields in Hungary. Crop Prot. 2018, 110, 236–244. [Google Scholar] [CrossRef]
- Nagy, Z. Dísznövényből haszonnövény lett a facélia. Agrofórum 2019, 30, 16–18. [Google Scholar]
- Krähmer, H.; Andreasen, C.; Economou-Antonaka, G.; Holec, J.; Kalivas, D.; Kolarova, M.; Novak, R.; Panozzo, S.; Pinke, G.; Salonen, J.; et al. Weed surveys and weed mapping in Europe: State of the art and future tasks. Crop Prot. 2020, 129, 105010. [Google Scholar] [CrossRef]
- Cohen, M.; Prenger, J.; DeBusk, W. Visible-near infrared reflectance spectroscopy for rapid, nondestructive assessment of wetland soil quality. J. Environ. Qual. 2005, 34, 1422–1434. [Google Scholar] [CrossRef]
- Viscarra Rossel, R.A.; Walvoort, D.J.J.; McBratney, A.B.; Janik, L.J.; Skjemstad, J.O. Visible, near infrared, mid infrared or combined diffuse reflectance spectroscopy for simultaneous assessment of various soil properties. Geoderma 2006, 131, 59–75. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Blackshaw, R.E.; Anderson, R.L.; Lemerle, D. Cultural weed management. In Non-Chemical Weed Management: Principles, Concepts and Technology; Upadhyaya, M.K., Blackshaw, R.E., Eds.; CAB International, Agriculture and Agri-Food Canada: Reading, UK, 2007; pp. 35–47. ISBN 978-1-84593-290-9. [Google Scholar]
- Cloutier, D.C.; Weide, E.Y.; Peruzzi, A.; Leblanc, M.L. Mechanical weed management. In Non-Chemical Weed Management: Principles, Concepts and Technology; Upadhyaya, M.K., Blackshaw, R.E., Eds.; CAB International, Agriculture and Agri-Food Canada: Reading, UK, 2007; pp. 111–134. ISBN 978-1-84593-290-9. [Google Scholar]
- Pinke, G.; Karácsony, P.; Czúcz, B.; Botta-Dukát, Z.; Lengyel, A. The influence of environment, management and site context on species composition of summer arable weed vegetation in Hungary. Appl. Veg. Sci. 2012, 15, 136–144. [Google Scholar] [CrossRef]
- Fox, J.; Monette, G. Generalized collinearity diagnostics. J. Am. Stat. Assoc. 1992, 87, 178–183. [Google Scholar] [CrossRef]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer: New York, NY, USA, 2011; ISBN 978-1-4419-7975-9. [Google Scholar]
- Lososová, Z.; Chytry, M.; Cimalová, S.; Kropác, Z.; Otypková, Z.; Pysek, P.; Tichy, L. Weed vegetation of arable land in Central Europe: Gradients of diversity and species composition. J. Veg. Sci. 2004, 15, 415–422. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org (accessed on 1 January 2020).
- Plate, T.; Heiberger, R. Abind: Combine Multidimensional Arrays. R Package Version 1.4-5. 2016. Available online: https://cran.r-project.org/package=abind (accessed on 21 July 2016).
- Fox, J.; Weisberg, S. An {R} Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.4.4. 2021. Available online: https://cran.r-project.org/package=DHARMa (accessed on 28 September 2021).
- Brooks, M.; Kristensen, K.; van Benthem, K.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.; Mächler, M.; Bolker, B. GlmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Hijmans, R.J. Raster: Geographic Data Analysis and Modeling. R Package Version 2.6-7. 2021. Available online: https://cran.r-project.org/package=raster (accessed on 13 November 2017).
- Pebesma, E. Simple features for R: Standardized support for spatial vector data. R J. 2018, 10, 439–446. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; et al. Vegan Community Ecology Package Version 2.5-7 November 2020. Available online: https://cran.r-project.org/package=vegan (accessed on 28 November 2020).
- Chen, H. VennDiagram: Generate High-Resolution Venn and Euler Plots. R Package Version 1.6.20. 2018. Available online: https://cran.r-project.org/package=VennDiagram (accessed on 28 March 2018).
- Andreasen, C.; Skovgaard, I.M. Crop and soil factors of importance for the distribution of plant species on arable fields in Denmark. Agric. Ecosyst. Environ. 2009, 133, 61–67. [Google Scholar] [CrossRef]
- Patzold, S.; Hbirkou, C.; Dicke, D.; Gerhards, R.; Welp, G. Linking weed patterns with soil properties: A long-term case study. Precis. Agric. 2020, 21, 569–588. [Google Scholar] [CrossRef]
- Vidotto, F.; Fogliatto, S.; Milan, M.; Ferrero, A. Weed communities in Italian maize fields as affected by pedo-climatic traits and sowing time. Eur. J. Agron. 2016, 74, 38–46. [Google Scholar] [CrossRef]
- Cimalová, S.; Lososová, Z. Arable weed vegetation of the northeastern part of the Czech Republic: Effects of environmental factors on species composition. Plant Ecol. 2009, 203, 45–57. [Google Scholar] [CrossRef]
- De Mol, F.; von Redwitz, C.; Gerowitt, B. Weed species composition of maize fields in Germany is influenced by site and crop sequence. Weed Res. 2015, 55, 574–585. [Google Scholar] [CrossRef]
- Fanfarillo, E.; Petit, S.; Dessaint, F.; Rosati, L.; Abbate, G. Species composition, richness, and diversity of weed communities of winter arable land in relation to geo-environmental factors: A gradient analysis in mainland Italy. Botany 2020, 98, 381–392. [Google Scholar] [CrossRef]
- Kästner, A.; Jäger, E.; Schubert, R. Handbuch der Segetalpflanzen Mitteleuropas; Springer: Vienna, Austria; New York, NY, USA, 2001. [Google Scholar]
- Andrade, J.; Satorre, E.; Ermacora, C.; Poggio, S. Weed communities respond to changes in the diversity of crop sequence composition and double cropping. Weed Res. 2017, 57, 148–158. [Google Scholar] [CrossRef]
- Geren, H.; Avcioglu, R.; Kaymakkavak, D. Effects of different row spacings on the seed yield and some other characteristics of phacelia (Phacelia tanacetifolia Bentham.) varieties. J. Food Agric. Environ. 2009, 7, 383–386. [Google Scholar]
- Okcu, M. Determination of the effects of different row spacing and seed quantity on yield and yield characteristics of phacelia (Phacelia tanacetifolia Bentham). Fresenius Environ. Bull. 2019, 28, 7630–7635. [Google Scholar]
- Szabó, R.; Horváth, E. A facélia (Phacelia tanacetifolia) gyomosodásának és a gyomirtás hatékonyságának vizsgálata. Georg. Agric. 2014, 19, 217–226. [Google Scholar]
- Pinke, G.; Kolejanisz, T.; Vér, A.; Nagy, K.; Milics, G.; Schlögl, G.; Bede-Fazekas, A.; Botta-Dukát, Z.; Czúcz, B. Drivers of Ambrosia artemisiifolia abundance in arable fields along the Austrian-Hungarian border. Preslia 2019, 91, 369–389. [Google Scholar] [CrossRef]
- Montagnani, C.; Gentili, R.; Smith, M.; Guarino, M.F.; Citterio, S. The worldwide spread, success, and impact of ragweed (Ambrosia spp.). Crit. Rev. Plant Sci. 2017, 36, 139–178. [Google Scholar] [CrossRef]
- Storkey, J.; Moss, S.R.; Cussans, J.W. Using assembly theory to explain changes in a weed flora in response to agricultural intensification. Weed Sci. 2010, 58, 39–46. [Google Scholar] [CrossRef]
- Bajwa, A.; Zulfiqar, U.; Sadia, S.; Bhowmik, P.; Chauhan, B. A global perspective on the biology, impact and management of Chenopodium album and Chenopodium murale: Two troublesome agricultural and environmental weeds. Environ. Sci. Pollut. Res. 2019, 26, 5357–5371. [Google Scholar] [CrossRef]
- Pinke, G.; Pál, R. Floristic composition and conservation value of the stubble-field weed community, dominated by Stachys annua in Western Hungary. Biologia 2009, 64, 279–291. [Google Scholar] [CrossRef]
- Fried, G.; Norton, L.R.; Reboud, X. Environmental and management factors determining weed species composition and diversity in France. Agric. Ecosyst. Environ. 2008, 128, 68–76. [Google Scholar] [CrossRef]
- Hanzlik, K.; Gerowitt, B. The importance of climate, site and management on weed vegetation in oilseed rape in Germany. Agric. Ecosyst. Environ. 2011, 141, 323–331. [Google Scholar] [CrossRef]
- Hofmeijer, M.; Melander, B.; Salonen, J.; Lundkvist, A.; Zarina, L.; Gerowitt, B. Crop diversification affects weed communities and densities in organic spring cereal fields in Northern Europe. Agric. Ecosyst. Environ. 2021, 308, 107251. [Google Scholar] [CrossRef]
- Strehlow, B.; de Mol, F.; Gerowitt, B. Herbicide intensity depends on cropping system and weed control target: Unraveling the effects in field experiments. Crop Prot. 2020, 129, 105011. [Google Scholar] [CrossRef]
- Christoffoleti, P.J.; Carvalho, S.J.; Nicolai, M.; Doohan, D.; VanGessel, M. Prevention strategies in weed management. In Non-Chemical Weed Management: Principles, Concepts and Technology; Upadhyaya, M.K., Blackshaw, R.E., Eds.; CAB International, Agriculture and Agri-Food Canada: Reading, UK, 2007; pp. 1–15. ISBN 978-1-84593-290-9. [Google Scholar]
- Souza, M.; Lins, H.; de Mesquita, H.; Teofilo, T.; Reginaldo, L.; Pereira, R.; Grangeiro, L.; Silva, D. Can irrigation systems alter the critical period for weed control in onion cropping? Crop Prot. 2021, 147, 105457. [Google Scholar] [CrossRef]
- Fagundez, J.; Olea, P.; Tejedo, P.; Mateo-Tomas, P.; Gomez, D. Irrigation and maize cultivation erode plant diversity within crops in Mediterranean dry cereal agro-ecosystems. Environ. Manag. 2016, 58, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Mitchell, J.; Lanini, W. Subsurface drip irrigation as a weed management tool for conventional and conservation tillage tomato (Lycopersicon esculentum Mill.) production in semi-arid agroecosystems. J. Sustain. Agric. 2007, 31, 91–112. [Google Scholar] [CrossRef]
- Buhler, D.; Stoltenberg, D.; Becker, R.; Gunsolus, J. Perennial weed populations after 14 years of variable tillage and cropping practices. Weed Sci. 1994, 42, 205–209. [Google Scholar] [CrossRef]
- Torresen, K.; Skuterud, R.; Tandsaether, H.; Hagemo, M. Long-Term Experiments with reduced tillage in spring cereals. I. Effects on weed flora, weed seedbank and grain yield. Crop Prot. 2003, 22, 185–200. [Google Scholar] [CrossRef]
- Armengot, L.; Berner, A.; Blanco-Moreno, J.; Mader, P.; Sans, F. Long-term feasibility of reduced tillage in organic farming. Agron. Sustain. Dev. 2015, 35, 339–346. [Google Scholar] [CrossRef]
- Govindasamy, P.; Sarangi, D.; Provin, T.; Hons, F.; Bagavathiannan, M. No-tillage altered weed species dynamics in a long-term (36-year) grain sorghum experiment in Southeast Texas. Weed Sci. 2020, 68, 476–484. [Google Scholar] [CrossRef]
- Mouazen, A.; Duerinckx, K.; Ramon, H.; Anthonis, J. Soil influences on the mechanical actions of a flexible spring tine during selective weed harrowing. Biosyst. Eng. 2007, 96, 7–18. [Google Scholar] [CrossRef]
- Magyar, L. A facélia mint gyomosító kultúrnövény és az ellene való védekezés újabb lehetősége őszi kalászosokban. Agrofórum Extra 2021, 89, 39–40. [Google Scholar]
- Colquhoun, J.; Rittmeyer, R.; Heider, D. Carrot weed management programs without linuron herbicide. Weed Technol. 2019, 33, 490–494. [Google Scholar] [CrossRef]
- Devine, M.; Vandenborn, W. Absorption, translocation and foliar activity of clopyralid and chlorosulfuron in Canada thistle (Cirsium arvense) and perennial sowthistle (Sonchus arvensis). Weed Sci. 1985, 33, 524–530. [Google Scholar] [CrossRef]
- Sakaliene, O.; Clay, S.; Koskinen, W.; Almantas, G. Early season weed suppression in buckwheat using clopyralid. Weed Technol. 2008, 22, 707–712. [Google Scholar] [CrossRef]
- Pinke, G.; Dunai, É.; Czúcz, B. Rise and fall of Stachys annua (L.) L. in the Carpathian basin: A historical review and prospects for its revival. Genet. Resour. Crop. Evol. 2021, 68, 3039–3053. [Google Scholar] [CrossRef]
- Wietzke, A.; van Waveren, C.; Bergmeier, E.; Meyer, S.; Leuschner, C. Current state and drivers of arable plant diversity in conventionally managed farmland in Northwest Germany. Diversity 2020, 12, 469. [Google Scholar] [CrossRef]
- Albrecht, H.; Cambecèdes, J.; Lang, M.; Wagner, M. Management options for the conservation of rare arable plants in Europe. Bot. Lett. 2016, 163, 389–415. [Google Scholar] [CrossRef]

| Variable (Unit) | Broad-Scale Survey (BS) Range/Values | Fine-Scale Survey (FS) Range/Values |
|---|---|---|
| ENVIRONMENTAL | ||
| Climate | ||
| Mean annual precipitation (mm) | 535–582 | 552–562 |
| Mean annual temperature (°C) | 10.07–10.47 | 10.25–10.47 |
| Soil | ||
| Soil pH (KCl) | 6.2–8.1 | 7–8 |
| Soil clay (%) | 8–41 | 11.8–38.1 |
| Soil N (g kg−1) a | 0.9–3.9 | 0.2–2.7 |
| Soil P (mg kg−1) b | 18.1–69.1 | 32.8–47.4 |
| Soil K (mmol kg−1) | 2.8–11.2 | 0.9–9.6 |
| Soil Ca (mmol kg−1) a | 78.8–461.6 | 137.4–330.8 |
| Soil Mg (mmol kg−1) a | 5.8–79.5 | 17.2–54.6 |
| Soil cation exchange capacity (mmol kg−1) a | 64–461 | 98.3–317.8 |
| Soil organic matter (%) b | 1.8–6.4 | 0.8–5.1 |
| NON-CHEMICAL MANAGEMENTc | ||
| Cultural | ||
| Farming type b | Conventional, organic | Conventional |
| Crop cover (%) | 10–100 | 50–100 |
| Crop dry weight (g 0.25 cm2–1) | Not measured | 49.65–251.85 |
| Seeding rate (kg ha−1) b | 4.7–12 | 5–12 |
| Crop row spacing (cm) b | 12–45 | 12–30 |
| Field size (ha) b | 0.2–71 | 0.22–15.45 |
| Cultivar a | Angélia, Balo, Faktotum, Júlia, Lilla, Liza, Mira. | Angélia, Júlia, Lilla |
| Date of sowing b | 27 February–15 April | 12–19 March |
| Preceding crop | Cereal, maize, miscellaneous, phacelia, rape, sunflower | Cereal, maize, soybean, rape |
| Irrigation (mm) | 0–35 | 0–35 |
| Organic manure (t ha−1) b | 0–50 | 0 |
| Amount of fertiliser (kg ha−1) | ||
| N b | 0–118 | 0–45 |
| P2O5 b | 0–90 | 0–45 |
| K2O a | 0–120 | 0–45 |
| Mechanical | ||
| Primary tillage depth (cm) b | 2–65 | 25–30 |
| Tillage system | No-tillage, ploughing | No-tillage, ploughing |
| Tine harrow (times) b | 0–1 | 0–1 |
| Cultivating tillage (times) b | 0–2 | 0 |
| Manual weed control (times) b | 0–1 | 0 |
| CHEMICAL WEED CONTROL | ||
| Herbicides | ||
| Linuron (g a.i. ha−1) | 0–810 | 0 |
| Clopyralid (g a.i. ha−1) | 0–240 | 0–150 |
| Quizalofop-P-ethyl (l a.i. ha−1) b | 0–0.75 | 0 |
| Quizalofop-P-terufil (g a.i. ha−1) b | 0–150 | 0 |
| Gross Effect | Net Effect | ||||||
|---|---|---|---|---|---|---|---|
| Factors | d.f. | Explained Variation (%) | Explained Variation (%) | F | p-Value | ||
| Soil pH | 1 | 3.083 | 0.0261 | 2.164 | 0.0187 | 5.172 | *** |
| Soil clay content | 1 | 2.598 | 0.0212 | 1.959 | 0.0165 | 4.683 | *** |
| Crop cover | 1 | 2.679 | 0.0220 | 1.927 | 0.0162 | 4.606 | *** |
| Precipitation | 1 | 2.507 | 0.0203 | 1.819 | 0.0150 | 4.348 | *** |
| Linuron | 1 | 2.188 | 0.0171 | 1.716 | 0.0139 | 4.101 | *** |
| Temperature | 1 | 1.531 | 0.0105 | 1.323 | 0.0097 | 3.163 | *** |
| Preceding crop | 5 | 4.413 | 0.0201 | 2.965 | 0.0092 | 1.418 | ** |
| Clopyralid | 1 | 2.273 | 0.0179 | 1.175 | 0.0081 | 2.809 | *** |
| Irrigation | 1 | 1.118 | 0.0063 | 1.013 | 0.0064 | 2.421 | *** |
| Tillage system | 1 | 1.019 | 0.0053 | 0.923 | 0.0054 | 2.205 | ** |
| Soil K content | 1 | 1.150 | 0.0066 | 0.840 | 0.0045 | 2.008 | ** |
| Ax 1 Score | Fit | Ax 1 Score | Fit | ||
|---|---|---|---|---|---|
| Soil pH (+ low, – high) | Soil clay (+ low, – high) | ||||
| Alopecurus myosuroides | 0.140 | 0.116 | Anagallis arvensis | −0.182 | 0.127 |
| Reseda lutea | −0.212 | 0.113 | Reseda lutea | −0.161 | 0.066 |
| Tripleurospermum inodorum | 0.080 | 0.102 | Kickxia elatine | −0.033 | 0.061 |
| Chenopodium polyspermum | 0.101 | 0.073 | Anagallis foemina | −0.070 | 0.061 |
| Stachys annua | −0.191 | 0.070 | Persicaria lapathifolia | −0.100 | 0.051 |
| Euphorbia falcata | −0.150 | 0.069 | Chenopodium polyspermum | −0.083 | 0.050 |
| Persicaria lapathifolia | 0.103 | 0.055 | Chenopodium album | 0.207 | 0.047 |
| Elymus repens | 0.062 | 0.036 | Euphorbia exigua | −0.051 | 0.043 |
| Mercurialis annua | −0.105 | 0.035 | Euphorbia falcata | −0.102 | 0.032 |
| Echinochloa crus−galli | 0.085 | 0.034 | Artemisia vulgaris | 0.015 | 0.029 |
| Crop cover (+ high, – low) | Precipitation (+ high, – low) | ||||
| Ambrosia artemisiifolia | 0.258 | 0.108 | Fallopia convolvulus | 0.226 | 0.097 |
| Kickxia elatine | 0.041 | 0.092 | Stachys annua | 0.157 | 0.047 |
| Anagallis foemina | 0.084 | 0.088 | Setaria viridis | 0.127 | 0.047 |
| Microrrhinum minus | 0.046 | 0.065 | Hyoscyamus niger | 0.037 | 0.044 |
| Anagallis arvensis | 0.118 | 0.054 | Ambrosia artemisiifolia | −0.160 | 0.042 |
| Chenopodium hybridum | −0.130 | 0.053 | Mercurialis annua | 0.114 | 0.041 |
| Chenopodium album | −0.208 | 0.047 | Amaranthus blitoides | 0.035 | 0.040 |
| Ajuga chamaepitys | 0.052 | 0.045 | Euphorbia exigua | 0.048 | 0.037 |
| Consolida regalis | 0.017 | 0.044 | Hibiscus trionum | −0.051 | 0.029 |
| Lathyrus tuberosus | 0.045 | 0.039 | Sinapis arvensis | 0.105 | 0.021 |
| Linuron (+ high, – low) | Temperature (+ low, – high) | ||||
| Anagallis arvensis | −0.121 | 0.056 | Setaria viridis | −0.172 | 0.085 |
| Polygonum aviculare | 0.173 | 0.049 | Ajuga chamaepitys | −0.059 | 0.059 |
| Chenopodium album | −0.191 | 0.040 | Euphorbia falcata | −0.132 | 0.054 |
| Chenopodium hybridum | −0.112 | 0.039 | Hordeum vulgare | −0.039 | 0.044 |
| Reseda lutea | 0.121 | 0.037 | Euphorbia exigua | 0.051 | 0.043 |
| Papaver rhoeas | −0.070 | 0.029 | Stachys annua | −0.150 | 0.043 |
| Convolvulus arvensis | 0.129 | 0.029 | Hibiscus trionum | −0.059 | 0.039 |
| Brassica napus | 0.042 | 0.022 | Capsella bursa−pastoris | 0.048 | 0.034 |
| Alopecurus myosuroides | 0.055 | 0.018 | Fallopia convolvulus | −0.121 | 0.028 |
| Fallopia convolvulus | 0.095 | 0.017 | Thlaspi arvense | −0.036 | 0.027 |
| Clopyralid (+ high, – low) | Irrigation (+ low, – high) | ||||
| Kickxia elatine | 0.042 | 0.096 | Solanum nigrum | −0.064 | 0.049 |
| Convolvulus arvensis | 0.220 | 0.084 | Datura stramonium | −0.070 | 0.048 |
| Euphorbia falcata | 0.123 | 0.047 | Chenopodium hybridum | −0.106 | 0.035 |
| Reseda lutea | 0.106 | 0.028 | Sinapis arvensis | −0.126 | 0.031 |
| Helianthus annuus | −0.038 | 0.019 | Lathyrus tuberosus | 0.037 | 0.027 |
| Anthemis austriaca | −0.039 | 0.019 | Mercurialis annua | −0.087 | 0.024 |
| Euphorbia exigua | 0.032 | 0.017 | Hordeum vulgare | 0.025 | 0.019 |
| Galium aparine | 0.027 | 0.014 | Anagallis arvensis | 0.065 | 0.016 |
| Medicago lupulina | −0.023 | 0.014 | Ambrosia artemisiifolia | 0.098 | 0.016 |
| Ajuga chamaepitys | 0.028 | 0.013 | Papaver rhoeas | −0.051 | 0.016 |
| Tillage (+ plough, – no tillage) | Soil K (+ high, – low) | ||||
| Mercurialis annua | 0.148 | 0.069 | Euphorbia falcata | −0.115 | 0.041 |
| Anthemis austriaca | −0.051 | 0.032 | Anagallis arvensis | −0.094 | 0.034 |
| Hordeum vulgare | −0.033 | 0.031 | Anagallis foemina | −0.050 | 0.032 |
| Avena fatua | −0.086 | 0.027 | Amaranthus blitoides | −0.031 | 0.031 |
| Datura stramonium | 0.048 | 0.023 | Lamium amplexicaule | −0.033 | 0.029 |
| Silene noctiflora | −0.043 | 0.021 | Panicum miliaceum | −0.078 | 0.029 |
| Panicum miliaceum | 0.067 | 0.021 | Reseda lutea | −0.100 | 0.025 |
| Cirsium arvense | −0.070 | 0.020 | Artemisia vulgaris | −0.013 | 0.024 |
| Lathyrus tuberosus | −0.032 | 0.020 | Ajuga chamaepitys | −0.037 | 0.023 |
| Euphorbia helioscopia | 0.042 | 0.018 | Sinapis arvensis | 0.106 | 0.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinke, G.; Giczi, Z.; Vona, V.; Dunai, É.; Vámos, O.; Kulmány, I.; Koltai, G.; Varga, Z.; Kalocsai, R.; Botta-Dukát, Z.; et al. Weed Composition in Hungarian Phacelia (Phacelia tanacetifolia Benth.) Seed Production: Could Tine Harrow Take over Chemical Management? Agronomy 2022, 12, 891. https://doi.org/10.3390/agronomy12040891
Pinke G, Giczi Z, Vona V, Dunai É, Vámos O, Kulmány I, Koltai G, Varga Z, Kalocsai R, Botta-Dukát Z, et al. Weed Composition in Hungarian Phacelia (Phacelia tanacetifolia Benth.) Seed Production: Could Tine Harrow Take over Chemical Management? Agronomy. 2022; 12(4):891. https://doi.org/10.3390/agronomy12040891
Chicago/Turabian StylePinke, Gyula, Zsolt Giczi, Viktória Vona, Éva Dunai, Ottilia Vámos, István Kulmány, Gábor Koltai, Zoltán Varga, Renátó Kalocsai, Zoltán Botta-Dukát, and et al. 2022. "Weed Composition in Hungarian Phacelia (Phacelia tanacetifolia Benth.) Seed Production: Could Tine Harrow Take over Chemical Management?" Agronomy 12, no. 4: 891. https://doi.org/10.3390/agronomy12040891
APA StylePinke, G., Giczi, Z., Vona, V., Dunai, É., Vámos, O., Kulmány, I., Koltai, G., Varga, Z., Kalocsai, R., Botta-Dukát, Z., Czúcz, B., & Bede-Fazekas, Á. (2022). Weed Composition in Hungarian Phacelia (Phacelia tanacetifolia Benth.) Seed Production: Could Tine Harrow Take over Chemical Management? Agronomy, 12(4), 891. https://doi.org/10.3390/agronomy12040891






