A One-Step Grafting Methodology Can Adjust Stem Morphology and Increase THCA Yield in Medicinal Cannabis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Single Step Grafting Method
2.3. Harvest Method
2.4. Cannabinoid Quantification
3. Results
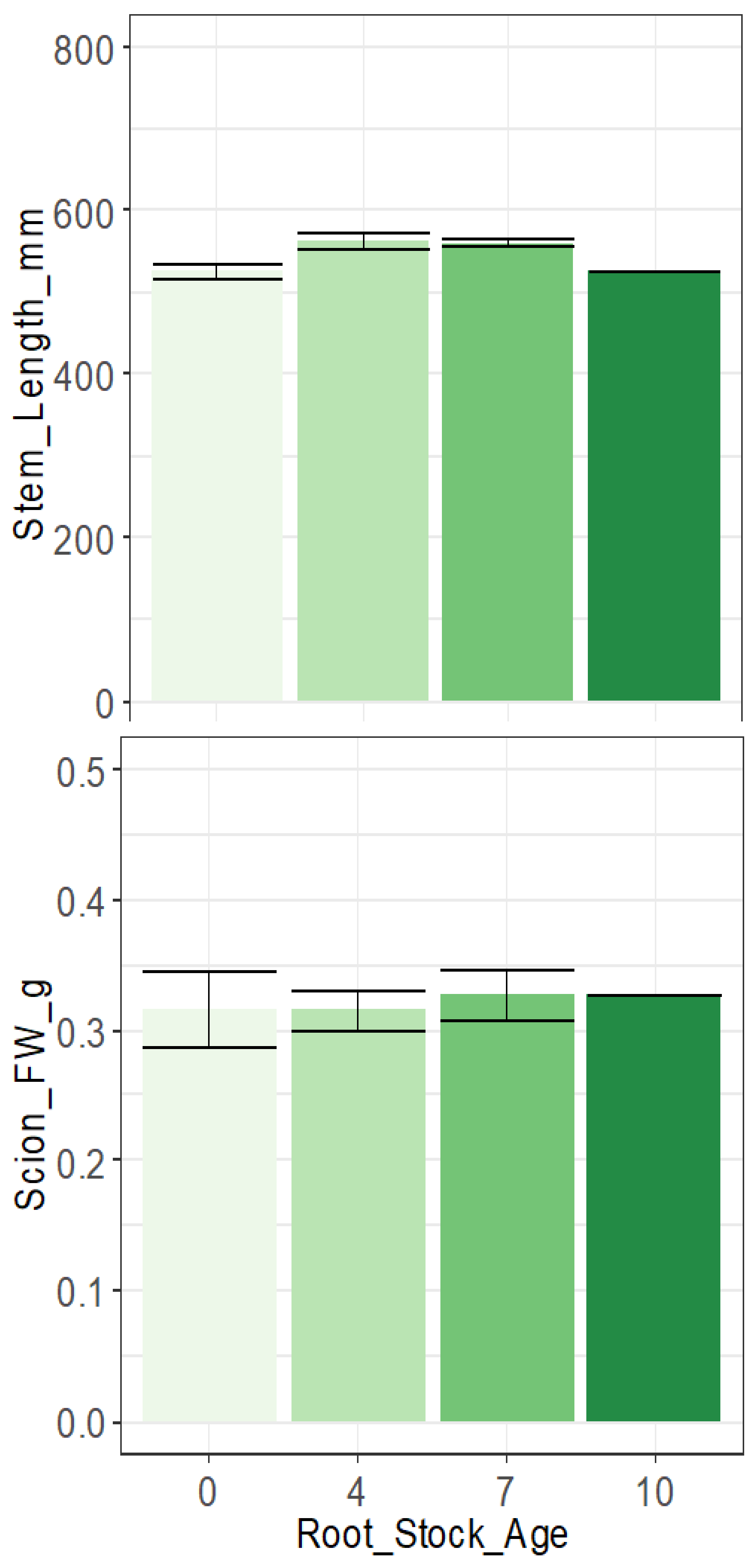
3.1. Rootstock Age
3.2. Rootstock Selection
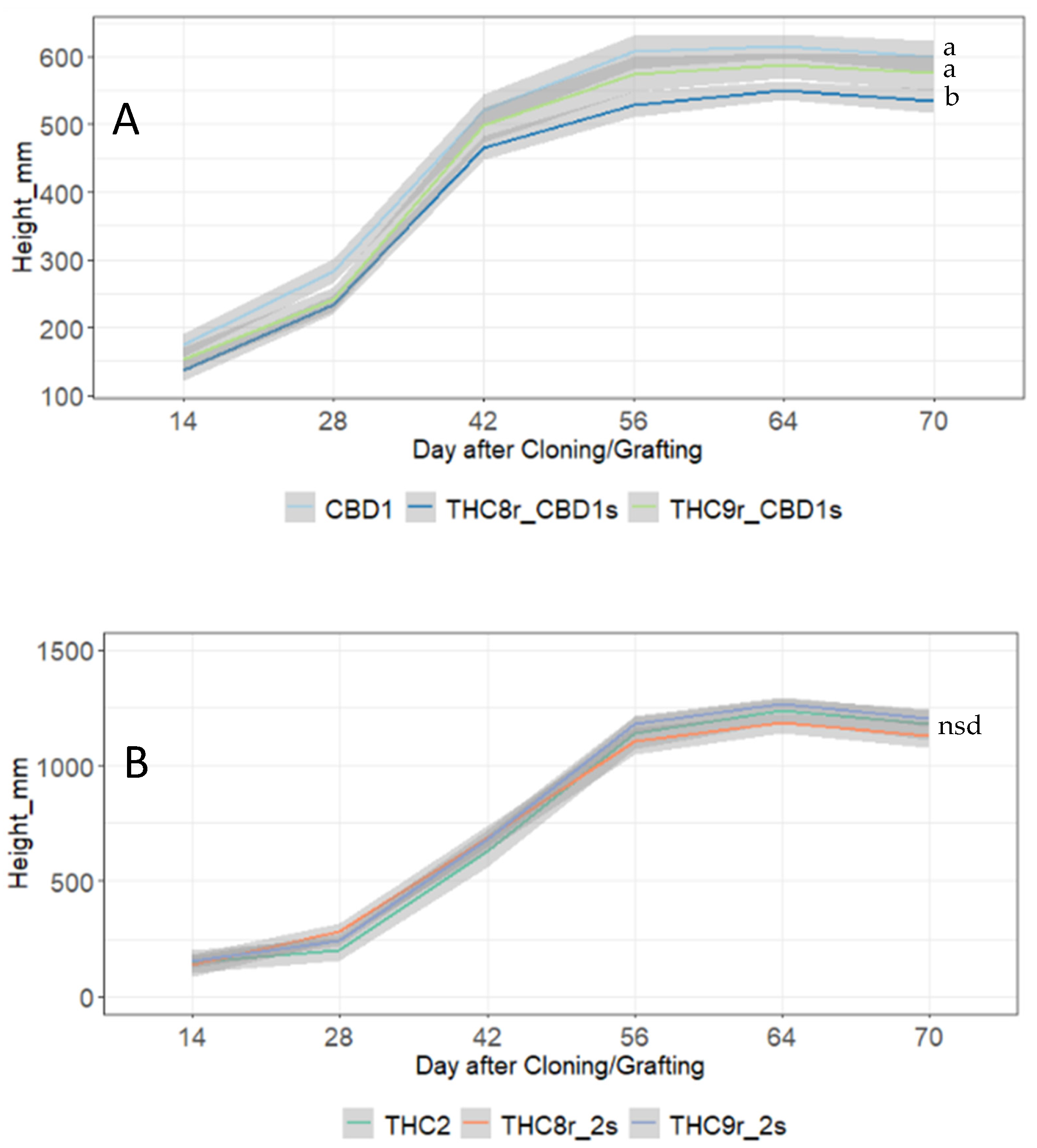
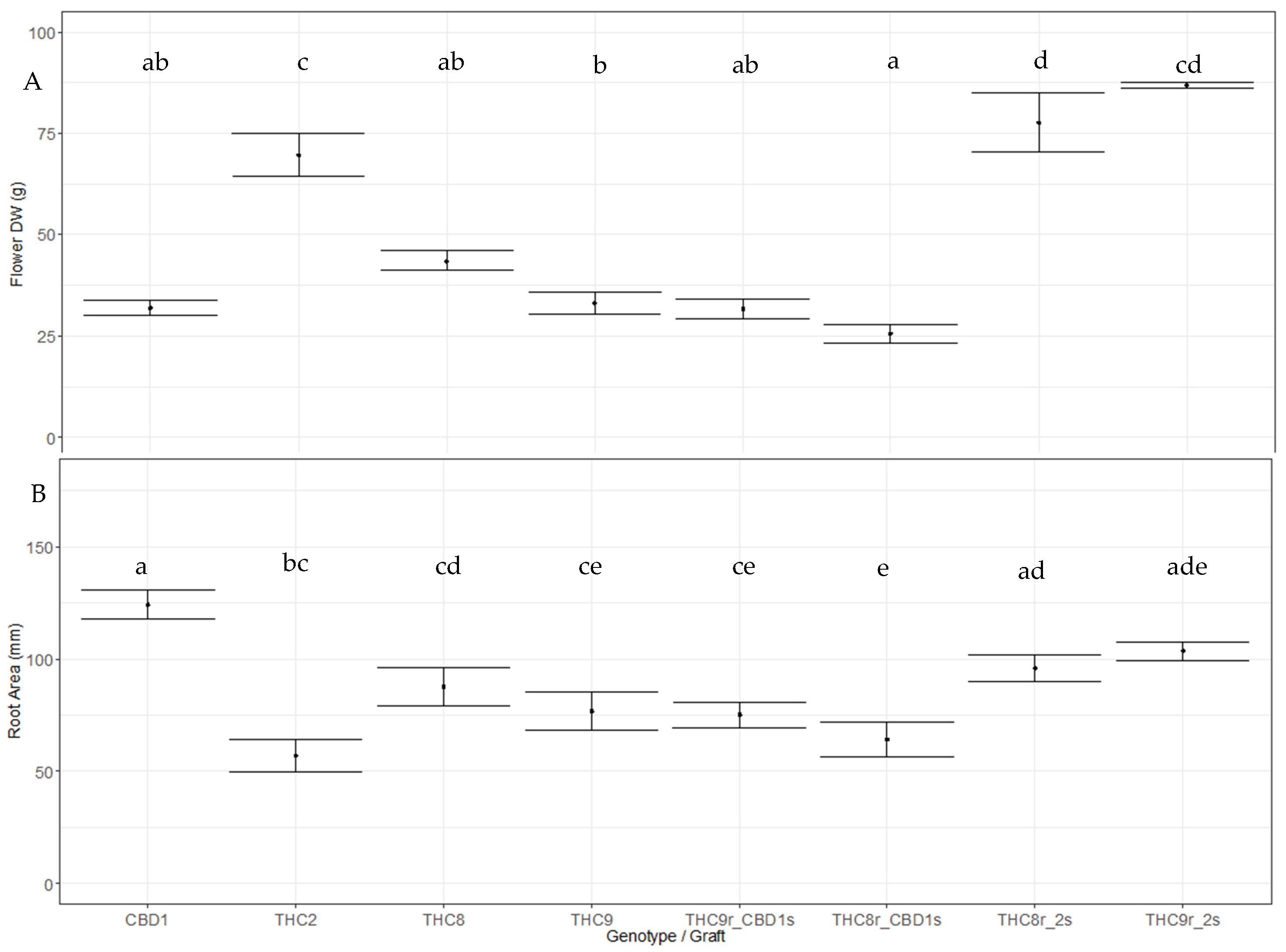
3.3. Impact of Grafting on Flowering Plant Performance
3.4. Cannabinoid Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parliament of Australia, Narcotic Drug Amendment Bill 2016. Available online: https://www.aph.gov.au/Parliamentary_Business/Bills_Legislation/Bills_Search_Results/Result?bId=r5609 (accessed on 20 March 2022).
- Australian Government Department of Health Office of Drug Control. Available online: https://www.odc.gov.au/summary-licences-granted (accessed on 20 March 2022).
- Flores-Sanchez, I.J.; Verpoorte, R. Secondary Metabolism in Cannabis. Phytochem. Rev. 2008, 7, 615–639. [Google Scholar] [CrossRef]
- Alger, B.E. Getting high on the endocannabinoid system. Cerebrum 2013, 2013, 14. [Google Scholar]
- Welling, M.T.; Liu, L.; Raymond, C.A.; Ansari, O.; King, G.J. Developmental Plasticity of the Major Alkyl Cannabinoid Chemotypes in a Diverse Cannabis Genetic Resource Collection. Front. Plant Sci. 2018, 9, 1510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulck, T.; Moller, B.L. Phytocannabinoids: Origins and Biosynthesis. Trends Plant Sci. 2020, 25, 985–1004. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.L.; Heblinski, M.; Absalom, N.L.; Hawkins, N.A.; Bowen, M.T.; Benson, M.J.; Zhang, F.; Bahceci, D.; Doohan, P.T.; Chebib, M.; et al. Cannabigerolic acid, a major biosynthetic precursor molecule in Cannabis, exhibits divergent effects on seizures in mouse models of epilepsy. Br. J. Pharmacol. 2021, 178, 4826–4841. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Lata, H.; ElSohly, M.A.; Walker, L.A.; Potter, D. Cannabis cultivation: Methodological issues for obtaining medical-grade product. Epilepsy Behav. 2017, 70, 302–312. [Google Scholar] [CrossRef]
- Mechoulam, R.; Ben-Shabat, S. From gan-zi-gun-nu to anandamide and 2-arachidonoylglycerol: The ongoing story of Cannabis. Nat. Prod. Rep. 1999, 16, 131–143. [Google Scholar] [CrossRef]
- Hoch, E.; Friemel, C.; Schneider, M.; Pogarell, O.; Hasan, A.; Preuss, U.W. Efficacy and safety of medicinal Cannabis: Results of the CaPRis study. Bundesgesundheitsblatt Gesundh. Gesundh. 2019, 62, 825–829. [Google Scholar] [CrossRef]
- Ali, S.; Scheffer, I.E.; Sadleir, L.G. Efficacy of cannabinoids in paediatric epilepsy. Dev. Med. Child Neurol. 2018, 61, 13. [Google Scholar] [CrossRef] [Green Version]
- Blake, A.; Wan, B.A.; Malek, L.; DeAngelis, C.; Diaz, P.; Lao, N.; Chow, E.; O’Hearn, S. A selective review of medical Cannabis in cancer pain management. Ann. Palliat. Med. 2017, 6, S215–S222. [Google Scholar] [CrossRef] [Green Version]
- Backer, R.; Schwinghamer, T.; Rosenbaum, P.; McCarty, V.; Bilodeau, S.E.; Lyu, D.; Ahmed, B.; Robinson, G.; Lefsrud, M.; Wilkins, O.; et al. Closing the Yield Gap for Cannabis: A Meta-Analysis of Factors Determining Cannabis Yield. Front. Plant. Sci. 2019, 10, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Measuring Yield. Cannabis Business Times. Available online: https://www.cannabisbusinesstimes.com/article/measuring-yield/ (accessed on 6 October 2016).
- Caulkins, J.P. Estimated Cost of Production for Legalized Cannabis; RAND Drug Policy Research Centre: Santa Monica, CA, USA, 2010. [Google Scholar]
- Nawaz, M.A.; Imtiaz, M.; Kong, Q.; Cheng, F.; Ahmed, W.; Huang, Y.; Bie, Z. Grafting: A Technique to Modify Ion Accumulation in Horticultural Crops. Front. Plant. Sci. 2016, 7, 1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-M.; Kubota, C.; Tsao, S.J.; Bie, Z.; Echevarria, P.H.; Morra, L.; Oda, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Thomas, H.R.; Frank, M.H. Connecting the pieces: Uncovering the molecular basis for long-distance communication through plant grafting. New Phytol. 2019, 223, 582–589. [Google Scholar] [CrossRef] [Green Version]
- Melnyk, C.W.; Meyerowitz, E.M. Plant grafting. Curr. Biol. 2015, 25, R183–R188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cervantes, J. The Cannabis Encyclopedia: The Definitive Guide to Cultivation & Consumption of Medical Marijuana; Van Patten Publishing: Vancouver, WA, USA, 2015. [Google Scholar]
- Deloitte, A. Economics, Pty Ltd. Modelling the Cost of Medicinal Cannabis. Department of Health. Office of Drug Control. 2016. Available online: https://www2.deloitte.com/content/dam/Deloitte/au/Documents/Economics/deloitte-au-modelling-cost-medicinal-cannabis-230916.pdf (accessed on 20 March 2022).
- Gorelick, J.; Bernstein, N. Chemical and Physical Elicitation for Enhanced Cannabinoid Production in Cannabis. In Cannabis sativa L.-Botany and Biotechnology; Chandra, S., Lata, H., ElSohly, M., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Saloner, A.; Bernstein, N. Nitrogen supply affects cannabinoid and terpenoid profile in medical Cannabis (Cannabis sativa L.). Ind. Crop. Prod. 2021, 167, 113516. [Google Scholar] [CrossRef]
- Magagnini, G.; Grassi, G.; Kotiranta, S. The Effect of Light Spectrum on the Morphology and Cannabinoid Content of Cannabis sativa L. Med. Cannabis Cannabinoids 2018, 1, 19–27. [Google Scholar] [CrossRef]
- Danziger, N.; Bernstein, N. Light matters: Effect of light spectra on cannabinoid profile and plant development of medical Cannabis (Cannabis sativa L.). Ind. Crop. Prod. 2021, 164, 113351. [Google Scholar] [CrossRef]
- Namdar, D.; Charuvi, D.; Ajjampura, V.; Mazuz, M.; Ion, A.; Kamara, I.; Koltai, H. LED lighting affects the composition and biological activity of Cannabis sativa secondary metabolites. Ind. Crop. Prod. 2019, 132, 177–185. [Google Scholar] [CrossRef]
- Danziger, N.; Bernstein, N. Plant architecture manipulation increases cannabinoid standardization in ‘drug-type’ medical Cannabis. Ind. Crop. Prod. 2021, 167, 113528. [Google Scholar] [CrossRef]
- Caplan, D.; Dixon, M.; Zheng, Y.B. Increasing Inflorescence Dry Weight and Cannabinoid Content in Medical Cannabis Using Controlled Drought Stress. Hortscience 2019, 54, 964–969. [Google Scholar] [CrossRef] [Green Version]
- Toth, J.A.; Smart, L.B.; Smart, C.D.; Stack, G.M.; Carlson, C.H.; Philippe, G.; Rose, J.K.C. Limited effect of environmental stress on cannabinoid profiles in high-cannabidiol hemp (Cannabis sativa L.). GCB Bioenergy 2021, 13, 1666–1674. [Google Scholar] [CrossRef]
- Westmoreland, F.M.; Kusuma, P.; Bugbee, B. Cannabis lighting: Decreasing blue photon fraction increases yield but efficacy is more important for cost effective production of cannabinoids. PLoS ONE 2021, 16, e0248988. [Google Scholar] [CrossRef] [PubMed]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 529. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Hewavitharana, A.K.; Gloerfelt-Tarp, F.; Nolan, M.; Barkla, B.J.; Purdy, S.; Kretzschmar, T. Simultaneous Quantification of 17 Cannabinoids in Cannabis Inflorescence by Liquid Chromatography-Mass Spectrometry. Separations 2022, 9, 85. [Google Scholar] [CrossRef]
- Team, R. RStudio: Integrated Development for R. RStudio; PBC: Boston, MA, USA, 2020. [Google Scholar]
- Kretzschmar, T.; Kohlen, W.; Sasse, J.; Borghi, L.; Schlegel, M.; Bachelier, J.B.; Martinoia, E.; Bours, R.; Bouwmeester, H.J. A petunia ABC protein controls strigolactone-dependent symbiotic signalling and branching. Nature 2012, 483, 341–344. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pagès, V.; Dun, E.A.; Pillot, J.-P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.-C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef]
- Mashiguchi, K.; Seto, Y.; Yamaguchi, S. Yamaguchi, Strigolactone biosynthesis, transport and perception. Plant J. 2021, 105, 335–350. [Google Scholar] [CrossRef]
- Xie, X.N.; Yoneyama, K.; Yoneyama, K. The Strigolactone Story. Annu. Rev. Phytopathol. 2010, 48, 93–117. [Google Scholar] [CrossRef] [Green Version]
- Bithell, S.L.; Condé, B.; Traynor, M.; Donald, E.C. Donald, Grafting for soilborne disease management in Australian vegetable production systems—A review. Australas. Plant Pathol. 2012, 42, 329–336. [Google Scholar] [CrossRef]
- Devi, P.; Tymon, L.; Keinath, A. Miles, Progress in grafting watermelon to manage Verticillium wilt. Plant Pathol. 2021, 70, 767–777. [Google Scholar] [CrossRef]
- Punja, Z.K.; Collyer, D.; Scott, C.; Lung, S.; Holmes, J.; Sutton, D. Pathogens and Molds Affecting Production and Quality of Cannabis sativa L. Front. Plant Sci. 2019, 10, 1120. [Google Scholar] [CrossRef] [Green Version]
- Kousik, C.S.; Ikerd, J.L.; Hassell, R. Powdery mildew resistant cucurbit rootstocks confer tolerance to grafted susceptible watermelon scions. Phytopathology 2015, 105, 75. [Google Scholar]
- Guan, W.J.; Zhao, X.; Hassell, R.; Thies, J. Defense Mechanisms Involved in Disease Resistance of Grafted Vegetables. Hortscience 2012, 47, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Jensen, P.J.; Rytter, J.; Detwiler, E.A.; Travis, J.W.; McNellis, T.W. Rootstock effects on gene expression patterns in apple tree scions. Plant Mol. Biol. 2003, 53, 493–511. [Google Scholar] [CrossRef] [PubMed]
- van Loon, L.C. Plant responses to plant growth-promoting rhizobacteria. Eur. J. Plant Pathol. 2007, 119, 243–254. [Google Scholar] [CrossRef] [Green Version]
- Wise, K.; Gill, H.; Selby-Pham, J. Willow bark extract and the biostimulant complex Root Nectar (R) increase propagation efficiency in chrysanthemum and lavender cuttings. Sci. Hortic. 2020, 263, 109108. [Google Scholar] [CrossRef]
- Hemphill, J.K.; Turner, J.C.; Mahlberg, P.G. Cannabinoid Content of Individual Plant Organs from Different Geographical Strains of Cannabis-Sativa, L. J. Nat. Prod. 1980, 43, 112–122. [Google Scholar] [CrossRef]
- Welling, M.T.; Liu, L.; Kretzschmar, T.; Mauleon, R.; Ansari, O.; King, G.J. An extreme-phenotype genome-wide association study identifies candidate cannabinoid pathway genes in Cannabis. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Welling, M.T.; Liu, L.; Shapter, T.; Raymond, C.A.; King, G.J. Characterisation of cannabinoid composition in a diverse Cannabis sativa L. germplasm collection. Euphytica 2016, 208, 463–475. [Google Scholar] [CrossRef] [Green Version]
- Sommano, S.R.; Chittasupho, C.; Ruksiriwanich, W.; Jantrawut, P. The Cannabis Terpenes. Molecules 2020, 25, 5792. [Google Scholar] [CrossRef] [PubMed]
- Shiponi, S.; Bernstein, N. The Highs and Lows of P Supply in Medical Cannabis: Effects on Cannabinoids, the Ionome, and Morpho-Physiology. Front. Plant. Sci. 2021, 12, 657323. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant. Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Variety | Chemotype | Rootstock or Scion | Experiment |
|---|---|---|---|
| CBD1 | High CBD | Scion | 1–4 |
| THC1 | High THC | Rootstock | 3 |
| THC2 | High THC | Rootstock and Scion | 3,4 |
| THC3 | High THC | Rootstock | 3 |
| THC6 | High THC | Rootstock | 3 |
| THC7 | High THC | Rootstock | 1–3 |
| THC8 | High THC | Rootstock | 3,4 |
| THC9 | High THC | Rootstock | 3,4 |
| Rootstock Age at Grafting (Days) | Grafts Taken | Transplanted | % Survival | |
|---|---|---|---|---|
| Experiment 1: | 10 | 6 | 2 | 33.3 |
| 7 | 6 | 5 | 83.3 | |
| 4 | 6 | 5 | 83.3 | |
| 0 | 6 | 6 | 100 | |
| Experiment 2: | 0 | 20 | 20 | 100 |
| Genotype | Maximum Height (mm) | Above Ground FW (g) |
|---|---|---|
| CBD1 | 445.83 +/− 7.79a | 139 +/− 4.0abc |
| THC9r_CBD1s | 430.83 +/− 8.31ab | 152 +/− 6.0c |
| THC1r_CBD1s | 445.83 +/− 2.71ab | 127 +/− 8.0abc |
| THC2r_CBD1s | 447.00 +/− 20.8ab | 129 +/− 10.0abc |
| THC3r_CBD1s | 426.67 +/− 7.15bc | 134 +/− 6.0abc |
| THC6r_CBD1s | 430.83 +/− 7.46ab | 143 +/− 6.0abc |
| THC7r_CBD1s | 442.50 +/− 8.24ab | 132 +/− 5.0ab |
| THC8r_CBD1s | 422.00 +/− 7.52bc | 132 +/− 6.0ab |
| Genotype/Graft | Total Branch Number | Branch Length (mm) | Nodes per Branch | Internode Length (mm) | Inflorescence Number |
|---|---|---|---|---|---|
| CBD1 | 8.875 +/− 0.31a | 243.54 +/− 6.40a | 5.08 +/− 0.06a | 49.70 +/− 1.44a | 30.50 +/− 0.35a |
| THC9r_CBD1s | 8.50 +/− 0.29a | 255.10 +/− 11.78a | 4.90 +/− 0.25ab | 55.28 +/− 1.35ab | 29.38 +/− 1.52ab |
| THC8r_CBD1s | 8.00 +/− 0.00a | 229.27 +/− 1.39a | 4.29 +/− 0.15b | 57.95 +/− 1.87b | 25.75 +/− 0.92b |
| THC2 | 12.625 +/− 0.63a | 521.15 +/− 38.42ab | 6.35 +/− 0.30ab | 85.56 +/− 2.72a | 38.13 +/− 1.80ab |
| THC9r_2s | 14.25 +/− 0.32a | 566.46 +/− 23.47c | 7.75 +/− 0.12c | 74.41 +/− 2.80b | 46.50 +/− 0.74c |
| THC8r_2s | 14.25 +/− 0.48a | 470.63 +/− 21.76b | 6.63 +/− 0.28b | 72.40 +/− 3.09b | 39.75 +/− 1.65b |
| Genotype/Graft | CBD1 | THC9r_CBD1s | THC8r_CBD1s | THC2 | THC9r_2s | THC8r_2s |
|---|---|---|---|---|---|---|
| CBDV | 8.75 +/− 0.44 | 7.95 +/− 0.43 | 7.80 +/− 0.53 | 0.70 +/− 0.35 | 0.00 +/− 0.00 | 0.00 +/− 0.00 |
| CBDVA | 282.98 +/− 12.38 | 235.04 +/− 16.57 | 283.18 +/− 6.36 | 14.84 +/− 8.84 | 28.08 +/− 3.38 | 4.97 +/− 3.07 |
| CBG | 274.59 +/− 9.30 | 235.98 +/− 8.93 | 276.44 +/− 11.48 | 413.99 +/− 28.78 | 403.96 +/− 14.66 | 375.80 +/− 10.37 |
| CBGA | 618.17 +/− 62.00 | 437.92 +/− 43.89 | 510.94 +/− 27.16 | 3507.16 +/− 132.40 | 3302.13 +/− 76.82 | 3158.43 +/− 133.87 |
| THCV | 2.99 +/− 0.45 | 3.10 +/− 0.13 | 2.91 +/− 0.19 | 18.21 +/− 4.87 | 16.67 +/− 1.49 | 14.48 +/− 1.28 |
| THCVA | 21.41 +/− 4.85 | 19.47 +/− 1.93 | 22.12 +/− 7.04 | 1497.00 +/− 156.43 | 1179.77 +/− 526.97 | 1753.37 +/− 142.74 |
| CBN | 1.45 +/− 0.66a | 2.53 +/− 0.83a | 5.99 +/− 0.22b | 4.63 +/− 1.33 | 5.14 +/− 0.50 | 5.20 +/− 0.82 |
| CBNA | 26.99 +/− 5.33 | 3.41 +/− 2.21 | 22.65 +/− 15.62 | 376.29 +/− 11.48 | 366.12 +/− 5.52 | 334.24 +/− 19.28 |
| CBC | 192.75 +/− 5.50 | 183.57 +/− 4.75 | 178.26 +/− 12.92 | 62.70 +/− 23.60 | 53.27 +/− 5.72 | 45.88 +/− 5.14 |
| CBCA | 2656.59 +/− 173.23 | 2421.79 +/− 208.73 | 3027.04 +/− 42.10 | 0.00 +/− 0.00 | 0.00 +/− 0.00 | 0.00 +/− 0.00 |
| CBLA | 121.67 +/− 18.89 | 507.50 +/− 259.57 | 355.98 +/− 128.22 | 852.97 +/− 35.81a | 1162.57 +/− 62.82b | 1178.88 +/− 31.66b |
| CBD | 2127.27 +/− 19.89 | 2317.93 +/− 110.48 | 1981.99 +/− 125.22 | 21.47 +/− 12.00 | 41.20 +/− 1.84 | 38.73 +/− 0.63 |
| CBDA | 76,504.48 +/− 1384.01a | 77,459.11 +/− 2784.75a | 86,119.56 +/− 1382.27b | 335.06 +/− 39.42 | 302.28 +/− 56.11 | 216.29 +/− 7.30 |
| THC | 526.71 +/− 4.95a | 476.23 +/− 10.48b | 491.86 +/− 14.58ab | 2455.09 +/− 642.03 | 2602.35 +/− 239.35 | 2233.05 +/− 166.03 |
| THCA | 3433.15 +/− 216.20 | 3064.04 +/− 184.21 | 3629.15 +/− 209.63 | 138,838.11 +/− 2522.18 | 149,386.51 +/− 6396.65 | 144,570.38 +/− 7562.30 |
| Genotype/Graft | CBD1 | THC9r_CBD1s | THC8r_CBD1s | THC2 | THC9r_2s | THC8r_2s |
|---|---|---|---|---|---|---|
| CBDV | 0.30 +/− 0.02 | 0.26 +/− 0.02 | 0.19 +/− 0.02 | 0.04 +/− 0.02 | 0.00 +/− 0.00 | 0.00 +/− 0.00 |
| CBDVA | 9.78 +/− 0.52 | 7.83 +/− 0.80 | 7.04 +/− 0.40 | 0.80 +/− 0.46 | 2.60 +/− 0.46 | 0.40 +/− 0.26 |
| CBG | 9.48 +/− 0.37 | 7.96 +/− 1.04 | 6.90 +/− 0.56 | 24.49 +/− 3.37a | 36.94 +/− 3.40b | 28.87 +/− 1.98ab |
| CBGA | 21.53 +/− 2.63 | 15.28 +/− 3.62 | 12.72 +/− 1.00 | 206.61 +/− 21.78 | 300.81 +/− 20.83 | 244.58 +/− 25.79 |
| THCV | 0.10 +/− 0.01 | 0.10 +/− 0.01 | 0.07 +/− 0.01 | 1.10 +/− 0.37 | 1.53 +/− 0.20 | 1.14 +/− 0.19 |
| THCVA | 0.73 +/− 0.16 | 0.66 +/− 0.10 | 0.57 +/− 0.19 | 88.89 +/− 15.25 | 100.75 +/− 43.89 | 136.25 +/− 18.49 |
| CBN | 0.05 +/− 0.02 | 0.08 +/− 0.02 | 0.15 +/− 0.01 | 0.26 +/− 0.06 | 0.46 +/− 0.04 | 0.40 +/− 0.06 |
| CBNA | 0.93 +/− 0.18 | 0.14 +/− 0.11 | 0.52 +/− 0.35 | 22.00 +/− 1.19 | 33.27 +/− 1.74 | 25.61 +/− 1.86 |
| CBC | 6.66 +/− 0.30 | 6.19 +/− 0.81 | 4.42 +/− 0.36 | 3.84 +/− 1.69 | 4.86 +/− 0.61 | 3.61 +/− 0.67 |
| CBCA | 92.52 +/− 10.02 | 80.52 +/− 8.53 | 75.55 +/− 5.28 | 0.00 +/− 0.00 | 0.00 +/− 0.00 | 0.00 +/− 0.00 |
| CBLA | 4.32 +/− 0.94 | 15.73 +/− 7.07 | 9.25 +/− 3.84 | 30.30 +/− 14.87 | 105.87 +/− 8.73 | 91.42 +/− 9.64 |
| CBD | 73.69 +/− 3.90a | 77.37 +/− 7.71a | 49.11 +/− 3.34b | 1.25 +/− 0.70 | 3.74 +/− 0.25 | 3.00 +/− 0.29 |
| CBDA | 2659.30 +/− 196.78 | 2608.15 +/− 324.99 | 2148.84 +/− 148.97 | 19.78 +/− 2.99 | 27.28 +/− 4.59 | 16.59 +/− 1.08 |
| THC | 18.24 +/− 0.92a | 15.96 +/− 1.72ab | 12.23 +/− 0.72b | 148.33 +/− 48.56 | 237.38 +/− 27.29 | 173.70 +/− 24.40 |
| THCA | 119.99 +/− 13.31 | 101.00 +/− 5.59 | 90.17 +/− 6.11 | 8158.95 +/− 703.47a | 13,622.48 +/− 1132.07b | 11,036.36 +/− 554.63ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purdy, S.J.; Hewavitharana, A.K.; Azman Halimi, R.; Magner, N.J.; Peterswald, T.J.; Trebilco, A.; Kretzschmar, T.; Hailstones, D. A One-Step Grafting Methodology Can Adjust Stem Morphology and Increase THCA Yield in Medicinal Cannabis. Agronomy 2022, 12, 852. https://doi.org/10.3390/agronomy12040852
Purdy SJ, Hewavitharana AK, Azman Halimi R, Magner NJ, Peterswald TJ, Trebilco A, Kretzschmar T, Hailstones D. A One-Step Grafting Methodology Can Adjust Stem Morphology and Increase THCA Yield in Medicinal Cannabis. Agronomy. 2022; 12(4):852. https://doi.org/10.3390/agronomy12040852
Chicago/Turabian StylePurdy, Sarah Jane, Amitha K. Hewavitharana, Razlin Azman Halimi, Nelson Joel Magner, Tyson James Peterswald, Amy Trebilco, Tobias Kretzschmar, and Deborah Hailstones. 2022. "A One-Step Grafting Methodology Can Adjust Stem Morphology and Increase THCA Yield in Medicinal Cannabis" Agronomy 12, no. 4: 852. https://doi.org/10.3390/agronomy12040852
APA StylePurdy, S. J., Hewavitharana, A. K., Azman Halimi, R., Magner, N. J., Peterswald, T. J., Trebilco, A., Kretzschmar, T., & Hailstones, D. (2022). A One-Step Grafting Methodology Can Adjust Stem Morphology and Increase THCA Yield in Medicinal Cannabis. Agronomy, 12(4), 852. https://doi.org/10.3390/agronomy12040852






