Effect of Genetics and Climate on Apple Sugars and Organic Acids Profiles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Trial, Plant Material, and Climate Data
2.2. Fruit Sampling, Individual Sugars, and Organic Acids Analysis
2.3. Data Analysis
2.4. Chemicals
3. Results
3.1. Accession and Year Effects
3.2. Individual Sugars and Organic Acids Profiles
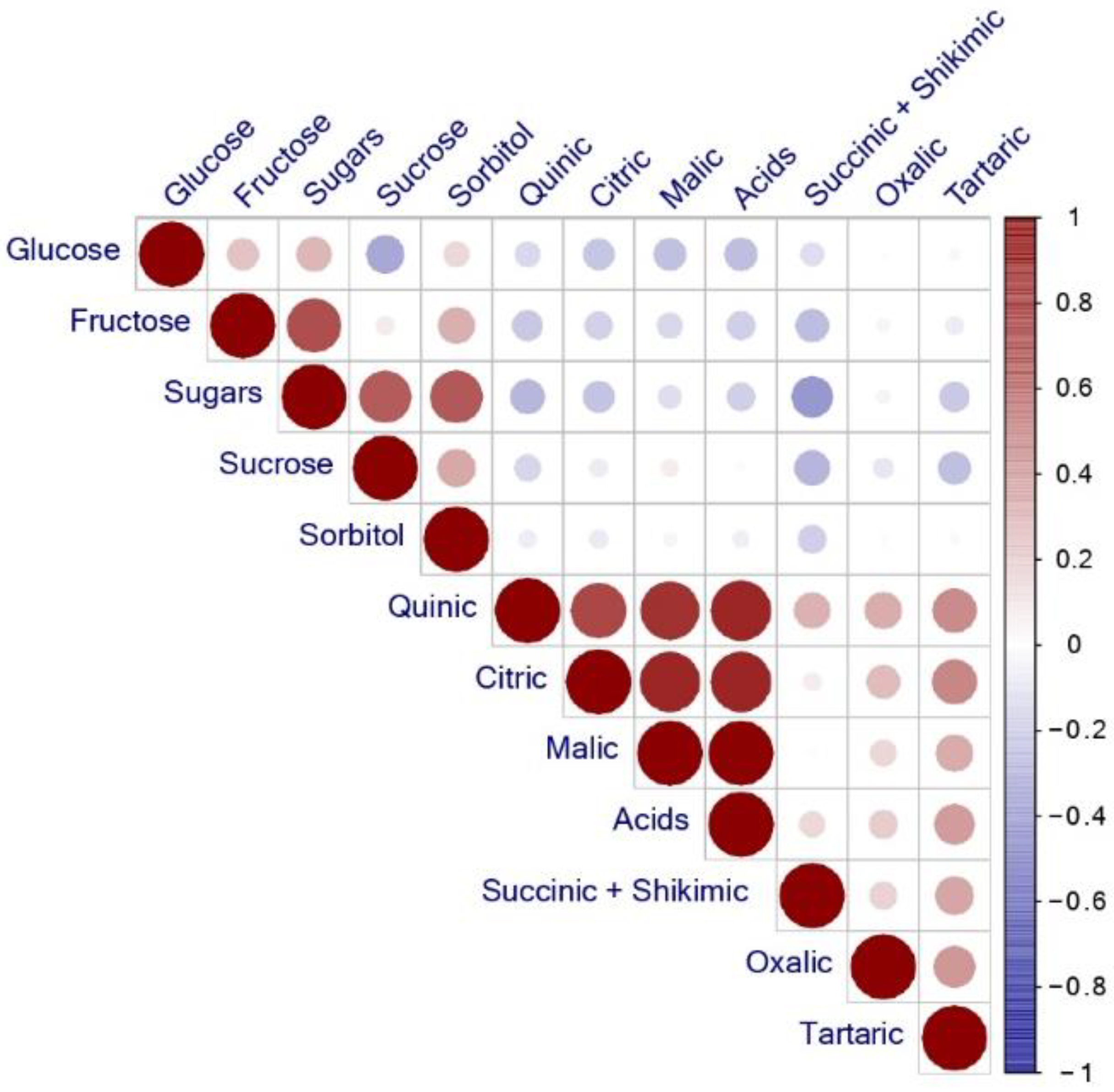
3.3. Pearson Correlation Coefficients
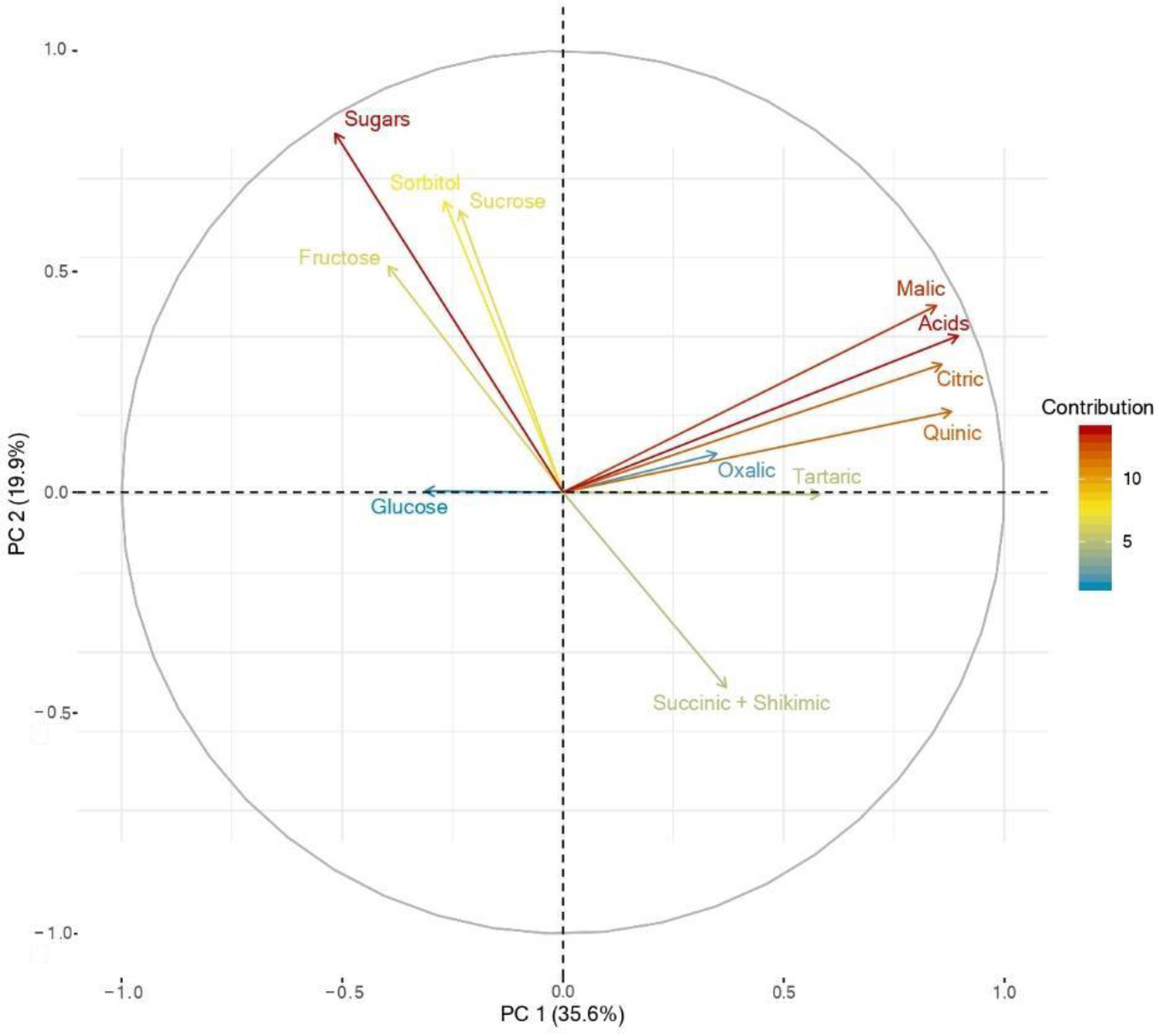
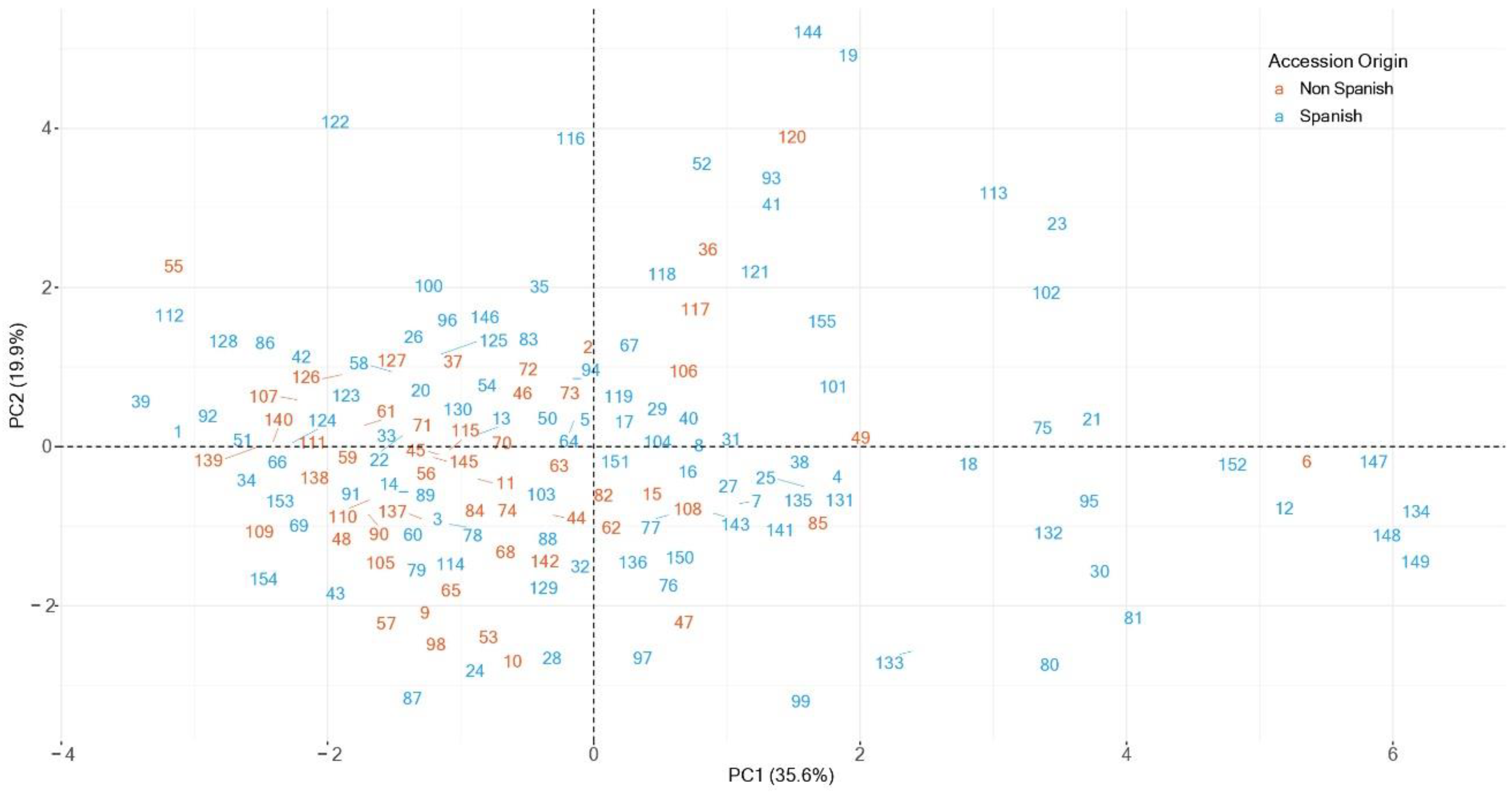
3.4. Principal Components Analysis
3.5. Mixed-Effect Model Results: Relationships between Climate and Genetic Origin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Mushtaq, R.; Wani, A.W.; Nayik, G.A. Apple. In Antioxidants in Fruits: Properties and Health Benefits; Nayik, G.A., Gull, A., Eds.; Springer Nature: Singapore, 2020; pp. 507–522. [Google Scholar]
- FAOSTAT. Food and Agricultural Organization. 2021. Available online: http://faostat.fao.org/ (accessed on 21 July 2021).
- Kim, I.; Ku, K.; Jeong, M.; Kim, S.S.; Mitchell, A.E.; Lee, J. A comparison of the chemical composition and antioxidant activity of several new early- to mid-season apple cultivars for a warmer climate with traditional cultivars. J. Sci. Food Agric. 2019, 99, 4712–4724. [Google Scholar] [CrossRef] [PubMed]
- Mignard, P.; Beguería, S.; Reig, G.; Font i Forcada, C.; Moreno, M.Á. Genetic origin and climate determine fruit quality and antioxidant traits on apple (Malus x domestica Borkh). Sci. Hortic. 2021, 285, 110142. [Google Scholar] [CrossRef]
- Verma, S.; Evans, K.; Guan, Y.; Luby, J.J.; Rosyara, U.R.; Howard, N.P.; Bassil, N.; Bink, M.C.A.M.; Van De Weg, W.E.; Peace, C.P. Two large-effect QTLs, Ma and Ma3, determine genetic potential for acidity in apple fruit: Breeding insights from a multi-family study. Tree Genet. Genomes 2019, 15, 18. [Google Scholar] [CrossRef] [Green Version]
- Zhen, Q.; Fang, T.; Peng, Q.; Liao, L.; Zhao, L.; Owiti, A.; Han, Y. Developing gene-tagged molecular markers for evaluation of genetic association of apple SWEET genes with fruit sugar accumulation. Hortic. Res. 2018, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Wei, X.; Miao, Z.; Hassan, H.; Song, Y.; Fan, M. Screening for antioxidant and antibacterial activities of phenolics from Golden Delicious apple pomace. Chem. Cent. J. 2016, 10, 47. [Google Scholar] [CrossRef] [Green Version]
- Gibney, E.; Milenkovic, D.; Combet, E.; Ruskovska, T.; Greyling, A.; González-Sarrías, A.; De Roos, B.; Tomás-Barberán, F.A.; Morand, C.; Rodríguez-Mateos, A. Factors influencing the cardiometabolic response to (poly)phenols and phytosterols: A review of the COST Action POSITIVe activities. Eur. J. Nutr. 2019, 58, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Ho, K.K.H.Y.; Ferruzzi, M.G.; Wightman, J.D. Potential health benefits of (poly)phenols derived from fruit and 100% fruit juice. Nutr. Rev. 2019, 78, 145–174. [Google Scholar] [CrossRef]
- Font i Forcada, C.; Reig, G.; Giménez, R.; Mignard, P.; Mestre, L.; Moreno, M.Á. Sugars and organic acids profile and antioxidant compounds of nectarine fruits influenced by different rootstocks. Sci. Hortic. 2019, 248, 145–153. [Google Scholar] [CrossRef]
- Jakobek, L.; Ištuk, J.; Buljeta, I.; Voća, S.; Žlabur, J.; Babojelić, M.S. Traditional, Indigenous Apple Varieties, a Fruit with Potential for Beneficial Effects: Their Quality Traits and Bioactive Polyphenol Contents. Foods 2020, 9, 52. [Google Scholar] [CrossRef] [Green Version]
- Minas, I.S.; Tanou, G.; Molassiotis, A. Environmental and orchard bases of peach fruit quality. Sci. Hortic. 2018, 235, 307–322. [Google Scholar] [CrossRef]
- Aprea, E.; Charles, M.; Endrizzi, I.; Corollaro, M.L.; Betta, E.; Biasioli, F.; Gasperi, F. Sweet taste in apple: The role of sorbitol, individual sugars, organic acids and volatile compounds. Sci. Rep. 2017, 7, 44950. [Google Scholar] [CrossRef] [PubMed]
- Briand, L.; Salles, C. Taste perception and integration. In Flavor; Woodhead Publishing: Sawston, UK, 2016; pp. 101–119. [Google Scholar]
- Iwanami, H.; Moriya, S.; Kotoda, N.; Mimida, N.; Takahashi-Sumiyoshi, S.; Abe, K. Mode of inheritance in fruit acidity in apple analysed with a mixed model of a major gene and polygenes using large complex pedigree. Plant Breed. 2012, 131, 322–328. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Cheng, L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘Honeycrisp’ apple flesh. Food Chem. 2010, 123, 1013–1018. [Google Scholar] [CrossRef]
- Charles, M.; Aprea, E.; Gasperi, F. Factors influencing sweet taste in apple. In Bioactive Molecules in Food; Mérillon, J.M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Yang, S.; Meng, Z.; Li, Y.; Chen, R.; Yang, Y.; Zhao, Z. Evaluation of Physiological Characteristics, Soluble Sugars, Organic Acids and Volatile Compounds in ‘Orin’ Apples (Malus domestica) at Different Ripening Stages. Molecules 2021, 26, 807. [Google Scholar] [CrossRef]
- Amri, R.; Font i Forcada, C.; Giménez, R.; Pina, A.; Moreno, M.Á. Biochemical Characterization and Differential Expression of PAL Genes Associated With “Translocated” Peach/Plum Graft-Incompatibility. Front. Plant Sci. 2021, 10, 622578. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, S.; Zhang, L.; Yang, J.; Wang, Z.; Ma, F.; Li, M. Carbohydrate metabolism and transport in apple roots under nitrogen deficiency. Plant Physiol. Biochem. 2020, 155, 455–463. [Google Scholar] [CrossRef]
- Ruan, Y.-L. Sucrose Metabolism: Gateway to Diverse Carbon Use and Sugar Signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, L.; Cui, W.; Zhang, C.; Li, D.; Ma, B.; Cheng, L.; Ruan, Y.-L.; Ma, F.; Li, M. Increased activity of MdFRK2, a high-affinity fructokinase, leads to upregulation of sorbitol metabolism and downregulation of sucrose metabolism in apple leaves. Hortic. Res. 2018, 5, 71. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Feng, F.; Cheng, L. Expression Patterns of Genes Involved in Sugar Metabolism and Accumulation during Apple Fruit Development. PLoS ONE 2012, 7, e33055. [Google Scholar] [CrossRef] [Green Version]
- Vallarino, J.G.; Osorio, S. Organic acids. In Postharvest Physiology and Biochemistry of Fruits and Vegetables; Woodhead Publishing: Sawston, UK, 2019; pp. 207–224. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Eprintsev, A.T. Organic Acids: The Pools of Fixed Carbon Involved in Redox Regulation and Energy Balance in Higher Plants. Front. Plant Sci. 2016, 7, 1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, H.; Dudareva, N. The Shikimate Pathway and Aromatic Amino Acid Biosynthesis in Plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Lorenzo, S.; Urrestarazu, J.; Ramos-Cabrer, A.; Miranda, C.; Pina, A.; Dapena, E.; Moreno, M.Á.; Errea, P.; Llamero, N.; Díaz-Hernández, M.; et al. Analysis of the genetic diversity and structure of the Spanish apple genetic resources suggests the existence of an Iberian genepool. Ann. Appl. Biol. 2017, 171, 424–440. [Google Scholar] [CrossRef]
- Urrestarazu, J.; Denancé, C.; Ravon, E.; Guyader, A.; Guisnel, R.; Feugey, L.; Poncet, C.; Lateur, M.; Houben, P.; Ordidge, M.; et al. Analysis of the genetic diversity and structure across a wide range of germplasm reveals prominent gene flow in apple at the European level. BMC Plant Biol. 2016, 16, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Ordidge, M.; Kirdwichai, P.; Baksh, M.F.; Venison, E.P.; Gibbings, J.G.; Dunwell, J.M. Genetic analysis of a major international collection of cultivated apple varieties reveals previously unknown historic heteroploid and inbred relationships. PLoS ONE 2018, 13, e0202405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swarup, S.; Cargill, E.J.; Crosby, K.; Flagel, L.; Kniskern, J.; Glenn, K.C. Genetic diversity is indispensable for plant breeding to improve crops. Crop Sci. 2020, 61, 839–852. [Google Scholar] [CrossRef]
- Font i Forcada, C.; Gradziel, T.M.; Gogorcena, Y.; Moreno, M.Á. Phenotypic diversity among local Spanish and foreign peach and nectarine [Prunus persica (L.) Batsch] accessions. Euphytica 2014, 197, 261–277. [Google Scholar] [CrossRef] [Green Version]
- Guajardo, V.; Solís, S.; Almada, R.; Saski, C.; Gasic, K.; Moreno, M.Á. Genome-wide SNP identification in Prunus rootstocks germplasm collections using Genotyping-by-Sequencing: Phylogenetic analysis, distribution of SNPs and prediction of their effect on gene function. Sci. Rep. 2020, 10, 1467. [Google Scholar] [CrossRef] [Green Version]
- McClure, K.A.; Gong, Y.; Song, J.; Vinqvist-Tymchuk, M.; Palmer, L.C.; Fan, L.; Burgher-MacLellan, K.; Zhang, Z.; Celton, J.-M.; Forney, C.F.; et al. Genome-wide association studies in apple reveal loci of large effect controlling apple polyphenols. Hortic. Res. 2019, 6, 107. [Google Scholar] [CrossRef] [Green Version]
- Muranty, H.; Denancé, C.; Feugey, L.; Crépin, J.-L.; Barbier, Y.; Tartarini, S.; Ordidge, M.; Troggio, M.; Lateur, M.; Nybom, H.; et al. Using whole-genome SNP data to reconstruct a large multi-generation pedigree in apple germplasm. BMC Plant Biol. 2020, 20, 2. [Google Scholar] [CrossRef] [Green Version]
- Vanderzande, S.; Howard, N.P.; Cai, L.; Linge, C.D.S.; Antanaviciute, L.; Bink, M.C.A.M.; Kruisselbrink, J.W.; Bassil, N.; Gasic, K.; Iezzoni, A.; et al. High-quality, genome-wide SNP genotypic data for pedigreed germplasm of the diploid outbreeding species apple, peach, and sweet cherry through a common workflow. PLoS ONE 2019, 14, e0210928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castel, L.; Pina, A.; Irisarri, P.; Errea, P. Sugar content and organic acid profiles of local apple cultivars recovered from mountain zones. J. Appl. Bot. Food Qual. 2020, 93, 217–224. [Google Scholar] [CrossRef]
- Reig, G.; Blanco, A.; Castillo, A.M.; Gogorcena, Y.; Moreno, M.Á. Phenotypic diversity of Spanish apple (Malus x domestica Borkh) accessions grown at the vulnerable climatic conditions of the Ebro Valley, Spain. Sci. Hortic. 2015, 185, 200–210. [Google Scholar] [CrossRef] [Green Version]
- Parajuli, R.; Thoma, G.; Matlock, M.D. Environmental sustainability of fruit and vegetable production supply chains in the face of climate change: A review. Sci. Total Environ. 2018, 650, 2863–2879. [Google Scholar] [CrossRef] [PubMed]
- Parry, M.L. Climate Change and World Agriculture; Routledge: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Beguería, S.; López-Moreno, J.I.; Lorente, A.; Seeger, M.; García-Ruiz, J.M. Assessing the Effect of Climate Oscillations and Land-use Changes on Streamflow in the Central Spanish Pyrenees. Ambio 2003, 32, 283–286. [Google Scholar] [CrossRef]
- Boudichevskaia, A.; Kumar, G.; Sharma, Y.; Kapoor, R.; Singh, A.K. Challenges and strategies for developing climate-smart apple varieties through genomic approaches. In Genomic Designing of Climate-Smart Fruit Crops; Springer: Cham, Switzerland, 2020; pp. 23–71. [Google Scholar]
- Cirilli, M.; Bassi, D.; Ciacciulli, A. Sugars in peach fruit: A breeding perspective. Hortic. Res. 2016, 3, 15067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, A.L.; Ahmed, S. Effects of climate change on fruit nutrition. In Fruit Crops; Elsevier: Amsterdam, The Netherlands, 2020; pp. 77–93. [Google Scholar] [CrossRef]
- Yuri, J.A.; Neira, A.; Quilodran, A.; Motomura, Y.; Palomo, I. Antioxidant activity and total phenolics concentration in apple peel and flesh is determined by cultivar and agroclimatic growing regions in Chile. J. Food Agric. Environ. 2009, 7, 513–517. [Google Scholar]
- Mills, T.; Behboudian, M.; Clothier, B. Water Relations, Growth, and the Composition of ‘Braeburn’ Apple Fruit under Deficit Irrigation. J. Am. Soc. Hortic. Sci. 1996, 121, 286–291. [Google Scholar] [CrossRef]
- Cakpo, C.B.; Vercambre, G.; Baldazzi, V.; Roch, L.; Dai, Z.; Valsesia, P.; Memah, M.-M.; Colombié, S.; Moing, A.; Gibon, Y.; et al. Model-assisted comparison of sugar accumulation patterns in ten fleshy fruits highlights differences between herbaceous and woody species. Ann. Bot. 2020, 126, 455–470. [Google Scholar] [CrossRef]
- Kumar, P.; Sethi, S.; Sharma, R.R.; Singh, S.; Saha, S.; Sharma, V.K.; Shashi, S.K.; Eldho, V. Influence of altitudinal variation on the physical and biochemical characteristics of apple (Malus domestica). Indian J. Agric. Sci. 2019, 89, 145–152. [Google Scholar]
- Li, M.; Ma, F.; Shang, P.; Zhang, M.; Hou, C.; Liang, D. Influence of light on ascorbate formation and metabolism in apple fruits. Planta 2009, 230, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, M.; Kobayashi, K. Climate change adaptation practices of apple growers in Nagano, Japan. Mitig. Adapt. Strat. Glob. Chang. 2011, 16, 865–877. [Google Scholar] [CrossRef]
- Li, M.; Guo, J.; He, J.; Xu, C.; Li, J.; Mi, C.; Tao, S. Possible impact of climate change on apple yield in Northwest China. Arch. Meteorol. Geophys. Bioclimatol. Ser. B 2019, 139, 191–203. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, H.; Ghalhari, G.F.; Baaghideh, M. Impacts of climate change on apple tree cultivation areas in Iran. Clim. Chang. 2018, 153, 91–103. [Google Scholar] [CrossRef]
- Gitea, M.A.; Gitea, D.; Tit, D.M.; Purza, L.; Samuel, A.D.; Bungau, S.; Badea, G.E.; Aleya, L. Orchard management under the effects of climate change: Implications for apple, plum, and almond growing. Environ. Sci. Pollut. Res. 2019, 26, 9908–9915. [Google Scholar] [CrossRef]
- R Core Team. R: A language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria. 2019. Available online: https://www.R-project.org/ (accessed on 8 March 2022).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models_. R Package Version 3.1-140. 2019. Available online: https://CRAN.R-project.org/package=nlme (accessed on 8 March 2022).
- Iglesias, I.; Carbo, J.; Bonany, J. Innovación Varietal manzana: Situación y perspectivas de futuro. Rev. Frutic. 2016, 52, 6–37. [Google Scholar]
- Begić-Akagić, A.; Spaho, N.; Gaši, F.; Drkenda, P.; Vranac, A.; Meland, M.; Besim, S. Sugar and organic acid profiles of the traditional and international apple cultivars for processing. J. Hyg. Eng. Des. 2014, 7, 190–196. [Google Scholar]
- Bureau, S.; Ścibisz, I.; Le Bourvellec, C.; Renard, C.M. Effect of Sample Preparation on the Measurement of Sugars, Organic Acids, and Polyphenols in Apple Fruit by Mid-infrared Spectroscopy. J. Agric. Food Chem. 2012, 60, 3551–3563. [Google Scholar] [CrossRef]
- Donahue, D.; Córdoba, G.R.; Elone, S.; Wallis, A.; Basedow, M. ‘Honeycrisp’ Bitter Pit Response to Rootstock and Region under Eastern New York Climatic Conditions. Plants 2021, 10, 983. [Google Scholar] [CrossRef]
- Font i Forcada, C.; Gogorcena, Y.; Moreno, M.Á. Agronomical Parameters, Sugar Profile and Antioxidant Compounds of “Catherine” Peach Cultivar Influenced by Different Plum Rootstocks. Int. J. Mol. Sci. 2014, 15, 2237–2254. [Google Scholar] [CrossRef]
- Iglesias, I.; Giné-Bordonaba, J.; Garanto, X.; Reig, G. Rootstock affects quality and phytochemical composition of ‘Big Top’ nectarine fruits grown under hot climatic conditions. Sci. Hortic. 2019, 256, 108586. [Google Scholar] [CrossRef]
- Gómez-Martínez, H.; Bermejo, A.; Zuriaga, E.; Badenes, M. Polyphenol content in apricot fruits. Sci. Hortic. 2020, 277, 109828. [Google Scholar] [CrossRef]
- Davey, M.W.; Auwerkerken, A.; Keulemans, J. Relationship of apple vitamin C and antioxidant contents to harvest date and postharvest pathogen infection. J. Sci. Food Agric. 2007, 87, 802–813. [Google Scholar] [CrossRef]
- Jing, C.; Feng, D.; Zhao, Z.; Wu, X.; Chen, X. Effect of environmental factors on skin pigmentation and taste in three apple cultivars. Acta Physiol. Plant. 2020, 42, 69. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S.; Gamsjäger, H. Phytochemical analysis by liquid chromatography of ten old apple varieties grown in Austria and their antioxidative activity. Eur. Food Res. Technol. 2020, 246, 437–448. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Zhao, Z. Effects of fruit bagging on anthocyanins, sugars, organic acids, and color properties of ‘Granny Smith’ and ‘Golden Delicious’ during fruit maturation. Eur. Food Res. Technol. 2012, 236, 329–339. [Google Scholar] [CrossRef]
- Busatto, N.; Matsumoto, D.; Tadiello, A.; Vrhovsek, U.; Costa, F. Multifaceted analyses disclose the role of fruit size and skin-russeting in the accumulation pattern of phenolic compounds in apple. PLoS ONE 2019, 14, e0219354. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-Z.; Dai, M.-S.; Zhang, S.-J.; Shi, Z.-B. Exploring Candidate Genes for Pericarp Russet Pigmentation of Sand Pear (Pyrus pyrifolia) via RNA-Seq Data in Two Genotypes Contrasting for Pericarp Color. PLoS ONE 2014, 9, e83675. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Nie, J.-Y.; Li, J.; Zhang, H.; Li, Y.; Farooq, S.; Bacha, S.A.S.; Wang, J. Evaluation of sugar and organic acid composition and their levels in highbush blueberries from two regions of China. J. Integr. Agric. 2020, 19, 2352–2361. [Google Scholar] [CrossRef]
- Forni, E.; Erba, M.; Maestrelli, A.; Polesello, A. Sorbitol and free sugar contents in plums. Food Chem. 1992, 44, 269–275. [Google Scholar] [CrossRef]
- Lu, Q.; Hao, M.; Wu, W.; Zhang, N.; Isaac, A.T.; Yin, J.; Zhu, X.; Du, L.; Yin, X. Antidiabetic cataract effects of GbE, rutin and quercetin are mediated by the inhibition of oxidative stress and polyol pathway. Acta Biochim. Pol. 2017, 65, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giné-Bordonaba, J.; Cantín, C.M.; Echeverría, G.; Ubach, D.; Larrigaudière, C. The effect of chilling injury-inducing storage conditions on quality and consumer acceptance of different Prunus persica cultivars. Postharvest Biol. Technol. 2016, 115, 38–47. [Google Scholar] [CrossRef]
- Keenan, D.F.; Valverde, J.; Gormley, R.; Butler, F.; Brunton, N.P. Selecting apple cultivars for use in ready-to-eat desserts based on multivariate analyses of physico-chemical properties. LWT 2012, 48, 308–315. [Google Scholar] [CrossRef]
- Bayarri, S.; Calvo, C.; Costell, E.; Durán, L. Influence of Color on Perception of Sweetness and Fruit Flavor of Fruit Drinks. Food Sci. Technol. Int. 2001, 7, 399–404. [Google Scholar] [CrossRef]
- Corollaro, M.L.; Aprea, E.; Endrizzi, I.; Betta, E.; Demattè, M.L.; Charles, M.; Bergamaschi, M.; Costa, F.; Biasioli, F.; Grappadelli, L.C.; et al. A combined sensory-instrumental tool for apple quality evaluation. Postharvest Biol. Technol. 2014, 96, 135–144. [Google Scholar] [CrossRef]
- Li, P.; Ma, F.; Cheng, L. Primary and secondary metabolism in the sun-exposed peel and the shaded peel of apple fruit. Physiol. Plant. 2012, 148, 9–24. [Google Scholar] [CrossRef]
- McGhie, T.K.; Hunt, A.M.; Barnett, L.E. Cultivar and Growing Region Determine the Antioxidant Polyphenolic Concentration and Composition of Apples Grown in New Zealand. J. Agric. Food Chem. 2005, 53, 3065–3070. [Google Scholar] [CrossRef]
- Dietrich, H.; Krüger-Steden, E.; Patz, C.D.; Will, F.; Rheinberger, A.; Hopf, I. Increase of sorbitol in pear and apple juice by water stress, a consequence of climatic change. Fruit Process. 2007, 6, 348–355. [Google Scholar]
- Zhang, J.Y.; Tian, R.R.; Dong, J.L.; Zhao, K.; Li, T.H.; Wang, T. Response and regulation of the S6PDH gene in apple leaves under osmotic stress. J. Hortic. Sci. Biotechnol. 2011, 86, 563–568. [Google Scholar] [CrossRef]
- Gao, Z.; Jayanty, S.; Beaudry, R.; Loescher, W. Sorbitol Transporter Expression in Apple Sink Tissues: Implications for Fruit Sugar Accumulation and Watercore Development. J. Am. Soc. Hortic. Sci. 2005, 130, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Pandey, V.P.; Awasthi, M.; Singh, S.; Tiwari, S.; Dwivedi, U.N. A Comprehensive Review on Function and Application of Plant Peroxidases. Biochem. Anal. Biochem. 2017, 6, 308. [Google Scholar] [CrossRef]
- Font i Forcada, C.; Guajardo, V.; Chin-Wo, S.R.; Moreno, M.Á. Association Mapping Analysis for Fruit Quality Traits in Prunus persica Using SNP Markers. Front. Plant Sci. 2019, 9, 2005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, M.; Roth, M.; Aranzana, M.J.; Auwerkerken, A.; Bink, M.; Denancé, C.; Dujak, C.; Durel, C.-E.; Font i Forcada, C.; Cantín, C.M.; et al. The apple REFPOP—A reference population for genomics-assisted breeding in apple. Hortic. Res. 2020, 7, 189. [Google Scholar] [CrossRef] [PubMed]
- Winfield, M.; Burridge, A.; Ordidge, M.; Harper, H.; Wilkinson, P.; Thorogood, D.; Copas, L.; Edwards, K.; Barker, G. Development of a minimal KASP marker panel for distinguishing genotypes in apple collections. PLoS ONE 2020, 15, e0242940. [Google Scholar] [CrossRef]
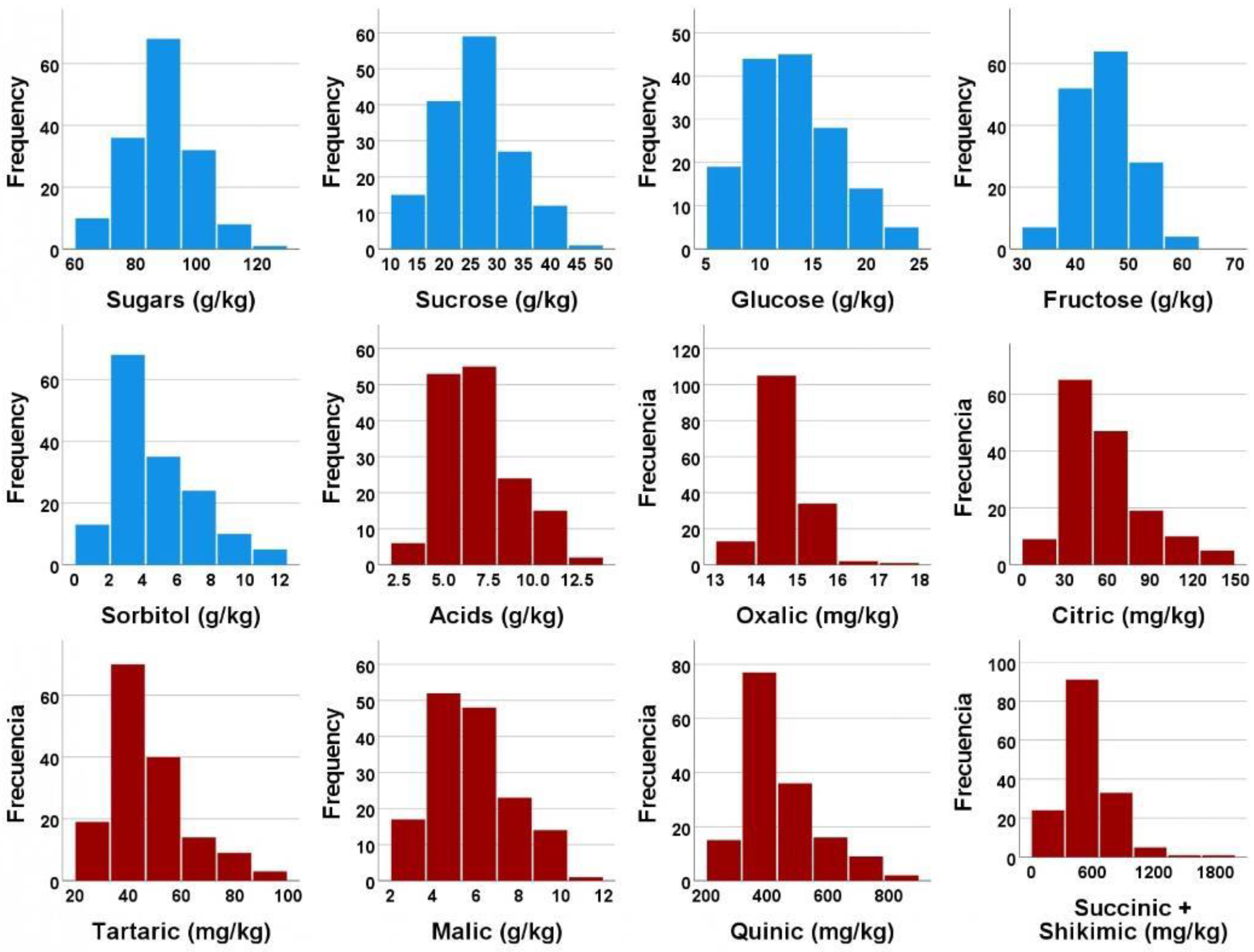
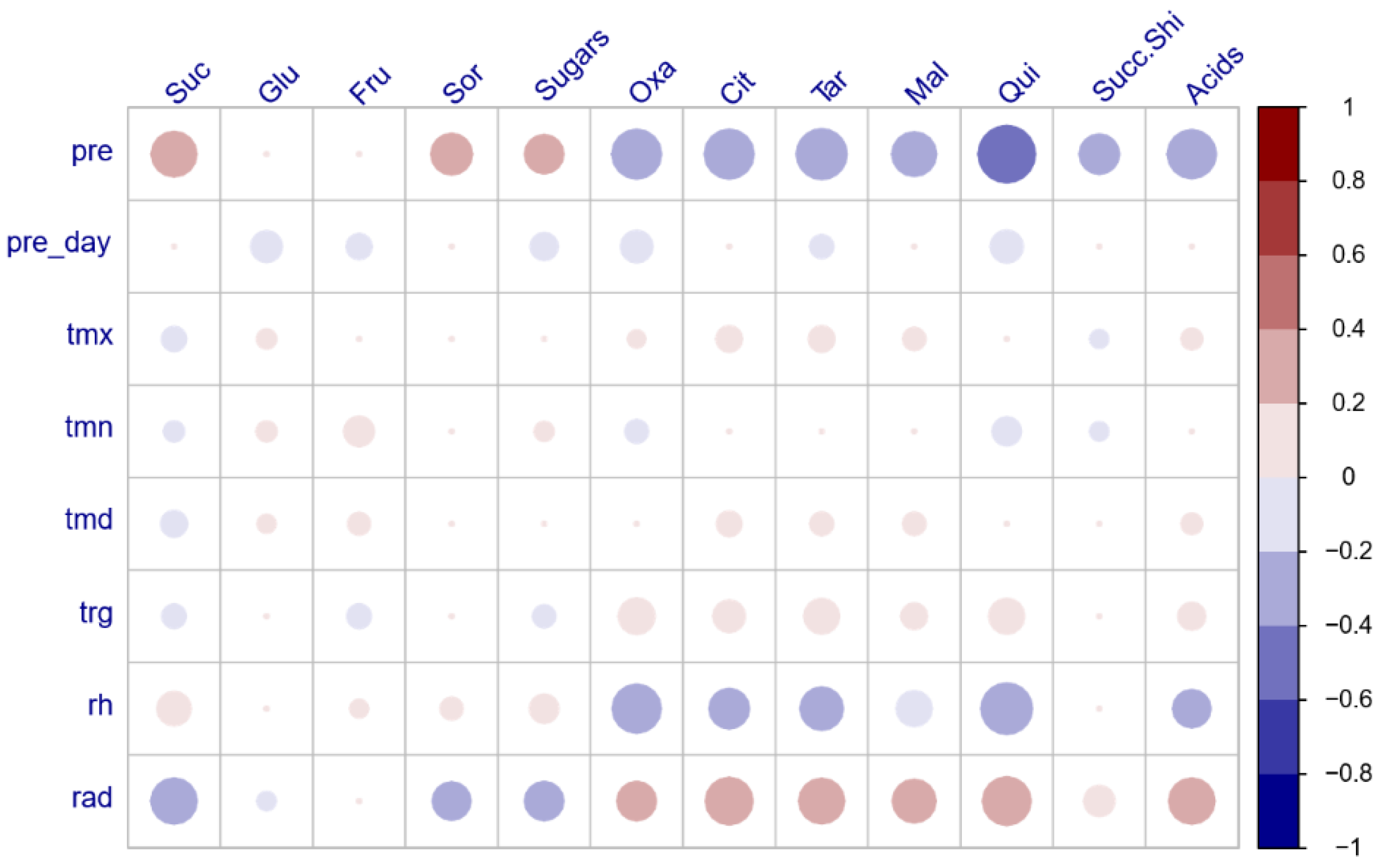
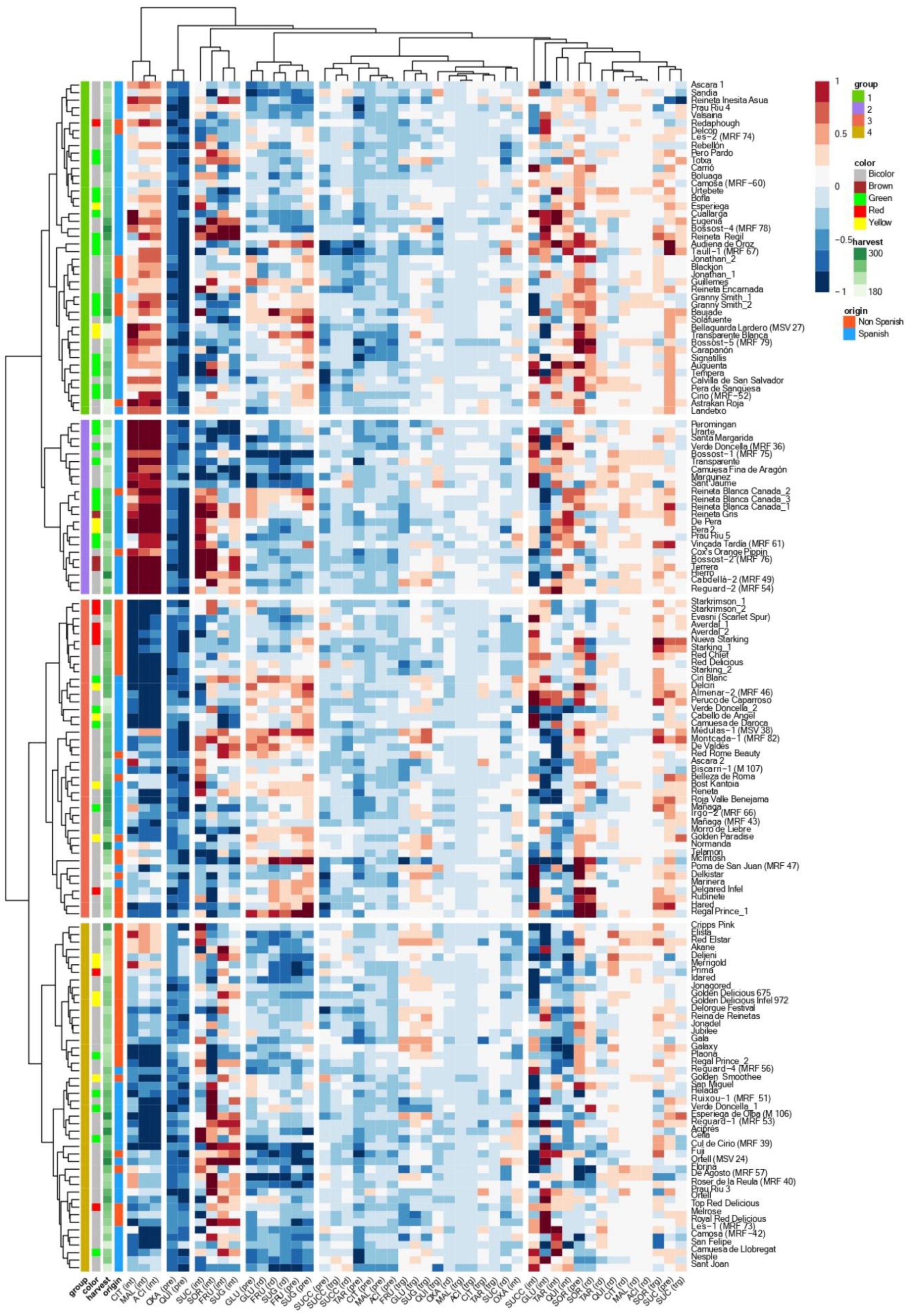
| Trait | Units | Minimum | Maximum | Mean | SD |
|---|---|---|---|---|---|
| Sugars | g kg−1 | 25.02 | 159.79 | 88.94 | 17.80 |
| Sucrose | g kg−1 | 3.26 | 61.18 | 25.75 | 9.17 |
| Glucose | g kg−1 | 2.18 | 37.56 | 13.16 | 5.68 |
| Fructose | g kg−1 | 14.63 | 87.40 | 45.38 | 8.96 |
| Sorbitol | g kg−1 | 0.48 | 26.23 | 4.65 | 3.41 |
| Acids | g kg−1 | 0.773 | 22.832 | 6.786 | 2.336 |
| Oxalic | g kg−1 | 0.010 | 0.020 | 0.015 | 0.001 |
| Citric | g kg−1 | 0.010 | 0.312 | 0.057 | 0.034 |
| Tartaric | g kg−1 | 0.017 | 0.271 | 0.047 | 0.021 |
| Malic | g kg−1 | 0.435 | 19.843 | 5.689 | 2.119 |
| Quinic | g kg−1 | 0.116 | 2.035 | 0.435 | 0.210 |
| Succinic + Shikimic | g kg−1 | 0.080 | 2.284 | 0.555 | 0.285 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mignard, P.; Beguería, S.; Giménez, R.; Font i Forcada, C.; Reig, G.; Moreno, M.Á. Effect of Genetics and Climate on Apple Sugars and Organic Acids Profiles. Agronomy 2022, 12, 827. https://doi.org/10.3390/agronomy12040827
Mignard P, Beguería S, Giménez R, Font i Forcada C, Reig G, Moreno MÁ. Effect of Genetics and Climate on Apple Sugars and Organic Acids Profiles. Agronomy. 2022; 12(4):827. https://doi.org/10.3390/agronomy12040827
Chicago/Turabian StyleMignard, Pierre, Santiago Beguería, Rosa Giménez, Carolina Font i Forcada, Gemma Reig, and María Ángeles Moreno. 2022. "Effect of Genetics and Climate on Apple Sugars and Organic Acids Profiles" Agronomy 12, no. 4: 827. https://doi.org/10.3390/agronomy12040827
APA StyleMignard, P., Beguería, S., Giménez, R., Font i Forcada, C., Reig, G., & Moreno, M. Á. (2022). Effect of Genetics and Climate on Apple Sugars and Organic Acids Profiles. Agronomy, 12(4), 827. https://doi.org/10.3390/agronomy12040827






