Chemical Profile, Elemental Composition, and Antimicrobial Activity of Plants of the Teucrium (Lamiaceae) Genus Growing in Moldova
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Extraction







2.2. Reagents and Apparatus
2.3. GC–MS Analysis
2.4. Neutron Activation Analysis
2.5. Antimicrobial Activity Assessment
2.6. Statistical Analysis
3. Results and Discussion
3.1. GC–MS Identification of Essential Oil Constituents
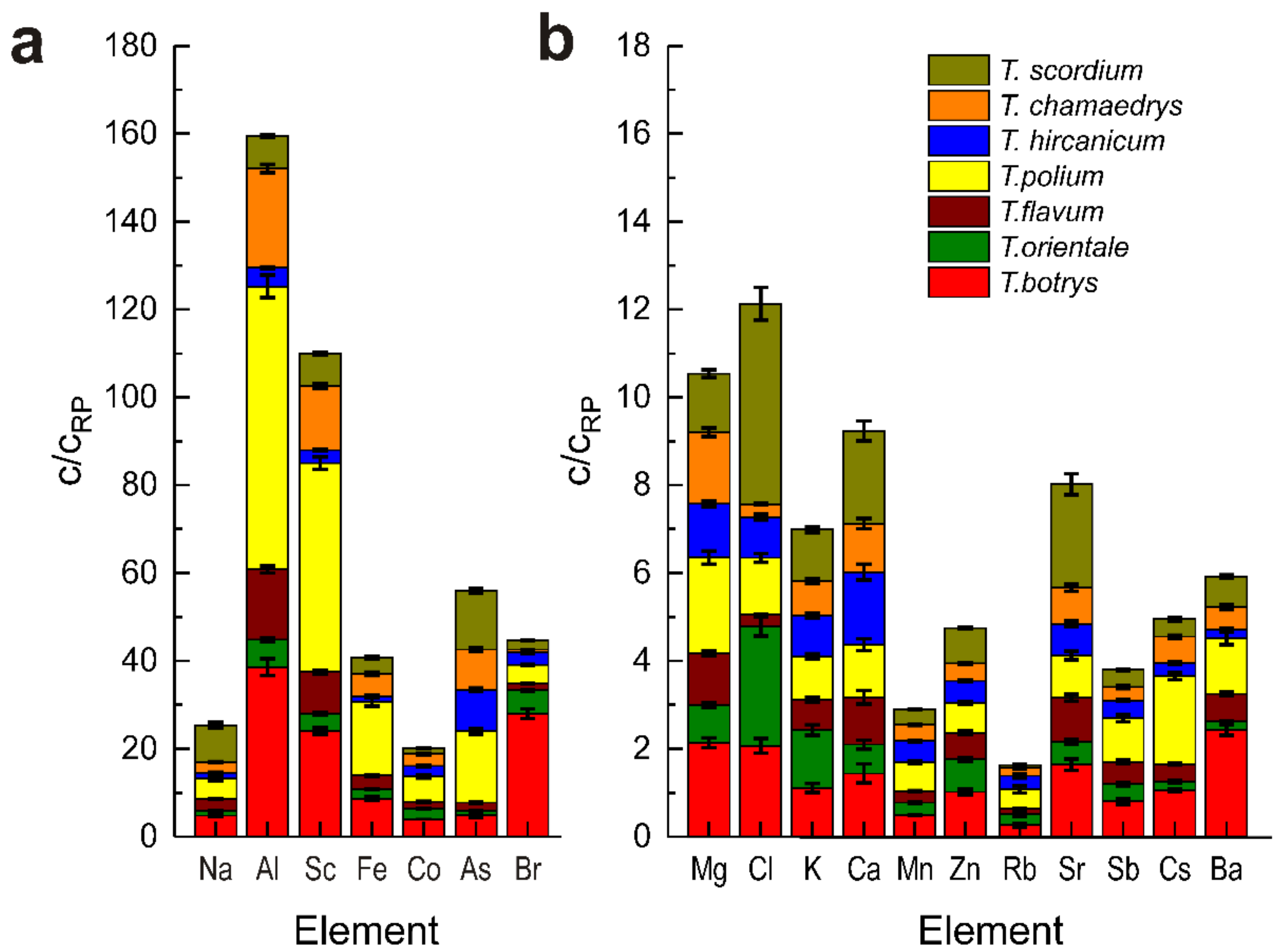
3.2. NAA Analysis of Elemental Content of Some Teucrium Species
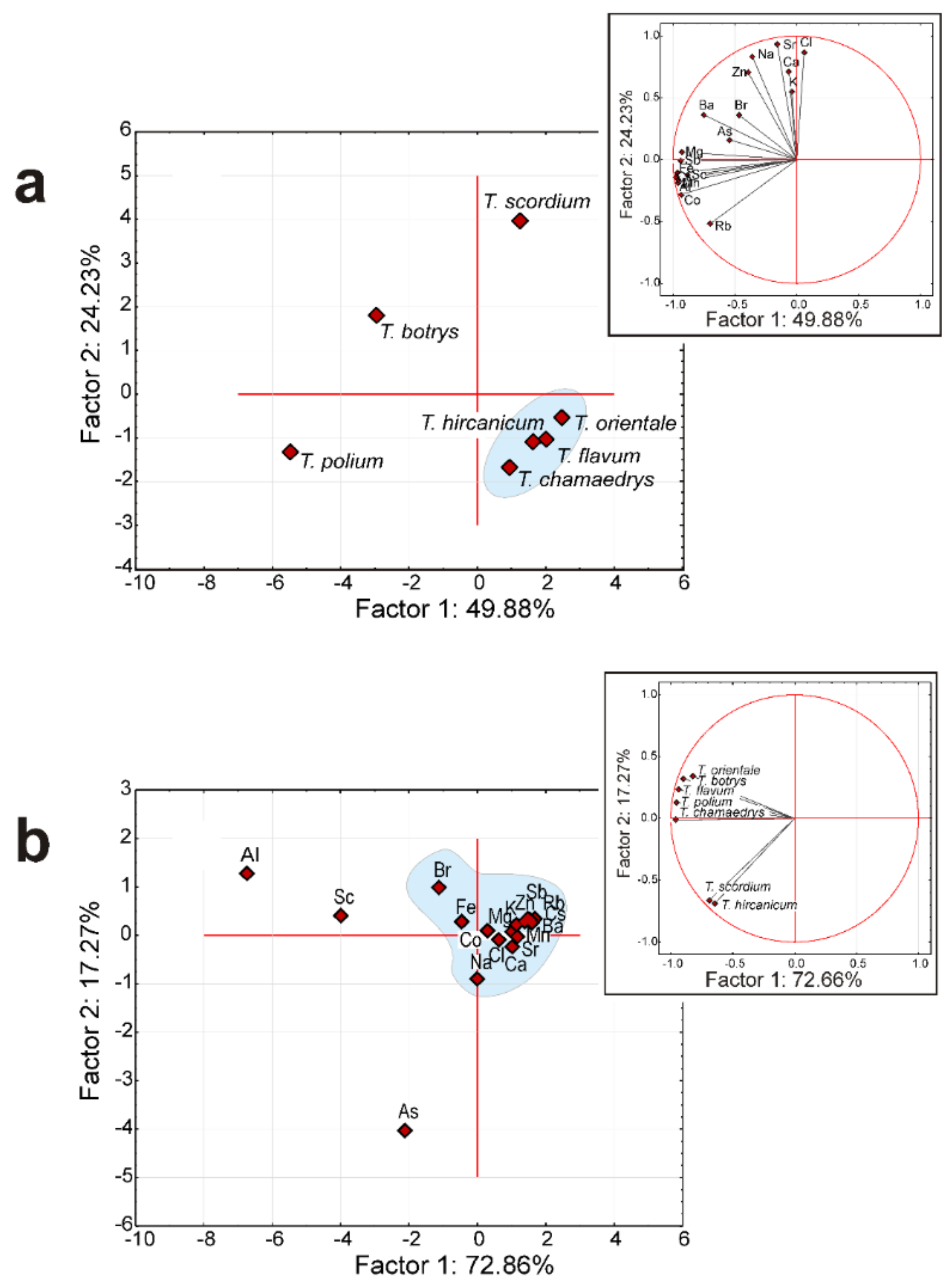
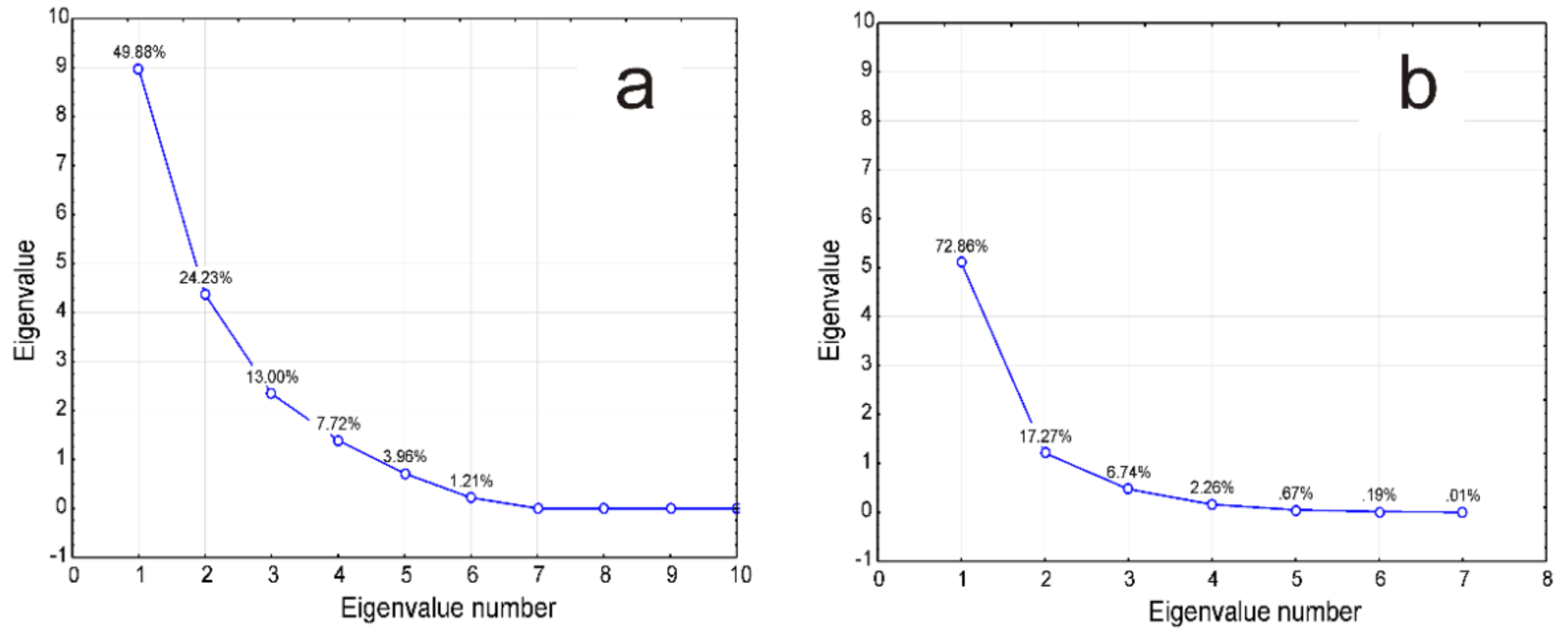
3.3. Statistical Analysis
3.4. Antimicrobial Activity Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATCC | American Type Culture Collection |
| CFU | Colony-forming unit |
| DNA | Deoxyribonucleic acid |
| GC–MS | Gas chromatography–mass spectrometry |
| MBC | Minimum bactericidal concentration |
| MFC | Minimum fungicidal concentration |
| MIC | Minimum inhibitory concentration |
| NAA | Neutron activation analysis |
| NCNM | National Collection of Non-Pathogenic Microorganisms |
| PCA | Principal component analysis |
| RNA | Ribonucleic acid |
| RP | Reference plants |
| RT | Retention time |
| SDA | Statistical data analysis |
References
- Cantino, P.D.; Harley, R.M.; Wagstaff, S.J. Genera of Labiatae: Status and classification. Adv. Labiate Sci. 1992, 11, 511–522. [Google Scholar]
- Ernst, W.R.; Ernst, W.R. Flora Europaea; California Botanical Society: Berkeley, CA, USA, 1969; Volume 20. [Google Scholar]
- Negru, A. Determinatór de Plante din Flora Republicii Moldova; Editura Universul: Chisinau, Moldova, 2007; ISBN 9789975470070. [Google Scholar]
- Rizk, A.M.; Hammouda, F.M.; Rimpler, H.; Kamel, A. Iridoids and flavonoids of Teucrium polium herb. Planta Med. 1986, 52, 87–88. [Google Scholar] [CrossRef]
- Suleiman, M.S.; Abdul-Ghani, A.S.; Al-Khalil, S.; Amin, R. Effect of Teucrium polium boiled leaf extract on intestinal motility and blood pressure. J. Ethnopharmacol. 1988, 22, 111–116. [Google Scholar] [CrossRef]
- Bruno, M.; Maggio, A.M.; Piozzi, F.; Puech, S.; Rosselli, S.; Simmonds, M.S.J. Neoclerodane diterpenoids from Teucrium polium subsp. polium and their antifeedant activity. Biochem. Syst. Ecol. 2003, 31, 1051–1056. [Google Scholar] [CrossRef]
- Frezza, C.; Venditti, A.; Matrone, G.; Serafini, I.; Foddai, S.; Bianco, A.; Serafini, M. Iridoid glycosides and polyphenolic compounds from Teucrium chamaedrys L. Nat. Prod. Res. 2018, 32, 1583–1589. [Google Scholar] [CrossRef]
- Grubešić, R.J.; Kremer, D.; Vladimir-Knežević, S.; Rodríguez, J.V. Analysis of polyphenols, phytosterols, and bitter principles in Teucrium L. species. Cent. Eur. J. Biol. 2012, 7, 542–550. [Google Scholar] [CrossRef]
- Esmaeili, M.A.; Yazdanparast, R. Hypoglycaemic effect of Teucrium polium: Studies with rat pancreatic islets. J. Ethnopharmacol. 2004, 95, 27–30. [Google Scholar] [CrossRef]
- Rasekh, H.R.; Khoshnood-Mansourkhani, M.J.; Kamalinejad, M. Hypolipidemic effects of Teucrium polium in rats. Fitoterapia 2001, 72, 937–939. [Google Scholar] [CrossRef]
- Parsaee, H.; Shafiee-Nick, R. Anti-spasmodic and anti-nociceptive effects of Teucrium polium aqueous extract. Iran. Biomed. J. 2006, 10, 145–149. [Google Scholar]
- Qabaha, K.; Hijawi, T.; Mahamid, A.; Mansour, H.; Naeem, A.; Abbadi, J.; Al-Rimawi, F. Anti-inflammatory and antioxidant activities of Teucrium polium leaf extract and its phenolic and flavonoids content. Asian J. Chem. 2021, 33, 881–884. [Google Scholar] [CrossRef]
- Bello, R.; Barrachina, M.D.; Martínez-Cuesta, M.A.; Esplugues, J.; Yúfera, E.P. Evaluation of the acute toxicity, analgesic and CNS activities of different species of Teucrium genus. Phyther. Res. 1995, 9, 277–280. [Google Scholar] [CrossRef]
- Raei, F.; Ashoori, N.; Eftekhar, F.; Yousefzadi, M. Chemical composition and antibacterial activity of Teucrium polium essential oil against urinary isolates of Klebsiella pneumoniae. J. Essent. Oil Res. 2014, 26, 65–69. [Google Scholar] [CrossRef]
- Stanković, M.S.; Mitrović, T.L.J.; Matić, I.Z.; Topuzović, M.D.; Stamenković, S.M. New values of Teucrium species: In Vitro study of cytotoxic activities of secondary metabolites. Not. Bot. Horti Agrobot. Cluj-Napoca 2015, 43, 41–46. [Google Scholar] [CrossRef]
- Tatjana, K.; Marina, M.; Aleksandra, T.; Tatjana, S.; Zorica, J.; Branislava, L. Cytotoxicity and antimicrobial activity of Teucrium scordium L. (Lamiaceae) extracts. Afr. J. Microbiol. Res. 2011, 5, 2950–2954. [Google Scholar] [CrossRef]
- Stankovic, M.S.; Curcic, M.G.; Zizic, J.B.; Topuzovic, M.D.; Solujic, S.R.; Markovic, S.D. Teucrium plant species as natural sources of novel anticancer compounds: Antiproliferative, proapoptotic and antioxidant properties. Int. J. Mol. Sci. 2011, 12, 4190–4205. [Google Scholar] [CrossRef]
- Javidnia, K.; Miri, R. Composition of the essential oil of Teucrium orientate L. ssp. orientate from Iran. J. Essent. Oil Res. 2003, 15, 118–119. [Google Scholar] [CrossRef]
- Formisano, C.; Rigano, D.; Senatore, F.; Bruno, M.; Maggio, A.; Piozzi, F. Chemical composition of the essential oil of Teucrium flavum ssp. flavum from Zakynthos, Greece. Rec. Nat. Prod. 2012, 6, 306–310. [Google Scholar]
- Presti, M.L.; Crupi, M.L.; Costa, R.; Dugo, G.; Mondello, L.; Ragusa, S.; Santi, L. Seasonal variations of Teucrium flavum L. essential oil. J. Essent. Oil Res. 2010, 22, 211–216. [Google Scholar] [CrossRef]
- Bezić, N.; Vuko, E.; Dunkić, V.; Ruščić, M.; Blažević, I.; Burčul, F. Antiphytoviral activity of sesquiterpene-rich essential oils from four croatian Teucrium species. Molecules 2011, 16, 8119–8129. [Google Scholar] [CrossRef]
- Kovacevic, N.N.; Lakusic, B.S.; Ristic, M.S. Composition of the essential oils of seven Teucrium species from serbia and montenegro. J. Essent. Oil Res. 2001, 13, 163–165. [Google Scholar] [CrossRef]
- Lograda, T.; Ramdani, M.; Chalard, P.; Figueredo, G.; Deghar, A. Chemical analysis and antimicrobial activity of Teucrium polium L. essential oil from Western Algeria. J. Med. Plants Res. 2013, 7, 897–902. [Google Scholar] [CrossRef]
- Hammami, S.; El Mokni, R.; Faidi, K.; Falconieri, D.; Piras, A.; Procedda, S.; Mighri, Z.; El Aouni, M.H. Chemical composition and antioxidant activity of essential oil from aerial parts of Teucrium flavum L. subsp. flavum growing spontaneously in Tunisia. Nat. Prod. Res. 2015, 29, 2336–2340. [Google Scholar] [CrossRef] [PubMed]
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Lyoussi, B.; Oumokhtar, B.; Abdellaoui, A. Phytochemistry, antioxidant and antibacterial activities of two Moroccan Teucrium polium L. subspecies: Preventive approach against nosocomial infections. Arab. J. Chem. 2020, 13, 3866–3874. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Zare, P.; Hassanzadeh, P.; Nosratpour, S. Effect of Teucrium polium essential oil on the physicochemical and sensory properties of probiotic yoghurt. J. Food Process. Preserv. 2014, 38, 880–888. [Google Scholar] [CrossRef]
- Ciocarlan, A.; Lupascu, L.; Aricu, A.; Dragalin, I.; Ciocarlan, N.; Zinicovscaia, I.; Slanina, V.; Yushin, N. Chemical Composition of the Essential Oil and Antimicrobial Properties of Crude Extract from Tanacetum corymbosum (L.) Shi. Bip. Chem. J. Mold. 2021, 16, 83–90. [Google Scholar] [CrossRef]
- Zinicovscaia, I.; Duliu, O.G.; Culicov, O.A.; Frontasyeva, M.V.; Sturza, R. Major and trace elements distribution in moldavian soils. Rom. Rep. Phys. 2018, 70, 701. [Google Scholar]
- Zinicovscaia, I.; Duliu, O.G.; Culicov, O.A.; Sturza, R.; Bilici, C.; Gundorina, S. Geographical Origin Identification of Moldavian Wines by Neutron Activation Analysis. Food Anal. Methods 2017, 10, 3523–3530. [Google Scholar] [CrossRef]
- Mothana, R.A.; Noman, O.M.; Al-Sheddi, E.S.; Khaled, J.M.; Al-Said, M.S.; Al-Rehaily, A.J. Chemical composition, in vitro antimicrobial, free-radical-scavenging and antioxidant activities of the essential oil of Leucas inflata Benth. Molecules 2017, 22, 367. [Google Scholar] [CrossRef]
- Marino, A.; Nostro, A.; Mandras, N.; Roana, J.; Ginestra, G.; Miceli, N.; Taviano, M.F.; Gelmini, F.; Beretta, G.; Tullio, V. Evaluation of antimicrobial activity of the hydrolate of Coridothymus capitatus (L.) Reichenb. Fil. (Lamiaceae) alone and in combination with antimicrobial agents. BMC Complement. Med. Ther. 2020, 20, 89. [Google Scholar] [CrossRef]
- Jollife, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 178. [Google Scholar]
- Moumni, S.; Elaissi, A.; Trabelsi, A.; Merghni, A.; Chraief, I.; Jelassi, B.; Chemli, R.; Ferchichi, S. Correlation between chemical composition and antibacterial activity of some lamiaceae species essential oils from Tunisia. BMC Complement. Med. Ther. 2020, 20, 103. [Google Scholar] [CrossRef] [PubMed]
- Jaradat, N.A. Review of the taxonomy, ethnobotany, phytochemistry, phytotherapy and phytotoxicity of germander plant (Teucrium polium L.). Asian J. Pharm. Clin. Res. 2015, 8, 13–19. [Google Scholar]
- Rahimi, M.A.; Nazeri, V.; Andi, S.A.; Sefidkon, F. Variation in essential oil composition of Teucrium hircanicum L. from Iran-A rich source of (E)-α-bergamotene. Nat. Prod. Res. 2019, 33, 1227–1232. [Google Scholar] [CrossRef]
- Bagci, E.; Yazgin, A.; Hayta, S.; Cakilcioglu, U. Composition of the essential Oil of Teucrium chamaedrys L. (Lamiaceae) from Turkey. J. Med. Plants Res. 2010, 4, 2588–2590. [Google Scholar]
- Aberumand, M.; Asgarpanah, J. Essential Oil Composition of Teucrium orientale subsp. glabrescens from Iran. Chem. Nat. Compd. 2017, 53, 381–382. [Google Scholar] [CrossRef]
- Kucukbay, F.Z.; Yildiz, B.; Kuyumcu, E.; Gunal, S. Chemical composition and antimicrobial activities of the essential oils of Teucrium orientale var. orientale and Teucrium orientale var. puberulens. Chem. Nat. Compd. 2011, 47, 833–836. [Google Scholar] [CrossRef]
- Markert, B. Establishing of “Reference Plant” for inorganic characterization of different plant species by chemical fingerprinting. Water Air Soil Pollut. 1992, 64, 533–538. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Al Mahmud, J.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium deficiency in plants: An urgent problem. Crop. J. 2016, 4, 83–91. [Google Scholar] [CrossRef]
- Maathuis, F.J.M. Sodium in plants: Perception, signalling, and regulation of sodium fluxes. J. Exp. Bot. 2014, 65, 849–858. [Google Scholar] [CrossRef]
- Chizzola, R.; Michitsch, H.; Franz, C. Monitoring of metallic micronutrients and heavy metals in herbs, spices and medicinal plants from Austria. Eur. Food Res. Technol. 2003, 216, 407–411. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Mousavi, S.R.; Shahsavari, M.; Rezaei, M. A general overview on manganese (Mn) importance for crops production. Aust. J. Basic Appl. Sci. 2011, 5, 1799–1803. [Google Scholar]
- Brown, P.H.; Cakmak, I.; Zhang, Q. Form and Function of Zinc Plants. In Zinc in Soils and Plants; Springer: Dordrecht, The Netherlands, 1993; pp. 93–106. [Google Scholar]
- World Health Organization. Pharmaceuticals Unit Quality control methods for medicinal plant materials. Geneva World Health Organ. 1998, 122, 1–84. [Google Scholar]
- Pavlova, D.; Karadjova, I. Chemical analysis of Teucrium species (Lamiaceae) growing on serpentine soils in Bulgaria. J. Plant Nutr. Soil Sci. 2012, 175, 891–899. [Google Scholar] [CrossRef]
- Zlatić, N.M.; Stanković, M.S.; Simić, Z.S. Secondary metabolites and metal content dynamics in Teucrium montanum L. and Teucrium chamaedrys L. from habitats with serpentine and calcareous substrate. Environ. Monit. Assess. 2017, 189, 110. [Google Scholar] [CrossRef]
- Piqué, N.; Miñana-Galbis, D.; Merino, S.; Tomás, J.M. Virulence factors of Erwinia amylovora: A review. Int. J. Mol. Sci. 2015, 16, 12836–12854. [Google Scholar] [CrossRef]
- Benada, M.; Boumaaza, B.; Boudalia, S.; Khaladi, O.; Guessas, B. Variability of Aggressiveness and Virulence of Erwinia carotovora subsp. Carotovorum Causing the Soft Rot on Potato Tubers in the Western of Algeria. Int. J. Plant Biol. 2018, 9, 7568. [Google Scholar] [CrossRef][Green Version]
- Tamir-Ariel, D.; Navon, N.; Burdman, S. Identification of genes in Xanthomonas campestris pv. vesicatoria induced during its interaction with tomato. J. Bacteriol. 2007, 189, 6359–6371. [Google Scholar] [CrossRef]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids lactones: Benefits to plants and people. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef] [PubMed]
- Jansen, B.J.M.; De Groot, A. The occurrence and biological activity of drimane sesquiterpenoids. Nat. Prod. Rep. 1991, 8, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Askun, T.; Tekwu, E.M.; Satil, F.; Modanlioglu, S.; Aydeniz, H. Preliminary antimycobacterial study on selected Turkish plants (Lamiaceae) against Mycobacterium tuberculosis and search for some phenolic constituents. BMC Complement. Altern. Med. 2013, 13, 365. [Google Scholar] [CrossRef] [PubMed]
| No. | RT * (min) | Component, Classification | Species | |||||
|---|---|---|---|---|---|---|---|---|
| TP | TH | TB | TCH | TF | TO | |||
| 1 | 4.28 | α-Thujene (C10 **) | 0.07 | - | - | - | - | - |
| 2 | 4.42 | α-Pinene (C10) | 5.27 | - | 3.03 | 1.69 | 12.38 | - |
| 3 | 4.72 | Camphene (C10) | 0.09 | - | - | - | - | - |
| 4 | 5.18 | β-Thujene (C10) | 0.16 | - | - | - | - | - |
| 5 | 5.27 | β-Pinene (C10) | 16.08 | - | 3.81 | 1.19 | 6.78 | - |
| 6 | 5.50 | β-Myrcene (C10) | 7.63 | - | 0.18 | - | 0.54 | 0.35 |
| 7 | 6.38 | (+)-Limonene (C10) | 5.82 | - | 3.29 | - | 4.64 | - |
| 8 | 6.55 | (E)-β-Ocimene (C10) | 0.23 | - | - | - | 2.87 | 2.35 |
| 9 | 6.81 | (Z)-β-Ocimene (C10) | 1.50 | - | - | - | 0.34 | 3.67 |
| 10 | 7.84 | Terpinolene (C10) | 0.11 | - | - | - | - | - |
| 11 | 8.11 | β-Linalool (C10) | 0.54 | 5.00 | 1.11 | - | - | - |
| 12 | 8.38 | 1-Octene-3-yl acetate | 0.44 | - | - | - | 0.49 | - |
| 13 | 8.64 | 3-Octyl acetate | - | - | - | - | 0.62 | - |
| 14 | 10.59 | α-Terpineol (C10) | 0.11 | - | - | - | - | - |
| 15 | 10.71 | Myrtenal (C10) | 0.14 | - | - | - | - | - |
| 16 | 12.03 | 2-Methylbutyl hexanoate | - | - | - | - | 1.39 | - |
| 17 | 13.02 | Bornyl acetate (C10) | - | - | 0.61 | - | - | - |
| 18 | 14.65 | α-Cubebene (C15) | - | - | - | - | 0.42 | 3.02 |
| 19 | 15.35 | Copaene (C15) | 0.31 | - | - | 0.80 | - | 3.33 |
| 20 | 15.59 | β-Bourbonene (C15) | 0.37 | - | 0.38 | 1.68 | 1.60 | 0.39 |
| 21 | 15.74 | β-Cubebone (C15) | 0.17 | - | - | 1.81 | - | 9.61 |
| 22 | 15.75 | β-Elemene (C15) | 0.20 | - | - | - | - | - |
| 23 | 16.45 | β-Caryophyllene (C15) | 0.28 | - | 54.06 | 40.95 | 6.99 | 16.47 |
| 24 | 16.84 | α-Bergamotene (C15) | - | 2.18 | - | - | 0.36 | 0.77 |
| 25 | 17.02 | β-Sesquiphellandrene (C15) | - | 0.98 | - | - | - | - |
| 26 | 17.30 | α-Caryophyllene (C15) | - | - | - | 9.14 | 3.83 | 6.28 |
| 27 | 17.32 | (Z)-β-Farnesene (C15) | 6.90 | 30.44 | 0.70 | - | - | - |
| 28 | 17.40 | Farnesane (C15) | - | 2.49 | - | - | - | - |
| 29 | 17.48 | Alloaromadendrene (C15) | 0.30 | - | 15.81 | 0.78 | 0.39 | 0.35 |
| 30 | 18.00 | α-Curcumene (C15) | - | 0.81 | - | - | - | - |
| 31 | 18.02 | Germacrene D (C15) | 32.43 | - | 1.63 | 22.09 | 15.47 | 13.86 |
| 32 | 18.12 | (+)-Valenune (C15) | 0.28 | - | - | - | - | - |
| 33 | 18.28 | (+)-Valencene (C15) | - | - | - | 0.78 | - | - |
| 34 | 18.36 | Bicyclogermacrene (C15) | 7.44 | - | 1.60 | 1.65 | 0.79 | 3.95 |
| 35 | 18.57 | δ-Guaiene (C15) | 0.47 | - | - | - | - | - |
| 36 | 18.61 | β-Bisabolene (C15) | - | 0.88 | - | - | 29.96 | - |
| 37 | 18.69 | Butylated hydroxytoluene | - | 9.22 | 0.50 | 1.63 | - | 14.97 |
| 38 | 18.78 | γ-Muurolene (C15) α-Himachalene (C15) | 0.67 | 38.21 | - | - | - | - |
| 39 | 18.87 | Seline-3,7[11]-diene (C15) | - | - | - | 5.98 | - | 0.57 |
| 40 | 18.97 | (-)-β-Cadinene (C15) | 0.34 | - | 0.58 | 6.55 | 0.70 | 3.79 |
| 41 | 19.18 | α-Patchoulene (C15) | - | 4.85 | - | - | - | - |
| 42 | 19.79 | γ-Elemene (C15) | 0.08 | - | - | - | - | 0.17 |
| 43 | 20.28 | (-)-Spathulenol (C15) | 0.10 | - | - | - | - | 0.10 |
| 44 | 20.44 | Caryophyllene oxide (C15) | 0.10 | - | - | 2.20 | 1.44 | 0.75 |
| 45 | 20.59 | Viridiflorol (C15) | - | - | - | - | 2.67 | - |
| 46 | 20.84 | Ledol (C15) | - | - | - | - | 1.00 | 0.21 |
| 47 | 21.73 | δ-Cadinol (C15) | 0.65 | - | 0.88 | - | - | 0.16 |
| 48 | 21.95 | α-Cadinol (C15) | - | - | - | - | 0.56 | 0.15 |
| 49 | 21.93 | β-Eudesmol (C15) | 1.05 | - | - | - | - | 0.28 |
| 50 | 22.01 | γ-Eudesmol (C15) | 1.81 | - | - | - | - | - |
| 51 | 22.57 | α-Bisabolol (C15) | - | - | - | - | 0.43 | 0.15 |
| 52 | 22.67 | (Z)-Lanceol (C15) | - | - | - | - | 0.39 | - |
| 53 | 24.85 | β-Selinene (C15) | 2.28 | - | - | - | - | - |
| 54 | 26.04 | Hexahydrofarnesyl acetone | - | 1.80 | - | - | - | 0.21 |
| 55 | 26.41 | Diisobutyl phthalate | - | - | - | - | 0.81 | - |
| 56 | 28.24 | Butyl-octyl phthalate | - | - | - | - | 0.53 | 0.56 |
| 57 | 30.11 | Phytol (C20) | - | - | - | - | 0.73 | 8.90 |
| 58 | 32.53 | Heptacosane (alkane) | - | 2.02 | - | - | 0.22 | 0.71 |
| 59 | 33.43 | Octacosane (alkane) | - | 1.04 | - | - | 0.20 | 0.68 |
| Element | T. polium | T. hircanicum | T. chamaedrys | T. scordium | T. flavum | T. orientale | T. botrys | RP [40] |
|---|---|---|---|---|---|---|---|---|
| Na | 696 ± 55 | 195 ± 15 | 361 ± 25 | 1260 ± 7 | 388 ± 15 | 174 ± 7 | 715 ± 29 | 150 |
| Mg | 4350 ± 160 | 2430 ± 145 | 3260 ± 195 | 2670 ± 160 | 2350 ± 117 | 1700 ± 100 | 4270 ± 213 | 200 |
| Al | 5160 ± 64 | 336 ± 13 | 1810 ± 72 | 594 ± 24 | 1280 ± 64 | 490 ± 25 | 3090 ± 150 | 80 |
| Cl | 2580 ± 200 | 1850 ± 150 | 575 ± 45 | 9130 ± 730 | 538 ± 43 | 5420 ± 430 | 4130 ± 330 | 2000 |
| K | 18,700 ± 1100 | 17,700 ± 1060 | 14,900 ± 890 | 22,500 ± 1350 | 13,200 ± 1190 | 25,100 ± 2250 | 21,100 ± 1900 | 19,000 |
| Ca | 12,000 ± 1200 | 16,500 ± 1650 | 11,000 ± 1100 | 21,100 ± 2110 | 10,700 ± 1605 | 6610 ± 990 | 14,400 ± 2160 | 10,000 |
| Sc | 0.95 ± 0.05 | 0.063 ± 0.003 | 0.29 ± 0.01 | 0.145 ± 0.01 | 0.18 ± 0.007 | 0.081 ± 0.004 | 0.48 ± 0.01 | 0.02 |
| Mn | 132 ± 5 | 96 ± 3.8 | 74 ± 3 | 69 ± 2.9 | 54 ± 1.6 | 55.0 ± 1.7 | 97 ± 2.9 | 200 |
| Fe | 2520 ± 150 | 193 ± 19 | 760 ± 50 | 535 ± 40 | 473 ± 33 | 316 ± 22 | 1300 ± 78 | 150 |
| Co | 1.16 ± 0.08 | 0.48 ± 0.03 | 0.53 ± 0.03 | 0.265 ± 0.02 | 0.3 ± 0.02 | 0.48 ± 0.02 | 0.79 ± 0.04 | 0.2 |
| Zn | 34.4 ± 1.4 | 25.4 ± 1.1 | 20.2 ± 0.8 | 40 ± 1.6 | 30 ± 1.8 | 37.0 ± 2 | 50 ± 3 | 50 |
| As | 1.63 ± 0.06 | 0.94 ± 0.03 | 0.92 ± 0.03 | 1.34 ± 0.05 | 0.19 ± 0.01 | 0.091 ± 0.01 | 0.5 ± 0.05 | 0.1 |
| Br | 16.8 ± 0.7 | 11.3 ± 0.4 | 2.7 ± 0.1 | 8.5 ± 0.3 | 6.2 ± 0.2 | 21.3 ± 1 | 112 ± 4.5 | 4 |
| Rb | 22.5 ± 3.4 | 15.0 ± 2.2 | 9.4 ± 1.4 | 2.8 ± 0.9 | 5.8 ± 1.0 | 12.3 ± 2 | 13 ± 2.2 | 50 |
| Sr | 48.0 ± 4.8 | 36.0 ± 3.6 | 41 ± 4.1 | 118 ± 12 | 50 ± 4.0 | 26.0 ± 2.3 | 82 ± 6.6 | 50 |
| Mo | 0.43 ± 0.04 | 1.1 ± 0.1 | 0.41 ± 0.04 | 0.97 ± 0.09 | n.d. | n.d. | n.d. | 0.5 |
| Sb | 0.1 ± 0.007 | 0.037 ± 0.003 | 0.027 ± 0.002 | 0.039 ± 0.003 | 0.04 ± 0.004 | 0.043 ± 0.004 | 0.084 ± 0.007 | 0.1 |
| Cs | 0.4 ± 0.02 | 0.058 ± 0.003 | 0.12 ± 0.007 | 0.078 ± 0.005 | 0.08 ± 0.004 | 0.04 ± 0.004 | 0.21 ± 0.008 | 0.2 |
| Ba | 50.5 ± 4.5 | 7.9 ± 0.7 | 20.3 ± 1.8 | 28 ± 2.2 | 25 ± 1.5 | 7.7 ± 0.6 | 97 ± 4.8 | 40 |
| Element | Present Study Range | T. chamaedrys [51] | T. montanum [51] | T. chamaedrys [50] | T. polium [50] | T. montanum [50] |
|---|---|---|---|---|---|---|
| Mg | 2430–4350 | 1124 | 2826 | 1800 | 2031 | 3068 |
| K | 13,200–25,100 | 16,065 | 8267 | - | - | - |
| Ca | 6610–21,100 | 6557 | 3400 | 5668 | 7174 | 4856 |
| Mn | 54–132 | 46.7 | 42.3 | 26 | 40 | 46.4 |
| Fe | 193–2520 | 331 | 1266 | 725 | 735 | 898 |
| Co | 0.27–1.16 | - | - | 8.2 | 1.7 | 2.3 |
| Zn | 20–50 | 38 | 25 | 31 | 36 | 44 |
| As | 0.5–1.63 | - | - | 0.23 | 0.96 | 0.7 |
| a | T. botrys | T. orientale | T. flavum | T. polium | T. hircanicum | T. chamaedrys | |
|---|---|---|---|---|---|---|---|
| T. orientale | 0.493 | ||||||
| T. flavum | 0.657 | 1.000 | |||||
| T. polium | 0.976 | 0.093 | 0.164 | ||||
| T. hircanicum | 0.518 | 1.000 | 1.000 | 0.102 | |||
| T. chamaedrys | 0.882 | 0.995 | 1.000 | 0.359 | 0.996 | ||
| T. scordium | 0.829 | 0.998 | 1.000 | 0.293 | 0.999 | 1.000 | |
| b | |||||||
| T. orientale | 0.038 | ||||||
| T. flavum | 0.048 | 0.899 | |||||
| T. polium | 0.812 | 0.040 | 0.054 | ||||
| T. hircanicum | 0.048 | 0.704 | 0.937 | 0.064 | |||
| T. chamaedrys | 0.085 | 0.800 | 0.912 | 0.071 | 0.962 | ||
| T. scordium | 0.376 | 0.194 | 0.268 | 0.496 | 0.275 | 0.438 |
| Test-Microorganisms | Species, Double Successive Dilutions (Mbc, Mfc, Μg/Ml) | |||||
|---|---|---|---|---|---|---|
| TP | TH | TB | TCH | TF | TO | |
| Bacillus subtilis NCNM BB-01 (4.8 × 108 CFU/mL) | 600 | 300 | 300 | 300 | 300 | 600 |
| Pseudomonas fluorescens NCNM -PFB-01 (4.8 × 108 CFU/mL) | 300 | 150 | 600 | 150 | 600 | 600 |
| Xanthomonas campestris NCNM BX-01 (4.8 × 108 CFU/mL) | 600 | 300 | 600 | 150 | 300 | 600 |
| Erwinia amylovora NCNM BE-01 (4.8 × 108 CFU/mL) | 600 | 150 | 600 | 300 | 150 | 150 |
| Erwinia carotovora NCNM 177 BE-03 (4.8 × 108 CFU/mL) | 300 | 150 | 600 | 300 | 300 | 600 |
| Candida utilis NCNM Y-22 (3.0 × 107 CFU/mL) | 300 | 150 | 150 | 300 | 300 | 300 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciocarlan, A.; Dragalin, I.; Aricu, A.; Lupascu, L.; Ciocarlan, N.; Vergel, K.; Duliu, O.G.; Hristozova, G.; Zinicovscaia, I. Chemical Profile, Elemental Composition, and Antimicrobial Activity of Plants of the Teucrium (Lamiaceae) Genus Growing in Moldova. Agronomy 2022, 12, 772. https://doi.org/10.3390/agronomy12040772
Ciocarlan A, Dragalin I, Aricu A, Lupascu L, Ciocarlan N, Vergel K, Duliu OG, Hristozova G, Zinicovscaia I. Chemical Profile, Elemental Composition, and Antimicrobial Activity of Plants of the Teucrium (Lamiaceae) Genus Growing in Moldova. Agronomy. 2022; 12(4):772. https://doi.org/10.3390/agronomy12040772
Chicago/Turabian StyleCiocarlan, Alexandru, Ion Dragalin, Aculina Aricu, Lucian Lupascu, Nina Ciocarlan, Konstantin Vergel, Octavian G. Duliu, Gergana Hristozova, and Inga Zinicovscaia. 2022. "Chemical Profile, Elemental Composition, and Antimicrobial Activity of Plants of the Teucrium (Lamiaceae) Genus Growing in Moldova" Agronomy 12, no. 4: 772. https://doi.org/10.3390/agronomy12040772
APA StyleCiocarlan, A., Dragalin, I., Aricu, A., Lupascu, L., Ciocarlan, N., Vergel, K., Duliu, O. G., Hristozova, G., & Zinicovscaia, I. (2022). Chemical Profile, Elemental Composition, and Antimicrobial Activity of Plants of the Teucrium (Lamiaceae) Genus Growing in Moldova. Agronomy, 12(4), 772. https://doi.org/10.3390/agronomy12040772








