Abstract
The phytoremediation of industrial crops is becoming popular for the revitalization of land contaminated by trace elements (TEs). This approach combines biomass production with the improvement of soil health. To implement phytoremediation and derive sufficient dry biomass, crop production must be adequately supported by agricultural practices, including the application of bioinoculants. The current study aims to test the influence of several plant growth-promoting bacteria (PGPB), isolated from TEs-contaminated soil—i.e., Stenotrophomonas maltophilia KP-13, Bacillus altitudinis KP-14, and Pseudomonas fluorescens KP-16 and their consortia on the phytoremediation of the industrial crop M. × giganteus cultivated in the same TEs-contaminated soil. Contrary to expectations, the effects of PGPB on the biomass harvest were low. The most significant increase was detected in leaf biomass treated with a consortium of tested PGPBs. More significant effects were detected in the uptake of individual TEs. The phytoparameters of translocation factor, comprehensive bioconcentration index and uptake index were used to characterize the behavior of the TEs; Cr; Mn; Ni; Cu; Zn; Sr; V; and Pb in the presence of isolates. Plants treated with PGPB strains accumulated minimal concentrations of Cu and Pb in their aboveground biomass, while a tendency for Zn accumulation in the leaves and stems, and Sr accumulation in the leaves was observed. The obtained results reveal the combinations of isolates that lead to the minimal uptake of TEs into the stems and the simultaneous increase in DW. This study provides more insight into the leading factors of phytoremediation supported by PGPB and can be helpful when M. × giganteus is grown on TEs-contaminated soils of different origins.
1. Introduction
Soils are often seriously degraded and polluted due to industrial, agricultural, and civil activities [1]. The primary sources of soil contamination are waste disposal, industrial and commercial activities, and storage [2]. Soil deterioration can damage several soil functions and lead to the contamination of surface and groundwater; in parallel, soil pollution and degradation pose constant risks to human health, food security, and the environment [3]. Land pollution has been recognized as a significant threat to soil health, affecting the soil’s ability to provide ecosystem services and produce safe and sufficient food, thus compromising global food security [1].
The inventory established by the European Environmental Agency defined 2.8 million potentially contaminated sites across Europe [4]. Trace elements (TEs) (34.8%), followed by mineral oils (23.8%), and polycyclic aromatic hydrocarbons (PAHs) (10.9%), are the primary pollutants detected in soil. TEs do not degrade by biological and physical processes and, thus, are persistent in the soil and pose a long-term environmental threat [5]. They are inadvertently introduced into soils by mining and military actions, smelting, warfare, fossil fuel consumption, waste disposal, irrigation, and the application of agrochemicals [1]. The standard remediation techniques for such contaminated soils are systematized as in situ and ex situ methods [6]. These techniques involve physical, chemical, biological, electrical, and thermal processes for rectifying soil contamination by containment (e.g., surface capping, encapsulation, and landfilling), immobilization (e.g., solidification, stabilization), or extraction (e.g., phytoextraction, electrokinetic, soil flushing, soil washing) [6]. Among these methods, phytoremediation—the application of plants and associated microorganisms—is considered a promising, environmentally friendly method for remediating large sites with relatively low concentrations of contaminants at shallow depths [7]. Compared with other techniques, phytoremediation is cost-effective and involves ecological benefits in addition to experiencing high public acceptance; however, it is time-consuming and often not efficient. However, when the use of plants for remediation is combined with utilization of their biomass for energy or bioproducts, the process becomes more attractive and profitable and is commonly interpreted as phytomanagement [8].
Phytotechnologies can offer environmentally friendly solutions for the revitalization of soil, the improvement of food safety, carbon sequestration, and the development of renewable energy sources, all of which contribute to sustainable, land use management [9]. In recent years, industrial energy crops have been proposed as effective agents of phytotechnology that can produce high-quality biomass and be cultivated in marginal lands while simultaneously improving the soil quality [10,11]. Of these crops, Miscanthus spp. is an effective plant with sufficient biomass for conversion to energy and different bioproducts [10,12,13,14]. The crop is characterized by high yield, cold tolerance, C4 photosynthesis, non-invasiveness, and a low requirement for fertilizers and herbicides; easy to harvest and handle [15]. Thus, this crop can be used in the application of phytotechnology to marginal land that is slightly or moderately contaminated; simultaneously, it is assured that commercial production can be carried out in a sustainable way [14]. The production has to be intensified via the application of different agricultural practices [13], incorporation of soil amendments [16], treatment of planting materials with plant growth regulators (PGRs) [17], and bio-inoculants [18,19].
Plant growth-promoting bacteria (PGPB) can replace fertilizers and stimulate plant growth and yield. Using microorganisms instead of synthetic chemicals provides a non-hazardous approach to plant health for increasing agricultural productivity and limiting the adverse effects of disease [20]. The beneficial effects of the interaction between plant and PGPB can be direct and indirect [21]. A direct mechanism means that plant growth-promoting substances mobilize mineral soil components [22], which influences plant development [23]. An indirect mechanism reduces certain plant diseases caused by pathogens, mitigates abiotic stress, or induces systemic resistance in the context of competition for nutrients and niches [24]. The most common and successful PGPBs belong mainly to the genera Agrobacterium; Azospirillum; Azotobacter; Bacillus; Burkholderia; Pseudomonas; Streptomyces; and Serratia [20].
Earlier, we studied the effects of a PGPB—Bacillus altitudinis strain KP-14—isolated from soil contaminated by TEs post-mining in Všebořice, the Czech Republic, on the production of M. × giganteus in the same soil, the experiment was carried out in greenhouse conditions. The results illustrated the positive influence of bacteria on the biomass parameters of M. × giganteus at harvest [25]. Other PGPB strains were isolated from the same soil, and differences in their abiotic stress tolerance were determined [25]; however, they were not tested as a possible agent for enhancing the cultivation of M. × giganteus in TEs-contaminated post-mining soil. We also hypothesized that these strains and their consortia could cumulatively affect the phytoremediation process. These were the hypotheses we attempted to verify by the experimental study described in this paper.
Recently, a novel approach to the evaluation of data pertaining to the phytoremediation potential was proposed [26], which allows the definition of the inter-factor effects on a multi-component environment by using the comprehensive bio-concentration index (CBCI) and a predictive index. The validation of this approach on a larger scale was another goal of the current study. The acquired data were expected to reveal which are the leading factors affecting the phytotechnology of Miscanthus spp. applied to TEs-contaminated soil assisted by microbial isolates.
2. Materials and Methods
2.1. Soil Collection
The experimental soil was taken in Všebořice, the suburb quarter of Ústí nad Labem, the Czech Republic (50°42′11.9″ N 13°58′32.1″ E). The locality is a former open, brown coal mining site that operated between 1958 and 1980 and is currently used as a landfill site. The soil sampling was carried out in a 5 m × 5 m testing square using the standard approach DSTU 4287:2004 [27]; five soil samples were taken at a depth of 0–30 cm and mixed using the envelope method. The collected soil was dried until a constant weight was reached and then passed through a sieve with a pore diameter of 2 mm to remove plant material and stones. The initial soil was stored at 4 °C until use in the pot experiment. The agrochemical parameters of the initial soil are presented in Table S1 [28].
2.2. Design of Experiment
2.2.1. Selection of Bacterial Strains and Compatibility Testing
Three PGPB isolates, i.e., Stenotrophomonas maltophilia KP-13 (NCBI accession no: DQ113454), Bacillus altitudinis KP-14 (NCBI accession no: MF511821), and Pseudomonas fluorescens KP-16 (NCBI accession no: DQ178227) had earlier been isolated from Všebořice soil and generally characterized [25]. The strains are publicly available in the Czech Collection of Microorganisms (CCM; https://ccm.sci.muni.cz/en (accessed on 6 March 2022)). Additionally, plant growth-promoting traits, i.e., P, K, and Zn solubilization, IAA, ammonia, HCN, and siderophore production were measured and are presented in Table 1. Compatibility tests were performed according to the method described by Sonkar et al. [29], to ensure that individual bacterial growth was not affected by the presence of other bacteria (Figure S1). These bacterial isolates were streaked adjacent to each other on nutrient agar plates.

Table 1.
Plant growth-promoting characteristics exhibited by the selected isolates: KP-13, KP-14, and KP-16 (modified from Pranaw et al. [25]).
2.2.2. Treatment of M. × giganteus Rhizomes by Individual PGPB Isolates and Their Consortia
The rhizomes were treated before planting, following the methodology explained in detail in [25]. Briefly, one rhizome with two or more buds was soaked in a bacterial suspension (108 cells mL−1) of selected isolate for 4–5 h. The rhizome was subsequently removed from the suspension, soaked on filter paper, and immediately planted into the pot. Distilled water was used in the control experiment.
A complete factorial pot experiment was designed with seven different PGPB rhizome treatments, as follows:
| Treatments | Description |
| A - | M. × giganteus uninoculated (control); |
| B - | M. × giganteus inoculated by Stenotrophomonas maltophilia KP-13; |
| C - | M. × giganteus inoculated by Bacillus altitudinis KP-14; |
| D - | M. × giganteus inoculated by Pseudomonas fluorescens KP-16; |
| E - | M. × giganteus inoculated by the mixture of KP-13 and KP-14 in a 1:1 ratio; |
| F - | M. × giganteus inoculated by the mixture of KP-14 and KP-16 in a 1:1 ratio; |
| G - | M. × giganteus inoculated by the mixture of KP-13 and KP-14 and KP-16 in a 1:1:1 ratio. |
Each variation of the treatment was replicated six times for a total of 42 pots; 5 kg of soil was gently placed in the pots (each one had a volume of 7 L). The planting material was M. × giganteus J.M. Greef and Deuter ex Hodkinson (Angiospermae: Poaceae). The rhizomes were three years old and received from the firm HVG druzstvo Handels and Vertriebsgenossenschaft, Cheb, the Czech Republic (https://agronaro.eu/ (accessed on 6 March 2022)).
Pots of planted rhizomes were kept outside under natural conditions in the open-air laboratory of the Institute of Crop Production, Chomutov, the Czech Republic, until harvest. Irrigation was conducted by providing tap water at regular intervals to maintain soil moisture. The biological parameters of plant height and the number of shoots were monitored monthly. The pot experiment started on 6 May 2020 and finished on 28 October 2020, when the plants became yellow.
2.3. Samples Collection at Harvest
M. × giganteus aboveground biomass (AGB) and roots were harvested at the end of vegetation. The samples were collected following DSTU ISO 11464:2007 [30]. The roots were thoroughly washed with running tap water to get rid of soil particles and then air-dried at room temperature until a constant weight; the cut AGB was dried similarly to the roots. The dry biomass weight (DW) was calculated for leaves, stems, and roots separately, following the procedure described in [16]. Each leaf, stem, and root sample was separately collected in a labelled plastic zip-lock bag and stored at room temperature until the chemical analysis was undertaken.
2.4. Analysis of TEs Content in Soil and Biomass
The soil samples were prepared for analysis according to DSTU ISO 11464:2007 [30] and explained in detail in [31]. Briefly, the soil sample was dried at 105 °C to a constant mass. The dry sample was put on a clean sheet of paper, and small stones, plant particles, and other inclusions were removed; larger soil clods were ground in a porcelain mortar and mixed with the main soil. Thoroughly mixed soil was placed on clean, square paper and divided into four equal parts using a spatula. Two opposing parts were discarded, and the remaining two were combined, remixed, and taken for further analysis. This average sample was additionally sieved (0.25 mm pore size), and larger particles were milled if necessary. The preparation of biomass samples (roots, stems, and leaves) was achieved following the standard DSTU ISO 11465-2001 [32].
The measurement of TEs content in soil and biomass was carried out using X-ray fluorescence analysis via an Elvax Light SDD Analyzer, Elvatech, Kyiv, Ukraine. The biomass samples were combusted at 400 °C for 4 h, cooled for 1 h in desiccators, weighed, and processed for analysis.
The device can detect chemical elements in a range of 11Na to 92U with high accuracy (0.01%). The time of data collection was 2 × 180 s for all samples. The limits of the absolute measuring error were ±0.05–0.2% (with the time for one measurement being 180 s). Three parallel measurements were undertaken for each sample. The concentration of TEs in the soil samples was determined in mg kg−1. The concentration of TEs in the biomass samples was determined in mass units in the ash and then recalculated into mg kg−1 based on the ash content of the initial biomass material. The concentration of TEs in the samples was expressed in mg kg−1 dry weight. For soil analysis, the samples (~2 g) were placed on ultra-thin (4 μm) polypropylene film (supplied with the device), which is transparent to X-rays, and accurately transferred into the device, where measurement was performed. For the biomass, combusted samples (ash) of roots, stems, and leaves (~0.5 g) were placed inside a plastic ring with a diameter of 1.25 cm, which was located on a similar thin polypropylene film, compacted using a glass rod, and then transferred into the device for measuring.
The measured concentrations of TEs in the research soil are presented in Table 2, and in the biomass in Table S1. A comparison of the TEs concentrations in the soil used in the current experiment (2020) and the previous experiment (2019) [31] is presented in Figure S2.

Table 2.
TEs’ concentrations in the research soil; depth of soil sampling: 0–30 cm.
2.5. Estimation of Phytoremediation Coefficients
The translocation factor (TLF) is the ratio of TE concentration in the plant aboveground biomass (AGB) (leaves and stems) to TE concentration in the roots and was calculated using the following equation [35]:
The comprehensive bioconcentration index (CBCI) is a predictive index used to assess the capacity for multi-TE accumulation of plants. The index was calculated using the following equation [26]:
where n is the total number of TEs, and i is a particular TE.
The bioconcentration factor (BCF) requested for CBCI estimation was calculated using the following equation [36]:
Uptake index (UI) is the total content of the TEs in the plant tissues (in milligrams) and was calculated using the formula [37]:
2.6. Statistical Analysis
Statistical data processing was conducted using RStudio software (version 1.3.959, R Studio PBC, 2020). First, a Two-Way repeated-measures analysis of variance (Two-Way RM ANOVA) was carried out to compare the changes in the growth dynamic (height) of sample groups during the experiment. One-Way ANOVA was performed to determine the significant difference between the plant’s physiological parameters (dry weight of leaves, stems, roots), TEs’ concentrations in soils, and TEs’ concentrations in plant tissues under different treatments. Then, when ANOVA identified a significant difference, Tukey HSD tests were performed for pairwise comparison of means. Finally, based on the Tukey HSD test, treatments were categorized (by letter, in descending gradation), and graphs were created. Significance was declared at p < 0.05.
3. Results and Discussion
3.1. Plant Physiological Parameters
The growth dynamics of M. × giganteus in TEs-contaminated soil in the presence of PGPB isolates and their consortia are presented in Figure 1. The graph shows that the majority of isolates did not significantly affect plant height, except P. fluorescens KP-16 (D treatment), which had an inhibiting effect. In the presence of S. maltophilia KP-13 (B), the consortium of B. altitudinis KP-14 and Pseudomonas fluorescens KP-16 (F), and a consortium of all studied isolates (G), the dynamics of growth for the inoculated plants were similar to those of non-inoculated plants. When the plant was treated with a consortium of S. maltophilia KP-13 and B. altitudinis KP-14 (E), its growth was linear, with equal increases in height. Up to September, the same linear growth tendency was observed under treatment D (P. fluorescens KP-16); however, in October, the growth slightly increased (Figure 1).
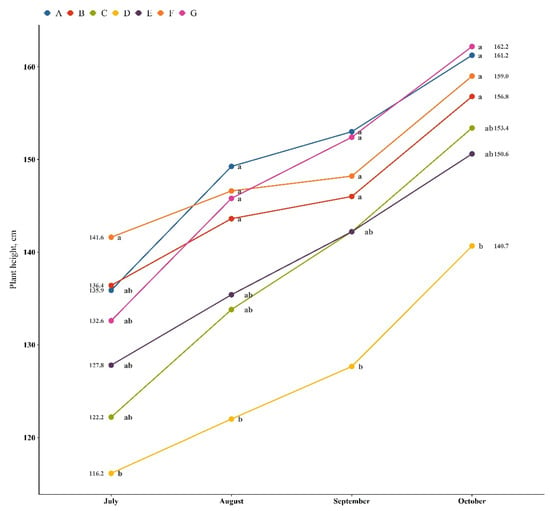
Figure 1.
The growth dynamic of M. × giganteus in TEs-contaminated soil supported by PGPB isolates. Different letters indicate the significant difference between treatments.
Nonetheless, the visible discrepancy related to the effectiveness of using the selected PGPB isolates for the enhancement of biomass was as expected. Usually, when PGPB isolates are upscaled from lab to field experiments, their utilization encounters several obstacles [38,39,40,41]. Biological and environmental factors, such as soil acidity, the application of pesticides and fertilizers in the background field, and the presence of salts and toxic elements, begin to have a greater impact. Additionally, new influencing factors appear, such as waterlogging and drought, extreme annual and diurnal variations in soil temperature, and adaptability to native soil microflora. In order to select isolates that are potentially more applicable under field conditions, an outdoor semi-field experiment was arranged using pots that were larger than those in the lab experiment [26]. This permitted us to mimic field conditions and evaluate the quality and efficacy of all isolated PGPBs (Table 2).
The values of M. × giganteus DW (in leaves, stems, and roots) after PGPB treatment are shown in Figure 2. It can be concluded that upon individual application (B, C, and D), the leaf biomass increased compared with the control (A) and was the highest under treatment C (Figure 2a). Upon treatment with the consortia of PGPBs (E, F, and G), the increase in leaf DW was greater in comparison with individual treatments (B, C, and D). The most effective combination was treatment E (a consortium of S. maltophilia KP-13 and B. altitudinis KP-14). However, the treatment of plants either with individual PGPB or their consortia did not significantly affect the stem biomass, except for the consortium of S. maltophilia KP-13 and B. altitudinis KP-14, in which there was inhibition (Figure 2b).
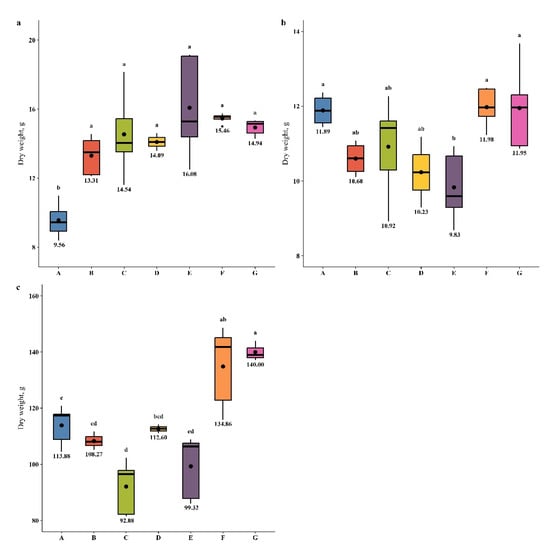
Figure 2.
M. × giganteus biomass productivity (DW) in the TEs-contaminated soil in the presence of PGPB strains for: (a) leaves; (b) stems; (c) roots. Different letters within one plant part indicate a significant difference between treatments.
Opposing results were recorded for the DW of roots (Figure 2c). The strongest effect was observed with combination G, and combination F showed a strong influence compared with individual treatments and the control. Treatment C increased the DW of M. × giganteus leaves (Figure 2a) but decreased the DW of roots (Figure 2c).
3.2. Phytoremediation Potential of M. × giganteus
Miscanthus spp. can be successfully cultivated in pure marginal soils, including those contaminated with TEs [11,42,43,44], oil products [16], pesticides [45], and a mixture of contaminants [46]. Various agricultural practices are utilized for enhancing plant development in pure soil, which include the incorporation of soil amendments (biochar [47]; biosolids and phosphates [48]; EDTA and citric acid [49]; and mycorrhizal fungi [50]), and the treatment of initial planting materials with plant growth regulators [17,51,52] and bioinoculants [25,26,53]. M. × giganteus growth in contaminated soil is accompanied by plant stress [54], and incorporating PGPBs can facilitate the adaptation of the plant to suboptimal soil conditions and promote plant advancement [25]. PGPBs produce different chemicals, amino acids, proteins, and antibiotics, which assist plants by resisting TEs’ toxicity [55,56] and can influence the phytoremediation process [57]. In our earlier study, we tested [28] the impact of the strain KP-14 on the phytoremediation of M. × giganteus in TEs-contaminated soil additionally enriched with Pb. This inoculant was selected based on its strong resistance to Pb [25].
Besides KP-14, other PGPB strains were isolated from Všebořice soil [25], and these can potentially impact the phytoremediation prospects, whether present individually or in the consortium (and thus possessing a cumulative effect).
3.2.1. Influence of PGPBs on Phytoremediation Parameters of Individual TEs
The calculated values of the coefficients: TLFs, UIs, and CBCIs for the studied TEs, i.e., Cr; Mn; Ni; Cu; Zn; Sr; V; and Pb, when M. × giganteus phytoremediation was conducted in the presence of inoculants are presented in Table S1. The results show that the Cu, Sr, and Mn accumulation in leaves significantly increased under treatment with individual PGPB and their consortia, while Zn and Pb accumulation decreased. In the case of Cu accumulation in leaves, a statistically significant increase (12%) was observed under treatment C, while the treatment with other PGPBs hardly affected the accumulation. Zn accumulation in the leaves significantly decreased by 16% and 15% in treatments F and G, respectively. Treatments B and D significantly increased the accumulation of Sr in leaves (by 13% and 16%, respectively). It can be assumed that B. altitudinis KP-14 can inhibit Sr accumulation in leaves since under consortia treatments (E, F, and G), no increased accumulation of this element was observed (Table S1). The Pb accumulation in leaves significantly decreased under individual treatments B and D, and consortium treatment F (by 17%, 20%, and 19%, respectively), while under treatment C, it slightly—but not significantly—increased, by 9%. This can explain why, in the presence of all three PGPBs (G), the Pb accumulation in leaves significantly increased by 16% (Table S1). The accumulation of Mn in leaves increased under individual treatment B and consortia treatment (F and G) by 28%, 21%, and 10%, respectively (Table S1).
The accumulation of Zn and Mn showed a similar tendency in both stems and leaves, which was opposite to that of Cu and Sr accumulation in stems (Table S1). The accumulation of Cu, Zn, and Sr in stems significantly decreased in almost all treatments (by 14–27%, 17–31%, and 23–49%, respectively), except for treatments F and D (for Cu only) (Table S1). Conversely, Mn accumulation in stems significantly increased in treatment D (by 14%), while a slight but not significant decrease was observed for this element in treatments B and E (Table S1).
All TEs initially present in the soil were detected in M. × giganteus roots (Table S1). Treatment with PGPBs mainly reduced TEs’ accumulation in the root system, an effect that was observed for V, Ni, Cu, Sr, and Mn (Table S1). Treatment C did not influence V accumulation in the roots, and its value was the same as for the control (A), though a significant decrease (by 26–55%) was detected for other treatments. Treatments D, F, and G increased Cr accumulation in the roots (by 85%, 88%, and 52%, respectively). In contrast, the accumulation of Sr under the same treatments was significantly decreased (by 33%, 27%, and 30%, correspondingly) (Table S1). Treatment D increased the accumulation of Ni in the roots (by 144%); however, treatments with consortia where P. fluorescens KP-16 was present (F and G) significantly decreased Ni accumulation (by 54% and 67%, respectively) (Table S1). All studied PGPBs reduced the Cu accumulation in the roots, but the most significant difference was observed under the treatments with consortia (E, F, and G) compared with non-inoculated plants (A) (28%, 35%, and 34%, respectively). The accumulation of Zn in the roots showed contrasting tendencies: treatment E (a consortium of S. maltophilia KP-13 and B. altitudinis KP-14) significantly increased Zn accumulation by 32%, while treatment G (a consortium of three PGPBs) decreased Zn accumulation by 41% (Table S1). Pb accumulation in the roots was not significantly affected by the treatment with PGPBs compared to the control (A); nevertheless, some differences were observed between treatment C (higher Pb accumulation compared to control) and treatments E and G (lower accumulation compared to control). Treatments with individual PGPBs and their consortia significantly decreased Mn accumulation in the roots by 20% to 51% (Table S1), with the exception being treatment B.
3.2.2. Comparison of Phytoremediation Parameters
The comparison of the phytoremediation assessed in the current study (2020 experiment) and an earlier study (2019 experiment) [25] is illustrated in Figure S2. The results show that the TLF values were higher in the 2020 experiment for almost all the TEs, while the levels of soil contamination by TEs were similar. The major differences between the designs of the 2020 and 2019 experiments were pot size. In 2019, a mesocosm study was conducted using pots containing 2 kg of soil (25 cm in height and 20 cm in diameter), whereas, in 2020, the pots contained 5 kg of soil (26 cm in height and 23 cm in diameter). Roots play a major role in the phytoremediation potential of any plant, and root development is typically impeded in small pots with low soil quantities [58,59]. When the plants were inoculated with PGPBs, their phytoremediation abilities were directly facilitated by increased root density [60,61]. In the 2020 experiment, the root biomass development was improved in the larger pots; as a result, it was around 3–4.5 times higher than in the 2019 experiment. This may be one of the main reasons for the increase in TLF values in the 2020 experiment.
The treatment of M. × giganteus with PGPBs and their consortia forced Cu, Sr, and Mn translocations from roots to leaves (Figure 3a). In the case of Zn translocation, when PGPBs were applied individually, three different behaviors were examined: treatment B increased the element translocation while treatment C had no effect, and treatment D caused a decrease. The most significant decrease in Zn translocation was observed under the treatment with the consortium (E); however, applying the same isolates separately did not cause a reduction. The translocation of Pb to leaves decreased in treatments A-D; however, it increased under the E and G treatments (by 1.3 and 1.6 times, respectively).
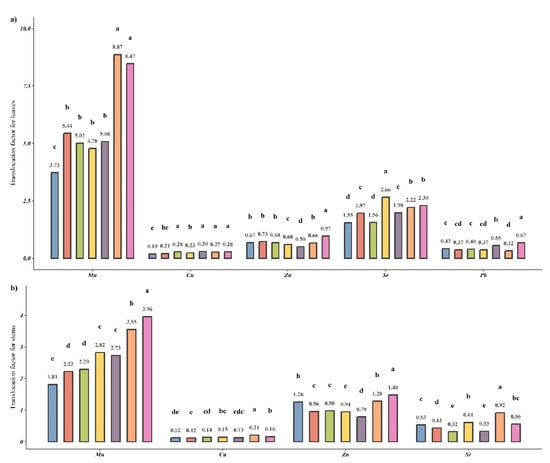
Figure 3.
TLFs of TEs accumulated in the AGB of M. × giganteus during growth in TEs-contaminated soil in the presence of PGPB strains for (a) leaves and (b) stems. Different letters within one TE indicate a significant difference between treatments.
Cu translocation into stems was slightly enhanced in treatments C (B. altitudinis KP-14) and D (P. fluorescens KP-16), while the application of these isolates in the consortium (F) increased translocation 1.8-fold. Contrarily, S. maltophilia KP-13 did not affect Cu translocation from roots to stems under any treatment in which this strain was presented, and it inhibited the influence of other PGPBs (Figure 3b). Zn translocation was reduced or remained the same under all treatments except G, wherein a slight increase was detected. Treatment D enhanced Sr translocation to AGB (Figure 3a,b), and Mn translocation increased gradually from A to G treatments.
3.2.3. Accessing Multi-Accumulation of TEs
The predictable comprehensive bioconcentration indexes (CBCI) were calculated following [26] to assess the capacity for multi-TEs accumulation in M. × giganteus under various PGPB treatments. The results, presented in Figure 4, show that in the case of treatments B, C, and E, it was possible to obtain less contaminated stem biomass, and this, therefore, has the potential to be utilized in bioproducts [62].
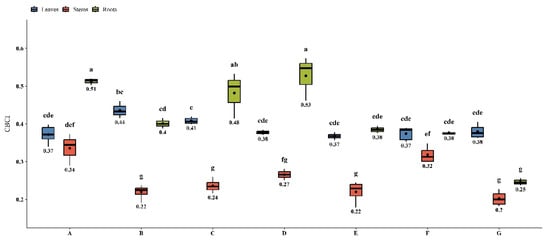
Figure 4.
Comprehensive bio-concentration indexes of M. × giganteus grown in TEs-contaminated soil in the presence of PGPB strains. Different letters indicate a significant difference between treatments.
For assessment of the ability of M. × giganteus to remediate contaminated soil at the field scale, the UI values were determined; the obtained results are presented in Table S2. The following tendencies were observed: treatment with individual PGPBs and their consortia increased the TEs’ accumulation in leaves and decreased it in stems. As such, the studied PGPBs can be utilized to enhance the phytoextraction of multi-TEs in the leaves with simultaneous production of less-contaminated stems. The potential quantity of TEs that can be extracted from one hectare of Všebořice soil in one year of cultivation was estimated and is presented in Figure 5. The results show that M. × giganteus seldom accumulates elements in its AGB; for studied TEs only, Zn accumulated in leaves and stems, and Sr accumulated in leaves. In contradiction to this, the plant accumulated minimal concentrations of Cu and Pb in its AGB, which was well developed, and as such, when Pb and Cu were the predominant contaminants in the target soil, the produced biomass can be safely recommended for conversion into bioproducts. The optimal treatment with PGPBs, resulting in enhanced phytoextraction and the production of less-contaminated stem biomass, is treatment E (a consortium of S. maltophilia KP-13 and B. altitudinis KP-14) (Figure 5).
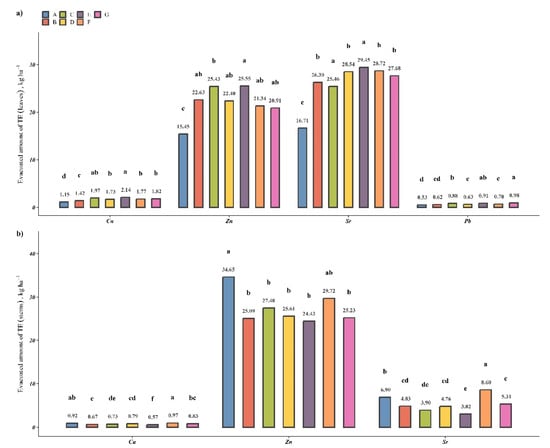
Figure 5.
The quantity of TEs potentially extracted by M. × giganteus from 1 ha in 1 year of cultivation for (a) leaves and (b) stems. Different letters within one TE indicate a significant difference between treatments.
4. Conclusions
Three PGPBs—Stenotrophomonas maltophilia KP-13, Bacillus altitudinis KP-14, and Pseudomonas fluorescens KP-16—isolated from TEs-contaminated soil in Všebořice, the Czech Republic, were tested individually and in consortia to determine their influence on the phytoremediation potential of M. × giganteus when crops were cultivated in the same contaminated soil. It was shown that the treatment of plants with a PGPBs’ consortium, comprising three test strains, enabled an increase in the produced biomass, and the effect was particularly apparent on the biomass of leaves and roots; however, this effect was only demonstrated to be statistically significant for the leaves. The phytoremediation behaviors of TEs detected in the soil—Cr; Mn; Ni; Cu; Zn; Sr; V; and Pb—were analyzed in terms of translocation factor, accumulation factor, and uptake by M. × giganteus. The treatment of plants with PGPBs and their consortia increased TEs’ accumulation in the leaves and decreased it in stems. When rhizomes were inoculated with Stenotrophomonas maltophilia KP-13, Bacillus altitudinis KP-14, and their consortia, less contaminated stem biomass was obtained. In the presence of the isolates, M. × giganteus showed a tendency to accumulate Zn in its leaves and stems, and Sr in its leaves. At the same time, plants treated with the strains accumulated minimal concentrations of Cu and Pb in their AGB, which were suitable for their processing into bioproducts. These results must be verified under the field conditions that prevail when M. × giganteus is produced commercially on a large scale.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy12040771/s1. Figure S1: Compatibility test to ensure that individual bacterial growth was not affected by the presence of other bacteria; Figure S2: Comparison of the TEs concentrations in control soil and soil treated with Bacillus altitudinis KP-14 from the 2019 and 2020 experiments. Different letters within one TE indicate a significant difference between experiments, Table S1: Measured TEs concentrations (mg kg−1) in M. × giganteus leaves, stems, and roots; Table S2: Uptake index of TEs (mg) in M. × giganteus leaves, stems, and roots calculated for a single plant.
Author Contributions
Conceptualization, V.P. and K.P.; methodology, K.P. and A.M.; software, A.M.; validation, V.P.; formal analysis, V.P., A.M., K.P. and P.S.; investigation, V.P., A.M., V.S. and K.P.; resources, V.P. and P.K.; data curation, V.S. and K.P.; writing—original draft preparation, V.P. and A.M.; writing—review and editing, V.P., A.M., K.P., V.S., P.K., J.T. and P.S.; visualization, A.M.; supervision, V.P.; project administration, V.P.; funding acquisition, V.P., P.K. and P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Czech-German project “MiscanValue - Cornet” (Reg. No. CZ.01.1.02/0.0/0.0/19_263/0018837) and CACTU (Reg. No. CZ.02.1.01/0.0/0.0/17049/0008397), co-financed by European Union from the European Regional Development Fund through the Operational Program Enterprise and Innovation for Competitiveness and Operational Program Research, Development, and Education, respectively.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Ethan Nicolas Duong, a former student of HTW Dresden and UJEP and Ing. Vojtech Vana, the Crop Research Institute for assistance during the Lab experiment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO and UNEP. Global Assessment of Soil Pollution: Report; FAO and UNEP: Rome, Italy, 2021; p. 846. [Google Scholar] [CrossRef]
- Janus, A.; Pelfrêne, A.; Heymans, S.; Deboffe, C.; Douay, F.; Waterlot, C. Elaboration, Characteristics and Advantages of Biochars for the Management of Contaminated Soils with a Specific Overview on Miscanthus Biochars. J. Environ. Manag. 2015, 162, 275–289. [Google Scholar] [CrossRef]
- Vamerali, T.; Bandiera, M.; Mosca, G. Field Crops for Phytoremediation of Metal-Contaminated Land. A Review. Environ. Chem. Lett. 2010, 8, 1–17. [Google Scholar] [CrossRef]
- EEA. European Environment Agency. Progress in Management of Contaminated Sites, CSI 015, DK-1050; Publications Office of the European Union: Copenhagen, Denmark, 2007. [Google Scholar]
- Suman, J.; Uhlik, O.; Viktorova, J.; Macek, T. Phytoextraction of Heavy Metals: A Promising Tool for Clean-Up of Polluted Environment? Front. Plant Sci. 2018, 9, 1476. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Wu, L.-H.; Li, N.; Cui, L.-Q.; Li, Z.; Jiang, J.-P.; Jiang, Y.-G.; Qiu, X.-Y.; Luo, Y.-M. Effect of planting densities on yields and zinc and cadmium uptake by Sedum plumbizincicola. Huan Jing Ke Xue Huanjing Kexue 2009, 30, 3422–3426. [Google Scholar]
- Evangelou, M.W.H.; Papazoglou, E.G.; Robinson, B.H.; Schulin, R. Phytomanagement: Phytoremediation and the Production of Biomass for Economic Revenue on Contaminated Land. In Phytoremediation: Management of Environmental Contaminants, Volume 1; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 115–132. ISBN 978-3-319-10395-2. [Google Scholar]
- Pandey, V.C.; Bauddh, K. Phytomanagement of Polluted Sites: Market Opportunities in Sustainable Phytoremediation; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 978-0-12-813913-4. [Google Scholar]
- Mench, M.; Lepp, N.; Bert, V.; Schwitzguébel, J.-P.; Gawronski, S.W.; Schröder, P.; Vangronsveld, J. Successes and Limitations of Phytotechnologies at Field Scale: Outcomes, Assessment and Outlook from COST Action 859. J. Soils Sediments 2010, 10, 1039–1070. [Google Scholar] [CrossRef]
- Nsanganwimana, F.; Pourrut, B.; Mench, M.; Douay, F. Suitability of Miscanthus Species for Managing Inorganic and Organic Contaminated Land and Restoring Ecosystem Services. A Review. J. Environ. Manag. 2014, 143, 123–134. [Google Scholar] [CrossRef]
- Rusinowski, S.; Krzyżak, J.; Sitko, K.; Kalaji, H.M.; Jensen, E.; Pogrzeba, M. Cultivation of C4 Perennial Energy Grasses on Heavy Metal Contaminated Arable Land: Impact on Soil, Biomass, and Photosynthetic Traits. Environ. Pollut. 2019, 250, 300–311. [Google Scholar] [CrossRef]
- Lewandowski, I.; Clifton-Brown, J.; Trindade, L.M.; van der Linden, G.C.; Schwarz, K.-U.; Müller-Sämann, K.; Anisimov, A.; Chen, C.-L.; Dolstra, O.; Donnison, I.S.; et al. Progress on Optimizing Miscanthus Biomass Production for the European Bioeconomy: Results of the EU FP7 Project OPTIMISC. Front. Plant Sci. 2016, 7, 1620. [Google Scholar] [CrossRef] [Green Version]
- Hauptvogl, M.; Kotrla, M.; Prčík, M.; Pauková, Ž.; Kováčik, M.; Lošák, T. Phytoremediation Potential of Fast-Growing Energy Plants: Challenges and Perspectives—A Review. Pol. J. Environ. Stud. 2019, 29, 505–516. [Google Scholar] [CrossRef] [Green Version]
- Erickson, L.E.; Pidlisnyuk, V. Phytotechnology with Biomass Production: Sustainable Management of Contaminated Sites, 1st ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2021; p. 242. ISBN 978-0-367-52280-3. [Google Scholar] [CrossRef]
- Bilandžija, N.; Jurišić, V.; Voća, N.; Leto, J.; Matin, A.; Grubor, M.; Krička, T. Energy Valorization of Miscanthus × giganteus Biomass: A Case Study in Croatia. J. Process. Energy Agric. 2017, 21, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Pidlisnyuk, V.; Herts, A.; Khomenchuk, V.; Mamirova, A.; Kononchuk, O.; Ust’ak, S. Dynamic of Morphological and Physiological Parameters and Variation of Soil Characteristics during Miscanthus × giganteus Cultivation in the Diesel-Contaminated Land. Agronomy 2021, 11, 798. [Google Scholar] [CrossRef]
- Nebeská, D.; Pidlisnyuk, V.; Stefanovska, T.; Trögl, J.; Shapoval, P.; Popelka, J.; Cerný, J.; Medkow, A.; Kvak, V.; Malinská, H. Impact of Plant Growth Regulators and Soil Properties on Miscanthus × giganteus Biomass Parameters and Uptake of Metals in Military Soils. Rev. Environ. Health 2019, 34, 283–291. [Google Scholar] [CrossRef]
- Liu, S.; Liu, H.; Chen, R.; Ma, Y.; Yang, B.; Chen, Z.; Liang, Y.; Fang, J.; Xiao, Y. Role of Two Plant Growth-Promoting Bacteria in Remediating Cadmium-Contaminated Soil Combined with Miscanthus floridulus (Lab.). Plants 2021, 10, 912. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, W.; Chao, Y.; Wang, S.; Tang, Y.-T.; Qiu, R.-L. Metal-Tolerant Enterobacter sp. Strain EG16 Enhanced Phytoremediation Using Hibiscus cannabinus via Siderophore-Mediated Plant Growth Promotion under Metal Contamination. Plant Soil 2017, 413, 203–216. [Google Scholar] [CrossRef]
- Fiodor, A.; Singh, S.; Pranaw, K. The Contrivance of Plant Growth Promoting Microbes to Mitigate Climate Change Impact in Agriculture. Microorganisms 2021, 9, 1841. [Google Scholar] [CrossRef]
- Naseem, H.; Ahsan, M.; Shahid, M.A.; Khan, N. Exopolysaccharides Producing Rhizobacteria and Their Role in Plant Growth and Drought Tolerance. J. Basic Microbiol. 2018, 58, 1009–1022. [Google Scholar] [CrossRef]
- Grover, M.; Bodhankar, S.; Sharma, A.; Sharma, P.; Singh, J.; Nain, L. PGPR Mediated Alterations in Root Traits: Way Toward Sustainable Crop Production. Front. Sustain. Food Syst. 2021, 4, 287. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Lata, C.; Tiwari, S.; Chauhan, A.S.; Mishra, S.K.; Agrawal, L.; Chakrabarty, D.; Nautiyal, C.S. Transcriptional Alterations Reveal Bacillus Amyloliquefaciens-Rice Cooperation under Salt Stress. Sci. Rep. 2019, 9, 11912. [Google Scholar] [CrossRef]
- Liu, J.; Fu, C.; Li, G.; Khan, M.N.; Wu, H. ROS Homeostasis and Plant Salt Tolerance: Plant Nanobiotechnology Updates. Sustainability 2021, 13, 3552. [Google Scholar] [CrossRef]
- Pranaw, K.; Pidlisnyuk, V.; Trögl, J.; Malinská, H. Bioprospecting of a Novel Plant Growth-Promoting Bacterium Bacillus altitudinis KP-14 for Enhancing Miscanthus × giganteus Growth in Metals Contaminated Soil. Biology 2020, 9, 305. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Xia, X.; Chu, J.; Wei, Y.; Shi, S.; Chang, E.; Yin, W.; Jiang, Z. The Evaluation of Heavy Metal Accumulation and Application of a Comprehensive Bio-Concentration Index for Woody Species on Contaminated Sites in Hunan, China. Environ. Sci. Pollut. Res. 2014, 21, 5076–5085. [Google Scholar] [CrossRef]
- DSTU 4287:2004. Soil Quality. Sampling; DP “UkrNDNC”: Kyiv, Ukraine, 2005; p. 9. [Google Scholar]
- Pidlisnyuk, V.; Mamirova, A.; Pranaw, K.; Shapoval, P.Y.; Trögl, J.; Nurzhanova, A. Potential Role of Plant Growth-Promoting Bacteria in Miscanthus × giganteus Phytotechnology Applied to the Trace Elements Contaminated Soils. Int. Biodeterior. Biodegrad. 2020, 155, 105103. [Google Scholar] [CrossRef]
- Sonkar, M.; Kumar, V.; Dutt, D. Use of Paper Mill Sludge and Sewage Sludge Powder as Nitrogen and Phosphorus Sources with Bacterial Consortium for the Treatment of Paper Industry Wastewater. Biocatal. Agric. Biotechnol. 2020, 30, 101843. [Google Scholar] [CrossRef]
- DSTU ISO 11464:2007. Soil Quality. Pretreatment of Samples for Physicochemical Analyses; DP “UkrNDNC”: Kyiv, Ukraine, 2009; p. 12. [Google Scholar]
- Pidlisnyuk, V.V.; Shapoval, P.; Zgorelec, Z.; Stefanovska, T.; Zhukov, O. Multiyear Phytoremediation and Dynamic of Foliar Metal(Loid)s Concentration during Application of Miscanthus × giganteus Greef et Deu to Polluted Soil from Bakar, Croatia. Environ. Sci. Pollut. Res. 2020, 27, 31446–31457. [Google Scholar] [CrossRef]
- DSTU ISO 11465-2001. Soil Quality. Determination of Dry Matter and Moisture Content by Mass Gravimetric Method (ISO 11465:1993, IDT); A.N. Sokolovsky Institute of Soil Science and Agrochemistry Ukrainian Academy of Agrarian Sciences: Kyiv, Ukraine, 2003. [Google Scholar]
- MEF Ministry of the Environment Finland. Government Decree on the Assessment of Soil Contamination and Remediation Needs (214/2007, 1 March 2007); Ministry of the Environment: Helsinki, Finland, 2007. [Google Scholar]
- MECR Ministry of the Environment of the Czech Republic. Decree Laying down Detailed Rules for the Protection of Quality of Agricultural Land and Amending Decree Specifying Some Details of Agricultural Land Resources Protection; Ministry of the Environment of the Czech Republic (MECR): Prague, Czech Republic, 2016. [Google Scholar]
- Yanqun, Z.; Yuan, L.; Jianjun, C.; Haiyan, C.; Li, Q.; Schvartz, C. Hyperaccumulation of Pb, Zn and Cd in Herbaceous Grown on Lead–Zinc Mining Area in Yunnan, China. Environ. Int. 2005, 31, 755–762. [Google Scholar] [CrossRef]
- Greger, M. Metal Availability and Bioconcentration in Plants. In Heavy Metal Stress in Plants: From Molecules to Ecosystems; Prasad, M.N.V., Ed.; Springer: Berlin/Heidelberg, Germay, 2004; pp. 1–27. ISBN 978-3-642-07268-0. [Google Scholar]
- Chorom, M.; Parnian, A.; Jaafarzadeh, N. Nickel Removal by the Aquatic Plant (Ceratophyllum demersum L.). Int. J. Environ. Sci. Dev. 2012, 3, 372. [Google Scholar] [CrossRef]
- Sessitsch, A.; Mitter, B. 21st Century Agriculture: Integration of Plant Microbiomes for Improved Crop Production and Food Security. Microb. Biotechnol. 2015, 8, 32–33. [Google Scholar] [CrossRef]
- Cardinale, M.; Ratering, S.; Suarez, C.; Zapata Montoya, A.M.; Geissler-Plaum, R.; Schnell, S. Paradox of Plant Growth Promotion Potential of Rhizobacteria and Their Actual Promotion Effect on Growth of Barley (Hordeum vulgare L.) under Salt Stress. Microbiol. Res. 2015, 181, 22–32. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Mazid, M.; Khan, T.A. Future of Bio-Fertilizers in Indian Agriculture: An Overview. Int. J. Agric. Food Res. 2015, 3, 10–23. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Stefanovska, T.; Lewis, E.E.; Erickson, L.E.; Davis, L.C. Miscanthus as a Productive Biofuel Crop for Phytoremediation. Crit. Rev. Plant Sci. 2014, 33, 1–19. [Google Scholar] [CrossRef]
- Alasmary, Z. Laboratory- to Field-Scale Investigations to Evaluate Phosphate Amendments and Miscanthus for Phytostabilization of Lead-Contaminated Military Sites. Ph.D. Thesis, Kansas State University, Manhattan, KS, USA, 2020. [Google Scholar]
- Šestak, I.; Bilandžija, N.; Perčin, A.; Fadljević, I.; Hrelja, I.; Zgorelec, Ž. Assessment of the Impact of Soil Contamination with Cadmium and Mercury on Leaf Nitrogen Content and Miscanthus Yield Applying Proximal Spectroscopy. Agronomy 2022, 12, 255. [Google Scholar] [CrossRef]
- Mamirova, A.; Pidlisnyuk, V.; Amirbekov, A.; Ševců, A.; Nurzhanova, A. Phytoremediation Potential of Miscanthus sinensis And. in Organochlorine Pesticides Contaminated Soil Amended by Tween 20 and Activated Carbon. Environ. Sci. Pollut. Res. 2021, 28, 16092–16106. [Google Scholar] [CrossRef]
- Nebeská, D.; Auer Malinská, H.; Erol, A.; Pidlisnyuk, V.; Kuráň, P.; Medžová, A.; Smaha, M.; Trögl, J. Stress Response of Miscanthus Plants and Soil Microbial Communities: A Case Study in Metals and Hydrocarbons Contaminated Soils. Appl. Sci. 2021, 11, 1866. [Google Scholar] [CrossRef]
- Guo, M.; Song, W.; Tian, J. Biochar-Facilitated Soil Remediation: Mechanisms and Efficacy Variations. Front. Environ. Sci. 2020, 8, 512512. [Google Scholar] [CrossRef]
- Alasmary, Z.; Hettiarachchi, G.M.; Roozeboom, K.L.; Davis, L.C.; Erickson, L.E.; Pidlisnyuk, V.; Stefanovska, T.; Trögl, J. Phytostabilization of a Contaminated Military Site Using Miscanthus and Soil Amendments. J. Environ. Qual. 2021, 50, 1220–1232. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, L.; Gu, J.; Zhao, J.; Fu, J. Citric Acid and EDTA on the Growth, Photosynthetic Properties and Heavy Metal Accumulation of Iris halophila Pall. Cultivated in Pb Mine Tailings. Int. Biodeterior. Biodegrad. 2018, 128, 15–21. [Google Scholar] [CrossRef]
- Damodaran, D.; Vidya Shetty, K.; Raj Mohan, B. Effect of Chelaters on Bioaccumulation of Cd (II), Cu (II), Cr (VI), Pb (II) and Zn (II) in Galerina vittiformis from Soil. Int. Biodeterior. Biodegrad. 2013, 85, 182–188. [Google Scholar] [CrossRef]
- Katelevsky, V. Efficiency of Influence of Foliar Treatment by Plant Growth Regulators on the Parameters of Miscanthus Biomass. Agrology 2020, 3, 19–24. [Google Scholar] [CrossRef]
- Ponomarenko, S.P.; Terek, O.I.; Grytsaenko, Z.M.; Babayants, O.V.; Moiseeva, T.V.; Wenxiu, H. Bioregulation of growth and development of plants: Plant growth regulators in crop science. In Bioregulation of Microbial-Plant Systems; Ponomarenko, S.P., Lutynska, H.O., Eds.; Nichlava: Kiev, Ukraine, 2010; pp. 251–291. [Google Scholar]
- Khan, W.-D.; Ramzani, P.M.A.; Anjum, S.; Abbas, F.; Iqbal, M.; Yasar, A.; Ihsan, M.Z.; Anwar, M.N.; Baqar, M.; Tauqeer, H.M.; et al. Potential of Miscanthus Biochar to Improve Sandy Soil Health, in Situ Nickel Immobilization in Soil and Nutritional Quality of Spinach. Chemosphere 2017, 185, 1144–1156. [Google Scholar] [CrossRef]
- Malinská, H.; Pidlisnyuk, V.; Nebeská, D.; Erol, A.; Medžová, A.; Trögl, J. Physiological Response of Miscanthus × giganteus to Plant Growth Regulators in Nutritionally Poor Soil. Plants 2020, 9, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, T.U.; Bano, A.; Naz, I. Alleviation of Heavy Metals Toxicity by the Application of Plant Growth Promoting Rhizobacteria and Effects on Wheat Grown in Saline Sodic Field. Int. J. Phytoremediation 2017, 19, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Oves, M.; Khan, M.S.; Zaidi, A. Chromium Reducing and Plant Growth Promoting Novel Strain Pseudomonas aeruginosa OSG41 Enhance Chickpea Growth in Chromium Amended Soils. Eur. J. Soil Biol. 2013, 56, 72–83. [Google Scholar] [CrossRef]
- Parihar, M.; Rakshit, A.; Chitara, M.K.; Jatav, H.S.; Rajput, V.D.; Singh, A.K.; Rana, K.; Jatav, S.S.; Anjum, M.; Minkina, T. Role of Plant-Associated Microbes in Phytoremediation of Heavy Metal Polluted Soils. In Heavy Metal Toxicity in Plants: Physiological and Molecular Adaptations; Aftab, T., Hakeem, K.R., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 157–169. ISBN 978-0-367-72507-5. [Google Scholar]
- Ghimire, B.; Hulbert, S.H.; Steber, C.M.; Garland-Campbell, K.; Sanguinet, K.A. Characterization of Root Traits for Improvement of Spring Wheat in the Pacific Northwest. Agron. J. 2020, 112, 228–240. [Google Scholar] [CrossRef]
- Poorter, H.; Bühler, J.; van Dusschoten, D.; Climent, J.; Postma, J.A.; Poorter, H.; Bühler, J.; van Dusschoten, D.; Climent, J.; Postma, J.A. Pot Size Matters: A Meta-Analysis of the Effects of Rooting Volume on Plant Growth. Funct. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.-D.; El-Alawi, Y.; Penrose, D.M.; Glick, B.R.; Greenberg, B.M. Responses of Three Grass Species to Creosote during Phytoremediation. Environ. Pollut. 2004, 130, 453–463. [Google Scholar] [CrossRef]
- Zainab, N.; Khan, A.A.; Azeem, M.A.; Ali, B.; Wang, T.; Shi, F.; Alghanem, S.M.; Hussain Munis, M.F.; Hashem, M.; Chaudhary, H.J. PGPR-Mediated Plant Growth Attributes and Metal Extraction Ability of Sesbania sesban L. in Industrially Contaminated Soils. Agronomy 2021, 11, 1820. [Google Scholar] [CrossRef]
- Pidlisnyuk, V.; Stefanovska, T.; Barbash, V.; Zelenchuk, T. Characteristics of Pulp Obtained from Miscanthus × giganteus Biomass Produced in Lead-Contaminated Soil. Cellul. Chem. Technol. 2021, 55, 271–280. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).