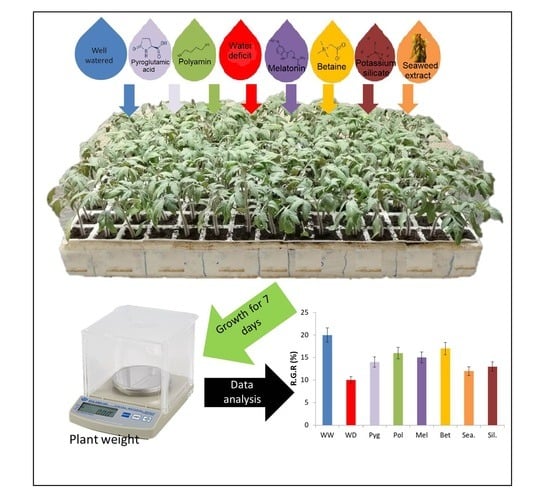
New Biostimulants Screening Method for Crop Seedlings under Water Deficit Stress
Abstract
:1. Introduction
2. Materials and Methods
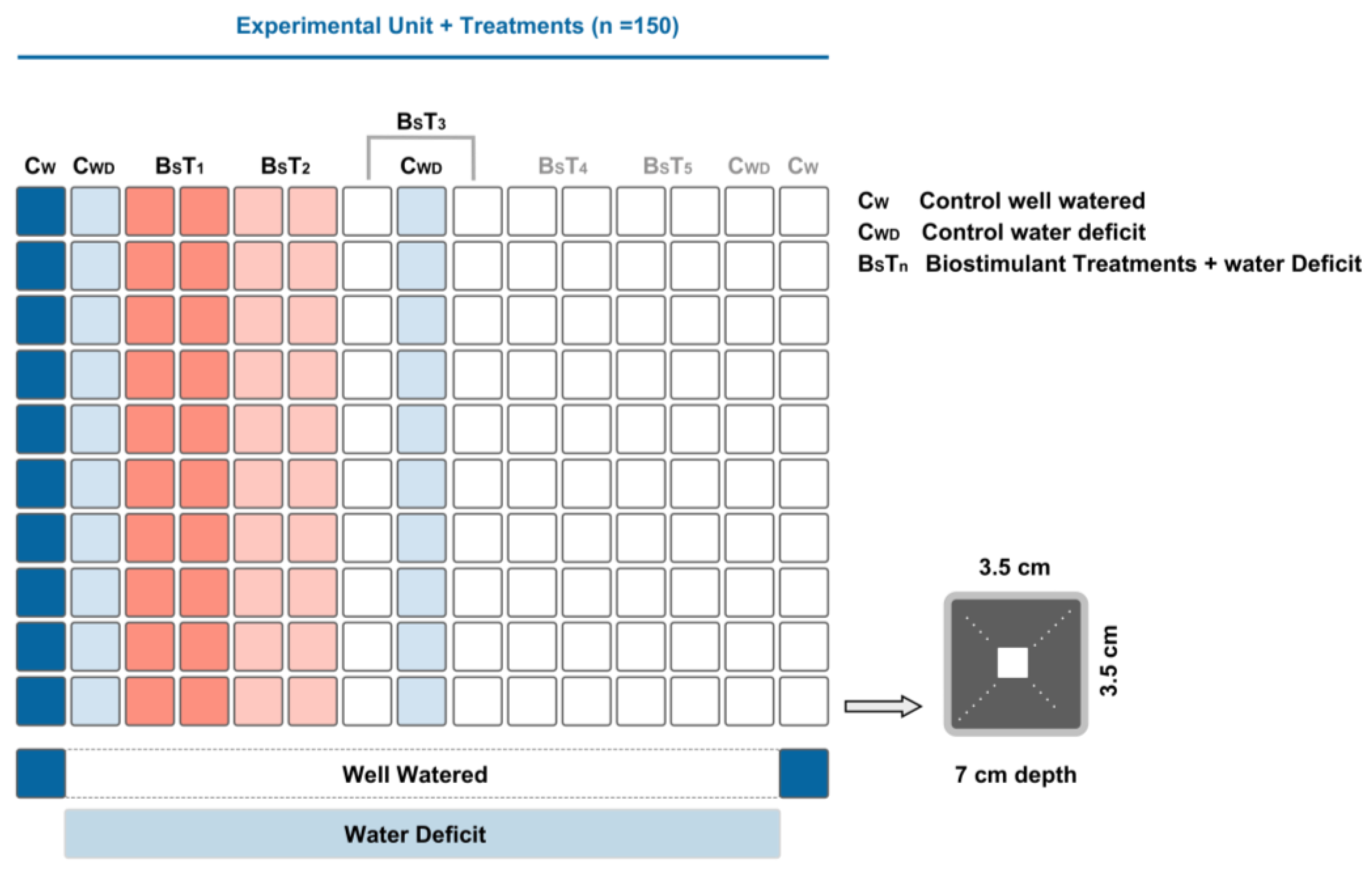
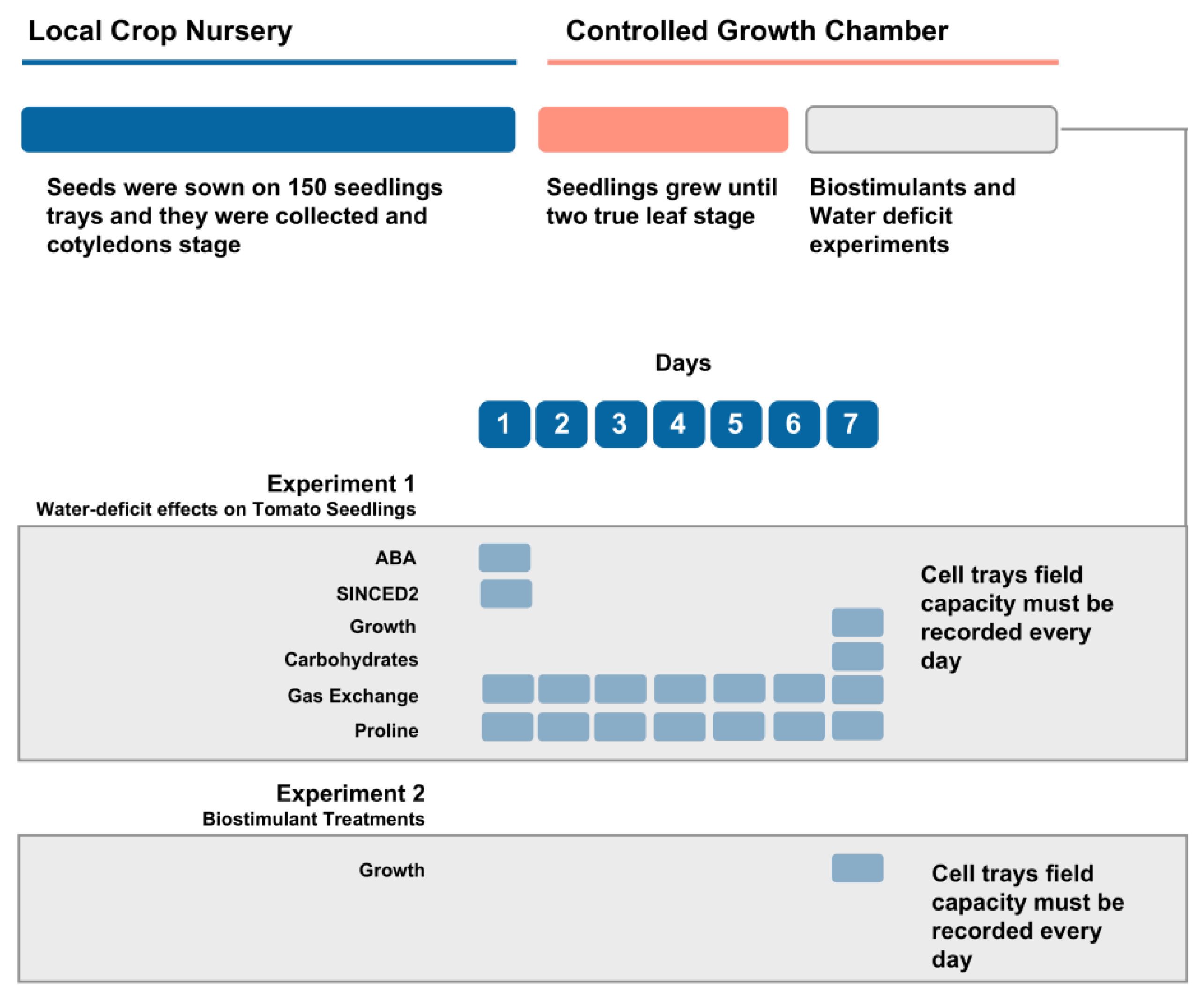
2.1. Plant Material and Experimental Conditions
2.2. Biostimulants Used in the Study
2.3. Growth and Gas Exchange Measurements
2.4. Biochemical and Molecular Measurements
2.5. Statistical Procedure
3. Results
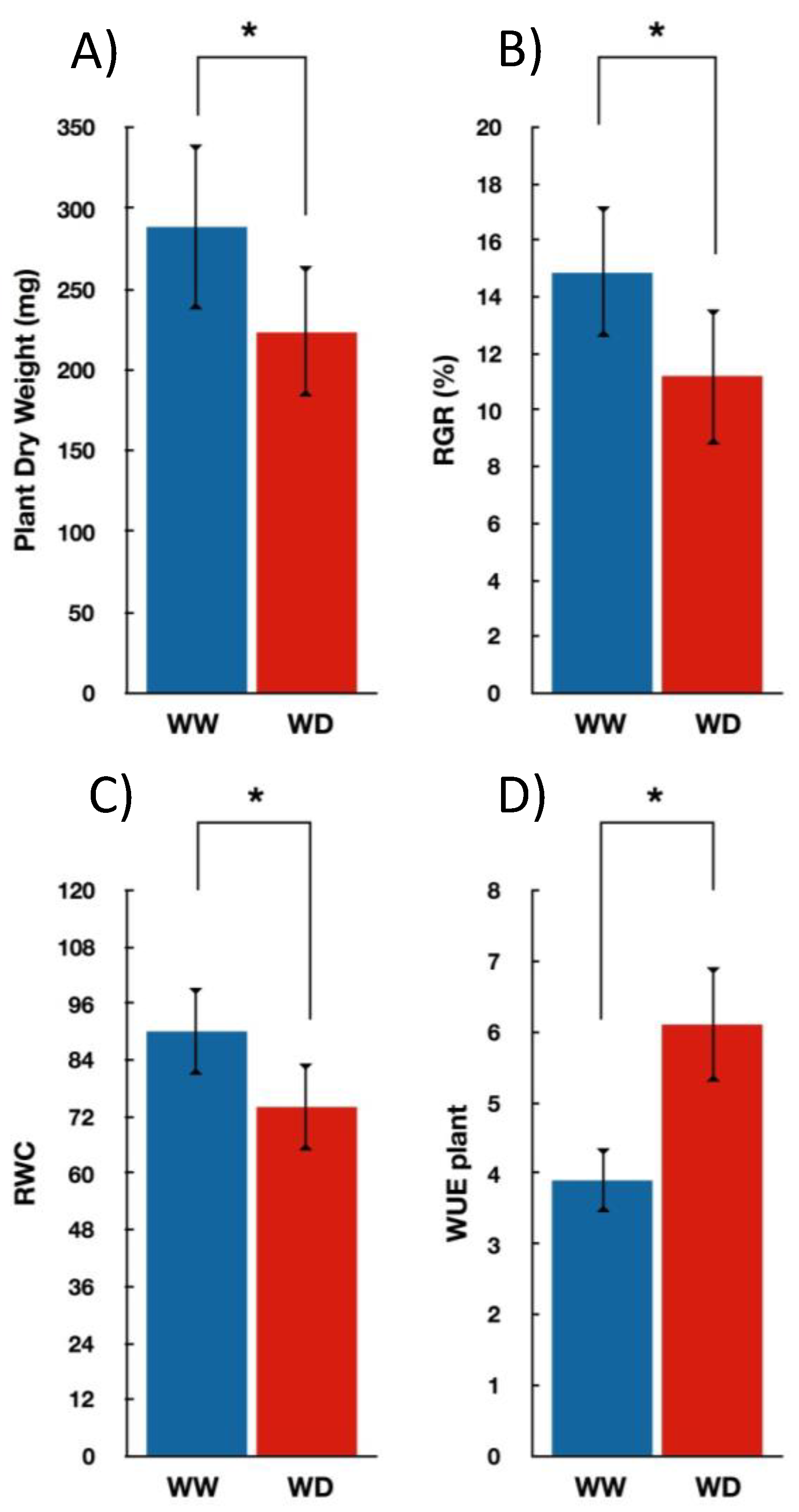
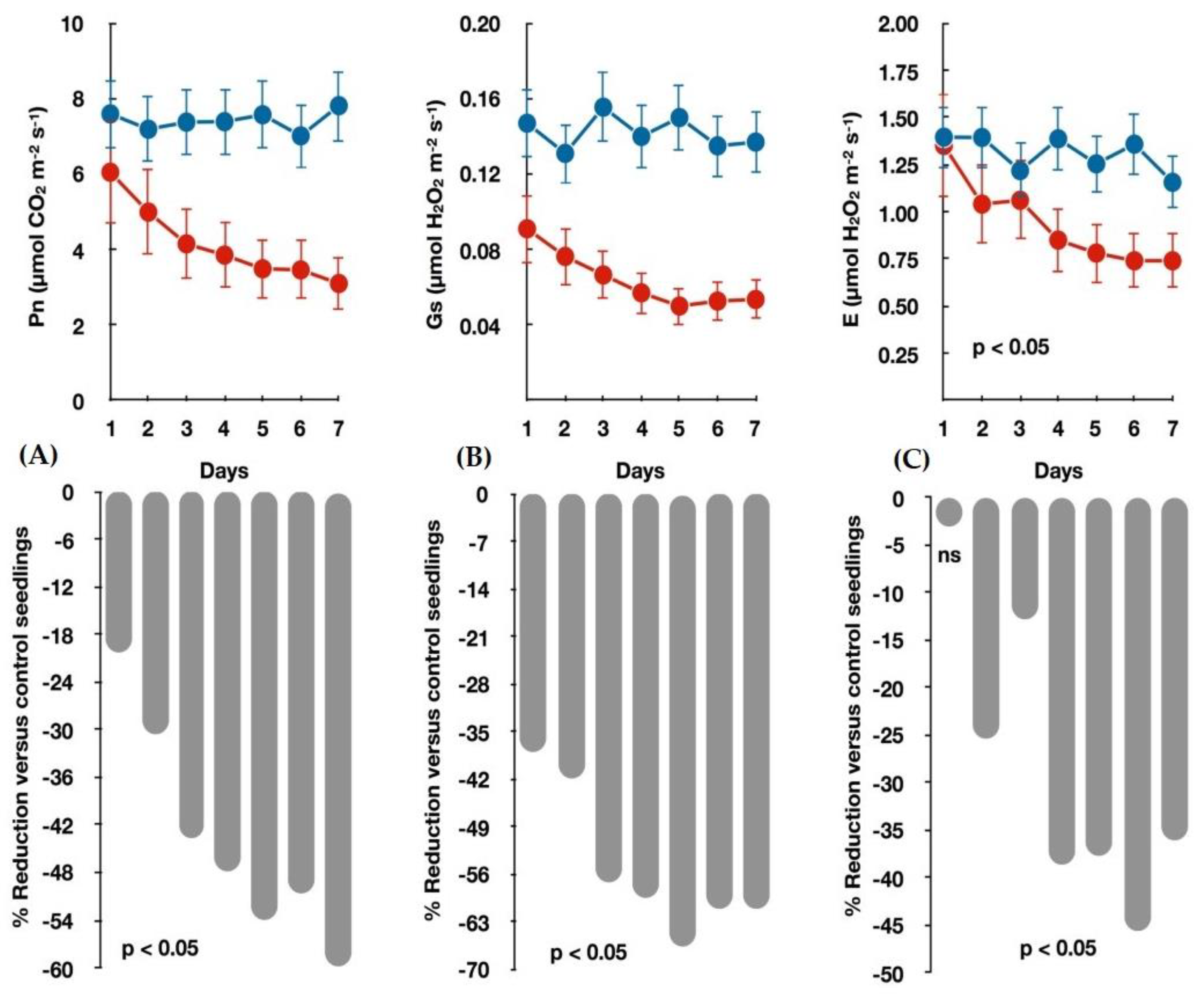
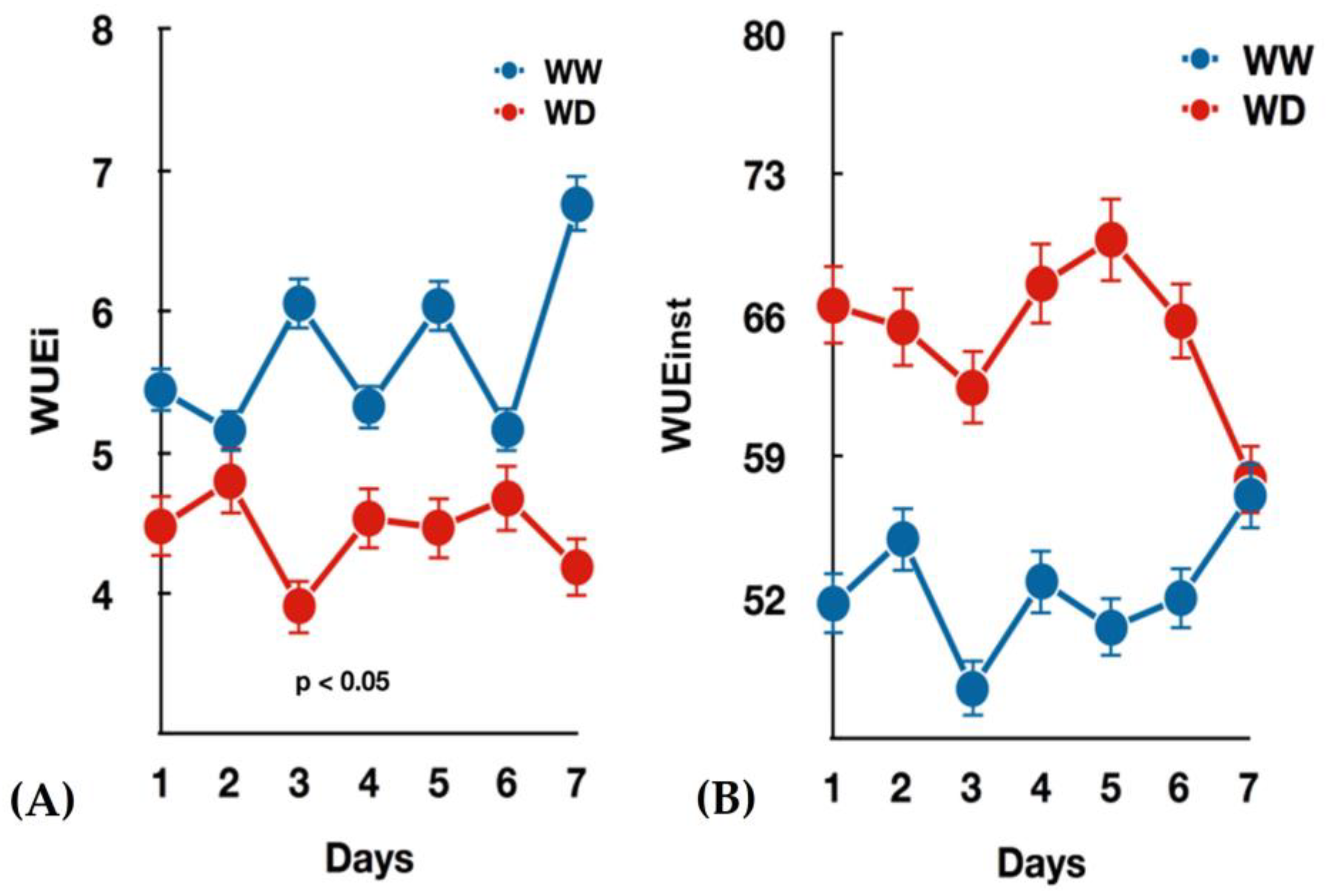
3.1. Seedlings Growth and Gas Exchange Measurements under Water-Deficit
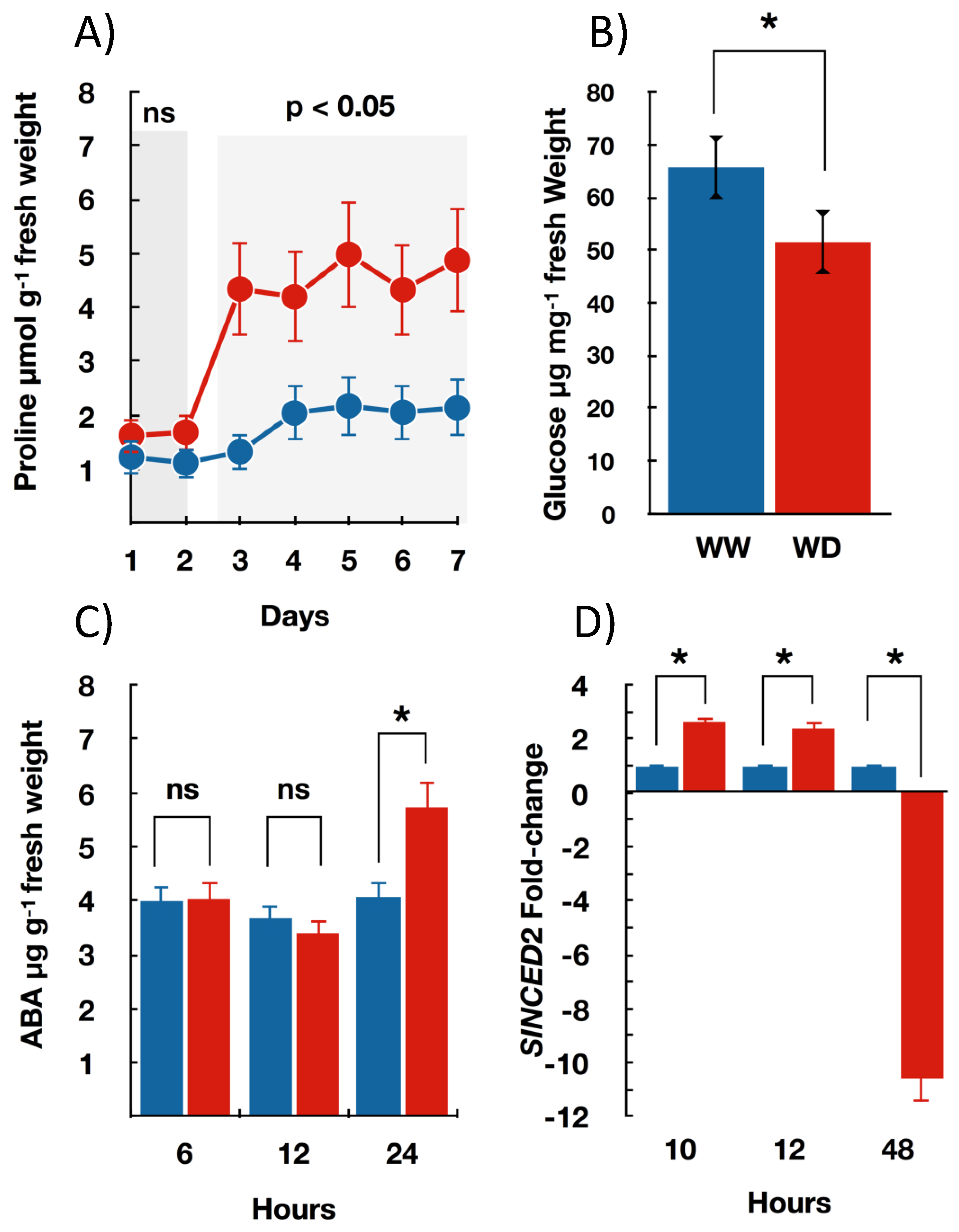
3.2. Seedlings Biochemical and Molecular Changes under Water Deficit
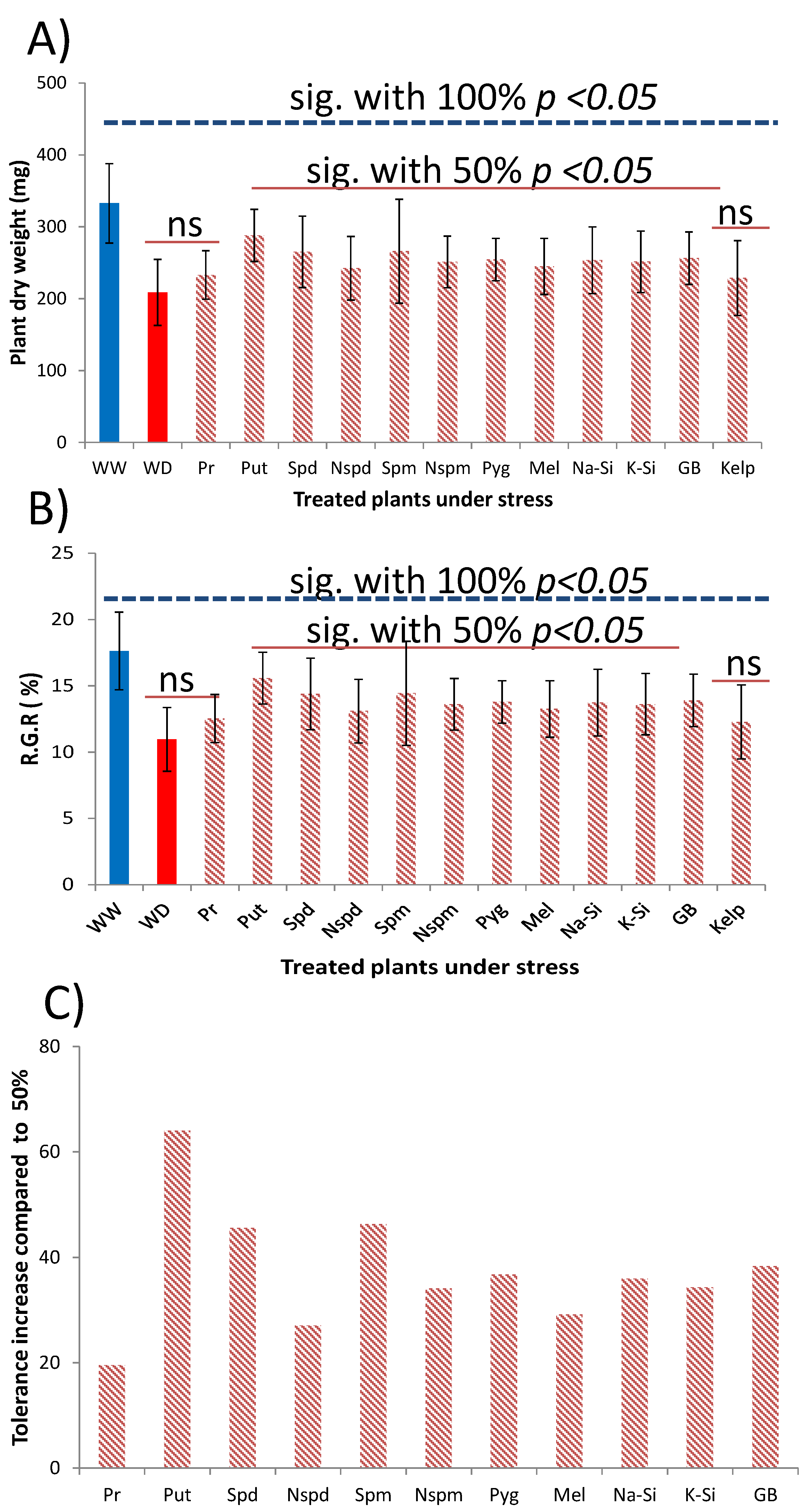
3.3. Method Simplification and Biostimulants Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- GAR Special Report on Drought. 2021. Available online: https://www.undrr.org/publication/gar-special-report-drought-2021 (accessed on 26 June 2021).
- Molotoks, A.; Smith, P.; Dawson, T.P. Impacts of land use, population, and climate change on global food security. Food Energy Secur. 2020, 10, e261. [Google Scholar] [CrossRef]
- Usman, M.; Farooq, M.; Wakeel, A.; Nawaz, A.; Alam Cheema, S.A.; Rehman, H.U.; Ashraf, I.; Sanaullah, M. Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total. Environ. 2020, 721, 137778. [Google Scholar] [CrossRef]
- Rahman, H.; Haque, K.S.; Khan, Z.H. A review on application of controlled released fertilizers influencing the sustainable agricultural production: A Cleaner production process. Environ. Technol. Innov. 2021, 23, 101697. [Google Scholar] [CrossRef]
- Mancosu, N.; Snyder, R.L.; Kyriakakis, G.; Spano, D. Water Scarcity and Future Challenges for Food Production. Water 2015, 7, 975–992. [Google Scholar] [CrossRef]
- Fischer, G.; Tubiello, F.N.; van Velthuizen, H.; Wiberg, D. Climate change impacts on irrigation water requirements: Effects of mitigation, 1990–2080. Technol. Forecast. Soc. Chang. 2007, 74, 1083–1107. [Google Scholar] [CrossRef] [Green Version]
- Eiovieno, P.; Punzo, P.; Eguida, G.; Emistretta, C.; Van Oosten, M.; Enurcato, R.; Bostan, H.; Ecolantuono, C.; Ecosta, A.; Ebagnaresi, P.; et al. Transcriptomic Changes Drive Physiological Responses to Progressive Drought Stress and Rehydration in Tomato. Front. Plant Sci. 2016, 7, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, M.; Skalicky, M.; Jahan, M.; Hossain, N.; Anwar, Z.; Nie, Z.; Alabdallah, N.; Brestic, M.; Hejnak, V.; Fang, X.-W. Spermine: Its Emerging Role in Regulating Drought Stress Responses in Plants. Cells 2021, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Zijian, X.; Jiachun, W.; Wentian, Z.; Tao, S.; Xiaohui, H. Abscisic acid alleviates harmful effect of saline–alkaline stress on tomato seedlings. Plant Physiol. Biochem. 2022, 175, 58–67. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, Z. Effects of endogenous abscisic acid, jasmonic acid, polyamines, and polyamine oxidase activity in tomato seedlings under drought stress. Sci. Hortic. 2013, 159, 172–177. [Google Scholar] [CrossRef]
- Lim, C.W.; Baek, W.; Jung, J.; Kim, J.-H.; Lee, S.C. Function of ABA in Stomatal Defense against Biotic and Drought Stresses. Int. J. Mol. Sci. 2015, 16, 15251–15270. [Google Scholar] [CrossRef] [Green Version]
- Ben Rejeb, K.; Abdelly, C.; Savouré, A. How reactive oxygen species and proline face stress together. Plant Physiol. Biochem. 2014, 80, 278–284. [Google Scholar] [CrossRef]
- Yang, R.; Yang, T.; Zhang, H.; Qi, Y.; Xing, Y.; Zhang, N.; Li, R.; Weeda, S.; Ren, S.; Ouyang, B.; et al. Hormone profiling and transcription analysis reveal a major role of ABA in tomato salt tolerance. Plant Physiol. Biochem. 2014, 77, 23–34. [Google Scholar] [CrossRef]
- García-García, A.L.; García-Machado, F.J.; Borges, A.A.; Morales-Sierra, S.; Boto, A.; Jiménez-Arias, D. Pure Organic Active Compounds Against Abiotic Stress: A Biostimulant Overview. Front. Plant Sci. 2020, 11, 1839. [Google Scholar] [CrossRef]
- Jiménez-Arias, D.; García-Machado, F.J.; Morales-Sierra, S.; García-García, A.L.; Herrera, A.J.; Valdés, F.; Luis, J.C.; Borges, A.A. A Beginner’s Guide to Osmoprotection by Biostimulants. Plants 2021, 10, 363. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.; Chaski, C.; Polyzos, N.; Petropoulos, S. Biostimulants Application: A Low Input Cropping Management Tool for Sustainable Farming of Vegetables. Biomolecules 2021, 11, 698. [Google Scholar] [CrossRef] [PubMed]
- Ben Mrid, R.; Benmrid, B.; Hafsa, J.; Boukcim, H.; Sobeh, M.; Yasri, A. Secondary metabolites as biostimulant and bioprotectant agents: A review. Sci. Total Environ. 2021, 777, 146204. [Google Scholar] [CrossRef]
- Andreotti, C. Management of Abiotic Stress in Horticultural Crops: Spotlight on Biostimulants. Agronomy 2020, 10, 1514. [Google Scholar] [CrossRef]
- Nephali, L.; Piater, L.A.; Dubery, I.A.; Patterson, V.; Huyser, J.; Burgess, K.; Tugizimana, F. Biostimulants for Plant Growth and Mitigation of Abiotic Stresses: A Metabolomics Perspective. Metabolites 2020, 10, 505. [Google Scholar] [CrossRef]
- Jiménez-Arias, D.; Morales-Sierra, S.; Borges, A.A.; Díaz, D.D. Biostimulant Nanoencapsulation: The New Keystone to Fight Hunger. J. Agric. Food Chem. 2020, 68, 7083–7085. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.D.E.S.; Oliveira, H.C.; Fraceto, L.F.; Santaella, C. Nanotechnology Potential in Seed Priming for Sustainable Agriculture. Nanomaterials 2021, 11, 267. [Google Scholar] [CrossRef]
- Jiménez-Árias, D.; García-Machado, F.J.; Morales-Sierra, S.; Garrido-Orduña, C.; Borges, A.A.; González, F.V.; Luis Jorge, J.C. Chapter 7-Vitamins and Environmental Stresses in Plants. In Plant Metabolites and Regulation Under Environmental Stress; Ahmad, P., Ahanger, M.A., Singh, V.P., Tripathi, D.K., Alam, P., Alyemeni, M.N., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 145–152. ISBN 978-0-12-812689-9. [Google Scholar]
- Jiménez-Arias, D.; Hernándiz, A.E.; Morales-Sierra, S.; García-García, A.L.; García-Machado, F.J.; Luis, J.C.; Borges, A.A. Applying Biostimulants to Combat Water Deficit in Crop Plants: Research and Debate. Agronomy 2022, 12, 571. [Google Scholar] [CrossRef]
- Shemi, R.; Wang, R.; Gheith, E.-S.M.S.; Hussain, H.A.; Hussain, S.; Irfan, M.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Effects of salicylic acid, zinc and glycine betaine on morpho-physiological growth and yield of maize under drought stress. Sci. Rep. 2021, 11, 3195. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Arias, D.; Machado, F.J.G.; Morales-Sierra, S.; Luis, J.C.; Suarez, E.; Hernández, M.; Valdés, F.; Borges, A.A. Lettuce plants treated with L-pyroglutamic acid increase yield under water deficit stress. Environ. Exp. Bot. 2018, 158, 215–222. [Google Scholar] [CrossRef] [Green Version]
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2020, 751, 141763. [Google Scholar] [CrossRef]
- Madende, M.; Hayes, M. Fish By-Product Use as Biostimulants: An Overview of the Current State of the Art, Including Relevant Legislation and Regulations within the EU and USA. Molecules 2020, 25, 1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No. 1069/2009 and (EC) No. 1107/2009 and Repealing Regulation (EC) No. 2003/2003 (Text with EEA Relevance). Volume 170. 2019. Available online: https://www.legislation.gov.uk/eur/2019/1009/contents (accessed on 13 February 2022).
- Campobenedetto, C.; Agliassa, C.; Mannino, G.; Vigliante, I.; Contartese, V.; Secchi, F.; Bertea, C. A Biostimulant Based on Seaweed (Ascophyllum nodosum and Laminaria digitata) and Yeast Extracts Mitigates Water Stress Effects on Tomato (Solanum lycopersicum L.). Agriculture 2021, 11, 557. [Google Scholar] [CrossRef]
- Turan, M.; Yildirim, E.; Ekinci, M.; Argin, S. Effect of Biostimulants on Yield and Quality of Cherry Tomatoes Grown in Fertile and Stressed Soils. HortScience 2021, 56, 414–423. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Gopalakrishnan, V.A.K.; Ghosh, A. Seaweed Biostimulants for Climate Change Adaptations in Dryland Agriculture in Semi-Arid Areas. In Climate Change Adaptations in Dryland Agriculture in Semi-Arid Areas; Poshiwa, X., Ravindra Chary, G., Eds.; Springer: Singapore, 2022; pp. 341–347. ISBN 9789811678615. [Google Scholar]
- Agliassa, C.; Mannino, G.; Molino, D.; Cavalletto, S.; Contartese, V.; Bertea, C.M.; Secchi, F. A new protein hydrolysate-based biostimulant applied by fertigation promotes relief from drought stress in Capsicum annuum L. Plant Physiol. Biochem. 2021, 166, 1076–1086. [Google Scholar] [CrossRef]
- Kaba, J.S.; Abunyewa, A.A.; Kugbe, J.; Kwashie, G.K.; Ansah, E.O.; Andoh, H. Arbuscular mycorrhizal fungi and potassium fertilizer as plant biostimulants and alternative research for enhancing plants adaptation to drought stress: Opportunities for enhancing drought tolerance in cocoa (Theobroma cacao L.). Sustain. Environ. 2021, 7, 1963927. [Google Scholar] [CrossRef]
- Goñi, O.; Quille, P.; O’Connell, S. Ascophyllum nodosum extract biostimulants and their role in enhancing tolerance to drought stress in tomato plants. Plant Physiol. Biochem. 2018, 126, 63–73. [Google Scholar] [CrossRef]
- Irani, H.; ValizadehKaji, B.; Naeini, M.R. Biostimulant-induced drought tolerance in grapevine is associated with physiological and biochemical changes. Chem. Biol. Technol. Agric. 2021, 8, 5. [Google Scholar] [CrossRef]
- Francesca, S.; Cirillo, V.; Raimondi, G.; Maggio, A.; Barone, A.; Rigano, M.M. A Novel Protein Hydrolysate-Based Biostimulant Improves Tomato Performances under Drought Stress. Plants 2021, 10, 783. [Google Scholar] [CrossRef]
- Sharma, S.; Chen, C.; Khatri, K.; Rathore, M.S.; Pandey, S.P. Gracilaria dura extract confers drought tolerance in wheat by modulating abscisic acid homeostasis. Plant Physiol. Biochem. 2019, 136, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Nephali, L.; Moodley, V.; Piater, L.; Steenkamp, P.; Buthelezi, N.; Dubery, I.; Burgess, K.; Huyser, J.; Tugizimana, F. A Metabolomic Landscape of Maize Plants Treated with a Microbial Biostimulant Under Well-Watered and Drought Conditions. Front. Plant Sci. 2021, 12, 362. [Google Scholar] [CrossRef] [PubMed]
- Silletti, S.; Di Stasio, E.; Van Oosten, M.; Ventorino, V.; Pepe, O.; Napolitano, M.; Marra, R.; Woo, S.; Cirillo, V.; Maggio, A. Biostimulant Activity of Azotobacter chroococcum and Trichoderma harzianum in Durum Wheat under Water and Nitrogen Deficiency. Agronomy 2021, 11, 380. [Google Scholar] [CrossRef]
- Othibeng, K.; Nephali, L.; Myoli, A.; Buthelezi, N.; Jonker, W.; Huyser, J.; Tugizimana, F. Metabolic Circuits in Sap Extracts Reflect the Effects of a Microbial Biostimulant on Maize Metabolism under Drought Conditions. Plants 2022, 11, 510. [Google Scholar] [CrossRef] [PubMed]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Reynaud, H.; Canaguier, R.; Trtílek, M.; Panzarová, K.; et al. Understanding the Biostimulant Action of Vegetal-Derived Protein Hydrolysates by High-Throughput Plant Phenotyping and Metabolomics: A Case Study on Tomato. Front. Plant Sci. 2019, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Saporta, R.; Bou, C.; Frías, V.; Mulet, J.M. A Method for a Fast Evaluation of the Biostimulant Potential of Different Natural Extracts for Promoting Growth or Tolerance against Abiotic Stress. Agronomy 2019, 9, 143. [Google Scholar] [CrossRef] [Green Version]
- Ugena, L.; Hýlová, A.; Podlešáková, K.; Humplík, J.F.; Doležal, K.; De Diego, N.; Spíchal, L. Characterization of Biostimulant Mode of Action Using Novel Multi-Trait High-Throughput Screening of Arabidopsis Germination and Rosette Growth. Front. Plant Sci. 2018, 9, 1327. [Google Scholar] [CrossRef] [Green Version]
- García-Maquilón, I.; Rodriguez, P.L.; Vaidya, A.S.; Lozano-Juste, J. A LuciferaseLuciferase ReporterReportersAssay to Identify Chemical Activators of ABAAbscisic Acid (ABA)Signaling. In Plant Chemical Genomics: Methods and Protocols; Hicks, G.R., Zhang, C., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; pp. 113–121. ISBN 978-1-07-160954-5. [Google Scholar]
- Arias, D.J.; Rodríguez, A.B.; Castro, A.B.; González, F.V.; Pérez, J.A.P.; Jorge, J.C.L. Use of Non-Prolinical Cyclic Amino Acids to Increase the Tolerance of Plants under Osmote Stress Conditions. U.S. Patent 16,085,465, 30 May 2019. [Google Scholar]
- Rodríguez, A.A.B.; Pérez, A.A.B.; Arias, D.J.; Rodríguez, V.M.; Rodríguez, M.E.; Jorge, J.C.L. Use of Menadione to Increase Saline Stress Tolerance in Plants. ES Patent ES 2332494 A1, 1 January 2011. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 114. [Google Scholar]
- Habibi, G. Silicon supplementation improves drought tolerance in canola plants. Russ. J. Plant Physiol. 2014, 61, 784–791. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Lee, D.-J. Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiol. Plant. 2009, 31, 937–945. [Google Scholar] [CrossRef]
- Zouari, M.; Hassena, A.B.; Trabelsi, L.; Rouina, B.B.; Decou, R.; Labrousse, P. Exogenous Proline-Mediated Abiotic Stress Tolerance in Plants: Possible Mechanisms. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants: Recent Advances and Future Perspectives; Hossain, M.A., Kumar, V., Burritt, D.J., Fujita, M., Mäkelä, P.S.A., Eds.; Springer: Cham, Switzerland, 2019; pp. 99–121. ISBN 978-3-030-27423-8. [Google Scholar]
- Liu, J.; Wang, W.; Wang, L.; Sun, Y. Exogenous melatonin improves seedling health index and drought tolerance in tomato. Plant Growth Regul. 2015, 77, 317–326. [Google Scholar] [CrossRef]
- Jiménez-Arias, D.; Pérez, J.A.; Luis, J.C.; Martín-Rodríguez, V.; Valdés-González, F.; Borges, A.A. Treating seeds in menadione sodium bisulphite primes salt tolerance in Arabidopsis by inducing an earlier plant adaptation. Environ. Exp. Bot. 2015, 109, 23–30. [Google Scholar] [CrossRef]
- Medrano, H.; Tomás, M.; Martorell, S.; Flexas, J.; Hernández, E.; Rosselló, J.; Pou, A.; Escalona, J.-M.; Bota, J. From leaf to whole-plant water use efficiency (WUE) in complex canopies: Limitations of leaf WUE as a selection target. Crop J. 2015, 3, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Arias, D.; Machado, F.J.G.; Morales-Sierra, S.; Suárez, E.; Pérez, J.A.; Luis, J.C.; Garrido-Orduña, C.; Herrera, A.J.; Valdés, F.; Sandalio, L.M.; et al. Menadione sodium bisulphite (MSB): Beyond seed-soaking. Root pretreatment with MSB primes salt stress tolerance in tomato plants. Environ. Exp. Bot. 2019, 157, 161–170. [Google Scholar] [CrossRef]
- Jiménez-Arias, D.; Borges, A.A.; Luis, J.C.; Valdés, F.; Sandalio, L.M.; Pérez, J.A. Priming effect of menadione sodium bisulphite against salinity stress in Arabidopsis involves epigenetic changes in genes controlling proline metabolism. Environ. Exp. Bot. 2015, 120, 23–30. [Google Scholar] [CrossRef]
- Kelen, M.; Çubuk Demiralay, E.; Şen, S.; Özkan, G. Separation of Abscisic Acid, Indole-3-Acetic Acid, Gibberellic Acid in 99 R (Vitis berlandieri x Vitis rupestris) and Rose Oil (Rosa damascena Mill.) by Reversed Phase Liquid Chromatography. Turk. J. Chem. 2004, 28, 603–610. [Google Scholar]
- Marchin, R.M.; Ossola, A.; Leishman, M.R.; Ellsworth, D.S. A Simple Method for Simulating Drought Effects on Plants. Front. Plant Sci. 2020, 10, 1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iqbal, M.S.; Singh, A.K.; Ansari, M.I. Effect of Drought Stress on Crop Production. In New Frontiers in Stress Management for Durable Agriculture; Rakshit, A., Singh, H.B., Singh, A.K., Singh, U.S., Fraceto, L., Eds.; Springer: Singapore, 2020; pp. 35–47. ISBN 9789811513220. [Google Scholar]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Jogawat, A.; Rahman, S.; Narayan, O.P. Secondary metabolites in the drought stress tolerance of crop plants: A review. Gene Rep. 2021, 23, 101040. [Google Scholar] [CrossRef]
- Zhu, M.; Meng, X.; Cai, J.; Li, G.; Dong, T.; Li, Z. Basic leucine zipper transcription factor SlbZIP1 mediates salt and drought stress tolerance in tomato. BMC Plant Biol. 2018, 18, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, K.; Kai, W.; Zhao, B.; Sun, Y.; Yuan, B.; Dai, S.; Li, Q.; Chen, P.; Wang, Y.; Pei, Y.; et al. SlNCED1 and SlCYP707A2: Key genes involved in ABA metabolism during tomato fruit ripening. J. Exp. Bot. 2014, 65, 5243–5255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, G.J.; Arndt, S.K. Osmotic Adjustment Under Drought Conditions. In Plant Responses to Drought Stress: From Morphological to Molecular Features; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 199–229. ISBN 978-3-642-32653-0. [Google Scholar]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Zarattini, M.; Forlani, G. Toward Unveiling the Mechanisms for Transcriptional Regulation of Proline Biosynthesis in the Plant Cell Response to Biotic and Abiotic Stress Conditions. Front. Plant Sci. 2017, 8, 927. [Google Scholar] [CrossRef] [Green Version]
- Balibrea, M.; Rus-Alvarez, A.; Bolarín, M.; Pérez-Alfocea, F. Fast changes in soluble carbohydrates and proline contents in tomato seedlings in response to ionic and non-ionic iso-osmotic stresses. J. Plant Physiol. 1997, 151, 221–226. [Google Scholar] [CrossRef]
- Ebeed, H.T.; Hassan, N.M.; Aljarani, A.M. Exogenous applications of Polyamines modulate drought responses in wheat through osmolytes accumulation, increasing free polyamine levels and regulation of polyamine biosynthetic genes. Plant Physiol. Biochem. 2017, 118, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Khan, A.L.; Shahzad, R.; Khan, M.A.; Bilal, S.; Khan, A.; Kang, S.-M.; Lee, I.-J. Exogenous melatonin induces drought stress tolerance by promoting plant growth and antioxidant defence system of soybean plants. AoB Plants 2021, 13, plab026. [Google Scholar] [CrossRef]
| Group | Molecule Name | Cas Number | Abbreviation | mM/(%) * |
|---|---|---|---|---|
| Hormones | Putrescine | 110-60-1 | Put | 1 |
| Espermidine | 124-20-9 | Esp | 0.1 | |
| Norspermidine | 56-18-8 | N-Esp | 1 | |
| Spermine | 71-44-3 | Spm | 1 | |
| Norspermine | 4605-14-5 | N-Spm | 0.1 | |
| Melatonin | 73-31-4 | Mel | 1 | |
| Amino Acids | Proline | 147-85-3 | Pr | 1 |
| Pyroglutamic acid | 98-79-3 | Pyg | 1 | |
| Β-Amino Butiric Acid | 541-48-0 | BABA | 1 | |
| Betaines | Glycine-Betaine | 590-46-5 | GB | 0.1 |
| Inorganic | Sodium silicilate | 6834-92-0 | Na-Si | 1 |
| Potassium silicilitate | 1312-76-1 | K-Si | 2 | |
| Seawed extract | Kelpak® (BASF; Ludwigshafen; Germany) | Kelp | (5) * | |
| Parameters | Variables | Tech./Inst. | Time | Cost * | Correlation ** |
|---|---|---|---|---|---|
| Growth | Dw | Low technical abilities from technicians and simple instrumentation | Relative low time, depending on the number of samples and treatments | Low | Dw vs the rest |
| RGR | |||||
| RWC | |||||
| WUEplant | |||||
| Gas exchange | Pn | High technical abilities from technicians and expensive instrumentation | Higher time requirements due to the number of samples and treatments | Low | p < 0.01/0.554 |
| Gs | p < 0.01/0.545 | ||||
| E | p < 0.01/0.537 | ||||
| WUE(i:inst) | Ns | ||||
| Metabolites/genes | Proline | High technical abilities from technicians, specific and expensive instrumentation | Demanding, depending on the chosen variable and samples per treatment | High | Ns |
| Carbohydrates | p < 0.01/0.548 | ||||
| ABA | |||||
| SlNC2 |
| Volume (mL) | Mg/Volume | Plants/Treatment * | mg/Treatment * | EUR/Treatment | DW ** | |
|---|---|---|---|---|---|---|
| WW | 5 | 0 | - | - | - | 260 ± 25 a |
| WD | 5 | 0 | - | - | - | 193 ± 32 b |
| BABA | 1 | 0.103 | 60 | 6.2 | 0.840 | 255 ± 49 ac |
| 3 | 0.309 | 60 | 18.5 | 2.521 | 248 ± 55 ac | |
| 5 | 0.515 | 60 | 30.9 | 4.202 | 260 ± 21 ac |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Arias, D.; Morales-Sierra, S.; Borges, A.A.; Herrera, A.J.; Luis, J.C. New Biostimulants Screening Method for Crop Seedlings under Water Deficit Stress. Agronomy 2022, 12, 728. https://doi.org/10.3390/agronomy12030728
Jiménez-Arias D, Morales-Sierra S, Borges AA, Herrera AJ, Luis JC. New Biostimulants Screening Method for Crop Seedlings under Water Deficit Stress. Agronomy. 2022; 12(3):728. https://doi.org/10.3390/agronomy12030728
Chicago/Turabian StyleJiménez-Arias, David, Sarai Morales-Sierra, Andrés A. Borges, Antonio J. Herrera, and Juan C. Luis. 2022. "New Biostimulants Screening Method for Crop Seedlings under Water Deficit Stress" Agronomy 12, no. 3: 728. https://doi.org/10.3390/agronomy12030728
APA StyleJiménez-Arias, D., Morales-Sierra, S., Borges, A. A., Herrera, A. J., & Luis, J. C. (2022). New Biostimulants Screening Method for Crop Seedlings under Water Deficit Stress. Agronomy, 12(3), 728. https://doi.org/10.3390/agronomy12030728