Seasonal Variation in Physiological Traits of Amazonian Coffea canephora Genotypes in Cultivation Systems with Contrasting Water Availability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Area and Coffee Cultivation
2.2. Experimental Design
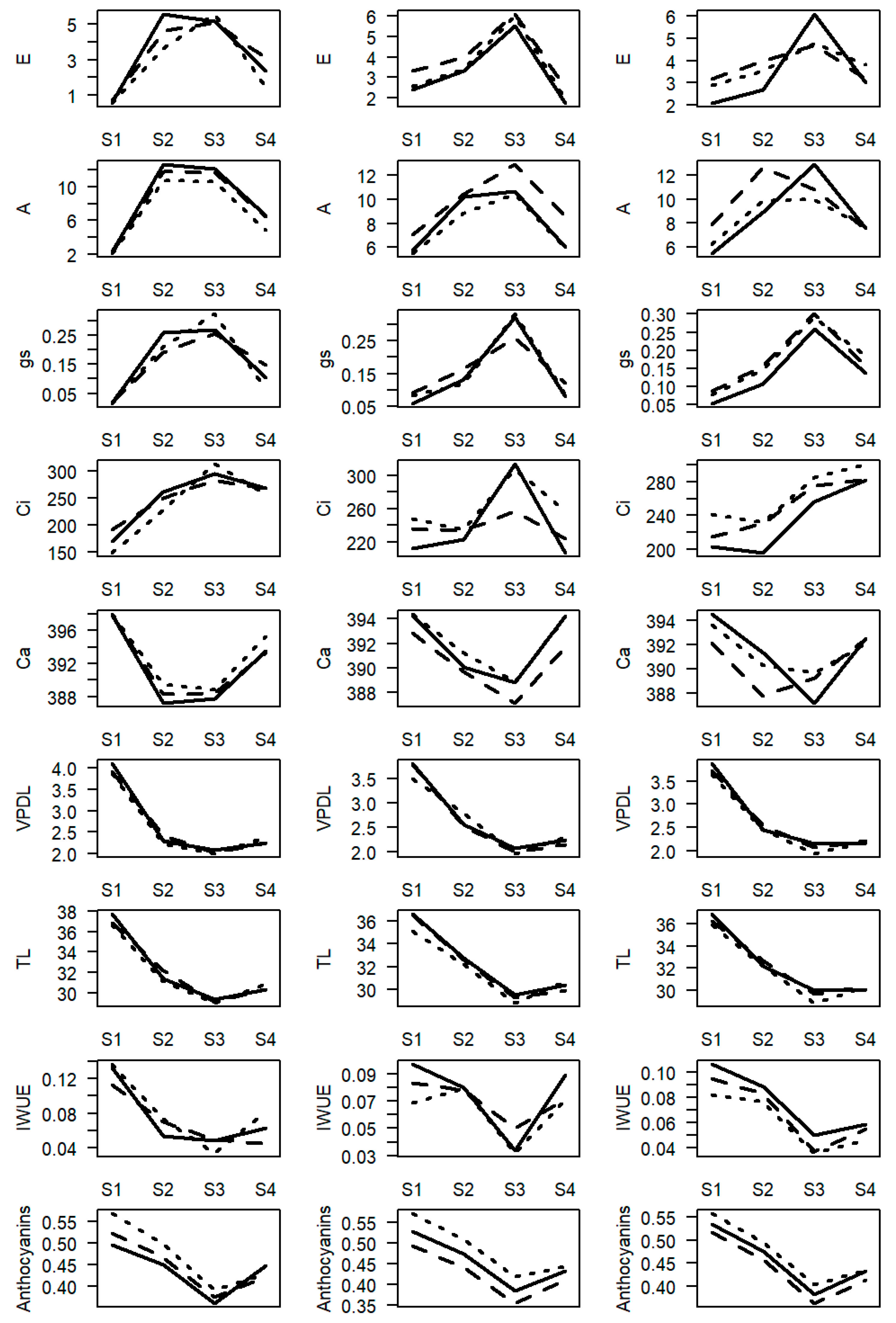
- S1—peak of the dry season, with evaluations carried out between 23 and 25 August 2019. During this period, trees were in pre-anthesis (Biologische Bundesanstalt, Bundessortenamt and Chemical Industry scale—BBCH—58,59 [48]) in the dryland farming system and at the beginning of anthesis (BBCH 61–65) in the irrigation and fertigation systems. In this season there is a reduced rate of vegetative growth.
- S2—beginning of the rainy season, with evaluations carried out between 21 and 24 November 2019. During this period, the accelerated growth of the fruit occurs (BBCH 73–75) and the resumption of the vegetative growth rates.
- S3—peak of the rainy season, with evaluations carried out between 28 February and 1 March 2020. During this period, the final stage of bean filling occurred (BBCH 77–79). At this season there are high rates of vegetative growth.
- S4—beginning of the dry season, with evaluations carried out between 26 and 28 June 2020. In this period, trees had been harvested 20 days before and, atypically, had only partially flowered (BBCH 58–65) (2020/2021 harvest). In this season vegetative growth is stopped (dryland farming system) or reduced (irrigation and fertigation systems).
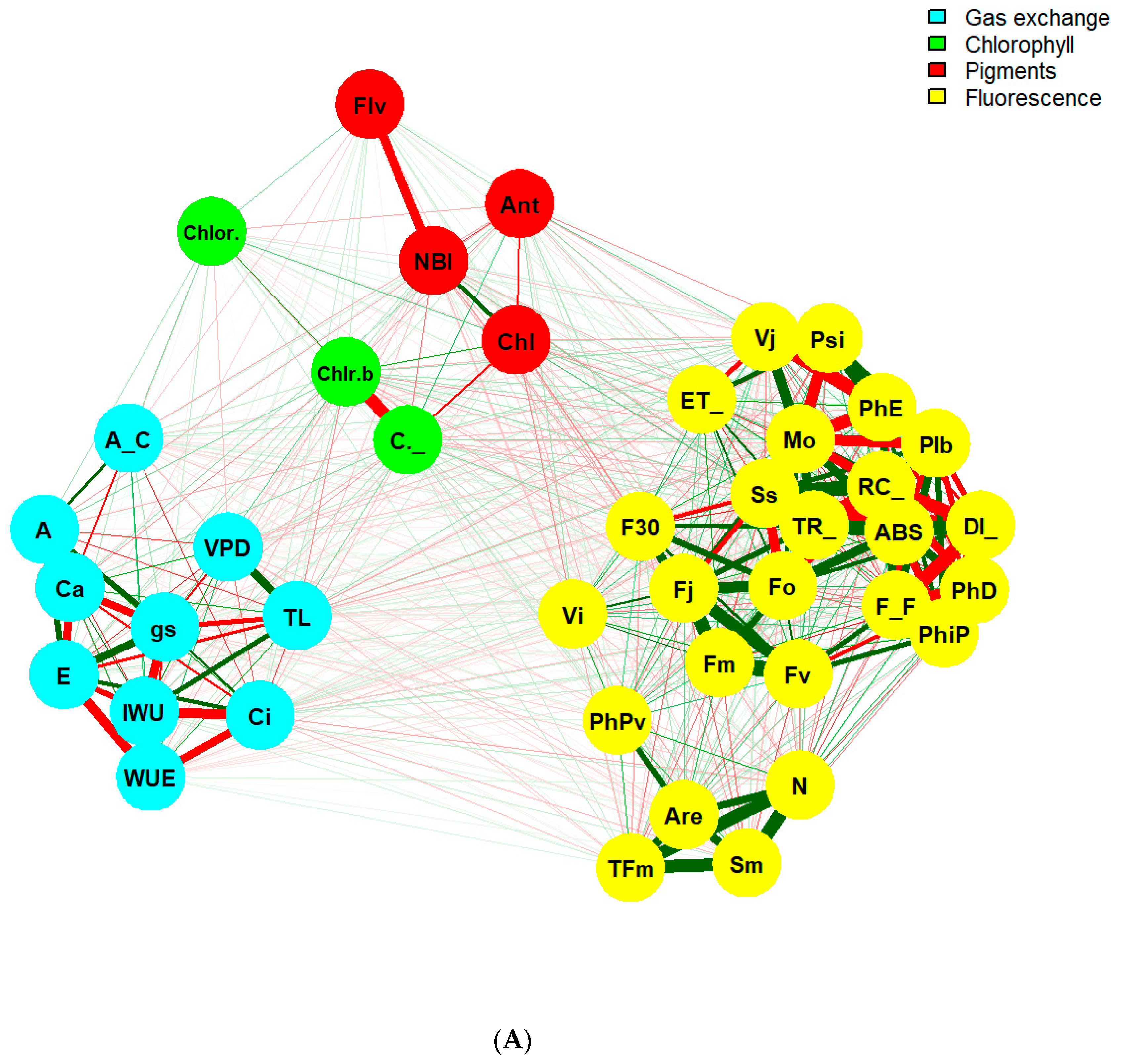
2.3. Assessment of Physiological Variables Associated with Photosynthesis
2.3.1. Leaf Pigment Index
2.3.2. Chlorophyll a Fluorescence
2.3.3. Gas Exchange
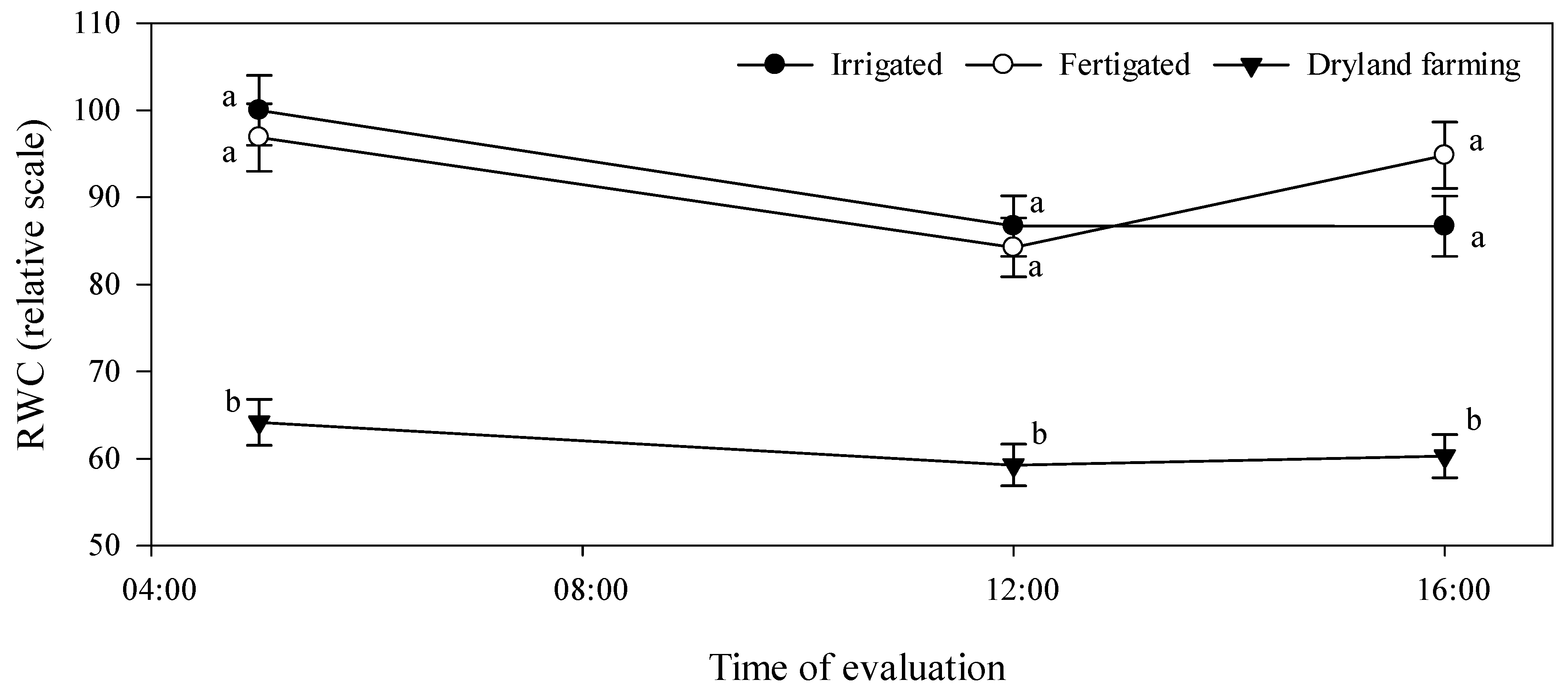
2.3.4. Relative Water Content (RWC) in Leaves
2.4. Vegetative Growth and Production
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Loubere, P. The Global Climate System. Nat. Educ. Knowl. 2012, 3, 24. [Google Scholar]
- Hartmann, D.L. Global Physical Climatology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780080918624. [Google Scholar]
- Venancio, L.P.; Filgueiras, R.; Mantovani, E.C.; do Amaral, C.H.; Da Cunha, F.F.; dos Santos Silva, F.C.; Althoff, D.; Dos Santos, R.A.; Cavatte, P.C. Impact of drought associated with high temperatures on Coffea canephora plantations: A case study in Espírito Santo State, Brazil. Sci. Rep. 2020, 10, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kath, J.; Byrareddy, V.M.; Craparo, A.; Nguyen-Huy, T.; Mushtaq, S.; Cao, L.; Bossolasco, L. Not so robust: Robusta coffee production is highly sensitive to temperature. Glob. Chang. Biol. 2020, 26, 3677–3688. [Google Scholar] [CrossRef] [PubMed]
- Da Matta, F.M.; Avila, R.; Cardoso, A.A.; Martins, S.C.V.; Ramalho, J.C. Physiological and Agronomic Performance of the Coffee Crop in the Context of Climate Change and Global Warming: A Review. J. Agric. Food Chem. 2018, 66, 5264–5274. [Google Scholar] [CrossRef]
- Oliosi, G.; Partelli, F.L.; Da Silva, C.A.; Dubberstein, D.; Gontijo, I.; Tomaz, M.A. Seasonal variation in leaf nutrient concentration of conilon coffee genotypes. J. Plant Nutr. 2020, 44, 74–85. [Google Scholar] [CrossRef]
- Franca, R.R. da Climatologia Das Chuvas Em Rondônia—Período 1981–2011. Rev. Geogr. 2015, 11, 44–58. [Google Scholar] [CrossRef]
- Dubreuil, V.; Fante, K.P.; Planchon, O.; Neto, J.L.S. Os tipos de climas anuais no Brasil: Uma aplicação da classificação de Köppen de 1961 a 2015. Rev. Fr.-Bras. Geogr. 2018, 37, 1–22. [Google Scholar] [CrossRef]
- Thioune, E.-H.; Strickler, S.; Gallagher, T.; Charpagne, A.; Decombes, P.; Osborne, B.; McCarthy, J. Temperature Impacts the Response of Coffea canephora to Decreasing Soil Water Availability. Trop. Plant Biol. 2020, 13, 236–250. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, W.P.; Filho, J.A.M.; da Silva, J.R.; de Assis, F.A.M.M.; Ferraz, T.M.; Ferreira, L.S.; Bezerra, L.B.D.S.; de Abreu, D.P.; Bernado, W.D.P.; Passos, L.C.; et al. Whole-canopy gas exchanges in Coffea sp. is affected by supra-optimal temperature and light distribution within the canopy: The insights from an improved multi-chamber system. Sci. Hortic. 2016, 211, 194–202. [Google Scholar] [CrossRef]
- Thioune, E.-H.; McCarthy, J.; Gallagher, T.; Osborne, B. A humidity shock leads to rapid, temperature dependent changes in coffee leaf physiology and gene expression. Tree Physiol. 2017, 37, 367–379. [Google Scholar] [CrossRef]
- Filho, J.A.M.; Rodrigues, W.P.; Baroni, D.F.; Pireda, S.; Campbell, G.; de Souza, G.A.R.; Filho, A.C.V.; Arantes, S.D.; Arantes, L.D.O.; da Cunha, M.; et al. Linking root and stem hydraulic traits to leaf physiological parameters in Coffea canephora clones with contrasting drought tolerance. J. Plant Physiol. 2021, 258–259, 153355. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic Response of Plants Under Different Abiotic Stresses: A Review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Grossiord, C.; Buckley, T.N.; Cernusak, L.A.; Novick, K.A.; Poulter, B.; Siegwolf, R.T.W.; Sperry, J.S.; McDowell, N.G. Plant responses to rising vapor pressure deficit. New Phytol. 2020, 226, 1550–1566. [Google Scholar] [CrossRef] [Green Version]
- da Silva, P.S.O.; Oliveira, L.F.G.; Gonzaga, M.I.S.; Sena, E.D.O.A.; Maciel, L.B.D.S.; Fiaes, M.P.; de Mattos, E.C.; Carnelossi, M.A.G. Effects of calcium particle films and natural shading on ecophysiological parameters of conilon coffee. Sci. Hortic. 2019, 245, 171–177. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Ronchi, C.P.; Maestri, M.; Barros, R.S. Ecophysiology of Coffee Growth and Production. Braz. J. Plant Physiol. 2007, 19, 485–510. [Google Scholar] [CrossRef] [Green Version]
- Tezara, W.; Guambi, L.A.D.; Chila, V.H.R.; Ortiz, R.N.; Ortega, M.J.B. Seasonal changes in gas exchange and yield of 21 genotypes of Coffea arabica. Bot. Sci. 2021, 100, 1000–1013. [Google Scholar] [CrossRef]
- Batista, K.D.; Araújo, W.L.; Antunes, W.C.; Cavatte, P.C.; Moraes, G.A.B.K.; Martins, S.C.V.; DaMatta, F.M. Photosynthetic limitations in coffee plants are chiefly governed by diffusive factors. Trees 2011, 26, 459–468. [Google Scholar] [CrossRef]
- Vieira, N.G.; Carneiro, F.A.; Sujii, P.S.; Alekcevetch, J.C.; Freire, L.P.; Vinecky, F.; Elbelt, S.; Silva, V.A.; DaMatta, F.M.; Ferrão, M.A.G.; et al. Different Molecular Mechanisms Account for Drought Tolerance in Coffea canephora var. Conilon. Trop. Plant Biol. 2013, 6, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Dubberstein, D.; Lidon, F.C.; Rodrigues, A.P.; Semedo, J.N.; Marques, I.; Rodrigues, W.; Gouveia, D.; Armengaud, J.; Semedo, M.C.; Martins, S.; et al. Resilient and Sensitive Key Points of the Photosynthetic Machinery of Coffea spp. to the Single and Superimposed Exposure to Severe Drought and Heat Stresses. Front. Plant Sci. 2020, 11, 1049. [Google Scholar] [CrossRef]
- Ávila, E.A.D.S.; Sousa, C.M.; Pereira, W.; De Melo, H.C.; Almeida, V.G.; Sarti, J.K. Relationship of gas exchanges in different phenological phases with coffee productivity in the Cerrado. Res. Soc. Dev. 2020, 9, e293974123. [Google Scholar] [CrossRef]
- Muñoz, C.A.U.; Bejarano, L.M.D.; Acuña, J.R. Effect of fruit load of the first coffee harvests on leaf gas exchange. Pesqui. Agropecuária Trop. 2021, 51, e69865. [Google Scholar] [CrossRef]
- Morais, L.E.; Cavatte, P.C.; Detmann, K.C.; Sanglard, L.M.V.P.; Ronchi, C.P.; DaMatta, F.M. Source strength increases with the increasing precociousness of fruit maturation in field-grown clones of conilon coffee (Coffea canephora) trees. Trees—Struct. Funct. 2012, 26, 1397–1402. [Google Scholar] [CrossRef]
- Almeida, W.L.; Ávila, R.T.; Pérez-Molina, J.P.; Barbosa, M.L.; Marçal, D.M.S.; de Souza, R.P.B.; Martino, P.B.; Cardoso, A.A.; Martins, S.C.V.; DaMatta, F.M. The interplay between irrigation and fruiting on branch growth and mortality, gas exchange and water relations of coffee trees. Tree Physiol. 2021, 41, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Panisset, J.S.; Libonati, R.; Gouveia, C.M.P.; Machado-Silva, F.; França, D.A.; França, J.R.A.; Peres, L.F. Contrasting patterns of the extreme drought episodes of 2005, 2010 and 2015 in the Amazon Basin. Int. J. Clim. 2018, 38, 1096–1104. [Google Scholar] [CrossRef]
- Jayakumar, M.; Rajavel, M.; Surendran, U.; Gopinath, G.; Ramamoorthy, K. Impact of climate variability on coffee yield in India—With a micro-level case study using long-term coffee yield data of humid tropical Kerala. Clim. Chang. 2017, 145, 335–349. [Google Scholar] [CrossRef]
- Byrareddy, V.; Kouadio, L.; Kath, J.; Mushtaq, S.; Rafiei, V.; Scobie, M.; Stone, R. Win-win: Improved irrigation management saves water and increases yield for robusta coffee farms in Vietnam. Agric. Water Manag. 2020, 241, 106350. [Google Scholar] [CrossRef]
- Sakai, E.; Barbosa, E.A.A.; de Carvalho Silveira, J.M.; Pires, R.C.D.M. Coffee productivity and root systems in cultivation schemes with different population arrangements and with and without drip irrigation. Agric. Water Manag. 2015, 148, 16–23. [Google Scholar] [CrossRef]
- Babou, C.; Mukharib, R.D.; Gokavi, K.M.N.; Manjunath, R.A.; Raghuramulu, Y. Influence of Micro Irrigation and Drip Fertigation Practices on Yield and Quality Parameters of Robusta Coffee (Coffea canephora). Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 701–706. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, A.L.T.; Santinato, R.; Drumond, L.C.D.; de Oliveira, C.B. Avaliação Do Uso de Fertilizantes Organominerais e Químicos Na Fertirrigação Do Cafeeiro Irrigado Por Gotejamento. Rev. Bras. Eng. Agrícola Ambient. 2007, 11, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, A.L.; Rocha, R.B.; Espindula, M.C.; Ramalho, A.R.; Júnior, J.R.V.; Alves, E.A.; Lunz, A.M.P.; Souza, F.D.F.; Costa, J.N.M.; Fernandes, C.D.F. Amazonian Robustas—New Coffea canephora coffee cultivars for the Western Brazilian Amazon. Crop Breed. Appl. Biotechnol. 2020, 20, e323420318. [Google Scholar] [CrossRef]
- da Silva, D.R.; da Silva, D.R.; Damaceno, J.B.D.; de Andrade, R.A.; Domingues, C.G.; da Silva, C.A.; Martins, J.K.D.; Traspadini, E.I.F.; Dubberstein, D.; Dias, J.R.M. Compatibility Test and Agronomic Performance of Coffee Genotypes (Coffea canephora Pierre ex Froehner) in the State of Rondônia, Brazil. J. Agric. Sci. 2019, 11, p162. [Google Scholar] [CrossRef]
- Barkhordarian, A.; Saatchi, S.S.; Behrangi, A.; Loikith, P.C.; Mechoso, C.R. A Recent Systematic Increase in Vapor Pressure Deficit over Tropical South America. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fontes, C.G.; Dawson, T.E.; Jardine, K.; McDowell, N.; Gimenez, B.O.; Anderegg, L.; Negrón-Juárez, R.; Higuchi, N.; Fine, P.V.A.; Araújo, A.C.; et al. Dry and hot: The hydraulic consequences of a climate change–type drought for Amazonian trees. Philos. Trans. R. Soc. B: Biol. Sci. 2018, 373, 20180209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kath, J.; Craparo, A.; Fong, Y.; Byrareddy, V.; Davis, A.P.; King, R.; Nguyen-Huy, T.; van Asten, P.J.A.; Marcussen, T.; Mushtaq, S.; et al. Vapour pressure deficit determines critical thresholds for global coffee production under climate change. Nat. Food 2022, 1–10. [Google Scholar] [CrossRef]
- Garavito, A.; Montagnon, C.; Guyot, R.; Bertrand, B. Identification by the DArTseq method of the genetic origin of the Coffea canephora cultivated in Vietnam and Mexico. BMC Plant Biol. 2016, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Montagnon, C.; Cubry, P.; Leroy, T. Amélioration génétique du caféier Coffea canephora Pierre: Connaissances acquises, stratégies et perspectives. Cah. Agric. 2012, 21, 143–153. [Google Scholar] [CrossRef]
- Dalazen, J.R.; Rocha, R.B.; Pereira, L.L.; Alves, E.A.; Espindula, M.C.; De Souza, C.A. Beverage quality of most cultivated Coffea canephora clones in the Western Amazon. Coffee Sci. 2020, 15, 1–10. [Google Scholar] [CrossRef]
- Martins, S.C.V.; Sanglard, M.L.; Morais, L.E.; Menezes-Silva, P.E.; Mauri, R.; Avila, R.T.; Vital, C.E.; Cardoso, A.A.; DaMatta, F.M. How do coffee trees deal with severe natural droughts? An analysis of hydraulic, diffusive and biochemical components at the leaf level. Trees—Struct. Funct. 2019, 33, 1679–1693. [Google Scholar] [CrossRef]
- Praxedes, S.C.; DaMatta, F.M.; Loureiro, M.E.; Ferrão, M.A.G.; Cordeiro, A.T. Effects of long-term soil drought on photosynthesis and carbohydrate metabolism in mature robusta coffee (Coffea canephora Pierre var. kouillou) leaves. Environ. Exp. Bot. 2006, 56, 263–273. [Google Scholar] [CrossRef]
- dos Santos, H.G.; Jacomine, P.K.T.; dos Anjos, L.H.C.; de Oliveira, V.Á.; Lumbreras, J.F.; Coelho, M.R.; de Almeida, J.A.; de Araújo Filho, J.C.; de Oliveira, J.B. (Eds.) Sistema Brasileiro de Classificação de Solos, 5th ed.; EMBRAPA: Brasilia, Brazil, 2018; ISBN 978-85-7035-800-4. [Google Scholar]
- INMET. Banco de Dados Meteorológicos Do INMET. Estação Meteorológica A-939. Available online: https://bdmep.inmet.gov.br/# (accessed on 24 February 2021).
- Moraes, J.R.D.S.C.D.; Rolim, G.D.S.; Martorano, L.G.; Aparecido, L.E.D.O.; de Oliveira, M.D.S.P.; Neto, J.T.D.F. Agrometeorological models to forecast açaí (Euterpe oleracea Mart.) yield in the Eastern Amazon. J. Sci. Food Agric. 2020, 100, 1558–1569. [Google Scholar] [CrossRef]
- Prezotti, L.C.; Guarçoni, A.M.; Bragança, S.M.; Lani, J.A. Conilon Coffee Liming and Fertilization. In Conilon Coffee: The Coffea Canephora Produced in Brazil; Ferrão, R.G., da Fonseca, A.F.A., Ferrão, M.A.G., de Muner, L.H., Eds.; INCAPER: Vitória, Brazil, 2019; pp. 421–438. [Google Scholar]
- Ferrão, R.G.; da Fonseca, A.F.A.; Ferrão, M.A.G.; de Muner, L.H. Conilon Coffee—The Coffea Canephora Produced in Brazil, 3rd ed.; INCAPER: Vitória, Brazil, 2019; ISBN 978-85-89274-32-6.
- Marcolan, A.L.; Espindula, M.C. (Eds.) Café Na Amazônia, 1st ed.; EMBRAPA: Brasilia, Brazil, 2015; ISBN 978-85-7035-469-3. [Google Scholar]
- Dalazen, J.R.; Rocha, R.B.; Espindula, M.C.; Dias, J.R.M.; Dalazen, J.R. Base Genética da Cafeicultura e Caracterização Dos Principais Clones Cultivados No Estado de Rondônia. In Café Conilon: Conhecimento Para Superar Desafios; Partelli, F.L., Espindula, M.C., Eds.; CAUFES: Alegre, Brazil, 2019; pp. 165–178. ISBN 978-85-54343-20-0. [Google Scholar]
- Salazar, B.; Joy Martinez Lagrimas, A.; Jose Santos, P.A.; Salazar, B.M.; Gunda, D.M.; Joy Lagrimas, A.M.; del Rosario, E.E. Profiling and Analysis of Reproductive Phenology of Four Coffee (Coffea Spp.) Species in the Philippines Using the BBCH Scale. Philipp. J. Crop Sci. 2019, 44, 10–19. [Google Scholar]
- Cerovic, Z.G.; Masdoumier, G.; Ben Ghozlen, N.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The Fluorescence Transient as a Tool to Characterize and Screen Photosynthetic Samples. In Probing Photosynthesis Mechanism, Regulation & Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor and Francis: London, UK, 2000; pp. 445–483. ISBN 9780748408214. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. ISBN 978-1-4020-3218-9. [Google Scholar]
- Villasenor Alva, J.A.; González-Estrada, E. A Generalization of Shapiro–Wilk’s Test for Multivariate Normality. In Communications in Statistics-Theory and Methods; Taylor and Francis: Boca Raton, FL, USA, 2009; Volume 38, pp. 1870–1883. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An Analysis of Transformations. J. R. Stat. Soc. Ser. B 1964, 26, 211–243. [Google Scholar] [CrossRef]
- Gabriel, K.R. Biometrika Trust the Biplot Graphic Display of Matrices with Application to Principal Component Analysis. Biometrika 1971, 58, 453–467. [Google Scholar] [CrossRef]
- da Silva, A.; Rêgo, E.R.D.; Pessoa, A.M.D.S.; Rêgo, M.M.D. Correlation network analysis between phenotypic and genotypic traits of chili pepper. Pesqui. Agropecu Bras. 2016, 51, 372–377. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.R. Biotools: Tools for Biometry and Applied Statistics in Agricultural Science 2017. Available online: https://cran.r-project.org/web/packages/biotools/index.html (accessed on 30 October 2022).
- Friendly, M.; Fox, J. Candisc: Visualizing Generalized Canonical Discriminant and Canonical Correlation Analysis 2017. Available online: https://cran.r-project.org/web/packages/candisc/index.html (accessed on 30 October 2022).
- Epskamp, S.; Cramer, A.O.J.; Waldorp, L.J.; Schmittmann, V.D.; Borsboom, D. qgraph: Network Visualizations of Relationships in Psychometric Data. J. Stat. Softw. 2012, 48, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Urban, L.; Aarrouf, J.; Bidel, L.P.R. Assessing the Effects of Water Deficit on Photosynthesis Using Parameters Derived from Measurements of Leaf Gas Exchange and of Chlorophyll a Fluorescence. Front. Plant Sci. 2017, 8, 2068. [Google Scholar] [CrossRef] [Green Version]
- Dubberstein, D.; Partelli, F.L.; Rafael, J.D.M.; Espindula, M.C. Influência da Adubação No Crescimento Vegetativo de Cafeeiros Na Amazônia Sul Ocidental. Coffee Sci. 2017, 12, 50–59. [Google Scholar] [CrossRef] [Green Version]
- Moustaka, J.; Tanou, G.; Giannakoula, A.; Adamakis, I.-D.S.; Panteris, E.; Eleftheriou, E.P.; Moustakas, M. Anthocyanin accumulation in poinsettia leaves and its functional role in photo-oxidative stress. Environ. Exp. Bot. 2020, 175, 104065. [Google Scholar] [CrossRef]
- Piccolo, E.; Landi, M.; Giordani, T.; Lorenzini, G.; Malorgio, F.; Massai, R.; Nali, C.; Pellegrini, E.; Rallo, G.; Remorini, D.; et al. Can anthocyanin presence ameliorate the photosynthetic performance of Prunus saplings subjected to polyethylene glycol-simulated water stress? Photosynthetica 2020, 58, 799–807. [Google Scholar] [CrossRef]
- Pucci, M.; Mandrone, M.; Chiocchio, I.; Mac Sweeney, E.; Tirelli, E.; Uberti, D.; Memo, M.; Poli, F.; Mastinu, A.; Abate, G. Different Seasonal Collections of Ficus carica L. Leaves Diversely Modulate Lipid Metabolism and Adipogenesis in 3T3-L1 Adipocytes. Nutrients 2022, 14, 2833. [Google Scholar] [CrossRef] [PubMed]
- Rakocevic, M.; Braga, K.S.M.; Batista, E.R.; Maia, A.H.N.; Scholz, M.B.S.; Filizola, H.F. The vegetative growth assists to reproductive responses of Arabic coffee trees in a long-term FACE experiment. Plant Growth Regul. 2020, 91, 305–316. [Google Scholar] [CrossRef]
- Tesfaye, S.G.; Ismail, M.R.; Ramlan, M.F.; Marziah, M.; Kausar, H. Effect of Soil Drying on Rate of Stress Development, LEAF Gas Exchange and Proline Accumulation in Robusta Coffee (Coffea Canephora Pierre ex Froehner) Clones. Exp. Agric. 2013, 50, 458–479. [Google Scholar] [CrossRef] [Green Version]
- Espindula, M.C.; Teixeira, A.L.; Rocha, R.B.; Ramalho, A.R.; Vieira Júnior, J.R.; Alves, E.A.; Diocleciano, J.M.; Lunz, A.M.P.; de Souza, F.F.; Costa, J.N.M.; et al. Novas Cultivares de Cafeeiros Coffea Canephora Para a Amazônia Ocidental Brasileira—Principais Características. Embrapa Rondônia-Comun. Técnico 2019, 413, 1–35. [Google Scholar]
- Martins Silva, F.; Soares de Maria de Medeiros, P. Impacto da Cafeicultura No Uso e Ocupação do Solo da Bacia do Rio Ribeirão Cacau-Ro. Ciência Geográfica 2020, 24, 619–634. [Google Scholar]
- Marengo, J.A.; Souza, C.A.J.; Thonicke, K.; Burton, C.; Halladay, K.; Betts, R.A.; Alves, L.M.; Soares, W.R. Changes in Climate and Land Use Over the Amazon Region: Current and Future Variability and Trends. Front. Earth Sci. 2018, 6, 228. [Google Scholar] [CrossRef]
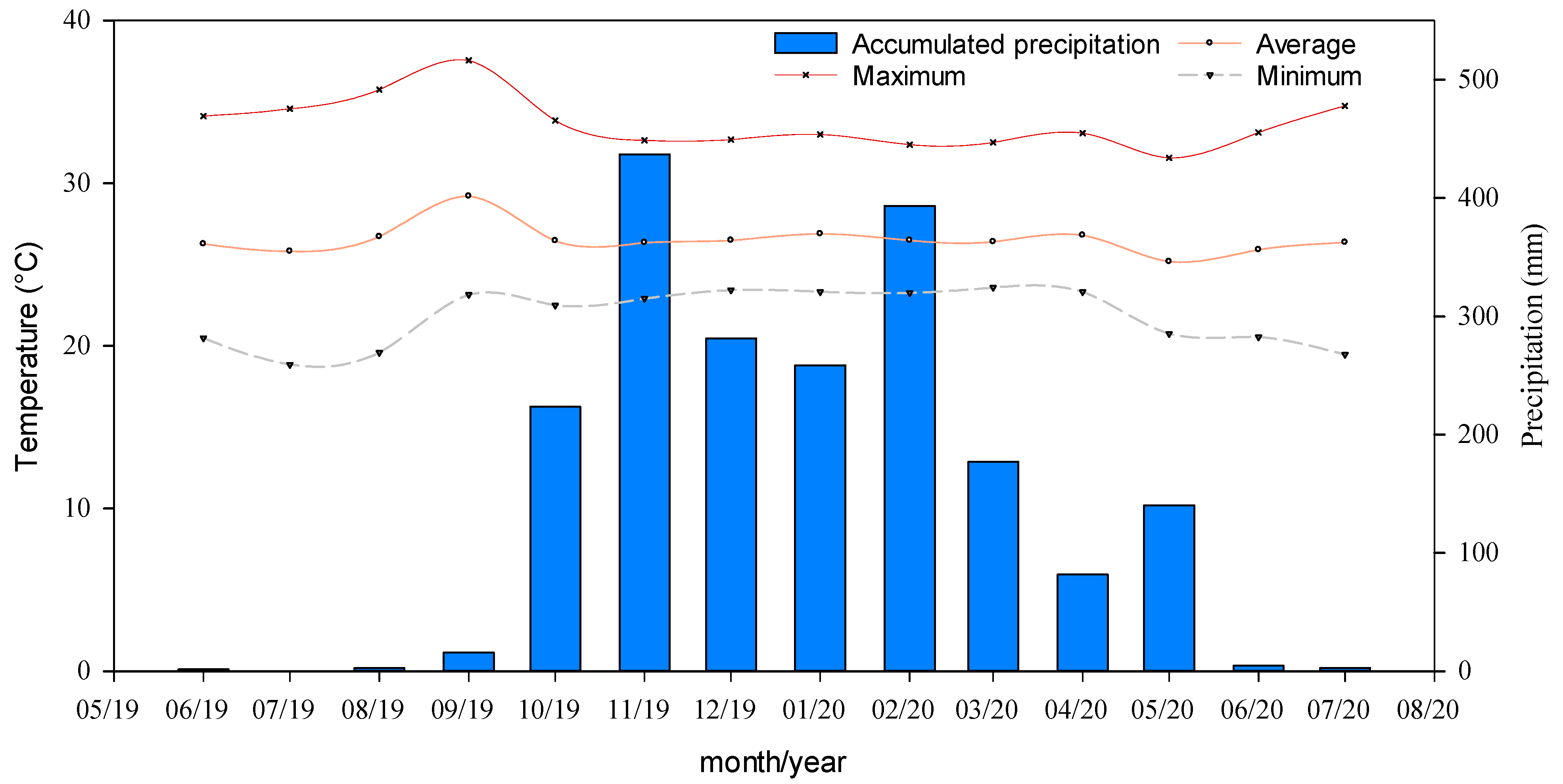


| Season | Rel. Air Humidity (%) | Temperature (°C) | VPD (kPa) | ||||
|---|---|---|---|---|---|---|---|
| Minimum | Average | Minimum | Average | Maximum | Average | Maximum | |
| S1 | 30.0 | 56.5 | 22.5 | 28.7 | 36.4 | 1.38 | 3.31 |
| S2 | 50.3 | 78.7 | 22.7 | 26.3 | 32.7 | 0.59 | 1.93 |
| S3 | 60.7 | 83.9 | 23.1 | 25.8 | 30.9 | 0.44 | 1.39 |
| S4 | 38.0 | 69.0 | 20.2 | 25.8 | 33.1 | 0.84 | 2.46 |
| Environmental Variables | Gas Exchange Variables | |||
|---|---|---|---|---|
| gs | Ci | TL | VPDL | |
| Te | −0.78 | −0.98 * | 0.99 * | 0.96 * |
| RH | 0.94 + | 0.92 + | −0.94 + | −0.94 + |
| VPD | −0.90 + | −0.93 + | 0.97 * | 0.97 * |
| Can. 1 | Green Coffee (MT/ha) | Dried Cherry Coffee (MT/ha) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | G1 | G2 | G3 | G1 | G2 | G3 | |
| Fertigation | −2.44 bB | 0.56 bA | −0.13 aA | 1.72 aC | 2.53 aB | 4.38 aA | 4.08 aB | 5.37 aB | 8.70 aA |
| Irrigation | −0.83 aC | 1.44 aA | 0.63 aB | 1.82 aA | 1.51 bA | 2.20 bA | 3.77 aA | 3.12 bA | 4.57 bA |
| Dryland farming | −1.06 aC | 1.36 aA | 0.46 aB | 0.45 bB | 0.17 cB | 1.29 cA | 0.94 bB | 0.39 cB | 2.69 cA |
| SVC | -- | 29.75% | 26.14% | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Custodio, A.M.; de Menezes Silva, P.E.; Santos, T.R.d.; Lourenço, L.L.; Avila, R.G.; da Silva, A.R.; de Lima e Silva, F.H.; Espindula, M.C.; Dias, J.R.M.; Silva, F.G. Seasonal Variation in Physiological Traits of Amazonian Coffea canephora Genotypes in Cultivation Systems with Contrasting Water Availability. Agronomy 2022, 12, 3197. https://doi.org/10.3390/agronomy12123197
Custodio AM, de Menezes Silva PE, Santos TRd, Lourenço LL, Avila RG, da Silva AR, de Lima e Silva FH, Espindula MC, Dias JRM, Silva FG. Seasonal Variation in Physiological Traits of Amazonian Coffea canephora Genotypes in Cultivation Systems with Contrasting Water Availability. Agronomy. 2022; 12(12):3197. https://doi.org/10.3390/agronomy12123197
Chicago/Turabian StyleCustodio, Aldo Max, Paulo Eduardo de Menezes Silva, Thiago Rodrigues dos Santos, Lucas Loram Lourenço, Roniel Geraldo Avila, Anderson Rodrigo da Silva, Fernando Higino de Lima e Silva, Marcelo Curitiba Espindula, Jairo Rafael Machado Dias, and Fabiano Guimarães Silva. 2022. "Seasonal Variation in Physiological Traits of Amazonian Coffea canephora Genotypes in Cultivation Systems with Contrasting Water Availability" Agronomy 12, no. 12: 3197. https://doi.org/10.3390/agronomy12123197
APA StyleCustodio, A. M., de Menezes Silva, P. E., Santos, T. R. d., Lourenço, L. L., Avila, R. G., da Silva, A. R., de Lima e Silva, F. H., Espindula, M. C., Dias, J. R. M., & Silva, F. G. (2022). Seasonal Variation in Physiological Traits of Amazonian Coffea canephora Genotypes in Cultivation Systems with Contrasting Water Availability. Agronomy, 12(12), 3197. https://doi.org/10.3390/agronomy12123197








