Recovery of Energy and Nutrients from Mycotoxin-Contaminated Food Products through Biological Treatments in a Circular Economy Perspective: A Review
Abstract
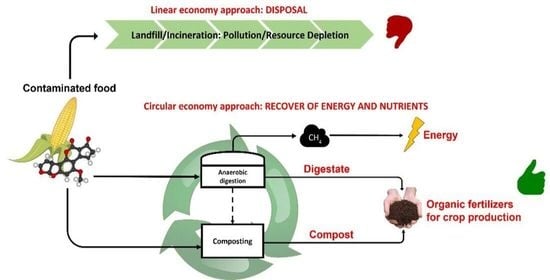
1. Introduction and Aim of the Review
2. Mycotoxins’ Generality: Classification, Biosynthesis, and Hazards
3. Mycotoxin Contamination of Food Products: Issues, Legislation, and Actual Disposal
4. Biological Treatments for Energy and Nutrients’ Recovery from Contaminated Food Products
4.1. Biological Treatments for Energy and Nutrients’ Recovery from Organic Wastes
4.2. AD of Contaminated Food Products
4.2.1. Biogas Production and Digestate Quality
4.2.2. Mycotoxins’ Fate during AD
4.3. Composting of Contaminated Products
4.3.1. Composting Process Evolution and Compost Quality
4.3.2. Mycotoxins’ Fate during Composting
5. Research Gaps and Future Challenges in Mycotoxins’ Degradation through Biological Treatments
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kharola, S.; Ram, M.; Kumar Mangla, S.; Goyal, N.; Nautiyal, O.P.; Pant, D.; Kazancoglu, Y. Exploring the Green Waste Management Problem in Food Supply Chains: A Circular Economy Context. J. Clean. Prod. 2022, 351, 131355. [Google Scholar] [CrossRef]
- Guo, B.; Yu, J.; Holbrook, C.C.; Cleveland, T.E.; Nierman, W.C.; Scully, B.T. Strategies in Prevention of Preharvest Aflatoxin Contamination in Peanuts: Aflatoxin Biosynthesis, Genetics and Genomics. Peanut Sci. 2009, 36, 11–20. [Google Scholar] [CrossRef]
- Yunus, A.W.; Razzazi-Fazeli, E.; Bohm, J. Aflatoxin B1 in Affecting Broiler’s Performance, Immunity, and Gastrointestinal Tract: A Review of History and Contemporary Issues. Toxins 2011, 3, 566–590. [Google Scholar] [CrossRef]
- Jia, K.; Yan, L.; Jia, Y.; Xu, S.; Yan, Z.; Wang, S. Afln Is Involved in the Biosynthesis. of Aflatoxin and Conidiation in Aspergillus Flavus. Toxins 2021, 13, 831. [Google Scholar] [CrossRef]
- Singh, R.; Hsieh, D.P.H. Aflatoxin Biosynthetic Pathway: Elucidation by Using Blocked Mutants of Aspergillus Parasiticus. Arch. Biochem. Biophys. 1977, 178, 285–292. [Google Scholar] [CrossRef]
- Cotty, P.J.; Jaime-Garcia, R. Influences of Climate on Aflatoxin Producing Fungi and Aflatoxin Contamination. Int. J. Food. Microbiol. 2007, 119, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Battilani, P.; Camardo Leggieri, M.; Rossi, V.; Giorni, P. AFLA-Maize, a Mechanistic Model for Aspergillus Flavus Infection and Aflatoxin B1 Contamination in Maize. Comput. Electron. Agric. 2013, 94, 38–46. [Google Scholar] [CrossRef]
- Carla, G.; Souza, S.; Feddern, V.; Heidtmann, R.; Santos Hackbart, H.C.; dos de Souza, M.M.; Santos Oliveira, M.; dos Garda-Buffon, J.; Gilberto, E.; Badiale-Furlong, E. Aflatoxins: Contamination, Analysis and Control. In Aflatoxins–Biochemistry and Molecular Biology; IntechOpen: London, UK, 2011. [Google Scholar]
- Medina, A.; Rodriguez, A.; Magan, N. Effect of Climate Change on Aspergillus Flavus and Aflatoxin B1 Production. Front. Microbiol. 2014, 5, 348. [Google Scholar] [CrossRef]
- Gacem, M.A.; Ould El Hadj-Khelil, A. Toxicology, Biosynthesis, Bio-Control of Aflatoxin and New Methods of Detection. Asian Pac. J. Trop. Biomed. 2016, 6, 808–814. [Google Scholar] [CrossRef]
- Waliyar, F.; Kumar, K.V.K.; Diallo, M.; Traore, A.; Mangala, U.N.; Upadhyaya, H.D.; Sudini, H. Resistance to Pre-Harvest Aflatoxin Contamination in ICRISAT’s Groundnut Mini Core Collection. Eur. J. Plant Pathol. 2016, 145, 901–913. [Google Scholar] [CrossRef]
- Samuel, S.M.; Aiko, V.; Panda, P.; Mehta, A. Aflatoxin B1 Occurrence, Biosynthesis and Its Degradation. J. Pure Appl. Microbiol. 2013, 7, 965–971. [Google Scholar]
- Long, X.D.; Zhao, D.; Wang, C.; Huang, X.Y.; Yao, J.G.; Ma, Y.; Wei, Z.H.; Liu, M.; Zeng, L.X.; Mo, X.Q.; et al. Genetic Polymorphisms in DNA Repair Genes XRCC4 and XRCC5 and Aflatoxin B1-Related Hepatocellular Carcinoma. Epidemiology 2013, 24, 571–581. [Google Scholar] [CrossRef] [PubMed]
- FAO. Food Outlook—Biannual Report on Global Food Markets, Food Outlook—Biannual Report on Global Food Markets. Available online: https://doi.org/10.4060/cb1993en (accessed on 8 February 2022).
- Strosnider, H.; Azziz-Baumgartner, E.; Banziger, M.; Bhat, R.V.; Breiman, R.; Brune, M.N.; DeCock, K.; Dilley, A.; Groopman, J.; Hell, K.; et al. Workgroup Report: Public Health Strategies for Reducing Aflatoxin Exposure in Developing Countries. Environ. Health Perspect. 2006, 114, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, N.J.; Bowers, E.; Hurburgh, C.; Wu, F. Potential Economic Losses to the US Corn Industry from Aflatoxin Contamination. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2016, 33, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Guclu, H. Aflatoxin Regulations in a Network of Global Maize Trade. PLoS ONE 2012, 7, e45151. [Google Scholar] [CrossRef]
- Binder, E.M.; Tan, L.M.; Chin, L.J.; Handl, J.; Richard, J. Worldwide occurrence of mycotoxins in commodities, feeds and feed ingredients. Anim. Feed Sci. Technol. 2007, 137, 265–282. [Google Scholar] [CrossRef]
- Piotrowska, M.; Slizewska, K.; Biernasiak, J. Mycotoxins in cereal and soybean-based food and feed. In Brazil: Soybean-Pest Resistance; IntechOpen: London, UK, 2013; pp. 185–230. [Google Scholar] [CrossRef]
- Battilani, P. Recent Advances in Modeling the Risk of Mycotoxin Contamination in Crops. Curr. Opin. Food Sci. 2016, 11, 10–15. [Google Scholar] [CrossRef]
- Van Egmond, H.P.; Jonker, M.A. Worldwide Regulations for Mycotoxins in Food and Feed in 2003: Summary of Study; Summary of study, carried out for the Food and Agriculture Organization (FAO); Food and Nutrition Paper No. 81; FAO: Rome, Italy, 2005. [Google Scholar]
- van Egmond, H.P.; Schothorst, R.C.; Jonker, M.A. Regulations Relating to Mycotoxins in Food. Anal. Bioanal. Chem. 2007, 389, 147–157. [Google Scholar] [CrossRef]
- Wu, F.; Khlangwiset, P. Health Economic Impacts and Cost-Effectiveness of Aflatoxin-Reduction Strategies in Africa: Case Studies in Biocontrol and Post-Harvest Interventions. Food Addit. Contam. Part A 2010, 27, 496–509. [Google Scholar] [CrossRef]
- Pinotti, L.; Ottoboni, M.; Giromini, C.; Dell’Orto, V.; Cheli, F. Mycotoxin Contamination in the EU Feed Supply Chain: A Focus on Cereal Byproducts. Toxins 2016, 8, 45. [Google Scholar] [CrossRef]
- European Union Commission Regulation (EU). No. 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in Foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- European Commission Directive. 2002/32/Ec of the European Parliament and of the Council of 7 May 2002 on Undesirable Substances in Animal Feed. Off. J. Eur. Comm. 2002, 269, 10–22. [Google Scholar]
- European Commission. Commission Regulation (EC) No 401/2006 of 23 February 2006 Laying down the Methods of Sampling and Analysis for the Official Control of the Levels of Mycotoxins in Foodstuffs; (Text with EEA Relevance). Off. J. Eur. Union 2006, 70, 12–34. [Google Scholar]
- European Commission. 2012/154/EU: Commission Recommendation of 15 March 2012 on the Monitoring of the Presence of Ergot Alkaloids in Feed and Food Text with EEA Relevance. Off. J. Eur. Union 2012, 70, 20–21. [Google Scholar]
- FDA U.S. Title 21–Food and Drugs Chapter I–Food and Drug Administration Department of Health and Human Services Subchapter B–Food for Human Consumption; FDA: Silver Spring, MD, USA, 2022. [Google Scholar]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A Review on Metabolism, Toxicity, Occurrence in Food, Occupational Exposure, and Detoxification Methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef]
- Verheecke, C.; Liboz, T.; Mathieu, F. Microbial Degradation of Aflatoxin B1: Current Status and Future Advances. Int. J. Food Microbiol. 2016, 237, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Jezkova, A.; Yuan, Z.; Pavlikova, L.; Dohnal, V.; Kuca, K. Biological Degradation of Aflatoxins. Drug Metab. Rev. 2009, 41, 1–7. [Google Scholar] [CrossRef]
- McCormick, S.P. Microbial Detoxification of Mycotoxins. J. Chem. Ecol. 2013, 39, 907–918. [Google Scholar] [CrossRef]
- Sangare, L.; Zhao, Y.; Folly, Y.M.; Innie, E.; Chang, J.; Li, J.; Selvaraj, J.N.; Xing, F.; Zhou, L.; Wang, Y.; et al. Aflatoxin B1 Degradation by a Pseudomonas Strain. Toxins 2014, 6, 3028. [Google Scholar] [CrossRef]
- Zhang, W.; Xue, B.; Li, M.; Mu, Y.; Chen, Z.; Li, J.; Shan, A. Screening a Strain of Aspergillus Niger and Optimization of Fermentation Conditions for Degradation of Aflatoxin B1. Toxins 2014, 6, 3157. [Google Scholar] [CrossRef]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human Aflatoxicosis in Developing Countries: A Review of Toxicology, Exposure, Potential Health Consequences, and Interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [CrossRef] [PubMed]
- Hariprasad, P.; Vipin, A.V.; Karuna, S.; Raksha, R.K.; Venkateswaran, G. Natural Aflatoxin Uptake by Sugarcane (Saccharum Officinaurum L.) and Its Persistence in Jaggery. Environ. Sci. Pollut. Res. 2015, 22, 6246–6253. [Google Scholar] [CrossRef] [PubMed]
- Snigdha, M.; Hariprasad, P.; Venkateswaran, G. Transport via Xylem and Accumulation of Aflatoxin in Seeds of Groundnut Plant. Chemosphere 2015, 119, 524–529. [Google Scholar] [CrossRef] [PubMed]
- MdQuadri, S.H.; Niranjan, M.; Chaluvaraju, K.; Shantaram, U.; Enamul, H. An Overview on Chemistry, Toxicity, Analysis and Control of Aflatoxins. Int. J. Chem. Life Sci. 2017, 2, 1071–1078. [Google Scholar]
- Vejerano, E.P.; Leon, E.C.; Holder, A.L.; Marr, L.C. Characterization of Particle Emissions and Fate of Nanomaterials during Incineration. Environ. Sci. Nano 2014, 1, 133–143. [Google Scholar] [CrossRef]
- Juodeikiene, G.; Cernauskas, D.; Trakselyte-Rupsiene, K.; Bartkiene, E.; Zadeike, D.; Banyte, G.; Santini, A. Acoustic-Based Screening Method for the Detection of Total Aflatoxin in Corn and Biological Detoxification in Bioethanol Production. Front. Microbiol. 2020, 11, 543. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xu, F.; Ge, X.; Li, Y. Improving the Sustainability of Organic Waste Management Practices in the Food-Energy-Water Nexus: A Comparative Review of Anaerobic Digestion and Composting. Renew. Sustain. Energy Rev. 2018, 89, 151–167. [Google Scholar] [CrossRef]
- Yang, L.; Ge, X.; Wan, C.; Yu, F.; Li, Y. Progress and Perspectives in Converting Biogas to Transportation Fuels. Renew. Sustain. Energy Rev. 2014, 40, 1133–1152. [Google Scholar] [CrossRef]
- Ge, X.; Yang, L.; Sheets, J.P.; Yu, Z.; Li, Y. Biological Conversion of Methane to Liquid Fuels: Status and Opportunities. Biotechnol. Adv. 2014, 32, 1460–1475. [Google Scholar] [CrossRef]
- Tambone, F.; Orzi, V.; D’Imporzano, G.; Adani, F. Solid and Liquid Fractionation of Digestate: Mass Balance, Chemical Characterization, and Agronomic and Environmental Value. Bioresour. Technol. 2017, 243, 1251–1256. [Google Scholar] [CrossRef]
- Sheets, J.P.; Yang, L.; Ge, X.; Wang, Z.; Li, Y. Beyond Land Application: Emerging Technologies for the Treatment and Reuse of Anaerobically Digested Agricultural and Food Waste. Waste Manag. 2015, 44, 94–115. [Google Scholar] [CrossRef] [PubMed]
- Alburquerque, J.A.; de la Fuente, C.; Campoy, M.; Carrasco, L.; Nájera, I.; Baixauli, C.; Caravaca, F.; Roldán, A.; Cegarra, J.; Bernal, M.P. Agricultural Use of Digestate for Horticultural Crop Production and Improvement of Soil Properties. Eur. J. Agron. 2012, 43, 119–128. [Google Scholar] [CrossRef]
- Cucina, M.; Tacconi, C.; Ricci, A.; Pezzolla, D.; Sordi, S.; Zadra, C.; Gigliotti, G. Evaluation of Benefits and Risks Associated with the Agricultural Use of Organic Wastes of Pharmaceutical Origin. Sci. Total Environ. 2018, 613–614, 773–782. [Google Scholar] [CrossRef] [PubMed]
- Solé-Bundó, M.; Cucina, M.; Folch, M.; Tàpias, J.; Gigliotti, G.; Garfí, M.; Ferrer, I. Assessing the Agricultural Reuse of the Digestate from Microalgae Anaerobic Digestion and Co-Digestion with Sewage Sludge. Sci. Total Environ. 2017, 586, 1–9. [Google Scholar] [CrossRef]
- Cucina, M.; Castro, L.; Escalante, H.; Ferrer, I.; Garfí, M. Benefits and Risks of Agricultural Reuse of Digestates from Plastic Tubular Digesters in Colombia. Waste Manag. 2021, 135, 220–228. [Google Scholar] [CrossRef]
- Tambone, F.; Orzi, V.; Zilio, M.; Adani, F. Measuring the Organic Amendment Properties of the Liquid Fraction of Digestate. Waste Manag. 2019, 88, 21–27. [Google Scholar] [CrossRef]
- Li, Y.; Park, S.Y.; Zhu, J. Solid-State Anaerobic Digestion for Methane Production from Organic Waste. Renew. Sustain. Energy Rev. 2011, 15, 821–826. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Dosta, J.; Romero-Güiza, M.S.; Fonoll, X.; Peces, M.; Astals, S. A Critical Review on Anaerobic Co-Digestion Achievements between 2010 and 2013. Renew. Sustain. Energy Rev. 2014, 36, 412–427. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Pandey, A.K.; Bundela, P.S.; Khan, J. Co-Composting of Organic Fraction of Municipal Solid Waste Mixed with Different Bulking Waste: Characterization of Physicochemical Parameters and Microbial Enzymatic Dynamic. Bioresour. Technol. 2015, 182, 200–207. [Google Scholar] [CrossRef]
- Lin, L.; Yang, L.; Xu, F.; Michel, F.C.; Li, Y. Comparison of Solid-State Anaerobic Digestion and Composting of Yard Trimmings with Effluent from Liquid Anaerobic Digestion. Bioresour. Technol. 2014, 169, 439–446. [Google Scholar] [CrossRef]
- Cucina, M.; Tacconi, C.; Sordi, S.; Pezzolla, D.; Gigliotti, G.; Zadra, C. Valorization of a Pharmaceutical Organic Sludge through Different Composting Treatments. Waste Manag. 2018, 74, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Kibler, K.M.; Reinhart, D.; Hawkins, C.; Motlagh, A.M.; Wright, J. Food Waste and the Food-Energy-Water Nexus: A Review of Food Waste Management Alternatives. Waste Manag. 2018, 74, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Adugna, G. A Review on Impact of Compost on Soil Properties, Water Use and Crop Productivity. Agric. Sci. Res. J. 2016, 4, 93–104. [Google Scholar]
- Nkoa, R. Agricultural Benefits and Environmental Risks of Soil Fertilization with Anaerobic Digestates: A Review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Congilosi, J.L.; Aga, D.S. Review on the Fate of Antimicrobials, Antimicrobial Resistance Genes, and Other Micropollutants in Manure during Enhanced Anaerobic Digestion and Composting. J. Hazard Mater. 2021, 405, 123634. [Google Scholar] [CrossRef]
- Sertillanges, N.; Haudin, C.S.; Bourdat-Deschamps, M.; Bernet, N.; Serre, V.; Danel, A.; Houot, S.; Patureau, D. Process Type Is the Key Driver of the Fate of Organic Micropollutants during Industrial Scale Treatment of Organic Wastes. Sci. Total Environ. 2020, 734, 139108. [Google Scholar] [CrossRef]
- Cheng, D.; Feng, Y.; Liu, Y.; Xue, J.; Li, Z. Dynamics of Oxytetracycline, Sulfamerazine, and Ciprofloxacin and Related Antibiotic Resistance Genes during Swine Manure Composting. J. Environ. Manag. 2019, 230, 102–109. [Google Scholar] [CrossRef]
- Mitchell, S.M.; Ullman, J.L.; Teel, A.L.; Watts, R.J.; Frear, C. The Effects of the Antibiotics Ampicillin, Florfenicol, Sulfamethazine, and Tylosin on Biogas Production and Their Degradation Efficiency during Anaerobic Digestion. Bioresour. Technol. 2013, 149, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Arikan, O.A.; Sikora, L.J.; Mulbry, W.; Khan, S.U.; Rice, C.; Foster, G.D. The Fate and Effect of Oxytetracycline during the Anaerobic Digestion of Manure from Therapeutically Treated Calves. Process. Biochem. 2006, 41, 1637–1643. [Google Scholar] [CrossRef]
- Zhang, J.N.; Yang, L.; Zhang, M.; Liu, Y.S.; Zhao, J.L.; He, L.Y.; Zhang, Q.Q.; Ying, G.G. Persistence of Androgens, Progestogens, and Glucocorticoids during Commercial Animal Manure Composting Process. Sci. Total Environ. 2019, 665, 91–99. [Google Scholar] [CrossRef]
- Sun, W.; Qian, X.; Gu, J.; Wang, X.J.; Duan, M.L. Mechanism and Effect of Temperature on Variations in Antibiotic Resistance Genes during Anaerobic Digestion of Dairy Manure. Sci. Rep. 2016, 6, 30237. [Google Scholar] [CrossRef]
- Riaz, L.; Wang, Q.; Yang, Q.; Li, X.; Yuan, W. Potential of Industrial Composting and Anaerobic Digestion for the Removal of Antibiotics, Antibiotic Resistance Genes and Heavy Metals from Chicken Manure. Sci. Total Environ. 2020, 718, 137414. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, Z.; Wang, J.J. Influence of Humic Substances on Bioavailability of Cu and Zn during Sewage Sludge Composting. Bioresour. Technol. 2011, 102, 8022–8026. [Google Scholar] [CrossRef]
- Salati, S.; D’Imporzano, G.; Panseri, S.; Pasquale, E.; Adani, F. Degradation of Aflatoxin B1 during Anaerobic Digestion and Its Effect on Process Stability. Int. Biodeterior. Biodegrad. 2014, 94, 19–23. [Google Scholar] [CrossRef]
- Giorni, P.; Pietri, A.; Bertuzzi, T.; Soldano, M.; Piccinini, S.; Rossi, L.; Battilani, P. Fate of Mycotoxins and Related Fungi in the Anaerobic Digestion Process. Bioresour. Technol. 2018, 265, 554–557. [Google Scholar] [CrossRef]
- Ferrara, M.; Haidukowski, M.; D’Imperio, M.; Parente, A.; de Angelis, E.; Monaci, L.; Logrieco, A.F.; Mulè, G. New Insight into Microbial Degradation of Mycotoxins during Anaerobic Digestion. Waste Manag. 2021, 119, 215–225. [Google Scholar] [CrossRef]
- Soldano, M.; Pietri, A.; Bertuzzi, T.; Fabbri, C.; Piccinini, S.; Gallucci, F.; Aureli, G. Anaerobic Digestion of Mycotoxin-Contaminated Wheat: Effects on Methane Yield and Contamination Level. Bioenergy Res. 2021, 14, 313–321. [Google Scholar] [CrossRef]
- de Gelder, L.; Audenaert, K.; Willems, B.; Schelfhout, K.; de Saeger, S.; de Boevre, M. Processing of Mycotoxin Contaminated Waste Streams through Anaerobic Digestion. Waste Manag. 2018, 71, 122–128. [Google Scholar] [CrossRef]
- Goux, X.; Bourget, L.; Giraud, F.; Cocco, E.; Guignard, C.; Hoffmann, L.; Delfosse, P. Deoxynivalenol Concentration Decrease during Mesophilic Anaerobic Digestion of Wheat Flour. In Proceedings of the Third International Symposium on Energy from Biomass and Waste, Venice, Italy, 10–12 November 2010. [Google Scholar]
- Tacconi, C.; Cucina, M.; Pezzolla, D.; Zadra, C.; Gigliotti, G. Effect of the Mycotoxin Aflatoxin B1 on a Semi-Continuous Anaerobic Digestion Process. Waste Manag. 2018, 78, 467–473. [Google Scholar] [CrossRef]
- Guan, S.; Ji, C.; Zhou, T.; Li, J.; Ma, Q.; Niu, T. Aflatoxin B1 Degradation by Stenotrophomonas Maltophilia and Other Microbes Selected Using Coumarin Medium. Int. J. Mol. Sci. 2008, 9, 1489–1503. [Google Scholar] [CrossRef]
- Farzaneh, M.; Shi, Z.Q.; Ghassempour, A.; Sedaghat, N.; Ahmadzadeh, M.; Mirabolfathy, M.; Javan-Nikkhah, M. Aflatoxin B1 Degradation by Bacillus Subtilis UTBSP1 Isolated from Pistachio Nuts of Iran. Food Control 2012, 23, 100–106. [Google Scholar] [CrossRef]
- Lahtinen, S.J.; Haskard, C.A.; Ouwenhand, A.C.; Salminen, S.J.; Ahokas, J.T. Binding of Aflatoxin B1 to Cell Wall Components of Lactobacillus Rhamnosus Strain GG. Food Addit. Contam. 2004, 21, 158–164. [Google Scholar] [CrossRef]
- Gonzalez-Gil, L.; Papa, M.; Feretti, D.; Ceretti, E.; Mazzoleni, G.; Steimberg, N.; Pedrazzani, R.; Bertanza, G.; Lema, J.M.; Carballa, M. Is Anaerobic Digestion Effective for the Removal of Organic Micropollutants and Biological Activities from Sewage Sludge? Water Res. 2016, 102, 211–220. [Google Scholar] [CrossRef]
- Samaras, V.G.; Stasinakis, A.S.; Thomaidis, N.S.; Mamais, D.; Lekkas, T.D. Fate of Selected Emerging Micropollutants during Mesophilic, Thermophilic and Temperature Co-Phased Anaerobic Digestion of Sewage Sludge. Bioresour. Technol. 2014, 162, 365–372. [Google Scholar] [CrossRef]
- Tacconi, C.; Cucina, M.; Zadra, C.; Gigliotti, G.; Pezzolla, D. Plant Nutrients Recovery from Aflatoxin B1 Contaminated Corn through Co-Composting. J. Environ. Chem. Eng. 2019, 7, 103046. [Google Scholar] [CrossRef]
- Akoto, E.Y.; Klu, Y.A.K.; Lamptey, M.; Asibuo, J.Y.; Davis, J.; Phillips, R.; Jordan, D.; Rhoads, J.; Hoistington, D.; Chen, J. Use of Peanut Meal as a Model Matrix to Study the Effect of Composting on Aflatoxin Decontamination. World Mycotoxin J. 2017, 10, 131–141. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of Animal Manures and Chemical Criteria for Compost Maturity Assessment. A Review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Adebo, O.A.; Njobeh, P.B.; Gbashi, S.; Nwinyi, O.C.; Mavumengwana, V. Review on Microbial Degradation of Aflatoxins. Crit. Rev. Food Sci. Nutr. 2017, 57, 3208–3217. [Google Scholar] [CrossRef]
- Said-Pullicino, D.; Kaiser, K.; Guggenberger, G.; Gigliotti, G. Changes in the Chemical Composition of Water-Extractable Organic Matter during Composting: Distribution between Stable and Labile Organic Matter Pools. Chemosphere 2007, 66, 2166–2176. [Google Scholar] [CrossRef]
- Cucina, M.; Zadra, C.; Marcotullio, M.C.; di Maria, F.; Sordi, S.; Curini, M.; Gigliotti, G. Recovery of Energy and Plant Nutrients from a Pharmaceutical Organic Waste Derived from a Fermentative Biomass: Integration of Anaerobic Digestion and Composting. J. Environ. Chem. Eng. 2017, 5, 3051–3057. [Google Scholar] [CrossRef]
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorat, A.; Brattebo, H.; Almås, Å.; Singh, B.R. Sewage Sludge Disposal Strategies for Sustainable Development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.B.; Zakaria, M.P.; Latif, P.A.; Saari, N. Degradation of Veterinary Antibiotics and Hormone during Broiler Manure Composting. Bioresour. Technol. 2013, 131, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, I.; Audenaert, K.; de Gelder, L. Biodegradation of Mycotoxins: Tales from Known and Unexplored Worlds. Front. Microbiol. 2016, 7, 561. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, C.; Zhang, D.; Zhao, M.; Zheng, D.; Lyu, Y.; Cheng, W.; Guo, P.; Cui, Z. Effective Degradation of Aflatoxin B1 Using a Novel Thermophilic Microbial Consortium TADC7. Bioresour. Technol. 2017, 224, 166–173. [Google Scholar] [CrossRef]
- Velmourougane, K.; Bhat, R.; Gopinandhan, T.N. Composting Coffee Wastes, a Potential Source of Ochratoxigenic Fungi and Ochratoxin A Contamination. World Mycotoxin J. 2012, 5, 373–376. [Google Scholar] [CrossRef]
- Ji, C.; Fan, Y.; Zhao, L. Review on Biological Degradation of Mycotoxins. Anim. Nutr. 2016, 2, 127–133. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Sarsaiya, S.; Wainaina, S.; Rajendran, K.; Kumar, S.; Quan, W.; Duan, Y.; Awasthi, S.K.; Chen, H.; Pandey, A.; et al. A Critical Review of Organic Manure Biorefinery Models toward Sustainable Circular Bioeconomy: Technological Challenges, Advancements, Innovations, and Future Perspectives. Renew. Sustain. Energy Rev. 2019, 111, 115–131. [Google Scholar] [CrossRef]
- Li, Y.; Manandhar, A.; Li, G.; Shah, A. Life Cycle Assessment of Integrated Solid State Anaerobic Digestion and Composting for On-Farm Organic Residues Treatment. Waste Manag. 2018, 76, 294–305. [Google Scholar] [CrossRef]
- Zeng, Y.; de Guardia, A.; Dabert, P. Improving Composting as a Post-Treatment of Anaerobic Digestate. Bioresour. Technol. 2016, 201, 293–303. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Restrepo, A.P.; Alburquerque, J.A.; Pérez-Murcia, M.D.; Paredes, C.; Moral, R.; Bernal, M.P. Recycling of Anaerobic Digestates by Composting: Effect of the Bulking Agent Used. J. Clean. Prod. 2013, 47, 61–69. [Google Scholar] [CrossRef]
- Cucina, M.; Tacconi, C.; Gigliotti, G.; Zadra, C. Integration of Anaerobic Digestion and Composting Allows Safety Recovery of Energy and Nutrients from AFB1 Contaminated Corn. J. Environ. Chem. Eng. 2022, 10, 108356. [Google Scholar] [CrossRef]
- Capcarova, M.; Zbynovska, K.; Kalafova, A.; Bulla, J.; Bielik, P. Environment Contamination by Mycotoxins and Their Occurrence in Food and Feed: Physiological Aspects and Economical Approach. J. Environ. Sci. Health B 2016, 51, 236–244. [Google Scholar] [CrossRef]
- Magnoli, A.P.; Poloni, V.L.; Cavaglieri, L. Impact of Mycotoxin Contamination in the Animal Feed Industry. Curr. Opin. Food Sci. 2019, 29, 99–108. [Google Scholar] [CrossRef]
- di Maria, F.; Sisani, F.; Gigliotti, G.; Pezzolla, D.; Tacconi, C.; Cucina, M.; Zadra, C. Environmental Consequences of the Treatment of Corn Contaminated by Aflatoxin B1 with Co-Digestion and Co-Composting in a Life Cycle Perspective. Environ. Sci. Pollut. Res. 2021, 28, 9267–9275. [Google Scholar] [CrossRef]
- Cucchiella, F.; D’Adamo, I.; Gastaldi, M. An Economic Analysis of Biogas-Biomethane Chain from Animal Residues in Italy. J. Clean. Prod. 2019, 230, 382–389. [Google Scholar] [CrossRef]
- Domingo, J.L.; Nadal, M. Domestic Waste Composting Facilities: A Review of Human Health Risks. Environ. Int. 2009, 35, 1232. [Google Scholar] [CrossRef]
- Schlosser, O.; Robert, S.; Noyon, N. Airborne Mycotoxins in Waste Recycling and Recovery Facilities: Occupational Exposure and Health Risk Assessment. Waste Manag. 2020, 105, 395–404. [Google Scholar] [CrossRef]
- FDA U.S. Guidance for Industry: Action Levels for Poisonous or Deleterious Substances in Human Food and Animal Feed; FDA: Silver Spring, MD, USA, 2000.
| Product | Final Consumer | EU a (µg kg−1) | USA b (µg kg−1) |
|---|---|---|---|
| Corn | Humans | 4 | 20 |
| Corn (to be sorted) | Humans | 10 | - |
| Groundnuts | Humans | 4 | 20 |
| Groundnuts (to be sorted) | Humans | 15 | - |
| Corn | Immature animals | 10 | 20 |
| Corn | Mature animals | 20 | 100 |
| Corn | Mature feedlot cattle | 20 | 300 |
| Corn | Dairy cattle | 5 | 20 |
| Milk | Humans | 0.05 | 0.5 |
| Milk | Infants | 0.025 | 0.5 |
| Disposal System | Advantages | Disadvantages |
|---|---|---|
| Landfilling | Low disposal costs | Potential soil and water contaminations; slow AFs’ removal |
| Burying | Low disposal costs | Potential soil and water contaminations; slow AFs’ removal; plant uptake of AFs |
| Incineration | Low disposal costs (for low-technology incineration systems); complete AFs’ removal | Potential air contamination (for low-technology incineration systems); requirements of energy, investments and specialized staff (for high-technology incineration systems) |
| Anaerobic digestion | Recovery of energy and nutrients (biomethane and digestate); moderate efficiency for AFs removal | Requirements of initial investments and specialized staff |
| Composting | Recovery of nutrients (compost); high efficiency for AFs’ removal | Requirements of energy |
| Mycotoxin | Anaerobic Digestion | Organic Substrate | Biogas Production (NL/kg TS) | Methane (% v/v) | Process Stability | References |
|---|---|---|---|---|---|---|
| AFB1 | Batch mesophilic | Corn grain | 579–617 | 57–60 | n.a. | [70] |
| CSTR mesophilic | Corn grain | 580 | 58 | VFA, VFA/alkalinity, ammonium-N in optimal range | ||
| AFB1 | CSTR mesophilic | Corn flour | 600–625 | 50–55 | VFA, VFA/alkalinity, pH in optimal range | [71] |
| FB1 + FB2 + FB3 | Batch mesophilic | Corn silage | 170–180 | 55 | pH in optimal range | [72] |
| Batch mesophilic | Wholewheat flour | 340 | 55 | n.a. | ||
| Batch mesophilic | Wheat bran | 330 | 55 | n.a. | ||
| DON + T-2 + HT-2 | Batch mesophilic | Wheat fine bran | 350 | 55 | n.a. | [73] |
| Batch mesophilic | Wheat semolina | 350 | 50 | n.a. | ||
| Batch mesophilic | Wheat fine middlings | 300 | 50 | n.a. | ||
| AFB1 + DON + ZEN + OTA + FB1 + T-2 + ergot alkaloid mix | Batch mesophilic | Corn grain | 500–550 | 55–60 | n.a. | [74] |
| Batch thermophilic | Corn grain | 580–620 | 55–60 | n.a. | ||
| DON + 3-ADON + 15-ADON + AOH + T-2 + ZEN + FB1 + FB2 + ENNB | CSTR mesophilic | Corn grain | 680 | 60–65 | VFA, VFA/alkalinity, pH in optimal range | |
| DON | Batch mesophilic | Wheat flour | 667.2–742.8 | 50–55 | n.a. | [75] |
| CSTR thermophilic | Corn grain | 690 | 60–65 | VFA, VFA/alkalinity, pH in optimal range | [76] | |
| AFB1 | CSTR mesophilic | Corn grain | 700–800 (25 µg kg−1 AFB1) | 60–65 | VFA, VFA/alkalinity, ammonium-N, and pH in optimal range | |
| CSTR mesophilic | Corn grain | 0 (100 µg kg−1 AFB1) | 0 | VFA accumulation and pH decrease to inhibiting values | ||
| AFB1 + DON + ZEN + OTA + FB1 + T-2 + ergot alkaloid mix | Batch mesophilic | Corn grain | 500–550 | 55–60 | n.a. | |
| Batch thermophilic | Corn grain | 580–620 | 55–60 | n.a. | ||
| DON + 3-ADON + 15-ADON + AOH + T-2 + ZEN + FB1 + FB2 + ENNB | CSTR mesophilic | Corn grain | 680 | 60–65 | VFA, VFA/alkalinity, pH in optimal range | |
| CSTR thermophilic | Corn grain | 690 | 60–65 | VFA, VFA/alkalinity, pH in optimal range |
| Mycotoxin | Initial Contamination (µg kg−1) | Anaerobic Digestion | Organic Substrate | Average Mycotoxin Removal | References |
|---|---|---|---|---|---|
| AFB1 | 0.54–110.0 | Batch mesophilic | Corn grain | 69–87% | [70] |
| 7.2 | CSTR mesophilic | Corn grain | 61% | ||
| AFB1 | 2–470 | CSTR mesophilic | Corn flour | 12–95% | [71] |
| FB1 + FB2 + FB3 + AFB1 | 241.5–13874 (FB1) + 866.5–3877 (FB2) + 42.5–3591 (FB3) + 251 (AFB1) | Batch mesophilic | Corn silage | 20–60% (FB1, FB2, FB3) 55% (AFB1) | [72] |
| DON + T-2 + HT-2 | 368–12,916 (DON) + 5–65 (T-2+HT-2) | Batch mesophilic | Wholewheat flour | 89.9% (DON) 100% (T-2, HT-2) | [73] |
| 368–12,916 (DON) + 5–65 (T-2 + HT-2) | Batch mesophilic | Wheat bran | 88.5% (DON) 100% (T-2, HT-2) | ||
| 368–12,916 (DON) + 5–65 (T-2 + HT-2) | Batch mesophilic | Wheat fine bran | 83.9% (DON) 100% (T-2, HT-2) | ||
| 368–12,916 (DON) + 5–65 (T-2 + HT-2) | Batch mesophilic | Wheat semolina | 82.1% (DON) 100% (T-2, HT-2) | ||
| 368–12,916 (DON) + 5–65 (T-2 + HT-2) | Batch mesophilic | Wheat fine middlings | 98.7% (DON) 100% (T-2, HT-2) | ||
| AFB1 + DON + ZEN + OTA + FB1 + T-2 + ergot alkaloid mix | 40 (AFB1) + 300 (DON) + 100 (ZEN) + 50 (OTA) + 100 (FB1) + 100 (T-2) + 40 (ergot alkaloid mix) | Batch mesophilic | Corn grain | >90% (AFB1, DON, ZEN, OTA, T-2) 70% (FB1) 64% (ergot alkaloid mix) | [74] |
| Batch thermophilic | Corn grain | >90% (AFB1, DON, ZEN, OTA, T-2) 85% (FB1) 98% (ergot alkaloid mix) | |||
| DON + 3-ADON + 15-ADON + AOH + T-2 + ZEN + FB1 + FB2 + ENNB | 4413 (DON) + 729 (3-ADON + 15-ADON) + 14 (AOH) + 28 (T-2) + 1052 (ZEN) + >80 (FB1 + FB2) + >80 (ENNB) | CSTR mesophilic | Corn grain | >99% | |
| CSTR thermophilic | Corn grain | >99% | |||
| DON | 1976–80,000 | Batch mesophilic | Wheat flour | 100% | [75] |
| AFB1 | 25 | CSTR mesophilic | Corn grain | 18.8% * | [76] |
| 100 | CSTR mesophilic | Corn grain | 37.2% * |
| Mycotoxin | Initial Contamination (µg kg−1) | Organic Substrate | Composting Process | Peak Temperature (°C) | Average Mycotoxin Removal | References |
|---|---|---|---|---|---|---|
| AFB1 | 100 | Corn grain and pig slurry | Pilot scale, passive aerated, static composting | 75.5 | 85.7% | [82] |
| Corn grain and organic fraction of municipal solid wastes | 74.8 | 97.3% | ||||
| AFB1 + AFB2 + AFG1 + AFG2 | 195.4 (AFB1) + 22.2 (AFB2) + 2.9 (AFG1) + 1.2 (AFG2) | Peanut meal | Laboratory scale, actively aerated, continuously mixed composting | 36.4 | 58.6% (AFB1) 54.5% (AFB2) 96.6% (AFG1) 83.3% (AFG2) | [83] |
| 2955 (total AF) | Peanut seeds, peanut shells, peanut leaves, and cowpea pods | Pilot scale, actively aerated, 3-times a week mixed composting | n.a. | 77% | ||
| OTA | 0.37–1.66 | Coffee pulp and husks + bulking material | Real scale, passive aerated, monthly mixed composting | n.a. | 400–600% * | [84] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cucina, M.; Tacconi, C. Recovery of Energy and Nutrients from Mycotoxin-Contaminated Food Products through Biological Treatments in a Circular Economy Perspective: A Review. Agronomy 2022, 12, 3198. https://doi.org/10.3390/agronomy12123198
Cucina M, Tacconi C. Recovery of Energy and Nutrients from Mycotoxin-Contaminated Food Products through Biological Treatments in a Circular Economy Perspective: A Review. Agronomy. 2022; 12(12):3198. https://doi.org/10.3390/agronomy12123198
Chicago/Turabian StyleCucina, Mirko, and Chiara Tacconi. 2022. "Recovery of Energy and Nutrients from Mycotoxin-Contaminated Food Products through Biological Treatments in a Circular Economy Perspective: A Review" Agronomy 12, no. 12: 3198. https://doi.org/10.3390/agronomy12123198
APA StyleCucina, M., & Tacconi, C. (2022). Recovery of Energy and Nutrients from Mycotoxin-Contaminated Food Products through Biological Treatments in a Circular Economy Perspective: A Review. Agronomy, 12(12), 3198. https://doi.org/10.3390/agronomy12123198