Foliar Application of Chitosan and Phosphorus Alleviate the Potato virus Y-Induced Resistance by Modulation of the Reactive Oxygen Species, Antioxidant Defense System Activity and Gene Expression in Potato
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Potato Cultivar
2.2. Source of the Virus Isolate
2.3. Plant Materials
2.4. Source of the Plant Nutrition
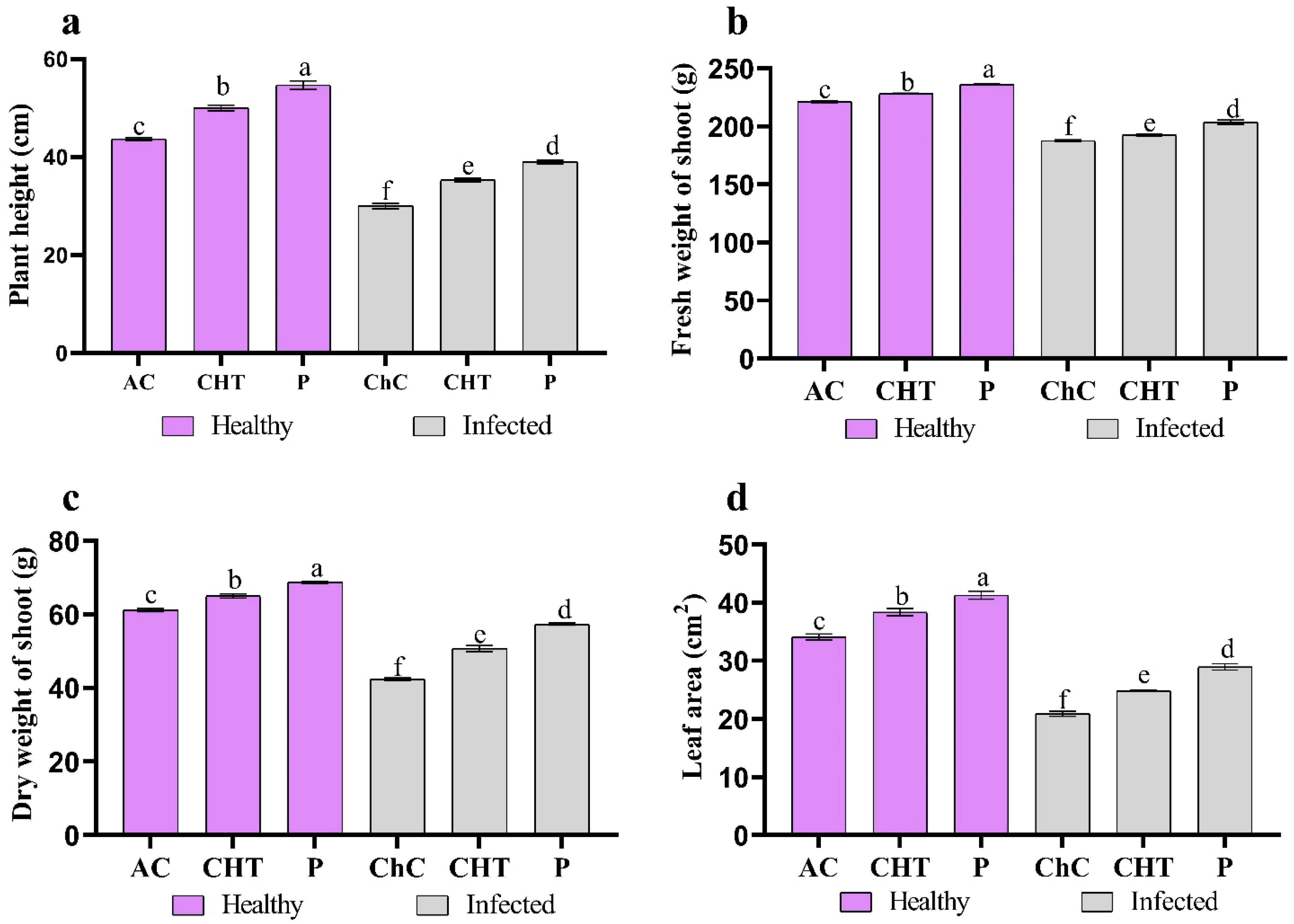
2.5. Growth Indices
2.6. Photosynthetic Characteristics and Chlorophyll Content
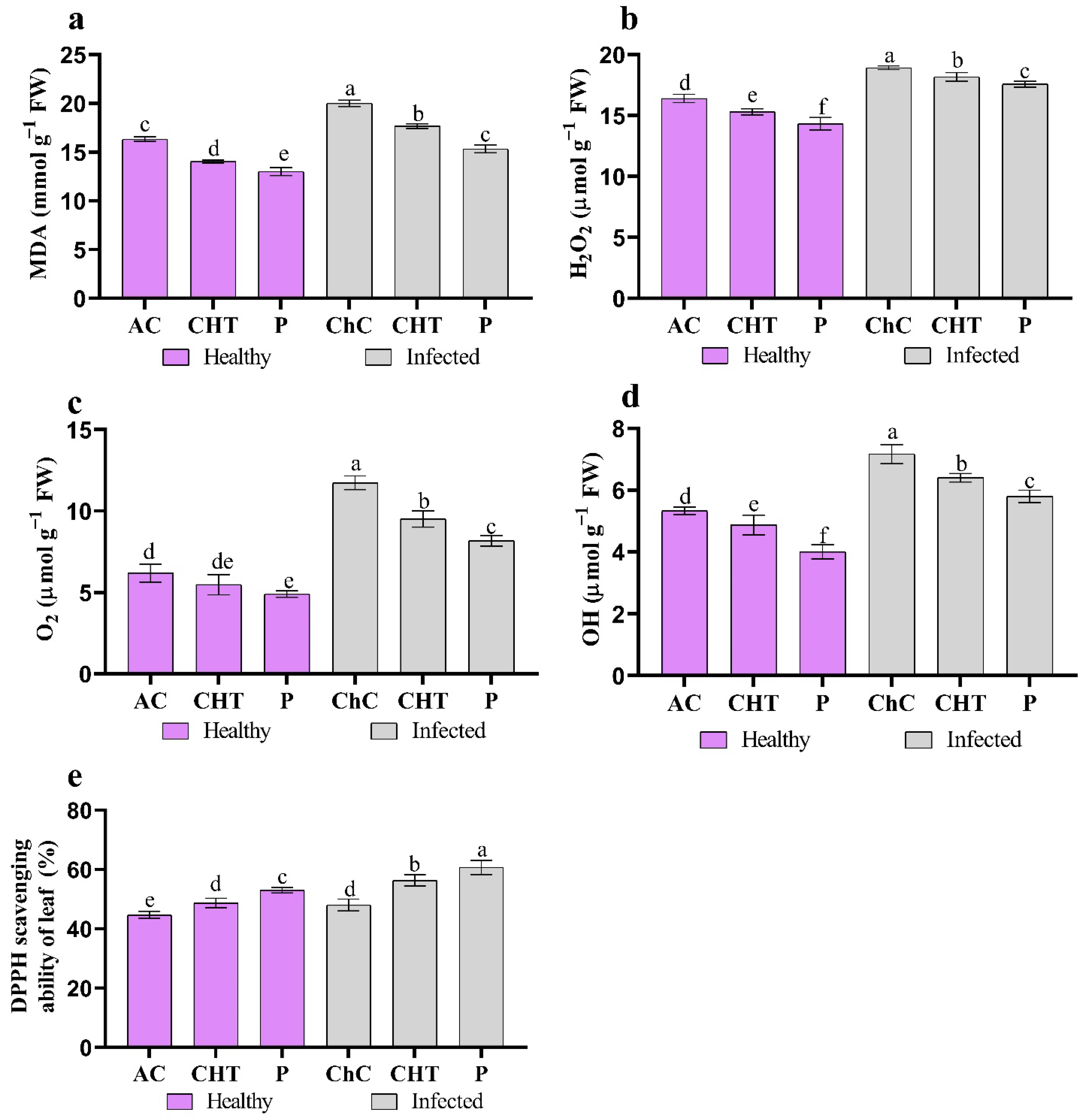
2.7. Oxidative Stress Markers
2.7.1. Proline
2.7.2. Glycine Betaine Analysis
2.7.3. Total Soluble Sugar Contents
2.7.4. Phenols
2.7.5. Glutathione (GSH)
2.7.6. Ascorbic Acid (AsA)
2.8. ROS Indicators
2.8.1. Lipid Peroxidation
2.8.2. Hydrogen Peroxide
2.8.3. Superoxide Anion
2.8.4. Hydroxyl Radical
2.8.5. DPPH Free Radical Scavenging Activity
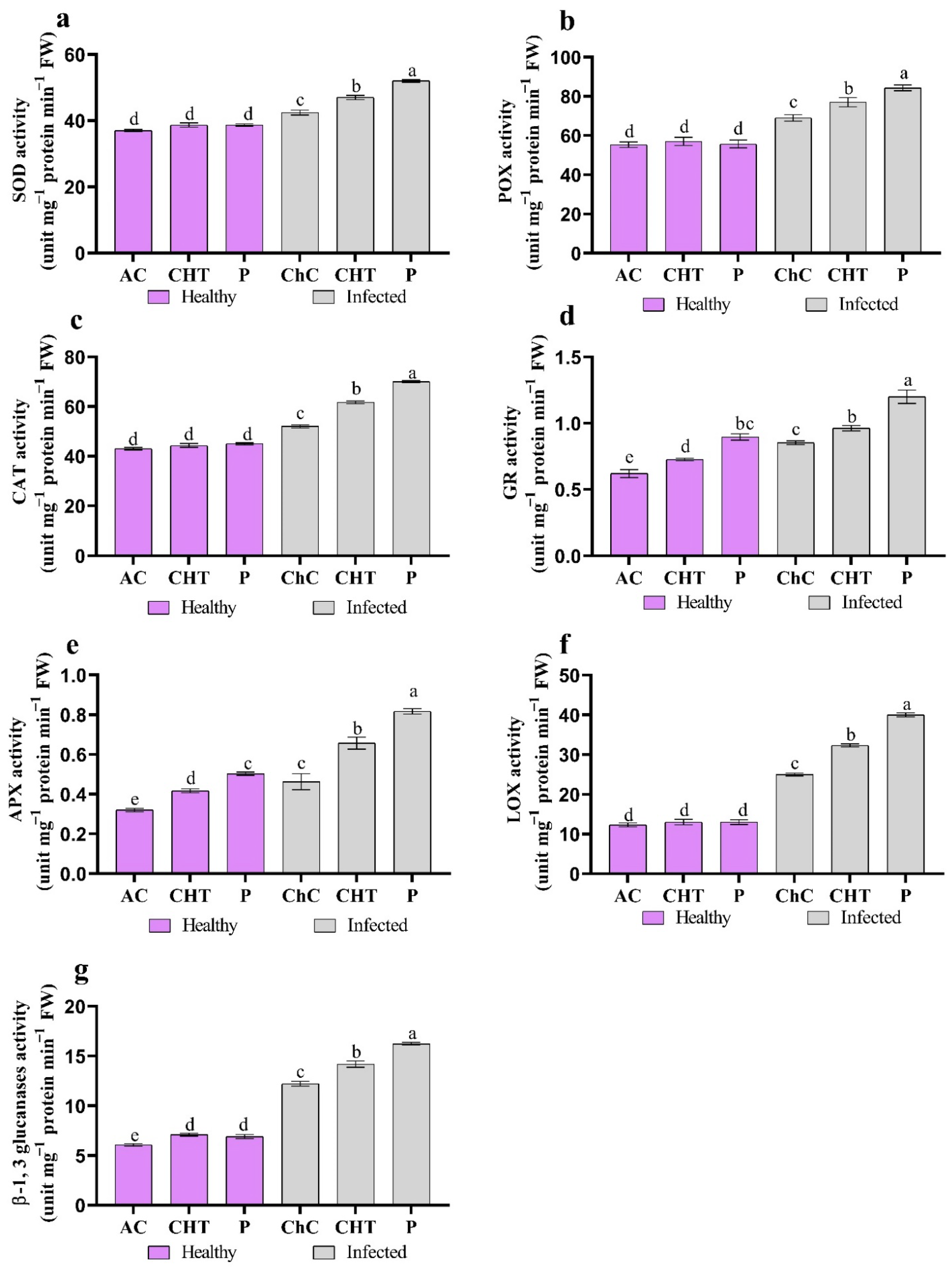
2.9. Extraction and Examination of Potato Leaves Were Utilized to Estimate Enzymes
2.10. Estimation of Hormonal Contents
2.11. Determination of Mineral Contents
2.12. qRT-PCR Analysis of the Related Genes Regulated in Potato Plants under Different Conditions
2.13. Statistical Analysis
3. Results
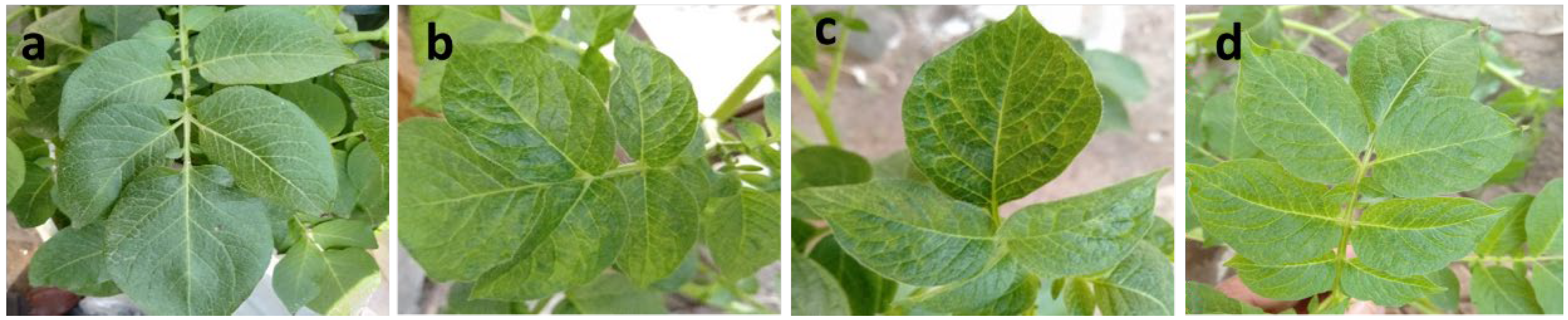
3.1. Disease Severity and Reduction of Virus Infectivity
3.2. Physiological and Biochemical Studies
3.2.1. Changes in Plant Growth
3.2.2. Chlorophyll Content and Photosynthetic Characteristics
3.2.3. Oxidative Stress Markers
3.2.4. ROS Indicators
3.2.5. Antioxidant Enzymes
3.2.6. Phytohormones Content
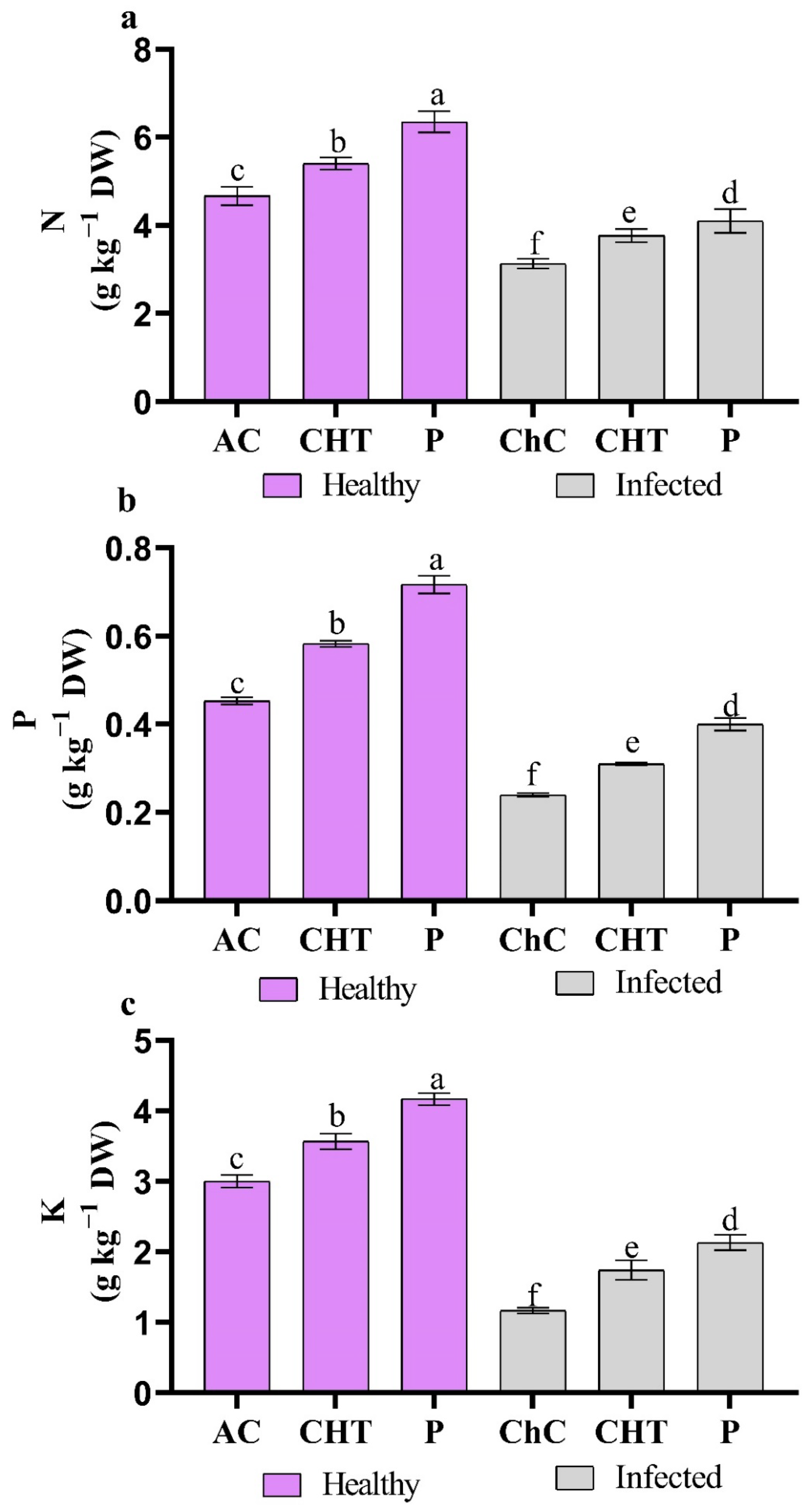
3.2.7. Minerals Content
3.2.8. Gene Expression
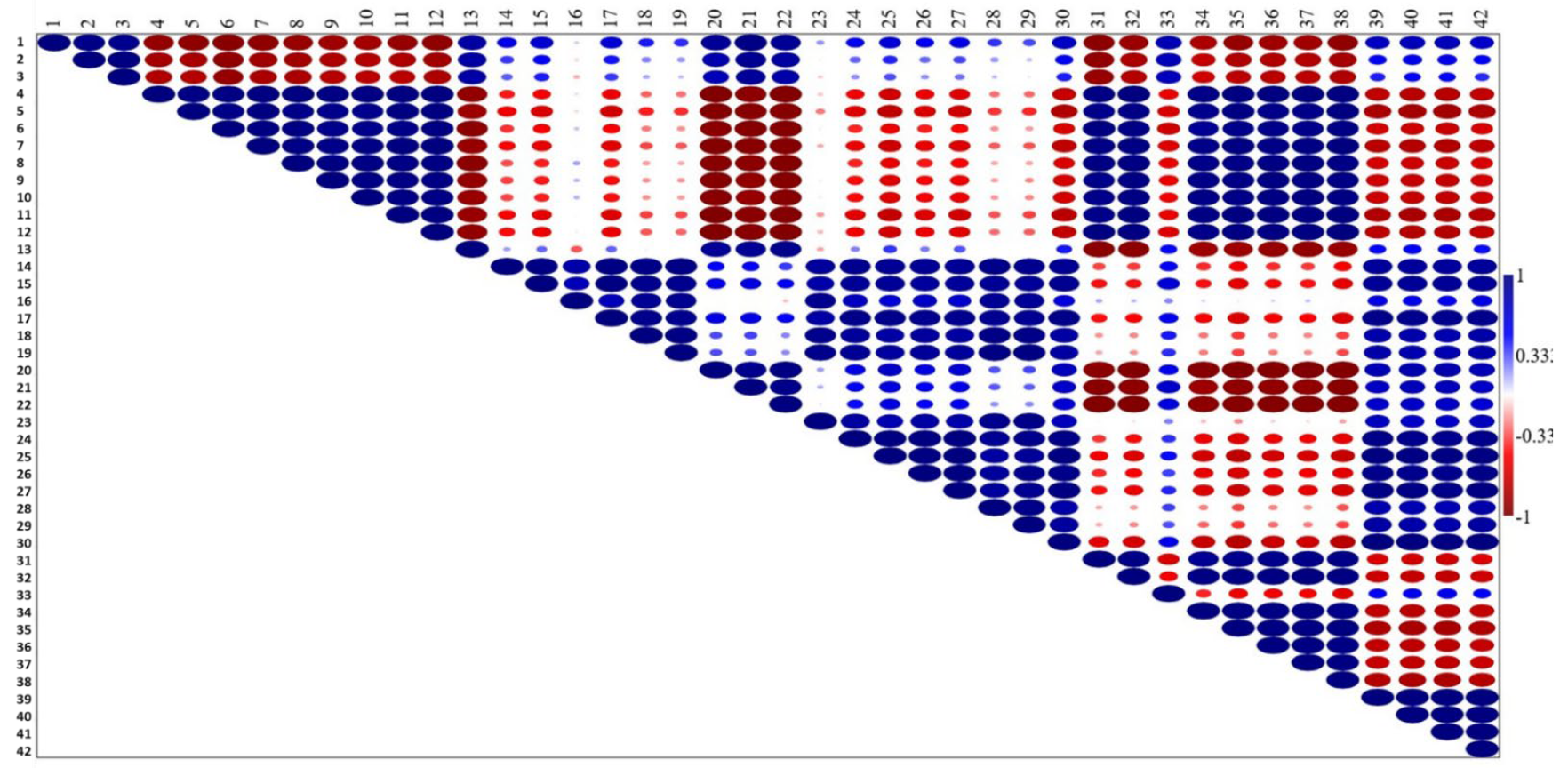
3.3. Correlation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Deloko, D.C.T.; Achiangia, N.P.; Chofong, N.G.; Mbulli, A.I.; Anoumaa, M.; Sama, L.F.; Fonkou, T. Prevalence of potato viruses on potato (Solanum tuberosum L.) grown in the Western Highlands of Cameroon. J. Agric. Food Res. 2021, 5, 100192. [Google Scholar] [CrossRef]
- Serrem, K.; Dunay, A.; Serrem, C.; Atubukha, B.; Oláh, J.; Illés, C.B. Paucity of nutrition guidelines and nutrient quality of meals served to kenyan boarding high school students. Sustainability 2020, 12, 3463. [Google Scholar] [CrossRef]
- Zaheer, K.; Akhtar, M.H. Potato production, usage, and nutrition—A review. Crit. Rev. Food Sci. Nutr. 2016, 56, 711–721. [Google Scholar] [CrossRef]
- Bond, J.K. Potato utilization and markets. In The Potato: Botany, Production and Uses; CABI: Wallingford, UK, 2014; pp. 29–44. [Google Scholar]
- Sofy, M.R.; Mancy, A.G.; Alnaggar, A.E.A.M.; Refaey, E.E.; Mohamed, H.I.; Elnosary, M.E.; Sofy, A.R. A polishing the harmful effects of Broad Bean Mottle Virus infecting broad bean plants by enhancing the immunity using different potassium concentrations. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12654. [Google Scholar] [CrossRef]
- Rubio, L.; Galipienso, L.; Ferriol, I. Detection of plant viruses and disease management: Relevance of genetic diversity and evolution. Front. Plant Sci. 2020, 11, 1092. [Google Scholar] [CrossRef] [PubMed]
- Sofy, A.R.; Mahfouze, S.A.; El-Enany, M.A. Isozyme markers for response of wild potato species to potato spindle tuber viroid Egyptian isolate. World Appl. Sci. J 2013, 27, 1010–1022. [Google Scholar]
- Sofy, A.; Mousa, A.; Soliman, A.; El-Dougdoug, K.A. The limiting of climatic factors and predicting of suitable habitat for citrus gummy bark disease occurrence using GIS. Int. J. Virol. 2012, 8, 165–177. [Google Scholar] [CrossRef]
- Jones, R.A.; Barbetti, M.J. Influence of climate change on plant disease infections and epidemics caused by viruses and bacteria. CABI Rev. 2012, 7, 1–33. [Google Scholar] [CrossRef]
- Abd El-Aziz, M.H. The importance of Potato virus Y Potyvirus. J. Plant Sci. Phytopathol. 2020, 4, 9–15. [Google Scholar]
- Pieklarz, K.; Modrzejewska, Z. Sars-Cov-2—The Latest Global Threat and the Prospect of COVID-19 Therapy with the Use of Chitosan. Prog. Chem. Appl. Chitin Its Deriv. 2021, 26, 41–60. [Google Scholar] [CrossRef]
- Barber, M.; Bertram, R.; Ride, J. Chitin oligosaccharides elicit lignification in wounded wheat leaves. Physiol. Mol. Plant Pathol. 1989, 34, 3–12. [Google Scholar] [CrossRef]
- Felix, G.; Regenass, M.; Boller, T. Specific perception of subnanomolar concentrations of chitin fragments by tomato cells: Induction of extracellular alkalinization, changes in protein phosphorylation, and establishment of a refractory state. Plant J. 1993, 4, 307–316. [Google Scholar] [CrossRef]
- Kaku, H.; Shibuya, N.; Xu, P.; Aryan, A.P.; Fincher, G.B. N-acetylchitooligosaccharides elicit expression of a single (1→ 3)-β-glucanase gene in suspension-cultured cells from barley (Hordeum vulgare). Physiol. Plant. 1997, 100, 111–118. [Google Scholar] [CrossRef]
- Nojiri, H.; Sugimori, M.; Yamane, H.; Nishimura, Y.; Yamada, A.; Shibuya, N.; Kodama, O.; Murofushi, N.; Omori, T. Involvement of jasmonic acid in elicitor-induced phytoalexin production in suspension-cultured rice cells. Plant Physiol. 1996, 110, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Téllez, L.I.; Gómez-Merino, F.C. Nutrient management in strawberry: Effects on yield, quality and plant health. In Strawberries: Cultivation, Antioxidant Properties and Health Benefits; Malone, N., Ed.; Nova Science Publishers: New York, NY, USA, 2014; pp. 239–267. [Google Scholar]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Functions of macronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 135–189. [Google Scholar]
- Gupta, N.; Debnath, S.; Sharma, S.; Sharma, P.; Purohit, J. Role of nutrients in controlling the plant diseases in sustainable agriculture. In Agriculturally Important Microbes for Sustainable Agriculture; Springer: Berlin/Heidelberg, Germany, 2017; pp. 217–262. [Google Scholar]
- Zörb, C.; Senbayram, M.; Peiter, E. Potassium in agriculture–status and perspectives. J. Plant Physiol. 2014, 171, 656–669. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Abd-Elsalam, K.A.; Tmam, A.M.; Sofy, M.R. Silver-based nanomaterials for plant diseases management: Today and future perspectives. In Silver Nanomaterials for Agri-Food Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 495–526. [Google Scholar]
- Gugerli, P.; Gehriger, W. Enzyme-linked immunosorbent assay (ELISA) for the detection of potato leafroll virus and potato virus Y in potato tubers after artificial break of dormancy. Potato Res. 1980, 23, 353–359. [Google Scholar] [CrossRef]
- Clark, M.F.; Adams, A. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef]
- Estefan, G.; Sommer, R.; Ryan, J. Methods of soil, plant, and water analysis. Man. West Asia North Afr. Reg. 2013, 3, 65–119. [Google Scholar]
- Sofy, A.R.; Attia, M.S.; Sharaf, A.; El-Dougdoug, K.A. Potential impacts of seed bacterization or salix extract in faba bean for enhancing protection against bean yellow mosaic disease. Nat. Sci. 2014, 12, 67–82. [Google Scholar]
- Yang, X.; Kang, L.; Tien, P. Resistance of tomato infected with cucumber mosaic virus satellite RNA to potato spindle tuber viroid. Ann. Appl. Biol. 1996, 129, 543–551. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circular. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Faizan, M.; Faraz, A.; Yusuf, M.; Khan, S.; Hayat, S. Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica 2018, 56, 678–686. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Grieve, C.; Grattan, S. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 1983, 70, 303–307. [Google Scholar] [CrossRef]
- Yemm, E.; Willis, A. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508. [Google Scholar] [CrossRef]
- Ziouti, A.; El Modafar, C.; Fleuriet, A.; El Boustani, E. Les polyphenols, marqueurs potentiels de la resistance du palmier dattier (Phoenix dactylifera L.) au Fausarium oxysporum f. sp. albedinis. Bull. De Liaison-Groupe Polyphen. 1992, 16, 346. [Google Scholar]
- Owens, C.; Belcher, R. A colorimetric micro-method for the determination of glutathione. Biochem. J. 1965, 94, 705. [Google Scholar] [CrossRef] [PubMed]
- Jagota, S.; Dani, H. A new colorimetric technique for the estimation of vitamin C using Folin phenol reagent. Anal. Biochem. 1982, 127, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Jabs, T.; Dietrich, R.A.; Dangl, J.L. Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Science 1996, 273, 1853–1856. [Google Scholar] [CrossRef]
- Babbs, C.F.; Pham, J.A.; Coolbaugh, R.C. Lethal hydroxyl radical production in paraquat-treated plants. Plant Physiol. 1989, 90, 1267–1270. [Google Scholar] [CrossRef]
- Shimada, K.; Fujikawa, K.; Yahara, K.; Nakamura, T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J. Agric. Food Chem. 1992, 40, 945–948. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Castillo, F.J.; Penel, C.; Greppin, H. Peroxidase release induced by ozone in Sedum album leaves: Involvement of Ca2+. Plant Physiol. 1984, 74, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cao, X.; Lu, Y.; Wang, X. Effects of rare earth metal ions and their EDTA complexes on antioxidant enzymes of fish liver. Bull. Environ. Contam. Toxicol. 2000, 65, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Jiang, M.; Zhang, J. Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J. Exp. Bot. 2002, 53, 2401–2410. [Google Scholar] [CrossRef] [PubMed]
- Todd, J.F.; Paliyath, G.; Thompson, J.E. Characteristics of a membrane-associated lipoxygenase in tomato fruit. Plant Physiol. 1990, 94, 1225–1232. [Google Scholar] [CrossRef]
- Abeles, F.B.; Forrence, L.E. Temporal and hormonal control of β-1, 3-glucanase in Phaseolus vulgaris L. Plant Physiol. 1970, 45, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Knegt, E.; Bruinsma, J. A rapid, sensitive and accurate determination of indolyl-3-acetic acid. Phytochemistry 1973, 12, 753–756. [Google Scholar] [CrossRef]
- Lee, B.; Martin, P.; Bangerth, F. The effect of sucrose on the levels of abscisic acid, indoleacetic acid and zeatin/zeatin riboside in wheat ears growing in liquid culture. Physiol. Plant. 1989, 77, 73–80. [Google Scholar] [CrossRef]
- Bremner, J. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960, 55, 11–33. [Google Scholar] [CrossRef]
- Page, A.; Miller, R.; Keeny, D. Methods of Soil Analysis. Part II. Chemical and Microbiological Methods; American Society of Agronomy: Madison, WI, USA, 1982. [Google Scholar]
- Houba, V.; Uittenbogaard, J.; Pellen, P. Wageningen evaluating programmes for analytical laboratories (WEPAL), organization and purpose. Commun. Soil Sci. Plant Anal. 1996, 27, 421–431. [Google Scholar] [CrossRef]
- Wang, X.; Tang, C.; Zhang, G.; Li, Y.; Wang, C.; Liu, B.; Qu, Z.; Zhao, J.; Han, Q.; Huang, L. cDNA-AFLP analysis reveals differential gene expression in compatible interaction of wheat challenged with Puccinia striiformis f. sp. tritici. BMC Genom. 2009, 10, 289. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Chirkov, S. The antiviral activity of chitosan. Appl. Biochem. Microbiol. 2002, 38, 1–8. [Google Scholar] [CrossRef]
- Kochkina, Z.; Chirkov, S. Influence of the chitosan oligomer on the phage particles and reproduction of phage 1-97A in the culture of Bacillus thuringiensis. Microbiology 2001, 70, 706–710. [Google Scholar] [CrossRef]
- Beffa, R.S.; Hofer, R.-M.; Thomas, M.; Meins, F., Jr. Decreased Susceptibility to Viral Disease of [beta]-1, 3-Glucanase-Deficient Plants Generated by Antisense Transformation. Plant Cell 1996, 8, 1001–1011. [Google Scholar] [CrossRef]
- Stavolone, L.; Lionetti, V. Extracellular matrix in plants and animals: Hooks and locks for viruses. Front. Microbiol. 2017, 8, 1760. [Google Scholar] [CrossRef] [PubMed]
- Agnieszka, S.; Robert, P.; Del, V.L.; Silvano, S.; Gianluca, C. Interactions among genotype, environment and agronomic practices on production and quality of storage onion (Allium cepa L.)–A review. Hortic. Sci. 2017, 44, 21–42. [Google Scholar]
- Watanabe, M.; Walther, D.; Ueda, Y.; Kondo, K.; Ishikawa, S.; Tohge, T.; Burgos, A.; Brotman, Y.; Fernie, A.R.; Hoefgen, R. Metabolomic markers and physiological adaptations for high phosphate utilization efficiency in rice. Plant Cell Environ. 2020, 43, 2066–2079. [Google Scholar] [CrossRef]
- Gabbani, M.; Abubaker, M.Y.; Irabi, A.I.; Elhassan, S. Amelioration of biotic stress induced by onion yellow dwarf potyvirus on onion seed crop using nutrition. ARJA 2018, 9, 1–14. [Google Scholar]
- Talbi Zribi, O.; Slama, I.; Trabelsi, N.; Hamdi, A.; Smaoui, A.; Abdelly, C. Combined effects of salinity and phosphorus availability on growth, gas exchange, and nutrient status of Catapodium rigidum. Arid Land Res. Manag. 2018, 32, 277–290. [Google Scholar] [CrossRef]
- Badawy, I.H.; Hmed, A.A.; Sofy, M.R.; Al-Mokadem, A.Z. Alleviation of Cadmium and Nickel Toxicity and Phyto-Stimulation of Tomato Plant L. by Endophytic Micrococcus luteus and Enterobacter cloacae. Plants 2022, 11, 2018. [Google Scholar] [CrossRef] [PubMed]
- De Bang, T.C.; Husted, S.; Laursen, K.H.; Persson, D.P.; Schjoerring, J.K. The molecular–physiological functions of mineral macronutrients and their consequences for deficiency symptoms in plants. New Phytol. 2021, 229, 2446–2469. [Google Scholar] [CrossRef]
- Zheng, K.; Lu, J.; Li, J.; Yu, Y.; Zhang, J.; He, Z.; Ismail, O.M.; Wu, J.; Xie, X.; Li, X. Efficiency of chitosan application against Phytophthora infestans and the activation of defence mechanisms in potato. Int. J. Biol. Macromol. 2021, 182, 1670–1680. [Google Scholar] [CrossRef] [PubMed]
- Campos-Soriano, L.; Bundó, M.; Bach-Pages, M.; Chiang, S.F.; Chiou, T.J.; San Segundo, B. Phosphate excess increases susceptibility to pathogen infection in rice. Mol. Plant Pathol. 2020, 21, 555–570. [Google Scholar] [CrossRef]
- Fouda, H.M.; Sofy, M.R. Effect of biological synthesis of nanoparticles from Penicillium chrysogenum as well as traditional salt and chemical nanoparticles of zinc on canola plant oil productivity and metabolic activity. Egypt. J. Chem. 2022, 65, 1–2. [Google Scholar] [CrossRef]
- Araujo, L.; Bispo, W.M.S.; Rios, V.S.; Fernandes, S.A.; Rodrigues, F.A. Induction of the Phenylpropanoid Pathway by Acibenzolar-S-Methyl and Potassium Phosphite Increases Mango Resistance to Ceratocystis fimbriata Infection. Plant Dis. 2015, 99, 447–459. [Google Scholar] [CrossRef]
- Zafar, Z.U.; Athar, H.-U.-R. Influence of different phosphorus regimes on disease resistance in two cotton (Gossypium hirsutum L.) cultivars differing in resistance to cotton leaf curl virus (clcuv). Pak. J. Bot. 2013, 45, 617–627. [Google Scholar]
- Chakraborty, M.; Hasanuzzaman, M.; Rahman, M.; Khan, M.A.R.; Bhowmik, P.; Mahmud, N.U.; Tanveer, M.; Islam, T. Mechanism of plant growth promotion and disease suppression by chitosan biopolymer. Agriculture 2020, 10, 624. [Google Scholar] [CrossRef]
- El-Sheshtawy, H.S.; Sofy, M.R.; Ghareeb, D.A.; Yacout, G.A.; Eldemellawy, M.A.; Ibrahim, B.M. Eco-friendly polyurethane acrylate (PUA)/natural filler-based composite as an antifouling product for marine coating. App. Microbiol. Biotechnol. 2021, 105, 7023–7034. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, C.; Zheng, H.; Sun, M.; Zhang, F.; Zhang, M.; Cui, F.; Lv, D.; Liu, L.; Guo, S. Antagonistic interaction between auxin and SA signaling pathways regulates bacterial infection through lateral root in Arabidopsis. Cell Rep. 2020, 32, 108060. [Google Scholar] [CrossRef]
- Singh, S.K.; Badgujar, G.; Reddy, V.R.; Fleisher, D.H.; Bunce, J.A. Carbon dioxide diffusion across stomata and mesophyll and photo-biochemical processes as affected by growth CO2 and phosphorus nutrition in cotton. J. Plant Physiol. 2013, 170, 801–813. [Google Scholar] [CrossRef]
- Rahoutei, J.; García-Luque, I.; Barón, M. Inhibition of photosynthesis by viral infection: Effect on PSII structure and function. Physiol. Plant. 2000, 110, 286–292. [Google Scholar] [CrossRef]
- Sofy, A.R.; Sofy, M.R.; Hmed, A.A.; Dawoud, R.A.; Alnaggar, A.E.-A.M.; Soliman, A.M.; El-Dougdoug, N.K. Ameliorating the adverse effects of Tomato mosaic tobamovirus infecting tomato plants in Egypt by boosting immunity in tomato plants using zinc oxide nanoparticles. Molecules 2021, 26, 1337. [Google Scholar] [CrossRef] [PubMed]
- Cacique, I.S.; Bispo, W.M.S.; Araujo, L.; Aucique-Pérez, C.E.; Rios, J.A.; Silva, L.C.; Rodrigues, F.Á. Potassium-modulated physiological performance of mango plants infected by Ceratocystis fimbriata. Bragantia 2017, 76, 521–535. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Dimkpa, C.O.; Pandey, R. Exploring phosphorus fertilizers and fertilization strategies for improved human and environmental health. Biol. Fertil. Soils 2020, 56, 299–317. [Google Scholar]
- Havlin, J.L.; Schlegel, A.J. Review of phosphite as a plant nutrient and fungicide. Soil Syst. 2021, 5, 52. [Google Scholar] [CrossRef]
- Hossain, M.B.; Ryu, K.S. Effect of foliar applied phosphatic fertilizer on absorption pathways, yield and quality of sweet persimmon. Sci. Hortic. 2009, 122, 626–632. [Google Scholar] [CrossRef]
- Farahat, G.; Salama, N.H.; Elgamal, I.S.; Ghazy, N.A.; Elsharkawy, M.M. Impact of humic acid, vigamax and potassium phosphate on control of cercospora leaf spot disease. Arch. Phytopathol. Plant Prot. 2022, 55, 1661–1685. [Google Scholar] [CrossRef]
- Hakmaoui, A.; Pérez-Bueno, M.; García-Fontana, B.; Camejo, D.; Jiménez, A.; Sevilla, F.; Barón, M. Analysis of the antioxidant response of Nicotiana benthamiana to infection with two strains of Pepper mild mottle virus. J. Exp. Bot. 2012, 63, 5487–5496. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.F.A.; Abu-Elsaoud, A.M.; Sofy, M.R.; Mohamed, H.I.; Soliman, M.H. Appraisal of kinetin spraying strategy to alleviate the harmful effects of UVC stress on tomato plants. Env. Sci. Poll. Res. 2022, 29, 52378–52398. [Google Scholar] [CrossRef]
- El-Sheshtawy, H.S.; Mahdy, H.M.; Sofy, A.R.; Sofy, M.R. Production of biosurfactant by Bacillus megaterium and its correlation with lipid peroxidation of Lactuca sativa. Egypt. J. Petrol. 2022, 31, 1–6. [Google Scholar] [CrossRef]
- Martín, J.A.; Sobrino-Plata, J.; Rodríguez-Calcerrada, J.; Collada, C.; Gil, L. Breeding and scientific advances in the fight against Dutch elm disease: Will they allow the use of elms in forest restoration? New For. 2019, 50, 183–215. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Kennedy, J.F.; Jiang, M.; Cai, Q.; Wu, X. Chitosan induces resistance to tuber rot in stored potato caused by Alternaria tenuissima. Int. J. Biol. Macromol. 2019, 140, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Maksoud, M.A.; Bekhit, M.; El-Sherif, D.M.; Sofy, A.R.; Sofy, M.R. Gamma radiation-induced synthesis of a novel chitosan/silver/Mn-Mg ferrite nanocomposite and its impact on cadmium accumulation and translocation in brassica plant growth. Int. J. Biol. Macromol. 2022, 194, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.F.; Sofy, M.R.; Mohamed, H.I.; Sofy, A.R.; Abdel-kader, H.A. Hydrogen Sulfide Modulates Salinity Stress in Common Bean Plants by Maintaining Osmolytes and Regulating Nitric Oxide Levels and Antioxidant Enzyme Expression. J. Soil Sci. Plant Nutr. 2022, 22, 3708–3726. [Google Scholar] [CrossRef]
- Sofy, A.R.; Abd El Haliem, N.F.; Refaey, E.E.; Hmed, A.A. Polyvalent phage CoNShP-3 as a natural antimicrobial agent showing lytic and antibiofilm activities against antibiotic-resistant coagulase-negative staphylococci strains. Foods 2020, 9, 673. [Google Scholar] [CrossRef]
- Zayed, M.S.; Taha, E.-K.A.; Hassan, M.M.; Elnabawy, E.-S.M. Enhance Systemic Resistance Significantly Reduces the Silverleaf Whitefly Population and Increases the Yield of Sweet Pepper, Capsicum annuum L. var. annuum. Sustainability 2022, 14, 6583. [Google Scholar] [CrossRef]
- Mas-Bargues, C.; Escrivá, C.; Dromant, M.; Borrás, C.; Viña, J. Lipid peroxidation as measured by chromatographic determination of malondialdehyde. Human plasma reference values in health and disease. Arch. Biochem. Biophys. 2021, 709, 108941. [Google Scholar] [CrossRef]
- Radwan, D.E.M.; Fayez, K.A.; Younis Mahmoud, S.; Lu, G. Modifications of antioxidant activity and protein composition of bean leaf due to Bean yellow mosaic virus infection and salicylic acid treatments. Acta Physiol. Plant. 2010, 32, 891–904. [Google Scholar] [CrossRef]
- Abdelaal, K.; Attia, K.A.; Niedbała, G.; Wojciechowski, T.; Hafez, Y.; Alamery, S.; Alateeq, T.K.; Arafa, S.A. Mitigation of Drought Damages by Exogenous Chitosan and Yeast Extract with Modulating the Photosynthetic Pigments, Antioxidant Defense System and Improving the Productivity of Garlic Plants. Horticulturae 2021, 7, 510. [Google Scholar] [CrossRef]
- Li, T.; Huang, Y.; Xu, Z.-S.; Wang, F.; Xiong, A.-S. Salicylic acid-induced differential resistance to the Tomato yellow leaf curl virus among resistant and susceptible tomato cultivars. BMC Plant Biol. 2019, 19, 173. [Google Scholar] [CrossRef] [PubMed]
- Vitti, A.; La Monaca, E.; Sofo, A.; Scopa, A.; Cuypers, A.; Nuzzaci, M. Beneficial effects of Trichoderma harzianum T-22 in tomato seedlings infected by Cucumber mosaic virus (CMV). BioControl 2015, 60, 135–147. [Google Scholar] [CrossRef]
- Mohammadi, M.A.; Han, X.; Zhang, Z.; Xi, Y.; Boorboori, M.; Wang-Pruski, G. Phosphite application alleviates Pythophthora infestans by modulation of photosynthetic and physio-biochemical metabolites in potato leaves. Pathogens 2020, 9, 170. [Google Scholar] [CrossRef]
- Abu-Shahba, M.S.; Mansour, M.M.; Mohamed, H.I.; Sofy, M.R. Effect of biosorptive removal of cadmium ions from hydroponic solution containing indigenous garlic peel and mercerized garlic peel on lettuce productivity. Sci. Hortic. 2022, 293, 110727. [Google Scholar] [CrossRef]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M. Arsenic uptake, toxicity, detoxification, and speciation in plants: Physiological, biochemical, and molecular aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef]
- Ganusova, E.E.; Rice, J.H.; Carlew, T.S.; Patel, A.; Perrodin-Njoku, E.; Hewezi, T.; Burch-Smith, T.M. Altered expression of a chloroplast protein affects the outcome of virus and nematode infection. Mol. Plant-Microbe Interact. 2017, 30, 478–488. [Google Scholar] [CrossRef]
- Iglesias, V.A.; Meins, F. Movement of plant viruses is delayed in a β-1, 3-glucanase-deficient mutant showing a reduced plasmodesmatal size exclusion limit and enhanced callose deposition. Plant J. 2000, 21, 157–166. [Google Scholar] [CrossRef]
- Dobnik, D.; Baebler, Š.; Kogovšek, P.; Pompe-Novak, M.; Štebih, D.; Panter, G.; Janež, N.; Morisset, D.; Žel, J.; Gruden, K. β-1, 3-glucanase class III promotes spread of PVYNTN and improves in planta protein production. Plant Biotechnol. Rep. 2013, 7, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wan, J.; Zhang, Y.; Pan, B.; Lo, I.M. Selective phosphate removal from water and wastewater using sorption: Process fundamentals and removal mechanisms. Environ. Sci. Technol. 2019, 54, 50–66. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.B.M.; Khan, M.M.A.; Siddiqui, H.; Jahan, A. Chitosan and its oligosaccharides, a promising option for sustainable crop production-a review. Carbohydr. Polym. 2020, 227, 115331. [Google Scholar] [CrossRef] [PubMed]
- Bairwa, D.D.; Modhvadia, J.; Prajapat, B.S. Effect of Phosphorus and Sulphur Fertilization on Yield and Quality of Wheat (Triticum aestivum L.). Int. J. Curr. Microbiol. Appl. Sci. 2020, 9, 633–638. [Google Scholar] [CrossRef]
- Wright, D.M.; Jordan, G.J.; Lee, W.G.; Duncan, R.P.; Forsyth, D.M.; Coomes, D.A. Do leaves of plants on phosphorus-impoverished soils contain high concentrations of phenolic defence compounds? Funct. Ecol. 2010, 24, 52–61. [Google Scholar] [CrossRef]
- Chandra, S.; Chakraborty, N.; Dasgupta, A.; Sarkar, J.; Panda, K.; Acharya, K. Chitosan nanoparticles: A positive modulator of innate immune responses in plants. Sci. Rep. 2015, 5, 15195. [Google Scholar] [CrossRef]
- Nell, M.; Voetsch, M.; Vierheilig, H.; Steinkellner, S.; Zitterl-Eglseer, K.; Franz, C.; Novak, J. Effect of phosphorus uptake on growth and secondary metabolites of garden sage (Salvia officinalis L.). J. Sci. Food Agric. 2009, 89, 1090–1096. [Google Scholar] [CrossRef]
- Agha, M.S.; Abbas, M.A.; Sofy, M.R.; Haroun, S.A.; Mowafy, A.M. Dual inoculation of Bradyrhizobium and Enterobacter alleviates the adverse effect of salinity on Glycine max seedling. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12461. [Google Scholar] [CrossRef]
- ALHaithloul, H.A.S.; Khan, M.I.; Musa, A.; Ghoneim, M.M.; ALrashidi, A.A.; Khan, I.; Azab, E.; Gobouri, A.A.; Sofy, M.R.; El-Sherbiny, M. Phytotoxic effects of Acacia saligna dry leachates on germination, seedling growth, photosynthetic performance, and gene expression of economically important crops. PeerJ 2022, 10, e13623. [Google Scholar] [CrossRef]
- Wu, H.; Liu, L.; Chen, Y.; Liu, T.; Jiang, Q.; Wei, Z.; Li, C.; Wang, Z. Tomato SlCER1-1 catalyzes the synthesis of wax alkanes which increases the drought tolerance and fruit storability. Hortic. Res. 2022, 9, uhac004. [Google Scholar] [CrossRef]
- Alazem, M.; Lin, N.-S. Antiviral roles of abscisic acid in plants. Front. Plant Sci. 2017, 8, 1760. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.F.; Sofy, M.R.; Mohamed, H.I.; Sofy, A.R.; Abdel-Kader, H.A. N-or/and P-deprived Coccomyxa chodatii SAG 216–2 extracts instigated mercury tolerance of germinated wheat seedlings. Plant and Soil 2022, 1–29. [Google Scholar] [CrossRef]
- Oide, S.; Bejai, S.; Staal, J.; Guan, N.; Kaliff, M.; Dixelius, C. A novel role of PR 2 in abscisic acid (ABA) mediated, pathogen-induced callose deposition in Arabidopsis thaliana. New Phytol. 2013, 200, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Picchi, V.; Rossoni, M.; Gomarasca, S.; Ludwig, N.; Gargano, M.; Faoro, F. Chitosan antitranspirant activity is due to abscisic acid-dependent stomatal closure. Environ. Exp. Bot. 2009, 66, 493–500. [Google Scholar] [CrossRef]
- Cataldo, E.; Fucile, M.; Mattii, G.B. Biostimulants in viticulture: A sustainable approach against biotic and abiotic stresses. Plants 2022, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.; Benton, J.; Wolf, B.; Mills, H.A. Plant Analysis Handbook. A Practical Sampling, Preparation, Analysis, and Interpretation Guide; Micro-Macro Publishing, Inc.: Athens, GA, USA, 1991. [Google Scholar]
- Mascia, T.; Cillo, F.; Fanelli, V.; Finetti-Sialer, M.M.; De Stradis, A.; Palukaitis, P.; Gallitelli, D. Characterization of the interactions between Cucumber mosaic virus and Potato virus Y in mixed infections in tomato. Mol. Plant-Microbe Interact. 2010, 23, 1514–1524. [Google Scholar] [CrossRef]
- Campos-Vargas, R.; Saltveit, M.E. Involvement of putative chemical wound signals in the induction of phenolic metabolism in wounded lettuce. Physiol. Plant. 2002, 114, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Feng, G.; Chen, K. Defense responses of harvested tomato fruit to burdock fructooligosaccharide, a novel potential elicitor. Postharvest Biol. Technol. 2009, 52, 110–116. [Google Scholar] [CrossRef]
- Mandal, S.; Mitra, A. Reinforcement of cell wall in roots of Lycopersicon esculentum through induction of phenolic compounds and lignin by elicitors. Physiol. Mol. Plant Pathol. 2007, 71, 201–209. [Google Scholar] [CrossRef]
- Azarabadi, S.; Abdollahi, H.; Torabi, M.; Salehi, Z.; Nasiri, J. ROS generation, oxidative burst and dynamic expression profiles of ROS-scavenging enzymes of superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidase (APX) in response to Erwinia amylovora in pear (Pyrus communis L). Eur. J. Plant Pathol. 2017, 147, 279–294. [Google Scholar] [CrossRef]
- Radwan, D.E.M.; Fayez, K.A.; Mahmoud, S.Y.; Hamad, A.; Lu, G. Salicylic acid alleviates growth inhibition and oxidative stress caused by zucchini yellow mosaic virus infection in Cucurbita pepo leaves. Physiol. Mol. Plant Pathol. 2006, 69, 172–181. [Google Scholar] [CrossRef]
- Du, L.; Chen, E.; Wu, T.; Ruan, Y.; Wu, S. Resveratrol attenuates hydrogen peroxide-induced aging through upregulation of autophagy in human umbilical vein endothelial cells. Drug Des. Dev. Ther. 2019, 13, 747. [Google Scholar] [CrossRef] [PubMed]




| Soil Analysis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Practical Size Distribution | ||||||||||
| Coarse sand (%) | Fine sand (%) | Silt (%) | Clay (%) | Texture class | ||||||
| 45.22 | 27.94 | 15.60 | 11.24 | Sandy loam | ||||||
| Moisture content (%) at: | pH 1:2.5 | ECe (dS m−1) | CEC (cmolc kg−1) | OC (%) | OM (%) | CaCO3 (%) | ||||
| FC | PWP | AW | ||||||||
| 13.01 | 4.30 | 8.70 | 7.71 | 1.74 | 3.21 | 0.19 | 0.33 | 2.01 | ||
| Soluble ions | ||||||||||
| Cations | Anions | |||||||||
| Ca++ | Mg++ | Na+ | K+ | CO3= | HCO3− | Cl− | SO4= | |||
| 2.10 | 2.55 | 12.55 | 0.30 | 0.00 | 2.47 | 11.5 | 3.53 | |||
| Available macronutrients | ||||||||||
| N | P | K | ||||||||
| 35 | 7.3 | 31 | ||||||||
| Compost analysis | ||||||||||
| pH (1:10) | EC | OC % | OM % | C/N ratio | Macronutrients % | |||||
| 6.90 | 2.95 | 22.10 | 38. 01 | 13.31 | N | P | K | |||
| 1.66 | 0.67 | 1.00 | ||||||||
| Macronutrients (mgL−1) | Micronutrients (mgL−1) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | P | K | Ca | Mg | S | Fe | Mn | Zn | Cu | B | Mo |
| 105 | 15.5 | 117 | 100 | 24 | 64 | 1.5 | 0.50 | 0.50 | 0.05 | 0.30 | 0.05 |
| Gene | Forward Primer (′5–′3) | Reverse Primer (′5–′3) |
|---|---|---|
| SOD | CCGACAAGCAGATTCCTCTC | CAGGAGCAATTAACCCTGGA |
| APX | GCCTTCTTCGCTGACTATGC | TCCAGCGAGCTTTTCAGAAT |
| Pathogenesis related 1 basic (PR-1b) | TGGTGATTTCACGGGGAGGGCA | TCCGCACACTTGTCCGCTTGCA |
| Lipoxygenase (LOX) | GAGTTCTCCTCATGGTGTTCGTTTA | AGTAGTCTGACACCCAACTT |
| Actin | GACAACGGAACTGCACGATC | TACGCTGAGCTTCATCACCA |
| Treatment | Percentage of Infection % | Disease Severity % | Virus Concentration (Index) |
|---|---|---|---|
| Challenge control (ChC) | 92 | 75.22 | 1.68 |
| CHT + V | 36 | 21.67 | 0.96 |
| P + V | 20 | 11.33 | 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Mokadem, A.Z.; Alnaggar, A.E.-A.M.; Mancy, A.G.; Sofy, A.R.; Sofy, M.R.; Mohamed, A.K.S.H.; Abou Ghazala, M.M.A.; El-Zabalawy, K.M.; Salem, N.F.G.; Elnosary, M.E.; et al. Foliar Application of Chitosan and Phosphorus Alleviate the Potato virus Y-Induced Resistance by Modulation of the Reactive Oxygen Species, Antioxidant Defense System Activity and Gene Expression in Potato. Agronomy 2022, 12, 3064. https://doi.org/10.3390/agronomy12123064
Al-Mokadem AZ, Alnaggar AE-AM, Mancy AG, Sofy AR, Sofy MR, Mohamed AKSH, Abou Ghazala MMA, El-Zabalawy KM, Salem NFG, Elnosary ME, et al. Foliar Application of Chitosan and Phosphorus Alleviate the Potato virus Y-Induced Resistance by Modulation of the Reactive Oxygen Species, Antioxidant Defense System Activity and Gene Expression in Potato. Agronomy. 2022; 12(12):3064. https://doi.org/10.3390/agronomy12123064
Chicago/Turabian StyleAl-Mokadem, Alshymaa Z., Abd El-Aleem M. Alnaggar, Ahmed G. Mancy, Ahmed R. Sofy, Mahmoud R. Sofy, Abdel Kareem S. H. Mohamed, Mostafa M. A. Abou Ghazala, Khaled M. El-Zabalawy, Noura F. G. Salem, Mohamed E. Elnosary, and et al. 2022. "Foliar Application of Chitosan and Phosphorus Alleviate the Potato virus Y-Induced Resistance by Modulation of the Reactive Oxygen Species, Antioxidant Defense System Activity and Gene Expression in Potato" Agronomy 12, no. 12: 3064. https://doi.org/10.3390/agronomy12123064
APA StyleAl-Mokadem, A. Z., Alnaggar, A. E.-A. M., Mancy, A. G., Sofy, A. R., Sofy, M. R., Mohamed, A. K. S. H., Abou Ghazala, M. M. A., El-Zabalawy, K. M., Salem, N. F. G., Elnosary, M. E., & Agha, M. S. (2022). Foliar Application of Chitosan and Phosphorus Alleviate the Potato virus Y-Induced Resistance by Modulation of the Reactive Oxygen Species, Antioxidant Defense System Activity and Gene Expression in Potato. Agronomy, 12(12), 3064. https://doi.org/10.3390/agronomy12123064








