Toxicity and Preventive Activity of Chitosan, Equisetum arvense, Lecithin and Salix Cortex against Plasmopara viticola, the Causal Agent of Downy Mildew in Grapevine
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Plasmopara Viticola Inoculum
2.2. Commercial Products
2.3. Preventive Effect Assay on Plasmopara Viticola
2.4. Toxicity Assay on P. viticola
2.5. Half Maximal Inhibitory Concentration (IC50)
2.6. Whole-Plant Greenhouse Protection Assay
2.7. Disease Severity Evaluation
2.8. Statistical Analysis
3. Results
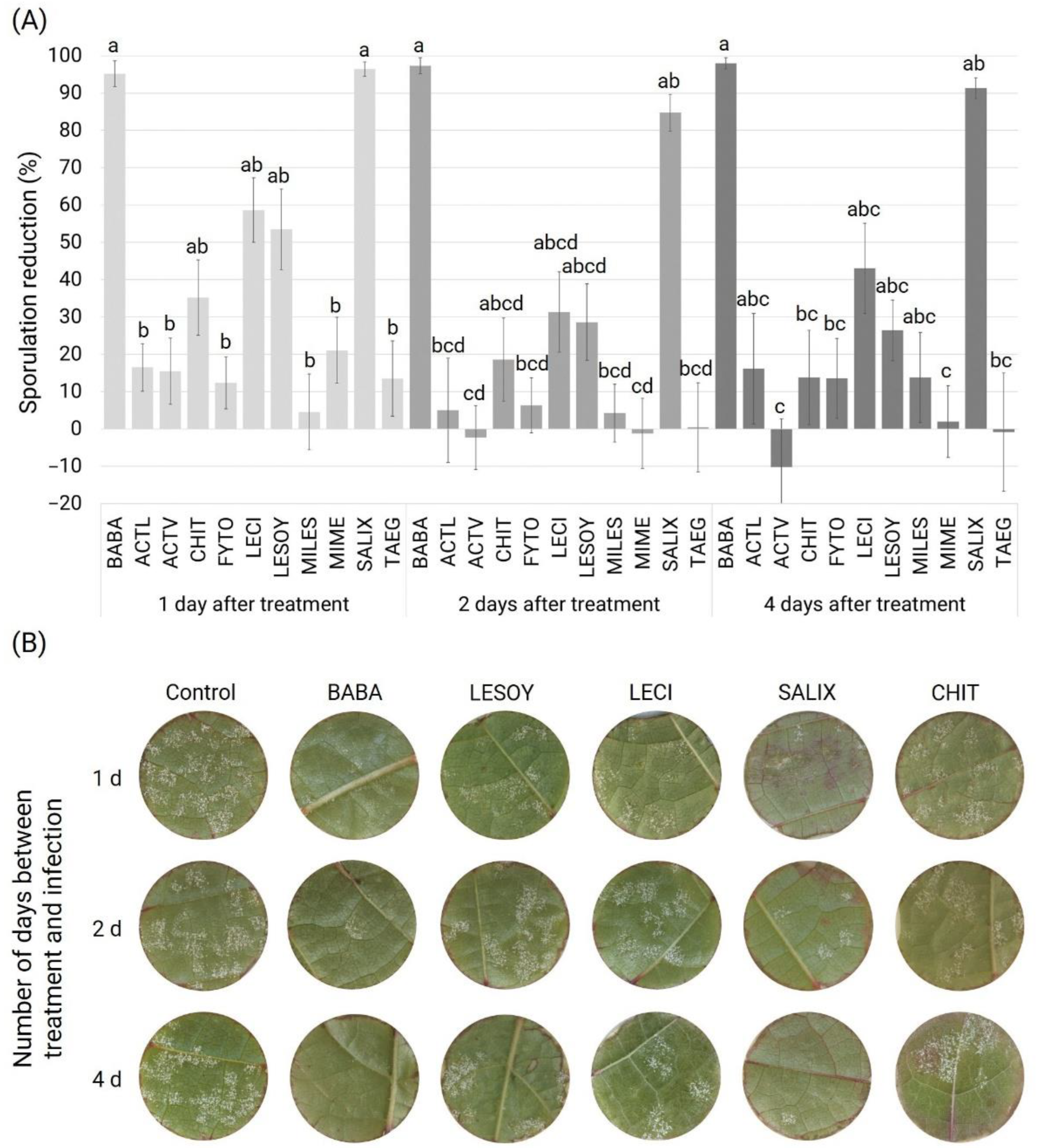
3.1. Preventive Assay
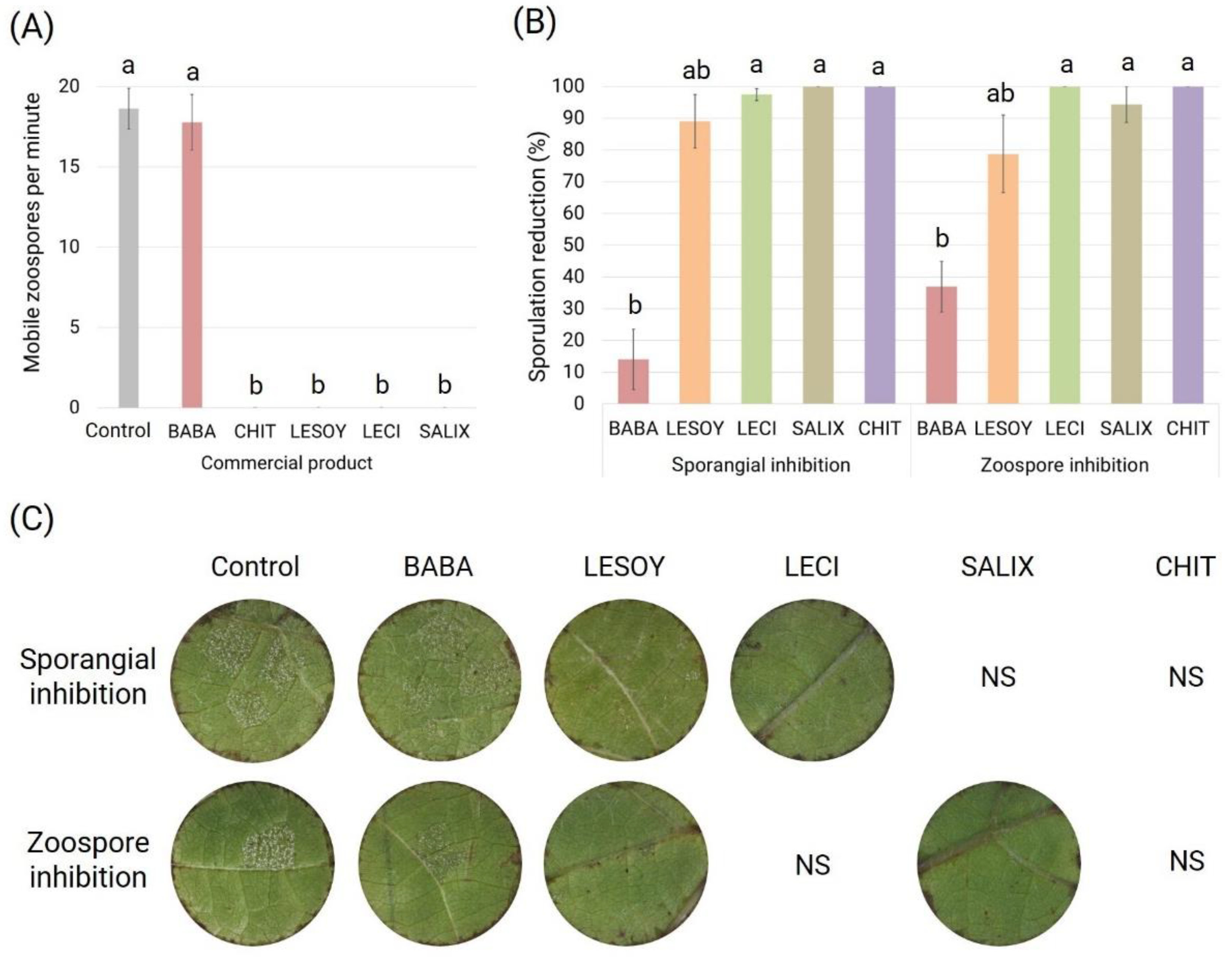
3.2. Toxicity Assay
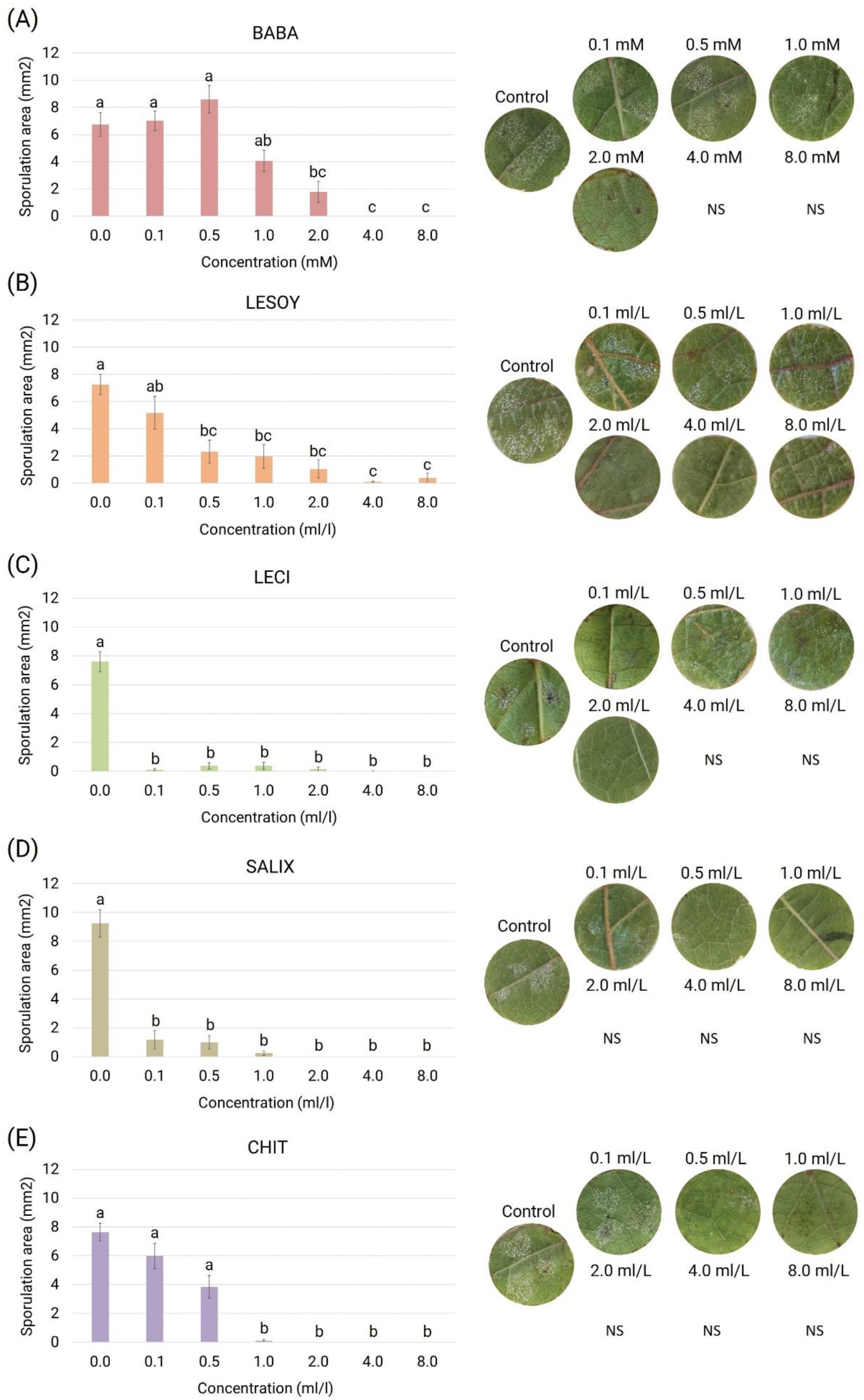
3.3. Half Maximal Inhibitory Concentration (IC50)
3.4. Whole-Plant Greenhouse Protection Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Organisation of Vine and Wine. State of the World Vine and Wine Sector 2021; International Organisation of Vine and Wine: Paris, France, 2022. [Google Scholar]
- Komárek, M.; Čadková, E.; Chrastný, V.; Bordas, F.; Bollinger, J.C. Contamination of Vineyard Soils with Fungicides: A Review of Environmental and Toxicological Aspects. Environ. Int. 2010, 36, 138–151. [Google Scholar] [CrossRef]
- Corio-Costet, M.F. Fungicide Resistance in Plasmopara viticola in France and Anti-Resistance Measures. In Fungicide Resistance in Crop Protection: Risk and Management; Thind, T.S., Ed.; CABI: Wallingford, UK, 2012; pp. 157–171. ISBN 978-1-84593-905-2. [Google Scholar]
- Massi, F.; Torriani, S.F.F.; Borghi, L.; Toffolatti, S.L. Fungicide Resistance Evolution and Detection in Plant Pathogens: Plasmopara viticola as a Case Study. Microorganisms 2021, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Commission Implementing Regulation (EU). Commission Implementing Regulation (EU) 2018/1981 of 13 December 2018 Renewing the Approval of the Active Substances Copper Compounds, as Candidates for Substitution, in Accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council Concerning the Placing of Plant Protection Products on the Market, and Amending the Annex to Commission Implementing Regulation (EU) No 540/2011. Off. J. Eur. Union 2018, 317, 16–20. [Google Scholar]
- Green Deal: Halving Pesticide Use by 2030. Available online: https://ec.europa.eu/eip/agriculture/en/news/green-deal-halving-pesticide-use-2030 (accessed on 5 October 2022).
- Berthon, J.Y.; Michel, T.; Wauquier, A.; Joly, P.; Gerbore, J.; Filaire, E. Seaweed and Microalgae as Major Actors of Blue Biotechnology to Achieve Plant Stimulation and Pest and Pathogen Biocontrol—A Review of the Latest Advances and Future Prospects. J. Agric. Sci. 2021, 159, 523–534. [Google Scholar] [CrossRef]
- Boulogne, I.; Petit, P.; Ozier-Lafontaine, H.; Desfontaines, L.; Loranger-Merciris, G. Insecticidal and Antifungal Chemicals Produced by Plants: A Review. Environ. Chem. Lett. 2012, 10, 325–347. [Google Scholar] [CrossRef]
- Pérez, M.; Falqué, E.; Domínguez, H. Antimicrobial Action of Compounds from Marine Seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef]
- Hou, S.; Liu, Z.; Shen, H.; Wu, D. Damage-Associated Molecular Pattern-Triggered Immunity in Plants. Front. Plant Sci. 2019, 10, 646. [Google Scholar] [CrossRef]
- Henry, G.; Thonart, P.; Ongena, M. PAMPs, MAMPs, DAMPs and Others: An Update on the Diversity of Plant Immunity Elicitors. Biotechnol. Agron. Soc. Environ. 2012, 16, 257–268. [Google Scholar]
- Quintana-Rodriguez, E.; Duran-Flores, D.; Heil, M.; Camacho-Coronel, X. Damage-Associated Molecular Patterns (DAMPs) as Future Plant Vaccines That Protect Crops from Pests. Sci. Hortic. 2018, 237, 207–220. [Google Scholar] [CrossRef]
- Héloir, M.C.; Adrian, M.; Brulé, D.; Claverie, J.; Cordelier, S.; Daire, X.; Dorey, S.; Gauthier, A.; Lemaître-Guillier, C.; Negrel, J.; et al. Recognition of Elicitors in Grapevine: From MAMP and DAMP Perception to Induced Resistance. Front. Plant Sci. 2019, 10, 1117. [Google Scholar] [CrossRef]
- Regulation (EC). Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC. Off. J. Eur. Union 2009, 309, 1–50. [Google Scholar]
- EU Pesticides Database—Active Substances. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/active-substances (accessed on 5 October 2022).
- International Organisation of Vine and Wine. The World Organic Vineyard; International Organisation of Vine and Wine: Paris, France, 2021. [Google Scholar]
- Kumari, S.; Kishor, R. Chitin and Chitosan: Origin, Properties, and Applications. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–33. ISBN 978-0-12-817970-3. [Google Scholar]
- Taylor, A.; Bonafos, R.; Chovelon, M.; Parvaud, C.E.; Furet, A.; Aveline, N.; Bertrand, C.; Marchand, P.A. Equisetum arvense (Horsetail) Extract: The First Approved Basic Substance Allowed for EU Crop Protection. Int. J. Bio-Resour. Stress Manag. 2022, 13, 566–577. [Google Scholar] [CrossRef]
- Marchand, P.A. Basic Substances under EC 1107/2009 Phytochemical Regulation: Experience with Non-Biocide and Food Products as Biorationals. J. Plant Prot. Res. 2016, 56, 312–318. [Google Scholar] [CrossRef]
- Deniau, M.G.; Bonafos, R.; Chovelon, M.; Parvaud, C.E.; Furet, A.; Bertrand, C.; Marchand, P.A. Willow Extract (Salix Cortex), a Basic Substance of Agronomical Interests. Int. J. Bio-Resour. Stress Manag. 2019, 10, 408–418. [Google Scholar] [CrossRef]
- Riseh, R.S.; Hassanisaadi, M.; Vatankhah, M.; Babaki, S.A.; Barka, E.A. Chitosan as a Potential Natural Compound to Manage Plant Diseases. Int. J. Biol. Macromol. 2022, 220, 998–1009. [Google Scholar] [CrossRef]
- El Hadrami, A.; Adam, L.R.; El Hadrami, I.; Daayf, F. Chitosan in Plant Protection. Mar. Drugs 2010, 8, 968. [Google Scholar] [CrossRef] [PubMed]
- De Bona, G.S.; Vincenzi, S.; De Marchi, F.; Angelini, E.; Bertazzon, N. Chitosan Induces Delayed Grapevine Defense Mechanisms and Protects Grapevine against Botrytis cinerea. J. Plant Dis. Prot. 2021, 128, 715–724. [Google Scholar] [CrossRef]
- Buzón-Durán, L.; Langa-Lomba, N.; González-García, V.; Casanova-Gascón, J.; Martín-Gil, J.; Pérez-Lebeña, E.; Martín-Ramos, P. On the Applicability of Chitosan Oligomers-Amino Acid Conjugate Complexes as Eco-Friendly Fungicides against Grapevine Trunk Pathogens. Agronomy 2021, 11, 324. [Google Scholar] [CrossRef]
- Vitalini, S.; Orlando, F.; Iriti, M. Field Study on the Efficacy of Plant Activators against Plasmopara viticola. Plant Fungal Res. 2020, 2, 2–7. [Google Scholar] [CrossRef]
- Aziz, A.; Trotel-Aziz, P.; Dhuicq, L.; Jeandet, P.; Couderchet, M.; Vernet, G. Chitosan Oligomers and Copper Sulfate Induce Grapevine Defense Reactions and Resistance to Gray Mold and Downy Mildew. Phytopathology 2006, 96, 1188–1194. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Mancini, V.; Carrasco-Quiroz, M.; Servili, A.; Gutiérrez-Gamboa, G.; Foglia, R.; Pérez-Álvarez, E.P.; Romanazzi, G. Chitosan and Laminarin as Alternatives to Copper for Plasmopara viticola Control: Effect on Grape Amino Acid. J. Agric. Food Chem. 2017, 65, 7379–7386. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Trotel-Aziz, P.; Conreux, A.; Dhuicq, L.; Jeandet, P.; Couderchet, M. Chitosan Induces Phytoalexin Synthesis, Chitinase and β-1,3-Glucanase Activities, and Resistance of Grapevine to Fungal Pathogens. In Macromolecules and Secondary Metabolites of Grapevine and Wines; Lavoisier, Intercept Ltd.: Paris, France, 2007. [Google Scholar]
- Andreu, V.; Levert, A.; Amiot, A.; Cousin, A.; Aveline, N.; Bertrand, C. Chemical Composition and Antifungal Activity of Plant Extracts Traditionally Used in Organic and Biodynamic Farming. Environ. Sci. Pollut. Res. 2018, 25, 29971–29982. [Google Scholar] [CrossRef] [PubMed]
- Langa-Lomba, N.; Buzón-Durán, L.; Martín-Ramos, P.; Casanova-Gascón, J.; Martín-Gil, J.; Sánchez-Hernández, E.; González-García, V. Assessment of Conjugate Complexes of Chitosan and Urtica Dioica or Equisetum arvense Extracts for the Control of Grapevine Trunk Pathogens. Agronomy 2021, 11, 976. [Google Scholar] [CrossRef]
- La Torre, A.; Righi, L.; Iovino, V.; Battaglia, V. Evaluation of Copper Alternative Products to Control Grape Downy Mildew in Organic Farming. J. Plant Pathol. 2019, 101, 1005–1012. [Google Scholar] [CrossRef]
- Pallag, A.; Bungau, S.; Tit, D.M.; Jurca, T.; Sirbu, V.; Honiges, A.; Horhogea, C. Comparative Study of Polyphenols, Flavonoids and Chlorophylls in Equisetum arvense L. Populations. Rev. Chim. 2016, 67, 530–533. [Google Scholar]
- Jolly, M.; Vidal, R.; Marchand, P.A. Lecithins: A Food Additive Valuable for Antifungal Crop Protection. Int. J. Econ. Plants 2018, 5, 104–107. [Google Scholar] [CrossRef]
- Llamazares-Miguel, D.; Bodin, E.; Laurens, M.; Corio-Costet, M.F.; Nieto, J.; Fernández-Navarro, J.R.; Mena-Petite, A.; Diez-Navajas, A.M. Genetic Regulation in Vitis vinifera by Approved Basic Substances against Downy Mildew. BIO Web Conf. 2022, 50, 03001. [Google Scholar] [CrossRef]
- Kast, W.K. Effects of Plant Extracts on Downy Mildew of Vine-Laboratory and Field Experiments [Wirkung von Pflanzenextrakten in Labor- und Freilandversuchen Gegen Rebenperonospora]. In Proceedings of the Ecofruit—10th International Conference on Cultivation Technique and Phytopathological Problems in Organic Fruit-Growing and Viticulture, Weinsberg, Germany, 4–7 February 2002; pp. 157–162. [Google Scholar]
- Marchand, P.A.; Isambert, C.A.; Jonis, M.; Parveaud, C.E.; Chovelon, M.; Gomez, C.; Lambion, J.; Ondet, S.J.; Aveline, N.; Molot, B.; et al. Évaluation Des Caractéristiques et de l’intérêt Agronomique de Préparations Simples de Plantes, Pour Des Productions Fruitières, Légumières et Viticoles Économes En Intrants. Innov. Agron. 2014, 34, 83–96. [Google Scholar]
- Peressotti, E.; Duchêne, E.; Merdinoglu, D.; Mestre, P. A Semi-Automatic Non-Destructive Method to Quantify Grapevine Downy Mildew Sporulation. J. Microbiol. Methods 2011, 84, 265–271. [Google Scholar] [CrossRef]
- EPPO. EPPO Standards—Guidelines for the Efficacy Evaluation of Plant Protection Products—PP 1/31(3) Plasmopara viticola. Bull. OEPP/EPPO Bull. 2001, 31, 313–317. [Google Scholar]
- Tinivella, F.; Hirata, L.M.; Celan, M.A.; Wright, S.A.I.; Amein, T.; Schmitt, A.; Koch, E.; van der Wolf, J.M.; Groot, S.P.C.; Stephan, D.; et al. Control of Seed-Borne Pathogens on Legumes by Microbial and Other Alternative Seed Treatments. Eur. J. Plant Pathol. 2009, 123, 139–151. [Google Scholar] [CrossRef]
- Suteu, D.; Rusu, L.; Zaharia, C.; Badeanu, M.; Daraban, G. Challenge of Utilization Vegetal Extracts as Natural Plant Protection Products. Appl. Sci. 2020, 10, 8913. [Google Scholar] [CrossRef]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of Secondary Metabolites in Plant Defense against Pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Cohen, Y.; Wang, W.; Ben-Daniel, B.H.; Ben-Daniel, Y. Extracts of Inula viscosa Control Downy Mildew of Grapes Caused by Plasmopara viticola. Phytopathology 2006, 96, 417–424. [Google Scholar] [CrossRef]
- Gabaston, J.; Cantos-Villar, E.; Biais, B.; Waffo-Teguo, P.; Renouf, E.; Corio-Costet, M.F.; Richard, T.; Mérillon, J.M. Stilbenes from Vitis vinifera L. Waste: A Sustainable Tool for Controlling Plasmopara viticola. J. Agric. Food Chem. 2017, 65, 2711–2718. [Google Scholar] [CrossRef]
- Gabaston, J.; Richard, T.; Biais, B.; Waffo-Teguo, P.; Pedrot, E.; Jourdes, M.; Corio-Costet, M.F.; Mérillon, J.-M. Stilbenes from Common Spruce (Picea abies) Bark as Natural Antifungal Agent against Downy Mildew (Plasmopara viticola). Ind. Crops Prod. 2017, 103, 267–273. [Google Scholar] [CrossRef]
- Schnee, S.; Queiroz, E.F.; Voinesco, F.; Marcourt, L.; Dubuis, P.H.; Wolfender, J.L.; Gindro, K. Vitis vinifera Canes, a New Source of Antifungal Compounds against Plasmopara viticola, Erysiphe necator, and Botrytis cinerea. J. Agric. Food Chem. 2013, 61, 5459–5467. [Google Scholar] [CrossRef]
- Krzyzaniak, Y.; Trouvelot, S.; Negrel, J.; Cluzet, S.; Valls, J.; Richard, T.; Bougaud, A.; Jacquens, L.; Klinguer, A.; Chiltz, A.; et al. A Plant Extract Acts Both as a Resistance Inducer and an Oomycide Against Grapevine Downy Mildew. Front. Plant Sci. 2018, 9, 1085. [Google Scholar] [CrossRef] [PubMed]
- Piękna-Grochala, J.; Kępczyńska, E. Induction of Resistance against Pathogens by β-Aminobutyric Acid. Acta Physiol. Plant. 2013, 35, 1735–1748. [Google Scholar] [CrossRef]
- Misato, T.; Homma, Y.; Kō, K. The Development of a Natural Fungicide, Soybean Lecithin. Neth. J. Plant Pathol. 1977, 83, 395–402. [Google Scholar] [CrossRef]
- Lachhab, N.; Sanzani, S.M.; Adrian, M.; Chiltz, A.; Balacey, S.; Boselli, M.; Ippolito, A.; Poinssot, B. Soybean and Casein Hydrolysates Induce Grapevine Immune Responses and Resistance against Plasmopara viticola. Front. Plant Sci. 2014, 5, 716. [Google Scholar] [CrossRef] [PubMed]
- Scholfield, C.R. Composition of Soybean Lecithin. J. Am. Oil Chem. Soc. 1981, 58, 889–892. [Google Scholar] [CrossRef]
- Liu, S.; Ruan, W.; Li, J.; Xu, H.; Wang, J.; Gao, Y.; Wang, J. Biological Control of Phytopathogenic Fungi by Fatty Acids. Mycopathologia 2008, 166, 93–102. [Google Scholar] [CrossRef]
- Wasternack, C.; Feussner, I. The Oxylipin Pathways: Biochemistry and Function. Annu. Rev. Plant Biol. 2018, 69, 363–386. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yang, J.; Li, X.; Zhang, Y. Salicylic Acid: Biosynthesis and Signaling. Annu. Rev. Plant Biol. 2021, 72, 761–791. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Maltese, F.; Figueiredo, A.; Rex, M.; Fortes, A.M.; Zyprian, E.; Pais, M.S.; Verpoorte, R.; Choi, Y.H. Alterations in Grapevine Leaf Metabolism upon Inoculation with Plasmopara viticola in Different Time-Points. Plant Sci. 2012, 191–192, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.; Monteiro, F.; Sebastiana, M. First Clues on a Jasmonic Acid Role in Grapevine Resistance against the Biotrophic Fungus Plasmopara viticola. Eur. J. Plant Pathol. 2015, 142, 645–652. [Google Scholar] [CrossRef]
- Guerreiro, A.; Figueiredo, J.; Sousa Silva, M.; Figueiredo, A. Linking Jasmonic Acid to Grapevine Resistance against the Biotrophic Oomycete Plasmopara viticola. Front. Plant Sci. 2016, 7, 565. [Google Scholar] [CrossRef]
- Avis, T.J.; Bélanger, R.R. Specificity and Mode of Action of the Antifungal Fatty Acid cis-9-Heptadecenoic Acid Produced by Pseudozyma flocculosa. Appl. Environ. Microbiol. 2001, 67, 956–960. [Google Scholar] [CrossRef]
- Bergsson, G.; Arnfinnsson, J.; Steingrímsson, Ó.; Thormar, H. In Vitro Killing of Candida albicans by Fatty Acids and Monoglycerides. Antimicrob. Agents Chemother. 2001, 45, 3209–3212. [Google Scholar] [CrossRef]
- Pobłocka-Olech, L.; Krauze-Baranowska, M.; Głód, D.; Kawiak, A.; Łojkowska, E. Chromatographic Analysis of Simple Phenols in Some Species from the Genus Salix. Phytochem. Anal. 2010, 21, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, B.; Kahlich, R.; Biegert, C.; Gleiter, C.H.; Heide, L. HPLC-MS/MS Analysis of Willow Bark Extracts Contained in Pharmaceutical Preparations. Phytochem. Anal. 2005, 16, 470–478. [Google Scholar] [CrossRef]
- Ramos, P.A.B.; Moreirinha, C.; Santos, S.A.O.; Almeida, A.; Freire, C.S.R.; Silva, A.M.S.; Silvestre, A.J.D. Valorisation of Bark Lipophilic Fractions from Three Portuguese Salix Species: A Systematic Study of the Chemical Composition and Inhibitory Activity on Escherichia coli. Ind. Crops Prod. 2019, 132, 245–252. [Google Scholar] [CrossRef]
- Ding, P.; Ding, Y. Stories of Salicylic Acid: A Plant Defense Hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef] [PubMed]
- da Rocha Neto, A.C.; Maraschin, M.; Di Piero, R.M. Antifungal Activity of Salicylic Acid against Penicillium expansum and Its Possible Mechanisms of Action. Int. J. Food Microbiol. 2015, 215, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, J.; Zhang, P.; Hasi, G.; Huang, Y.; Lu, J.; Zhang, Y. Response of Phytohormones and Correlation of SAR Signal Pathway Genes to the Different Resistance Levels of Grapevine against Plasmopara viticola Infection. Plant Physiol. Biochem. 2016, 107, 56–66. [Google Scholar] [CrossRef]
- Burdziej, A.; Bellée, A.; Bodin, E.; Valls Fonayet, J.; Magnin, N.; Szakiel, A.; Richard, T.; Cluzet, S.; Corio-Costet, M.F. Three Types of Elicitors Induce Grapevine Resistance against Downy Mildew via Common and Specific Immune Responses. J. Agric. Food Chem. 2021, 69, 1781–1795. [Google Scholar] [CrossRef]
- Harm, A.; Kassemeyer, H.-H.; Seibicke, T.; Regner, F. Evaluation of Chemical and Natural Resistance Inducers against Downy Mildew (Plasmopara viticola) in Grapevine. Am. J. Enol. Vitic. 2011, 62, 184–192. [Google Scholar] [CrossRef]
- Maia, A.J.; Botelho, R.V.; Faria, C.M.D.R.; Leite, C.D. Ação de quitosana sobre o desenvolvimento de Plasmopara viticola e Elsinoe ampelina, in vitro e em videiras cv. Isabel. Summa Phytopathol. 2010, 36, 203–209. [Google Scholar] [CrossRef][Green Version]
- Prakongkha, I.; Sompong, M.; Wongkaew, S.; Athinuwat, D.; Buensanteai, N. Changes in Salicylic Acid in Grapevine Treated with Chitosan and BTH against Sphaceloma ampelinum, the Causal Agent of Grapevine Anthracnose. Afr. J. Microbiol. Res. 2013, 7, 557–563. [Google Scholar] [CrossRef]
- Rial Otero, R.; Cancho Grande, B.; Arias Estévez, M.; López Periago, E.; Simal Gándara, J. Procedure for the Measurement of Soil Inputs of Plant-Protection Agents Washed off through Vineyard Canopy by Rainfall. J. Agric. Food Chem. 2003, 51, 5041–5046. [Google Scholar] [CrossRef] [PubMed]
- Dagostin, S.; Ferrari, A.; Pertot, I. Efficacy Evaluation of Biocontrol Agents against Downy Mildew for Copper Replacement in Organic Grapevine Production in Europe. In Proceedings of the IOBC/WPRS Integrated Protection in Viticulture, Boario Terme, Italy, 20–22 October 2005; pp. 15–21. [Google Scholar]
- Dagostin, S.; Formolo, T.; Pertot, I. Replacement of Copper in Organic Viticulture: Efficacy Evaluation of New Natural Fungicides against Downy Mildew. In Proceedings of the IOBC/WPRS Integrated Protection in Viticulture, Sicily, Italy, 25–27 October 2007; pp. 87–90. [Google Scholar]
- Romanazzi, G.; Mancini, V.; Foglia, R.; Marcolini, D.; Kavari, M.; Piancatelli, S. Use of Chitosan and Other Natural Compounds Alone or in Different Strategies with Copper Hydroxide for Control of Grapevine Downy Mildew. Plant Dis. 2021, 105, 3261–3268. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Global Warming of 1.5 °C: An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2018. [Google Scholar]
- Gitea, M.A.; Gitea, D.; Tit, D.M.; Purza, L.; Samuel, A.D.; Bungău, S.; Badea, G.E.; Aleya, L. Orchard Management under the Effects of Climate Change: Implications for Apple, Plum, and Almond Growing. Environ. Sci. Pollut. Res. 2019, 26, 9908–9915. [Google Scholar] [CrossRef] [PubMed]
- Droulia, F.; Charalampopoulos, I. Future Climate Change Impacts on European Viticulture: A Review on Recent Scientific Advances. Atmosphere 2021, 12, 495. [Google Scholar] [CrossRef]
- Salinari, F.; Giosuè, S.; Tubiello, F.N.; Rettori, A.; Rossi, V.; Spanna, F.; Rosenzweig, C.; Gullino, M.L. Downy Mildew (Plasmopara viticola) Epidemics on Grapevine under Climate Change. Glob. Chang. Biol. 2006, 12, 1299–1307. [Google Scholar] [CrossRef]
- Bove, F.; Savary, S.; Willocquet, L.; Rossi, V. Simulation of Potential Epidemics of Downy Mildew of Grapevine in Different Scenarios of Disease Conduciveness. Eur. J. Plant Pathol. 2020, 158, 599–614. [Google Scholar] [CrossRef]

| Product | Company | Abbreviation | Composition | Category |
|---|---|---|---|---|
| - | Sigma-Aldrich | BABA | 2 mM β-aminobutyric acid | - |
| Actileaf | Agrichem Bio | ACTL | Cerevisane® (S. cerevisiae strain LAS117) 94.1% w/w | LRAS |
| Activane | LIDA Plant Research | ACTV | Free aminoacids 6% | Biostimulant |
| Biofender Fusarum | Econatur | CHIT | Chitosan hydrochloride 1% | BS |
| Biofender Lectum | Econatur | LECI | Soy lecithin 25% + E. arvense extract 15% | Mixture of BS |
| Biofender Salix | Econatur | SALIX | Salix cortex extract 42% + chitosan hydrochloride 0.5% | Mixture of BS |
| Fytosave | LIDA Plant Research | FYTO | COS-OGA 1.25% w/v | LRAS |
| Lesoy | Idai Nature | LESOY | Soy lecithin 20% | BS |
| Miles | Servalesa | MILES | 2.00% w/v E. arvense L. | BS |
| Mimetic | Idai Nature | MIME | Mn 1%, Zn 1% and M. tenuiflora and Q. robur extracts | Biostimulant |
| Taegro | Syngenta | TAEG | Bacillus amyloliquefaciens strain FZB24 13% w/w | LRAS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llamazares De Miguel, D.; Mena-Petite, A.; Díez-Navajas, A.M. Toxicity and Preventive Activity of Chitosan, Equisetum arvense, Lecithin and Salix Cortex against Plasmopara viticola, the Causal Agent of Downy Mildew in Grapevine. Agronomy 2022, 12, 3139. https://doi.org/10.3390/agronomy12123139
Llamazares De Miguel D, Mena-Petite A, Díez-Navajas AM. Toxicity and Preventive Activity of Chitosan, Equisetum arvense, Lecithin and Salix Cortex against Plasmopara viticola, the Causal Agent of Downy Mildew in Grapevine. Agronomy. 2022; 12(12):3139. https://doi.org/10.3390/agronomy12123139
Chicago/Turabian StyleLlamazares De Miguel, Diego, Amaia Mena-Petite, and Ana María Díez-Navajas. 2022. "Toxicity and Preventive Activity of Chitosan, Equisetum arvense, Lecithin and Salix Cortex against Plasmopara viticola, the Causal Agent of Downy Mildew in Grapevine" Agronomy 12, no. 12: 3139. https://doi.org/10.3390/agronomy12123139
APA StyleLlamazares De Miguel, D., Mena-Petite, A., & Díez-Navajas, A. M. (2022). Toxicity and Preventive Activity of Chitosan, Equisetum arvense, Lecithin and Salix Cortex against Plasmopara viticola, the Causal Agent of Downy Mildew in Grapevine. Agronomy, 12(12), 3139. https://doi.org/10.3390/agronomy12123139






