Antifeeding, Toxic, and Growth-Reducing Activity of trans-Anethole and S-(+)-Carvone against Larvae of the Gypsy Moth Lymantria dispar (L.)
Abstract
:1. Introduction
2. Materials and Methods
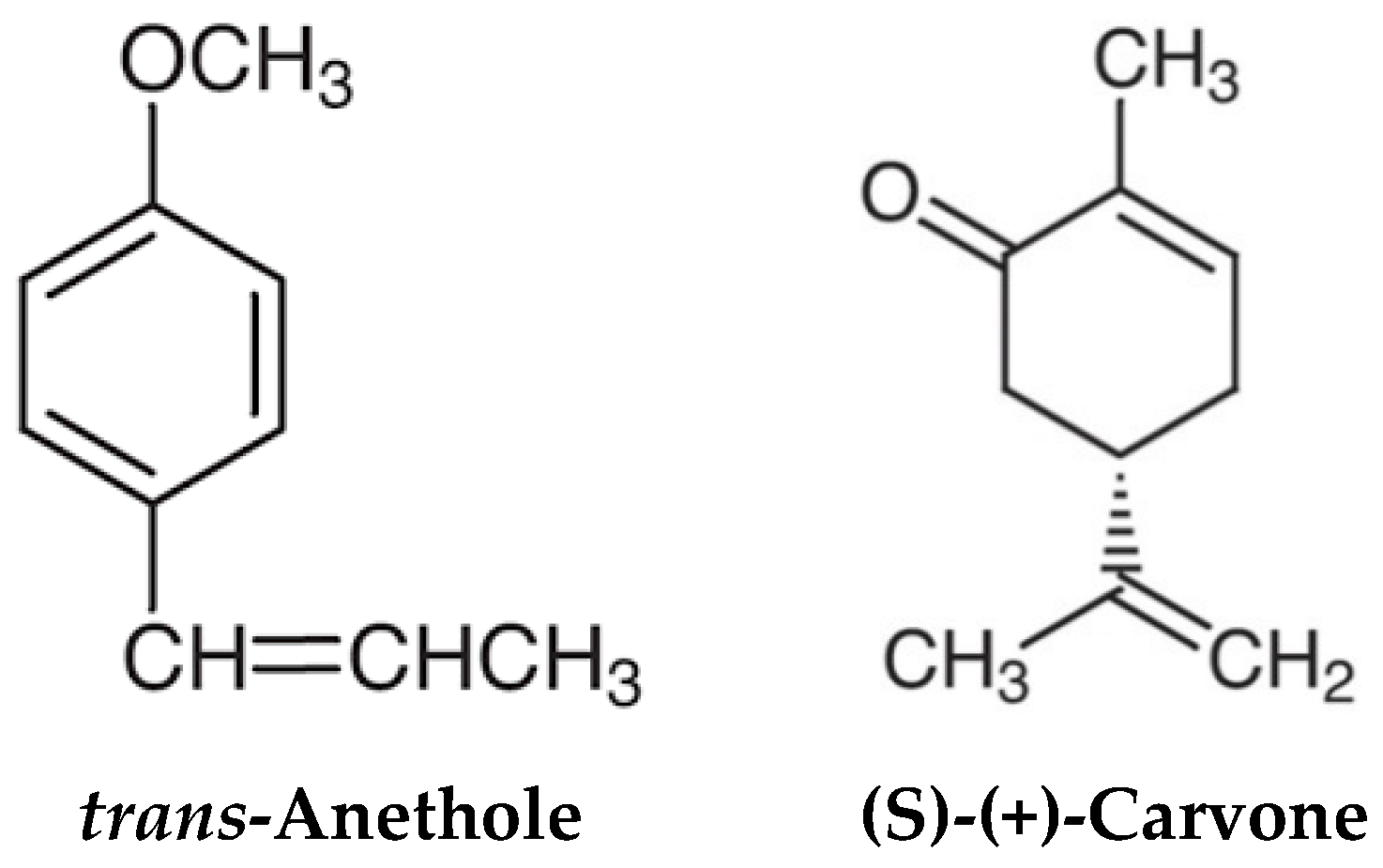
2.1. Chemicals
2.2. GML Rearing
2.3. Antifeeding Activity
2.4. Toxicity and Molting after Digestive and Contact Application of Chemicals
2.5. Growth and Nutritional Indices
2.6. Statistical Analysis
3. Results
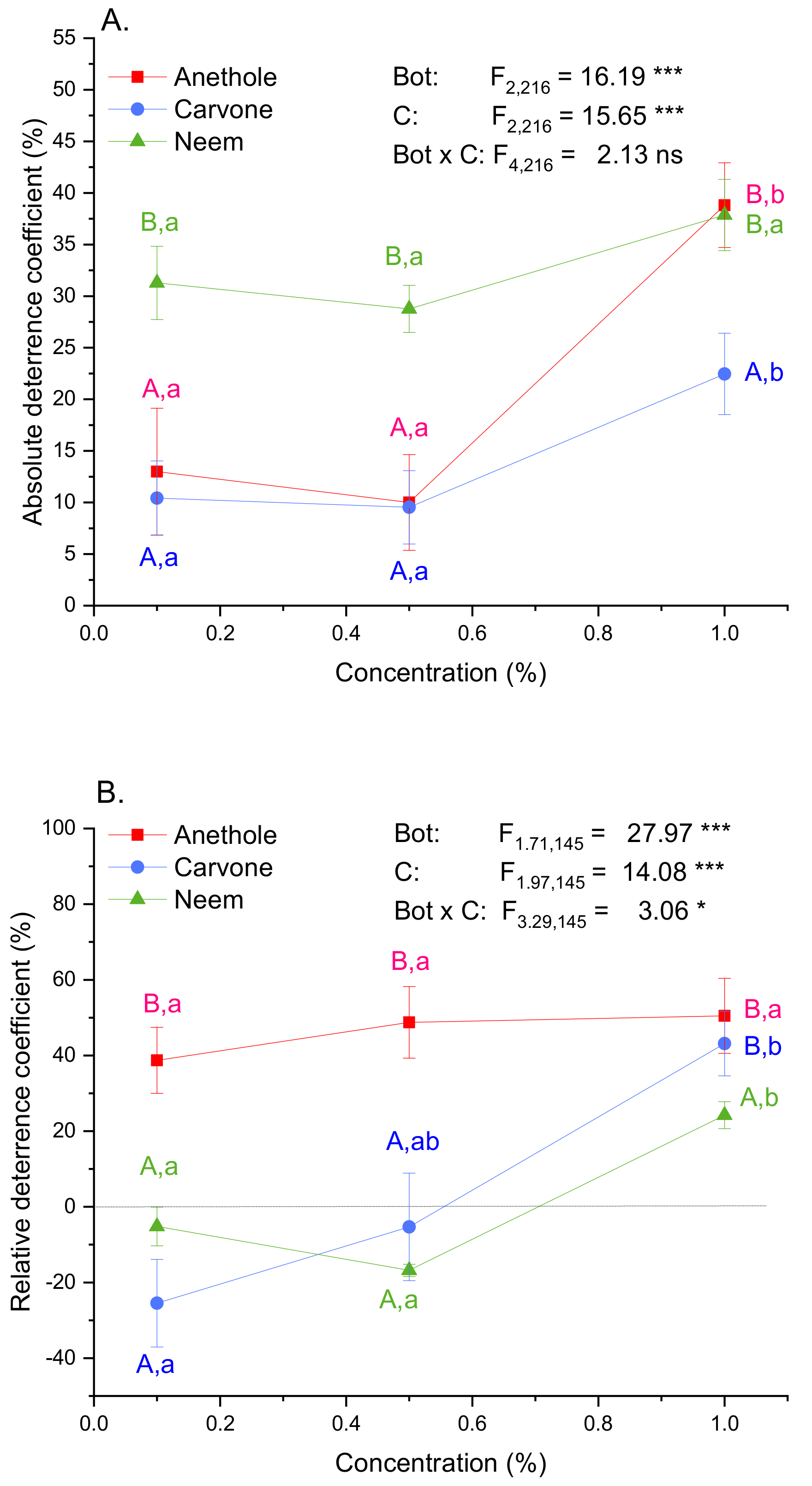
3.1. Feeding Deterrence
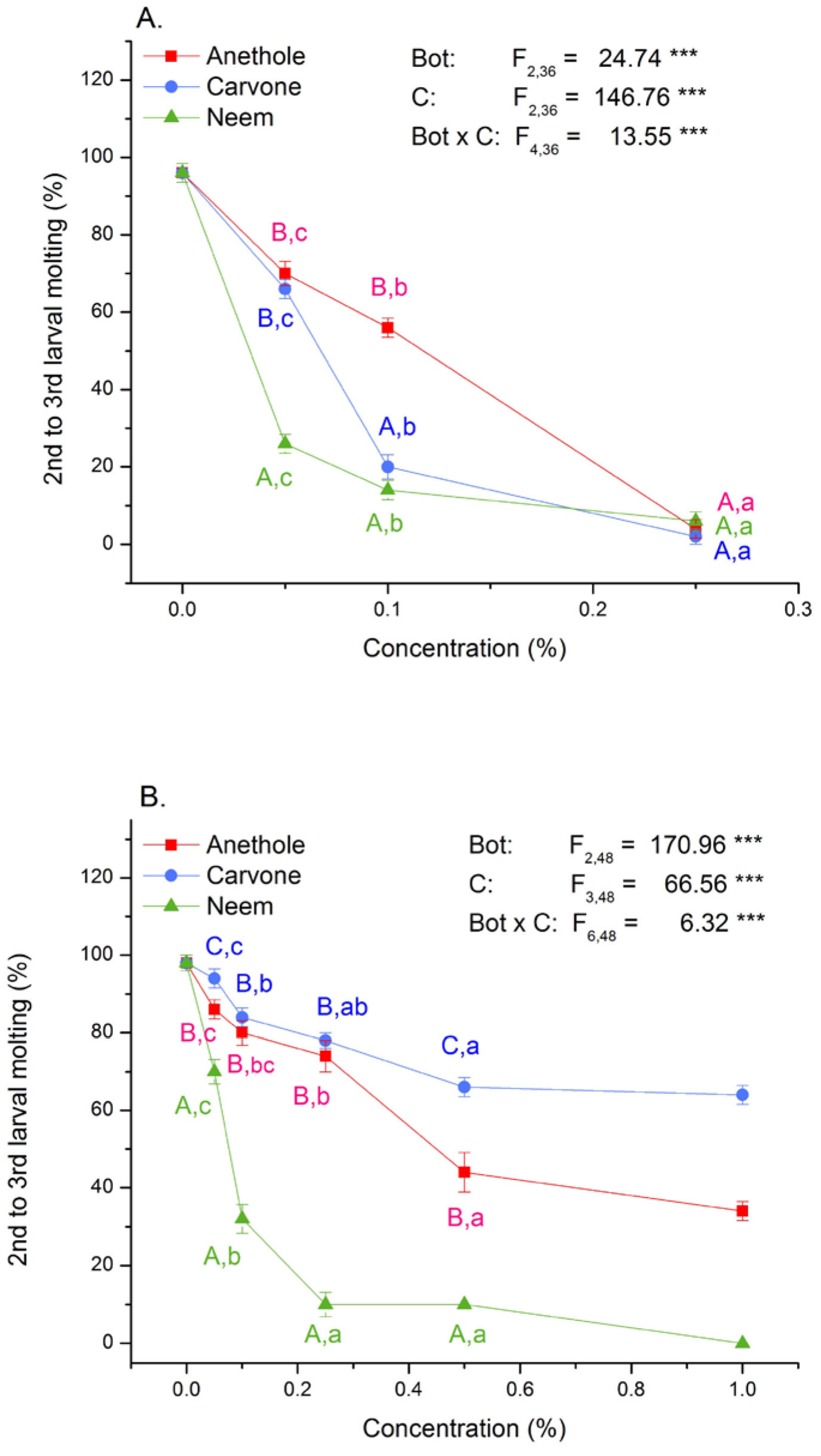
3.2. Toxicity and Molting Reducing Effects
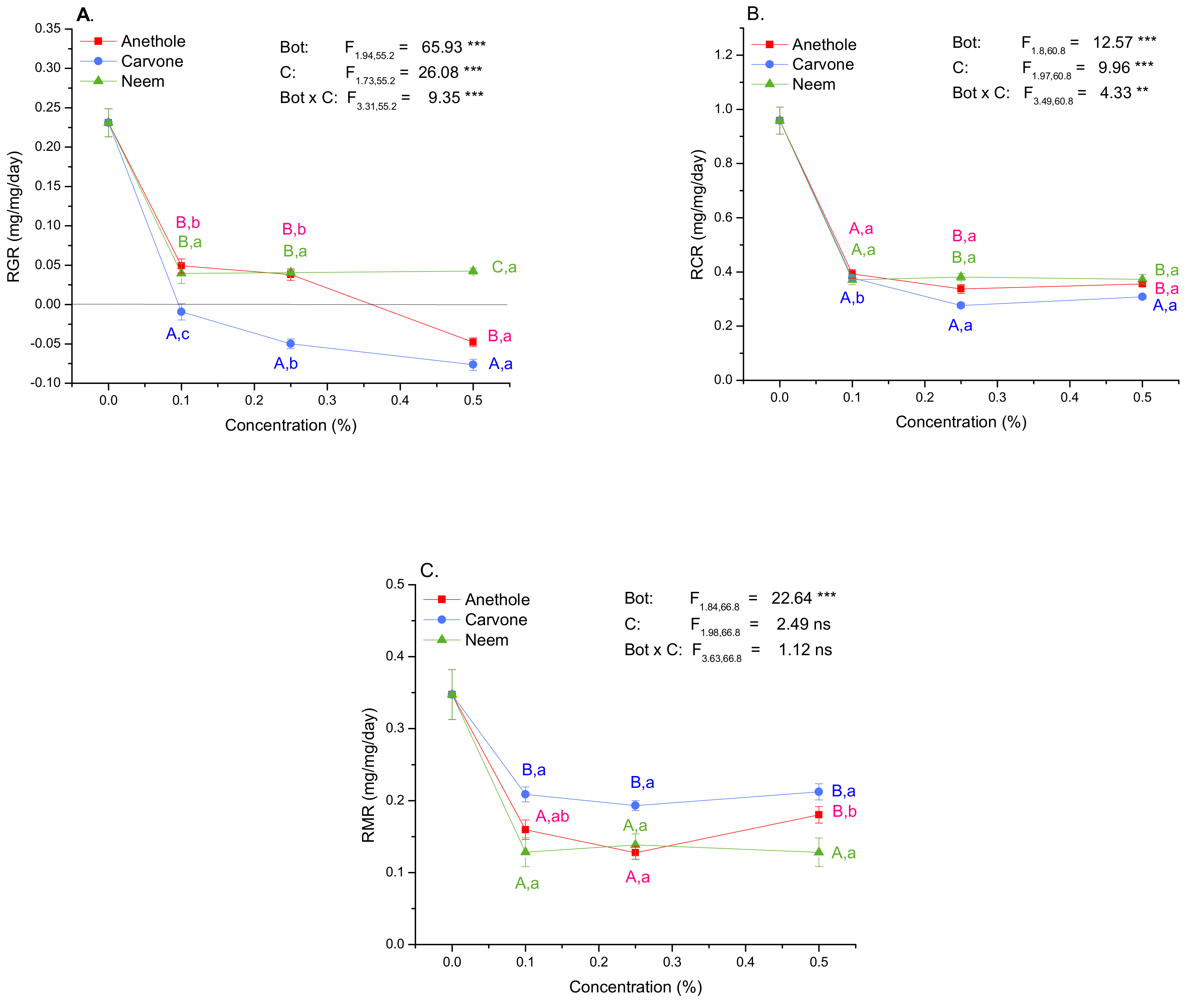
3.3. Larval Growth and Food Consumption, Assimilation, and Metabolization
3.4. Growth and Nutritional Indices
4. Discussion
4.1. Antifeeding Activity Depends on the Applied Assay and Compound Concentration
4.2. Mortality and Molting Are Differently Affected by Oral and Contact Application of Compounds
4.3. Carvone Is the Most Effective Growth-Reducing Compound
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Area Consumed (mm2) | ||||
|---|---|---|---|---|
| Botanical | Conc (%) | SE | p | |
| Anethole | 0.1 | 128.12 | 13.70 | 0.7279 |
| 0.5 | 127.77 | 9.92 | 0.7073 | |
| 1.0 | 68.19 | 6.70 | ˂0.0001 | |
| Carvone | 0.1 | 123.77 | 9.17 | 0.4658 |
| 0.5 | 125.71 | 8.73 | 0.5812 | |
| 1.0 | 98.15 | 9.17 | 0.0020 | |
| Neem | 0.1 | 79.52 | 6.35 | ˂0.0001 |
| 0.5 | 81.36 | 3.88 | ˂0.0001 | |
| 1.0 | 68.56 | 6.13 | ˂0.0001 | |
| Control | 0.0 | 144.08 | 9.71 | |
| ANOVA | F9,240 = 10.63 | ˂0.0001 | ||
| Control Area Consumed (mm2) | Treated Area Consumed (mm2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Botanical | Conc (%) | ±SE | ±SE | t | df | p | Activity | ||
| Anethole | 0.1 | 103.91 | 9.21 | 51.53 | 9.03 | 4.11 | 24 | ˂0.001 | D |
| 0.5 | 140.16 | 16.07 | 40.43 | 7.10 | 5.15 | 24 | ˂0.001 | D | |
| 1.0 | 128.98 | 13.98 | 33.43 | 5.98 | 5.50 | 24 | ˂0.001 | D | |
| Carvone | 0.1 | 66.05 | 11.40 | 117.43 | 12.73 | −2.32 | 24 | 0.029 | A |
| 0.5 | 80.40 | 13.20 | 85.19 | 12.90 | 0.25 | 24 | 0.803 | N | |
| 1.0 | 85.30 | 6.03 | 40.53 | 9.29 | 4.04 | 24 | 0.001 | D | |
| Neem | 0.1 | 177.62 | 7.24 | 208.96 | 14.32 | 1.36 | 24 | 0.186 | N |
| 0.5 | 234.71 | 6.21 | 328.93 | 6.26 | −10.33 | 24 | ˂0.001 | A | |
| 1.0 | 266.69 | 7.22 | 166.70 | 10.16 | 6.96 | 24 | ˂0.001 | D | |
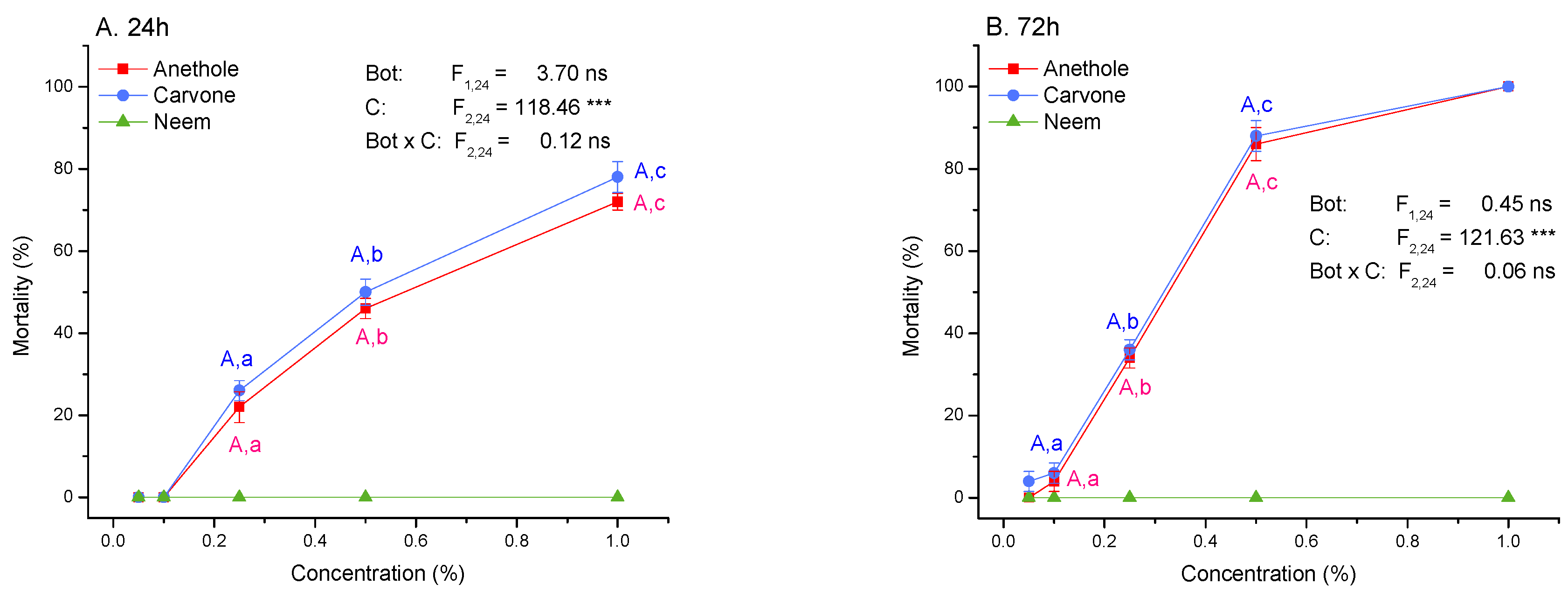


| Contact Application | Digestive Application | ||||||
|---|---|---|---|---|---|---|---|
| Percentage of Molting | Percentage of Molting | ||||||
| Botanical | Conc (%) | ±SE | p | ±SE | p | ||
| Anethole | 0.05 | 86 | 2.45 | ˂0.001 | 70 | 3.16 | ˂0.001 |
| 0.10 | 80 | 3.16 | ˂0.001 | 56 | 2.45 | ˂0.001 | |
| 0.25 | 74 | 4.00 | ˂0.001 | 4 | 2.45 | ˂0.001 | |
| 0.50 | 44 | 5.10 | ˂0.001 | 0 | - | ||
| 1.00 | 34 | 2.45 | ˂0.001 | 0 | - | ||
| Carvone | 0.05 | 94 | 2.45 | 0.403 | 66 | 2.45 | ˂0.001 |
| 0.10 | 84 | 2.45 | ˂0.001 | 20 | 3.16 | ˂0.001 | |
| 0.25 | 78 | 2.00 | ˂0.001 | 2 | 2.00 | ˂0.001 | |
| 0.50 | 66 | 2.45 | ˂0.001 | 0 | - | ||
| 1.00 | 64 | 2.45 | ˂0.001 | 0 | - | ||
| Neem | 0.05 | 70 | 3.16 | ˂0.001 | 26 | 2.45 | ˂0.001 |
| 0.10 | 32 | 3.74 | ˂0.001 | 14 | 2.45 | ˂0.001 | |
| 0.25 | 10 | 3.16 | ˂0.001 | 6 | 2.45 | ˂0.001 | |
| 0.50 | 8 | 2.00 | ˂0.001 | 0 | 0 | ||
| 1.00 | 0 | 0 | 0 | 0 | |||
| Control | 0.00 | 98 | 2.00 | 96 | 2.45 | ||
| ANOVA | F14,60 = 57.16 | ˂0.001 | F9,40 = 68.85 | ˂0.001 | |||
| MG | mc | ma | mm | ||
|---|---|---|---|---|---|
| Botanical | Conc (%) | p | p | p | p |
| Anethole | 0.10 | <0.001 | <0.001 | <0.001 | <0.001 |
| 0.25 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 0.50 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Carvone | 0.10 | <0.001 | <0.001 | <0.001 | 0.042 |
| 0.25 | <0.001 | <0.001 | <0.001 | 0.001 | |
| 0.50 | <0.001 | <0.001 | <0.001 | 0.043 | |
| Neem | 0.10 | 0.001 | <0.001 | <0.001 | <0.001 |
| 0.25 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 0.50 | <0.001 | <0.001 | <0.001 | <0.001 | |
| ANOVA | F5.57,53.2 = 40.10 | F6.05,58.1 = 16.68 | F6.54,63.2 = 12.75 | F7.14,69.6 = 16.86 | |
| <0.001 | <0.001 | <0.001 | <0.001 |
| RGR | RCR | RMR | ECI | AD | ECD | MC | ||
|---|---|---|---|---|---|---|---|---|
| Botanical | Conc (%) | p | p | p | p | p | p | p |
| Anethole | 0.10 | <0.001 | <0.001 | <0.001 | 0.001 | 0.013 | 0.025 | 0.025 |
| 0.25 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.014 | 0.014 | |
| 0.50 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Carvone | 0.05 | <0.001 | <0.001 | 0.007 | <0.001 | <0.001 | <0.001 | <0.001 |
| 0.10 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 0.25 | <0.001 | <0.001 | 0.030 | <0.001 | <0.001 | <0.001 | <0.001 | |
| Neem | 0.05 | 0.001 | <0.001 | <0.001 | 0.003 | <0.001 | 0.156 | 0.156 |
| 0.10 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |
| 0.25 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.086 | 0.086 | |
| ANOVA | F5.83,55.8 = 40.96 | F6.34,61.1 = 14.23 | F7.35,71.9 = 11.33 | F5.79,55.4 = 38.00 | F6.8,66 = 9.45 | F5.66,54.1 = 30.75 | F5.66,54.1 = 30.75 | |
| <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
References
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Aukema, J.E.; Leung, B.; Kovacs, K.; Chivers, C.; Britton, K.O.; Englin, J.; Frankel, S.J.; Haight, R.G.; Holmes, T.P.; Liebhold, A.M.; et al. Economic impacts of non-native forest insects in the continental United States. PLoS ONE 2011, 6, e24587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, J.; Fountain, M.; Markó, V.; Nagy, C. Arthropod ecosystem services in apple orchards and their economic benefits. Ecol. Entomol. 2015, 40, 82–96. [Google Scholar] [CrossRef]
- Holmes, S.B.; MacQuarrie, C.J. Chemical control in forest pest management. Can. Entomol. 2016, 148, S270–S295. [Google Scholar] [CrossRef] [Green Version]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, D. Environmental and economic costs of the application of pesticides primarily in the United States. Environ. Dev. Sustain. 2005, 7, 229–252. [Google Scholar] [CrossRef]
- Horowitz, A.R.; Ellsworth, P.C.; Ishaaya, I. Biorational pest control—An overview. In Biorational Control of Arthropod Pests; Ishaaya, I., Horowitz, A.R., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 1–20. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Dayan, F.E.; Duke, S.O. Natural products as sources for new pesticides. J. Nat. Prod. 2012, 75, 1231–1242. [Google Scholar] [CrossRef]
- Pavela, R. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects—A review. Plant Protect Sci. 2016, 52, 229–241. [Google Scholar]
- Zhang, Z.; Sun, X.; Luo, Z.; Gao, Y.; Chen, Z. The manipulation mechanism of “push–pull” habitat management strategy and advances in its application. Acta Ecol. Sin. 2013, 33, 94–101. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Gad, H.A.; Ramadan, G.R.; El-Bakry, A.M.; El-Sabrout, A.M. Monoterpenes: Chemistry, insecticidal activity against stored product insects and modes of action—A review. Int. J. Pest Manag. 2021, 1–23. [Google Scholar] [CrossRef]
- Khalequzzaman, M.; Nahar, J. Relative toxicity of some insecticides and azadirachtin against four crop infesting aphid species. Univ. J. Zool. Rajshahi Univ. 2008, 27, 31–34. [Google Scholar] [CrossRef] [Green Version]
- Nerio, L.S.; Olivero-Verbel, J.; Stashenko, E.E. Repellent activity of essential oils from seven aromatic plants grown in Colombia against Sitophilus zeamais Motschulsky (Coleoptera). J. Stored Prod. Res. 2009, 45, 212–214. [Google Scholar] [CrossRef]
- Hernández-Lambraño, R.; Caballero-Gallardo, K.; Olivero-Verbel, J. Toxicity and antifeedant activity of essential oils from three aromatic plants grown in Colombia against Euprosterna elaeasa and Acharia fusca (Lepidoptera: Limacodidae). Asian Pac. J. Trop. Biomed. 2014, 4, 695–700. [Google Scholar] [CrossRef] [Green Version]
- Liebhold, A.M.; Gottschalk, K.W.; Muzika, R.M.; Montgomery, M.E.; Young, R.; O’Day, K.; Kelley, B. Suitability of North American Tree Species to the Gypsy Moth: A Summary of Field and Laboratory Tests; U.S. Department of Agriculture Forest Service NE Forest Experimental Station General Technical Bulletin NE-211; U.S. Department of Agriculture: Washington, DC, USA, 1995; p. 34.
- Arai, T.; Yaginuma, K.; Toyoshima, S.; Ito, T.; Takanashi, M. Damage of Lymantria dispar and Lymantria mathura aurora in apple orchards. Annu. Rep. Soc. Plant Prot. North Jpn. 2010, 61, 220–224. [Google Scholar]
- Bigsby, K.M.; Ambrose, M.J.; Tobin, P.C.; Sills, E.O. The cost of gypsy moth sex in the city. Urban For. Urban Green. 2014, 13, 459–468. [Google Scholar] [CrossRef]
- Marović, R.; Maravić, M.; Jančić, G.; Lazarev, V. Gypsy moth outbreaks in Serbia. In Gypsy Moth Outbreaks in Serbia; Adamović, Ž., Ed.; The Entomological Society of Serbia: Belgrade, Serbia, 1998; pp. 1–12. [Google Scholar]
- Davidson, C.B.; Gottschalk, K.W.; Johnson, J.E. Tree mortality following defoliation by the European gypsy moth (Lymantria dispar L.) in the United States: A review. For. Sci. 1999, 45, 74–84. [Google Scholar]
- Milanović, S.; Mihajlović, L.; Karadžić, D.; Jankovsky, L.; Aleksić, P.; Janković-Tomanić, M.; Lazarević, J. Effects of pedunculate oak tree vitality on gypsy moth preference and performance. Arch. Biol. Sci. 2014, 66, 1659–1672. [Google Scholar] [CrossRef]
- Miller, J.; Hanson, P.; Dowell, R. The potential of gypsy moth as a pest of fruit and nut crops. Calif. Agric. 1987, 41, 10–12. [Google Scholar]
- Mihajlović, L. The gypsy moth (Lymantria dispar L.) (Lepidoptera, Lymantridae) in Serbia. Forestry 2008, 60, 1–26. (In Serbian) [Google Scholar]
- Sundararaj, R. Relevance of botanicals for the management of forest insect pests of India. In Basic and Applied Aspects of Biopesticides; Sahayaraj, K., Ed.; Springer: New Delhi, India, 2014; pp. 155–179. [Google Scholar] [CrossRef]
- Kovanci, O.B. Feeding and oviposition deterrent activities of microencapsulated cardamom oleoresin and eucalyptol against Cydia pomonella. Chil. J. Agric. Res. 2016, 76, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Pour, S.A.; Shahriari, M.; Zibaee, A.; Mojarab-Mahboubkar, M.; Sahebzadeh, N.; Hoda, H. Toxicity, antifeedant and physiological effects of trans-anethole against Hyphantria cunea Drury (Lep: Arctiidae). Pestic. Biochem. Phys. 2022, 185, 105135. [Google Scholar] [CrossRef] [PubMed]
- Kostić, M.; Popović, Z.; Brkić, D.; Milanović, S.; Sivčev, I.; Stanković, S. Larvicidal and antifeedant activity of some plant-derived compounds to Lymantria dispar L. (Lepidoptera: Limantriidae). Bioresour. Technol. 2008, 99, 7897–7901. [Google Scholar] [CrossRef] [PubMed]
- Gvozdenac, S.M.; Inđić, D.V.; Vuković, S.M.; Grahovac, M.S.; Tanasković, S.T. Antifeeding activity of several plant extracts against against Lymantria dispar L. (Lepidoptera: Lymantriidae) larvae. Pestic. Phytomed. 2012, 27, 305–311. [Google Scholar] [CrossRef]
- Moretti, M.D.; Sanna-Passino, G.; Demontis, S.; Bazzoni, E. Essential oil formulations useful as a new tool for insect pest control. AAPS Pharm. Sci. Tech. 2002, 3, 64–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popović, Z.; Kostić, M.; Stanković, S.; Milanović, S.; Sivčev, I.; Kostić, I.; Kljajić, P. Ecologically acceptable usage of derivatives of essential oil of sweet basil, Ocimum basilicum, as antifeedants against larvae of the gypsy moth, Lymantria dispar. J. Insect. Sci. 2013, 13, T161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kostić, I.; Petrović, O.; Milanović, S.; Popović, Z.; Stanković, S.; Todorović, G.; Kostić, M. Biological activity of essential oils of Athamanta haynaldii and Myristica fragrans to gypsy moth larvae. Ind. Crops Prod. 2013, 41, 17–20. [Google Scholar] [CrossRef]
- Devrnja, N.; Kostić, I.; Lazarević, J.; Savić, J.; Ćalić, D. Evaluation of tansy essential oil as a potential “green” alternative for gypsy moth control. Environ. Sci. Pollut. Res. 2020, 27, 11958–11967. [Google Scholar] [CrossRef]
- Kostić, I.; Lazarević, J.; Šešlija Jovanović, D.; Kostić, M.; Marković, T.; Milanović, S. Potential of essential oils from anise, dill and fennel seeds for the gypsy moth control. Plants 2021, 10, 2194. [Google Scholar] [CrossRef]
- Nikolić, B.M.; Milanović, S.D.; Milenković, I.L.; Todosijević, M.M.; Đorđević, I.Ž.; Brkić, M.Z.; Mitić, Z.S.; Marin, P.D.; Tešević, V.V. Bioactivity of Chamaecyparis lawsoniana (A. Murray) Parl. and Thuja plicata Donn ex D. Don essential oils on Lymantria dispar (Linnaeus, 1758) (Lepidoptera: Erebidae) larvae and Phytophthora de Bary 1876 root pathogens. Ind. Crops Prod. 2022, 178, 114550. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Zhang, B.W.; Yang, J.; Zou, C.S.; Li, T.; Zhang, G.C.; Chen, G.S. Detoxification, antioxidant, and digestive enzyme activities and gene expression analysis of Lymantria dispar larvae under carvacrol. J. Asia-Pac. Entomol. 2021, 24, 208–216. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, J.; Zhou, L.; Wang, J.; Gong, Y.; Chen, X.; Guo, Z.; Wang, Q.; Jiang, W. Antifungal activity of the essential oil of Illicium verum fruit and its main component trans-anethole. Molecules 2010, 15, 7558–7569. [Google Scholar] [CrossRef]
- Senatore, F.; Oliviero, F.; Scandolera, E.; Taglialatela-Scafati, O.; Roscigno, G.; Zaccardelli, M.; De Falco, E. Chemical composition, antimicrobial and antioxidant activities of anethole-rich oil from leaves of selected varieties of fennel [Foeniculum vulgare Mill. ssp. vulgare var. azoricum (Mill.) Thell]. Fitoterapia 2013, 90, 214–219. [Google Scholar] [CrossRef]
- Ben-Khalifa, N.E.; Chaieb, I.; Laarif, A.; Haouala, R. Insecticidal activity of six Apiaceae essential oils against Spodoptera littoralis Biosduval (Lepidoptera: Noctuidae). J. New Sci. 2018, 55, 3603–3609. [Google Scholar]
- Johnson, A.J.; Venukumar, V.; Varghese, T.S.; Viswanathan, G.; Leeladevi, P.S.; Remadevi, R.K.S.; Baby, S. Insecticidal properties of Clausena austroindica leaf essential oil and its major constituent, trans-anethole, against Sitophilus oryzae and Tribolium castaneum. Ind. Crops Prod. 2022, 182, 114854. [Google Scholar] [CrossRef]
- Mathela, C.S.; Padalia, R.C.; Chanotiya, C.S.; Tiwari, A. Carvone rich Mentha longifolia (Linn.): Chemical variation and commercial potential. J. Essent. Oil Bear. Plants 2005, 8, 130–133. [Google Scholar] [CrossRef]
- Porfírio, E.M.; Melo, H.M.; Pereira, A.M.G.; Cavalcante, T.T.A.; Gomes, G.A.; Carvalho, M.G.D.; Costa, R.A.; Júnior, F.E.A.C. In vitro antibacterial and antibiofilm activity of Lippia alba essential oil, citral, and carvone against Staphylococcus aureus. Sci. World J. 2017, 2017, 4962707. [Google Scholar] [CrossRef] [Green Version]
- Mutlu-Ingok, A.; Karbancioglu-Guler, F. Cardamom, cumin, and dill weed essential oils: Chemical compositions, antimicrobial activities, and mechanisms of action against Campylobacter spp. Molecules 2017, 22, 1191. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, A.K.; Prajapati, V.; Kumar, S. Bioactivities of l-carvone, d-carvone, and dihydrocarvone toward three stored product beetles. J. Econ. Entomol. 2003, 96, 1594–1601. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.; Mohamed, M.I.; Badawy, M.E.; El-arami, S.A. Fumigant and contact toxicities of monoterpenes to Sitophilus oryzae (L.) and Tribolium castaneum (Herbst) and their inhibitory effects on acetylcholinesterase activity. J. Chem. Ecol. 2009, 35, 518–525. [Google Scholar] [CrossRef]
- Mondal, M.; Khalequzzaman, M. Toxicity of naturally occurring compounds of plant essential oil against Tribolium castaneum (Herbst). J. Biol. Sci. 2010, 10, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Shahriari, M.; Sahebzadeh, N.; Sarabandi, M.; Zibaee, A. Oral Toxicity of Thymol, α-Pinene, Diallyl Disulfide and Trans-Anethole, and Their Binary Mixtures against Tribolium castaneum Herbst Larvae (Coleoptera: Tenebrionidae). Jordan J. Biol. Sci. 2016, 9, 213–219. [Google Scholar]
- Kanda, D.; Kaur, S.; Koul, O. Effect of keto-compounds from essential oils on the growth and reproductive performance of Tribolium castaneum (Herbst). Biopestic. Int. 2016, 12, 37–43. [Google Scholar]
- Kanda, D.; Kaur, S.; Koul, O. A comparative study of monoterpenoids and phenylpropanoids from essential oils against stored grain insects: Acute toxins or feeding deterrents. J. Pest Sci. 2017, 90, 531–545. [Google Scholar] [CrossRef]
- Rosa, J.S.; Oliveira, L.; Sousa, R.M.O.F.; Escobar, C.B.; Fernandes-Ferreira, M. Bioactivity of some Apiaceae essential oils and their constituents against Sitophilus zeamais (Coleoptera: Curculionidae). Bull. Entomol. Res. 2020, 110, 406–416. [Google Scholar] [CrossRef]
- Hummelbrunner, L.A.; Isman, M.B. Acute, sublethal, antifeedant, and synergistic effects of monoterpenoid essential oil compounds on the tobacco cutworm, Spodoptera litura (Lep., Noctuidae). J. Agric. Food Chem. 2001, 49, 715–720. [Google Scholar] [CrossRef]
- El-Minshawy, A.M.; Abdelgaleil, S.A.; Gadelhak, G.G.; Al-Eryan, M.A.; Rabab, R.A. Effects of monoterpenes on mortality, growth, fecundity, and ovarian development of Bactrocera zonata (Saunders) (Diptera: Tephritidae). Environ. Sci. Pollut. Res. 2018, 25, 15671–15679. [Google Scholar] [CrossRef]
- Al-Nagar, N.M.; Abou-Taleb, H.K.; Shawir, M.S.; Abdelgaleil, S.A. Comparative toxicity, growth inhibitory and biochemical effects of terpenes and phenylpropenes on Spodoptera littoralis (Boisd.). J. Asia-Pac. Entomol. 2020, 23, 67–75. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.; Al-Nagar, N.; Abou-Taleb, H.K.; Shawir, M.S. Effect of monoterpenes, phenylpropenes and sesquiterpenes on development, fecundity and fertility of Spodoptera littoralis (Boisduval). Int. J. Trop. Insect Sci. 2022, 42, 245–253. [Google Scholar] [CrossRef]
- Morgan, E.D. Azadirachtin, a scientific gold mine. Bioorg. Med. Chem. 2009, 17, 4096–4105. [Google Scholar] [CrossRef]
- Markovic, I.; Norris, D.M.; Nordheim, E.V. Gypsy moth (Lymantria dispar) larval development and survival to pupation on diet plus extractables from green ash foliage. Entomol. Exp. Appl. 1997, 84, 247–254. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Shim, J.K.; Hwang, H.S.; Bunch, H.; Lee, K.Y. Acaricidal effects of methyl benzoate against Tetranychus urticae Koch (Acari: Tetranychidae) on common crop plants. Pest Manag. Sci. 2020, 76, 2347–2354. [Google Scholar] [CrossRef]
- Wilcox, D.; Dove, B.; McDavid, D.; Greer, D. Image Tool Copyright UTHSCSA 1996–2002; University of Texas Health Science Center (UTHSCSA): San Antonio, TX, USA, 1996. [Google Scholar]
- Nawrot, J.; Błoszyk, E.; Harmatha, J.; Novotny’, L.; Drozdz, B. Action of antifeedants of plant origin onbeetles infesting stored products. Acta Entomol. Bohemoslov. 1986, 83, 327–335. [Google Scholar]
- Waldbauer, G.P. The consumption and utilization of food by insects. Adv. Insect Phys. 1968, 5, 229–288. [Google Scholar]
- Scriber, J.M.; Slansky, F., Jr. The nutritional ecology of immature insects. Annu. Rev. Entomol. 1981, 26, 183–211. [Google Scholar] [CrossRef]
- Farrar, R.R.; Barbour, J.D.; Kennedy, G.G. Quantifying food consumption and growth in insects. Ann. Entomol. Soc. Am. 1989, 82, 593–598. [Google Scholar] [CrossRef]
- Howell, D.C. Simple analysis of variance. In Statistical Methods for Psychology, 8th ed.; Wadsworth, Cengage Learning: Belmont, CA, USA, 2013; pp. 325–368. [Google Scholar]
- Finney, D.J. Probit Analysis, 3rd ed.; Cambridge University Press: Cambridge, UK, 1971. [Google Scholar]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Martemyanov, V.V.; Domrachev, D.V.; Pavlushin, S.V.; Belousova, I.A.; Bakhvalov, S.A.; Tkachev, A.V.; Glupov, V.V. Induction of terpenoid synthesis in leaves of silver birch after defoliation caused by gypsy moth caterpillars. Dokl. Biol. Sci. Proc. Acad. Sci. USSR Biol. Sci. Sect. 2010, 435, 407–410. [Google Scholar] [CrossRef]
- Powell, J.S.; Raffa, K.F. Fate of conifer terpenes in a polyphagous folivore: Evidence for metabolism by gypsy moth (Lepidoptera: Lymantriidae). J. Entomol. Sci. 2003, 38, 583–601. [Google Scholar] [CrossRef]
- Markovic, I.; Norris, D.M.; Phillips, J.K.; Webster, F.X. Volatiles involved in the nonhost rejection of Fraxinus pennsylvanica by Lymantria dispar larvae. J. Agric. Food Chem. 1996, 44, 929–935. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Singh, V.K.; Kedia, A.; Das, S.; Dubey, N.K. Essential oils and their bioactive compounds as eco-friendly novel green pesticides for management of storage insect pests: Prospects and retrospects. Environ. Sci. Pollut. Res. 2021, 28, 18918–18940. [Google Scholar] [CrossRef] [PubMed]
- Alkan, M.; Ertürk, S. Insecticidal efficacy and repellency of trans-anethole against four stored-product insect pests. J. Agric. Sci. 2020, 26, 64–70. [Google Scholar] [CrossRef]
- Kłyś, M.; Izdebska, A.; Malejky-Kłusek, N. Repellent Effect of the caraway Carum carvi L. on the rice weevil Sitophilus oryzae L. (Coleoptera, Dryophthoridae). Insects 2020, 11, 836. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Liu, J.; Zhao, Z.; Yan, X.; Wang, W.; Wei, J. Chemical compounds emitted from Mentha spicata repel Aromia bungii females. Insects 2022, 13, 244. [Google Scholar] [CrossRef] [PubMed]
- Dambolena, J.S.; Zunino, M.P.; Herrera, J.M.; Pizzolitto, R.P.; Areco, V.A.; Zygadlo, J.A. Terpenes: Natural products for controlling insects of importance to human health—A structure-activity relationship study. Psyche J. Entomol. 2016, 2016, 4595823. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, G.D.B.; Giurfa, M. A comparative analysis of neural taste processing in animals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 2171–2180. [Google Scholar] [CrossRef] [Green Version]
- Deletre, E.; Schatz, B.; Bourguet, D.; Chandre, F.; Williams, L.; Ratnadass, A.; Martin, T. Prospects for repellent in pest control: Current developments and future challenges. Chemoecology 2016, 26, 127–142. [Google Scholar] [CrossRef]
- Enan, E.E. Molecular and pharmacological analysis of an octopamine receptor from American cockroach and fruit fly in response to plant essential oils. Arch. Insect Biochem. Physiol. 2005, 59, 161–171. [Google Scholar] [CrossRef]
- Zaio, Y.P.; Gatti, G.; Ponce, A.A.; Saavedra Larralde, N.A.; Martinez, M.J.; Zunino, M.P.; Zygadlo, J.A. Cinnamaldehyde and related phenylpropanoids, natural repellents, and insecticides against Sitophilus zeamais (Motsch.). A chemical structure-bioactivity relationship. J. Sci. Food Agric. 2018, 98, 5822–5831. [Google Scholar] [CrossRef]
- Wilson, A.; Butler, J.F.; Withycombe, D.; Mookherjee, B.D.; Katz, I.; Schrankel, K.R. Use of D-Carvone as Mosquito Attractant. U.S. Patent 4970068, 13 November 1990. Available online: https://patentimages.storage.googleapis.com/dd/ca/1a/5efd45c9b821cd/US4970068.pdf (accessed on 17 October 2022).
- Su, T.; Mulla, M.S. Oviposition bioassay responses of Culex tarsalis and Culex quinquefasciatus to neem products containing azadirachtin. Entomol. Exp. Appl. 1999, 91, 337–345. [Google Scholar] [CrossRef]
- Moon, S.R.; Cho, S.R.; Jeong, J.W.; Shin, Y.H.; Yang, J.O.; Ahn, K.S.; Yoon, C.; Kim, G.H. Attraction response of spot clothing wax cicada, Lycorma delicatula (Hemiptera: Fulgoridae) to spearmint oil. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 558–567. [Google Scholar]
- Jaastad, G.; Knudsen, G.K.; Kobro, S.; Bäckman, A.C.; Witzgall, P.; Bengtsson, M. Attractive Plant Volatiles as a Control Method against Apple Fruit Moth (Argyresthia conjugella Zell.)? In Proceedings of the Ecofruit—11th International Conference on Cultivation Technique and Phytopathological Problems in Organic Fruit-Growing, Weinsberg, Germany, 3–5 February 2004; pp. 29–34. [Google Scholar]
- Tóth, M.; Furlan, L.; Szarukán, I.; Vuts, J. Development of a female-targeted attractant for the click beetle, Agriotes ustulatus Schwarz. Acta Phytopathol. Entomol. Hung. 2011, 46, 235–245. [Google Scholar] [CrossRef]
- Simmonds, M.S.J.; Blaney, W.M.; Ley, S.V.; Anderson, J.C.; Toogood, P.L. Azadirachtin: Structural requirements for reducing growth and increasing mortality in lepidopterous larvae. Entomol. Exp. Appl. 1990, 55, 169–181. [Google Scholar] [CrossRef]
- Jiang, Z.L.; Akhtar, Y.; Zhang, X.; Bradbury, R.; Isman, M.B. Insecticidal and feeding deterrent activities of essential oils in the cabbage looper, Trichoplusia ni (Lepidoptera: Noctuidae). J. Appl. Entomol. 2012, 136, 191–202. [Google Scholar] [CrossRef]
- Herrera, J.M.; Zunino, M.P.; Dambolena, J.S.; Pizzolitto, R.P.; Gañan, N.A.; Lucini, E.I.; Zygadlo, J.A. Terpene ketones as natural insecticides against Sitophilus zeamais. Ind. Crops Prod. 2015, 70, 435–442. [Google Scholar] [CrossRef]
- Shahriari, M.; Zibaee, A.; Sahebzadeh, N.; Shamakhi, L. Effects of α-pinene, trans-anethole, and thymol as the essential oil constituents on antioxidant system and acetylcholine esterase of Ephestia kuehniella Zeller (Lepidoptera: Pyralidae). Pestic. Biochem. Physiol. 2018, 150, 40–47. [Google Scholar] [CrossRef]
- López, M.D.; Pascual-Villalobos, M.J. Are monoterpenoids and phenylpropanoids efficient inhibitors of acetylcholinesterase from stored product insect strains? Flavour. Fragr. J. 2015, 30, 108–112. [Google Scholar] [CrossRef]
- Li, S.G.; Li, M.Y.; Huang, Y.Z.; Hua, R.M.; Lin, H.F.; He, Y.J.; Wei, L.L.; Liu, Z.Q. Fumigant activity of Illicium verum fruit extracts and their effects on the acetylcholinesterase and glutathione S-transferase activities in adult Sitophilus zeamais. J. Pest Sci. 2013, 86, 677–683. [Google Scholar] [CrossRef]
- Murfadunnisa, S.; Vasantha-Srinivasan, P.; Ganesan, R.; Senthil-Nathan, S.; Kim, T.J.; Ponsankar, A.; Kumar, S.D.; Chandramohan, D.; Krutmuang, P. Larvicidal and enzyme inhibition of essential oil from Spheranthus amaranthroids (Burm.) against lepidopteran pest Spodoptera litura (Fab.) and their impact on non-target earthworms. Biocatal. Agric. Biotechnol. 2019, 21, 101324. [Google Scholar] [CrossRef]
- Thanigaivel, A.; Chanthini, K.M.P.; Karthi, S.; Vasantha-Srinivasan, P.; Ponsankar, A.; Sivanesh, H.; Stanley-Raja, V.; Shyam-Sundar, N.; Narayanan, K.R.; Senthil-Nathan, S. Toxic effect of essential oil and its compounds isolated from Sphaeranthus amaranthoides Burm. f. against dengue mosquito vector Aedes aegypti Linn. Pestic. Biochem. Physiol. 2019, 160, 163–170. [Google Scholar] [CrossRef]
- Martinez, S.S.; Van Emden, H.F. Growth disruption, abnormalities and mortality of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae) caused by azadirachtin. Neotrop. Entomol. 2001, 30, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Taffar, A.; Yezli-Touiker, S.; Bendjedid, H.; Soltani, N. Evaluation of azadirachtin, a biopesticides, on growth, development and cuticle secretion of Mediterranean flour moth, Ephestia kuehniella Zeller. J. Entomol. Res. 2021, 45, 436–443. [Google Scholar] [CrossRef]
- Mordue, A.J.; Morgan, E.D.; Nisbet, A.J.; Gilbert, L.I.; Gill, S.S. Azadirachtin, a natural product in insect control. In Insect Control: Biological and Synthetic Agents; Gilbert, L.I., Gill, S.S., Eds.; Academic Press: London, UK, 2010; pp. 185–197. [Google Scholar]
- Swidan, M.H.; Kheirallah, D.A.; Osman, S.E.I.; Nour, F.E. Impact of certain natural insecticides on the morphological and biochemical characteristics of khapra beetle, Trogoderma granarium everts. Int. J. Zool. Investig. 2016, 2, 147–166. [Google Scholar]
- Erler, F.; Tunç, İ. Monoterpenoids as fumigants against greenhouse pests: Toxic, development and reproduction-inhibiting effects/Monoterpenoide als Begasungsmittel gegen Gewächshausschädlinge: Toxizität, Wirkung auf Entwicklung und Reproduktion. Z. Pflanzenkrankh. Pflanzenschutz J. Plant Dis. Prot. 2005, 112, 181–192. [Google Scholar]
- Soonwera, M.; Moungthipmalai, T.; Aungtikun, J.; Sittichok, S. Combinations of plant essential oils and their major compositions inducing mortality and morphological abnormality of Aedes aegypti and Aedes albopictus. Heliyon 2022, 8, e09346. [Google Scholar] [CrossRef]
- Nishida, R. Chemical ecology of insect–plant interactions: Ecological significance of plant secondary metabolites. Biosci. Biotechnol. Biochem. 2014, 78, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Meisner, J.; Kehat, M. The response of Earias insulana Boisd. larvae to phagodeterrent (–)-carvone incorporated in an artificial diet 1. Z. Angew. Entomol. 1980, 90, 80–82. [Google Scholar] [CrossRef]
- Sousa, R.M.O.; Rosa, J.S.; Oliveira, L.; Cunha, A.; Fernandes-Ferreira, M. Activities of Apiaceae essential oils and volatile compounds on hatchability, development, reproduction and nutrition of Pseudaletia unipuncta (Lepidoptera: Noctuidae). Ind. Crops Prod. 2015, 63, 226–237. [Google Scholar] [CrossRef] [Green Version]
- Abdelgaleil, S.A.; Abou-Taleb, H.K.; Al-Nagar, N.; Shawir, M.S. Antifeedant, growth regulatory and biochemical effects of terpenes and phenylpropenes on Spodoptera littoralis Boisduval. Int. J. Trop. Insect Sci. 2020, 40, 423–433. [Google Scholar] [CrossRef]
- Hashem, A.S.; Awadalla, S.S.; Zayed, G.M.; Maggi, F.; Benelli, G. Pimpinella anisum essential oil nanoemulsions against Tribolium castaneum—Insecticidal activity and mode of action. Environ. Sci. Pollut. Res. 2018, 25, 18802–18812. [Google Scholar] [CrossRef]
- Derbalah, A.; Keratum, A.; Darweesh, M.; Elebiary, M.; Hegazy, F. New trends for controlling Sitophilus oryzae concerning adult mortality, offspring production, mode of action, and grain quality. J. Verbrauch. Lebensm. J. Consum. Prot. Food Saf. 2021, 16, 343–351. [Google Scholar] [CrossRef]
- Shahriari, M.; Sahebzadeh, N.; Zibaee, A. Biochemical response of Mediterranean flour moth, Ephestia kuehniella Zeller (Lep.: Pyralidae) to the toxicity of trans-anethole. Plant Prot. (Sci. J. Agric.) 2022, 45, 121–136. [Google Scholar]
- da Cunha, F.A.B.; Wallau, G.L.; Pinho, A.I.; Nunes, M.E.M.; Leite, N.F.; Tintino, S.R.; da Costa, G.M.; Athayde, M.L.; Boligon, A.A.; Coutinho, H.D.M.; et al. Eugenia uniflora leaves essential oil induces toxicity in Drosophila melanogaster: Involvement of oxidative stress mechanisms. Toxicol. Res. 2015, 4, 634–644. [Google Scholar] [CrossRef]
- Ding, C.Y.; Ma, Y.M.; Li, B.; Wang, Y.; Zhao, L.; Peng, J.N.; Li, M.Y.; Liu, S.; Li, S.G. Identification and functional analysis of differentially expressed genes in Myzus persicae (Hemiptera: Aphididae) in response to trans-anethole. J. Insect Sci. 2022, 22, 3. [Google Scholar] [CrossRef]
- Petrović, M.; Popović, A.; Kojić, D.; Šućur, J.; Bursić, V.; Aćimović, M.; Malenčić, Ð.; Stojanović, T.; Vuković, G. Assessment of toxicity and biochemical response of Tenebrio molitor and Tribolium confusum exposed to Carum carvi essential oil. Entomol. Gen. 2019, 38, 333–348. [Google Scholar] [CrossRef]
- Passreiter, C.M.; Wilson, J.; Andersen, R.; Isman, M.B. Metabolism of thymol and trans-anethole in larvae of Spodoptera litura and Trichoplusia ni (Lepidoptera: Noctuidae). J. Agric. Food Chem. 2004, 52, 2549–2551. [Google Scholar] [CrossRef]
- Marumoto, S.; Okuno, Y.; Hagiwara, Y.; Miyazawa, M. Biotransformation of (+)-Carvone and (−)-Carvone by the Common Cutworm Spodoptera litura Larvae. J. Oleo Sci. 2018, 67, 1253–1257. [Google Scholar] [CrossRef]



| Traits | Formulae | Units |
|---|---|---|
| Mass gain | MG = m2 − m0 | mg |
| Amount of consumed food | mc = d2 − d0 | mg |
| Amount of assimilated food | ma = mc − me | mg |
| Amount of metabolized food | mm = ma − MG | mg |
| Relative growth rate | RGR = MG/(2 × m0) | mg/mg/day |
| Relative consumption rate | RCR = mc/(2 × m0) | mg/mg/day |
| Relative metabolic rate | RMR = mm/(2 × m0) | mg/mg/day |
| The efficiency of conversion of ingested food (gross growth efficiency) | ECI = MG/mc × 100 | % |
| Approximate digestibility (assimilation efficiency) | AD = ma/mc × 100 | % |
| The efficiency of conversion of digested food (net growth efficiency) | ECD = MG/ma × 100 | % |
| Metabolic cost | MC = 100 − ECD | % |
| Botanical | Period (h) | Slope ± SE (CI) | LC30 a (%) (CI) | LC50 a (%) (CI) | LC95 a (%) (CI) | χ2 (df) | p |
|---|---|---|---|---|---|---|---|
| Anethole | 24 | 2.25 ± 0.45 (1.36; 3.14) | 0.323 (0.216; 0.408) | 0.552 (0.441; 0.711) | 2.970 (1.744; 9.699) | 0.001 (1) | 0.980 |
| 48 | 3.02 ± 0.37 (2.30; 3.74) | 0.274 (0.221; 0.324) | 0.409 (0.346; 0.483) | 1.433 (1.076; 2.228) | 0.793 (2) | 0.673 | |
| 72 | 4.40 ± 0.53 (3.37; 5.44) | 0.220 (0.184; 0.252) | 0.289 (0.252; 0.330) | 0.683 (0.562; 0.911) | 1.857 (2) | 0.395 | |
| 96 | 8.42 ± 0.94 (6.58; 10.26) | 0.198 (0.170; 0.228) | 0.261 (0.231; 0.298) | 0.456 (0.401; 0.537) | 5.754 (3) | 0.124 | |
| 120 | 7.97 ± 1.38 (5.27; 10.67) | 0.177 (0.096; 0.264) | 0.243 (0.173; 0.375) | 0.450 (0.335; 0.805) | 7.155 (3) | 0.067 | |
| Carvone | 24 | 2.35 ± 0.46 (1.45; 3.24) | 0.287 (0.188; 0.365) | 0.481 (0.382; 0.600) | 2.411 (1.503; 6.623) | 0.083 (1) | 0.774 |
| 48 | 3.05 ± 0.36 (2.34; 3.76) | 0.242 (0.195; 0.287) | 0.360 (0.305; 0.424) | 1.247 (0.949; 1.884) | 0.398 (2) | 0.820 | |
| 72 | 4.19 ± 0.49 (3.23; 5.15) | 0.205 (0.172; 0.237) | 0.274 (0.238; 0.314) | 0.677 (0.553; 0.910) | 2.802 (2) | 0.246 | |
| 96 | 8.34 ± 0.94 (6.50; 10.18) | 0.189 (0.161; 0.218) | 0.252 (0.223; 0.289) | 0.449 (0.395; 0.532) | 5.662 (3) | 0.129 | |
| 120 | 8.85 ± 1.10 (6.70; 11.00) | 0.187 (0.154; 0.219) | 0.247 (0.215; 0.283) | 0.432 (0.380; 0.514) | 3.858 (2) | 0.145 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostić, I.; Milanović, S.; Kostić, M.; Šešlija Jovanović, D.; Ćalić, D.; Jankovský, L.; Lazarević, J. Antifeeding, Toxic, and Growth-Reducing Activity of trans-Anethole and S-(+)-Carvone against Larvae of the Gypsy Moth Lymantria dispar (L.). Agronomy 2022, 12, 3049. https://doi.org/10.3390/agronomy12123049
Kostić I, Milanović S, Kostić M, Šešlija Jovanović D, Ćalić D, Jankovský L, Lazarević J. Antifeeding, Toxic, and Growth-Reducing Activity of trans-Anethole and S-(+)-Carvone against Larvae of the Gypsy Moth Lymantria dispar (L.). Agronomy. 2022; 12(12):3049. https://doi.org/10.3390/agronomy12123049
Chicago/Turabian StyleKostić, Igor, Slobodan Milanović, Miroslav Kostić, Darka Šešlija Jovanović, Dušica Ćalić, Libor Jankovský, and Jelica Lazarević. 2022. "Antifeeding, Toxic, and Growth-Reducing Activity of trans-Anethole and S-(+)-Carvone against Larvae of the Gypsy Moth Lymantria dispar (L.)" Agronomy 12, no. 12: 3049. https://doi.org/10.3390/agronomy12123049
APA StyleKostić, I., Milanović, S., Kostić, M., Šešlija Jovanović, D., Ćalić, D., Jankovský, L., & Lazarević, J. (2022). Antifeeding, Toxic, and Growth-Reducing Activity of trans-Anethole and S-(+)-Carvone against Larvae of the Gypsy Moth Lymantria dispar (L.). Agronomy, 12(12), 3049. https://doi.org/10.3390/agronomy12123049








