Plant Growth Promoting Filamentous Fungi and Their Application in the Fertilization of Pastures for Animal Consumption
Abstract
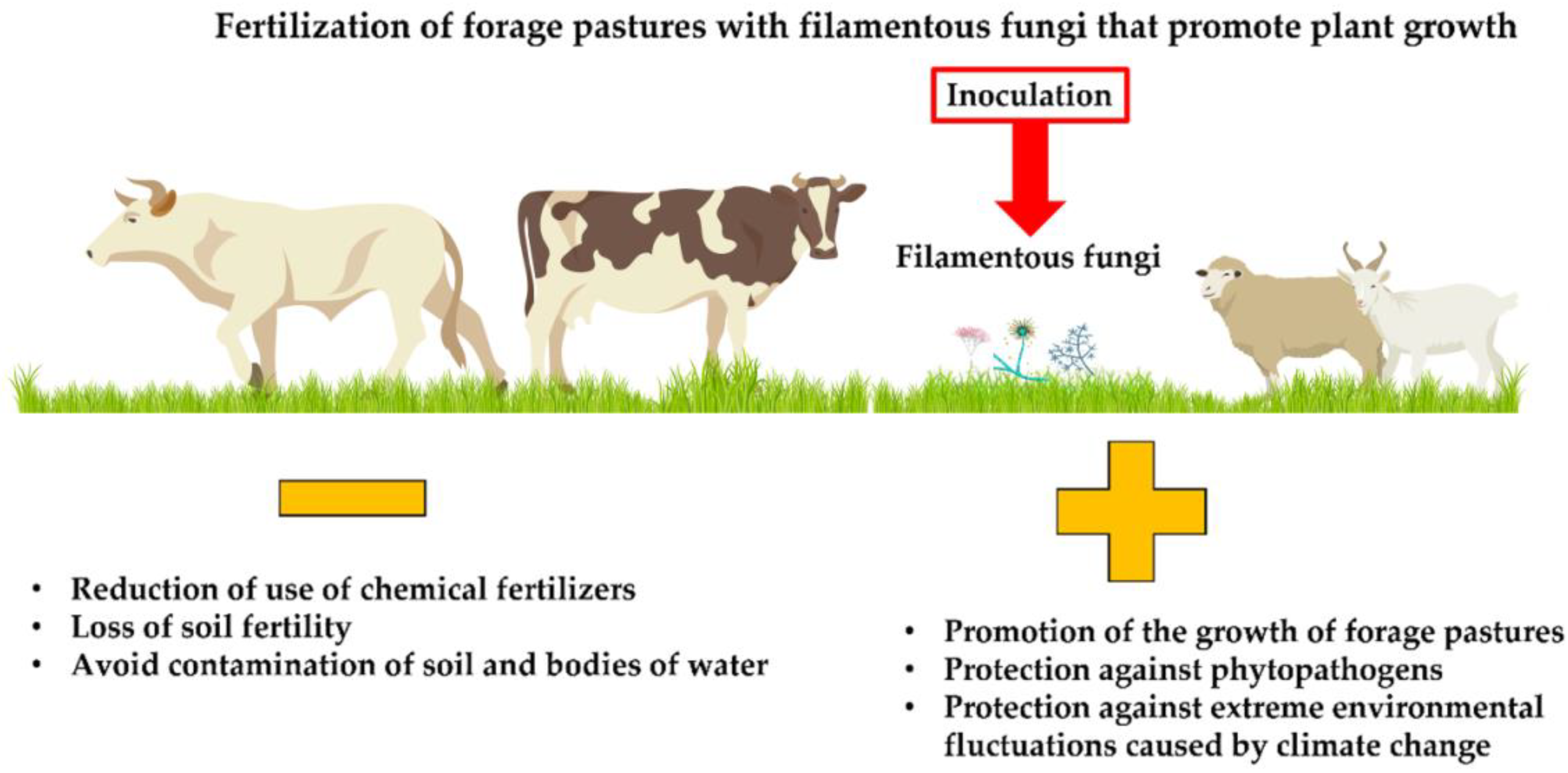
:1. Introduction
2. Interactions of Plants and Fungi
2.1. Pathogenesis
2.2. Commensalism
2.3. Mutualism
3. Plant Growth Promoting Filamentous Fungi
3.1. Trichoderma
3.2. Penicillium
3.3. Aspergillus
3.4. Fusarium
3.5. Other Fungal Genera
3.6. Filamentous Fungi That Promote Plant Growth of Gramineae
4. Importance of Pastures for Animal Consumption
Filamentous Fungi That Promote Plant Growth—A Sustainable Alternative for Pasture Production
| Trichoderma | Pastures | Benefit | References |
|---|---|---|---|
| Four strains of T. atroviride | Perennial ryegrass | The inoculation increased the dry weight of shoots and roots and controlled four phytopathogens (Rhizoctonia solani, Sclerotinia trifoliorum, Fusarium culmorum, and Pythium ultimum) | [166] |
| T. viride | Raygrass (Lolium perenne) | Trichoderma inoculation increased green matter and dry matter | [170] |
| T. harzianum (Trichozam®) | Brachiaria híbrido cv. Mulato | The inoculation produced the highest dry matter production (145.0 kg/ha/day), but it was not significantly different from the control (131.7 kg/ha/day) | [163] |
| T. harzianum + Azospirillum sp. | Marandú grass (Brachiaria brizantha) Guinea grass (Panicum maximum) | Trichoderma inoculation produced greater root development and a higher percentage of dry matter compared to the control | [165] |
| T. atroviride | Prairie grass (Bromus wildenowii Kunth) | Trichoderma inoculation had no effect on seed yield; however, it significantly reduced root infection by Gaeumannomyces graminis var. tritici | [171] |
| Mixture of T. atroviride | The sterile hybrid grass Miscanthus × giganteus | Trichoderma inoculation increased the chlorophyll content in the leaves as well as the digestibility of the dry material for cattle | [142] |
| Tricosave® (T. harzianum) + Bradyrhizobium sp. | Hybrid Tifton 85 (Cynodon dactylon) | Trichoderma inoculation increased grass biomass compared to the control | [164] |
| T. harzianum Rifai | Lolium perenne L. Lolium multiflorum Lam. (perennial ryegrass) | Trichoderma improved the growth of both grasses and increased the lengths of the perennial ryegrass leaves | [162] |
5. Perspectives and Conclusions
- (1)
- Use fungal isolates from the rhizospheres of the grasses to be fertilized to produce inocula, looking for fungal strains associated with particular forage grasses.
- (2)
- Use inocula consisting of a single fungal strain, of combinations of several fungal strains, and of combinations of fungal strains with other beneficial microorganisms.
- (3)
- Use different forms of inocula and inoculation.
- (4)
- Consider the efficiency of a fungal inoculum with respect to germination and plant growth.
- (5)
- Determine the effects of external factors on the viability of fungal inocula.
- (6)
- Determine the nutritional contents of forage grasses inoculated with fungal strains.
- (7)
- Consider the production costs of the fungal inocula.
- (8)
- Establish quality standards for fungal bioformulations for forage grasses.
- (9)
- Train farmers in the use of fungal bioformulations for forage grasses.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Isaac, S. Fungal Plant Interaction; Chapman and Hall Press: London, UK, 1992; pp. 1–2. [Google Scholar]
- Nanjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Diversity, taxonomy, and phylogeny of the fungi. Biol. Rev. 2019, 94, 2101–2137. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulus, C.J.; Mims, C.W.; Blackwell, M.M. Introductory Mycology, 4th ed.; Wiley: Oakland, CA, USA, 1996; pp. 1–18. [Google Scholar]
- Ruiz, J. El asombroso reino de los hongos. Av. Perspect. 2001, 20, 275–281. [Google Scholar]
- Webster, J.; Weber, R.W.S. Introduction to Fungi, 3rd ed.; Cambridge University Press: New York, NY, USA, 2007; pp. 1–7. [Google Scholar]
- Aristegui, B. El reino de los hongos. Rev. Iberoam. Micol. 2002, 8, 1–4. [Google Scholar]
- Mata, M. Costa Rica Mushrooms; Instituto Nacional de Biodiversidad (INBio): Heredia, Costa Rica, 2003; Volume 1, pp. 1–5. [Google Scholar]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Major ecological adaptations and evolutionary transitions. Biol. Rev. 2019, 94, 1443–1476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naranjo-Ortiz, M.A.; Gabaldón, T. Fungal evolution: Cellular, genomic and metabolic complexity. Biol. Rev. Camb. Philos. Soc. 2020, 95, 1198–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawksworth, D.L.; Lücking, R. Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol. Spectr. 2017, 5, 1–17. [Google Scholar] [CrossRef]
- Treseder, K.K.; Lennon, J.T. Fungal traits that drive ecosystem dynamics on land. Microbiol. Mol. Biol. Rev. 2015, 79, 243–262. [Google Scholar] [CrossRef] [Green Version]
- Nonzom, S.; Sumbali, G. Fate of mitosporic soil fungi in cold deserts: A review. Am. Int. J. Res. Form. Appl. Nat. Sci. 2015, 9, 1–9. [Google Scholar]
- Sun, J.Z.; Liu, X.Z.; McKenzie, E.H.C.; Jeewon, R.; Liu, J.K.; Zhang, X.L.; Zhao, Q.; Hyde, K.D. Fungicolous fungi: Terminology, diversity, distribution, evolution and species checklist. Fungal Divers. 2019, 95, 337–430. [Google Scholar] [CrossRef]
- Gostinčar, C.; Grube, M.; de Hoog, S.; Zalar, P.; Gunde-Cimerman, N. Extremotolerance in fungi: Evolution on the edge. FEMS Microbiol. Ecol. 2010, 71, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Selbmann, L.; Egidi, E.; Isola, D.; Onofri, S.; Zucconi, Z.; de Hoog, G.S.; Chinaglia, S.; Testa, L.; Tosi, S.; Balestrazzi, A.; et al. Biodiversity, evolution and adaptation of fungi in extreme environments. Plant Biosyst. 2013, 147, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Cuevas Moreno, J.A. Los hongos: Héroes y villanos de la prosperidad humana. Rev. Digit. Univ. 2016, 17, 10. [Google Scholar]
- Ashraf, S.; Zuhaib, M. Fungal biodiversity: A potential tool in plant disease management. In Management of Microbial Resources in the Environment; Malik, A., Grohmann, E., Alves, M., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 69–90. [Google Scholar]
- Chambergo, F.S.; Valencia, E.Y. Fungal biodiversity to biotechnology. Appl. Microbiol. Biot. 2016, 100, 2567–2577. [Google Scholar] [CrossRef]
- Meyer, V.; Andersen, M.R.; Brakhage, A.A.; Braus, G.H.; Caddick, M.X.; Cairns, T.C.; de Vries, R.P.; Haarmann, T.; Hansen, K.; Hertz-Fowler, C.; et al. Current challenges of research on filamentous fungi in relation to human welfare and a sustainable bio-economy: A white paper. Fungal Biol. Biotechnol. 2016, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Leitão, A.L.; Enguita, F.J. Gibberellins in Penicillium strains: Challenges for endophyte-plant host interactions under salinity stress. Microbiol. Res. 2016, 183, 8–18. [Google Scholar] [CrossRef]
- Waghunde, R.R.; Shelake, R.M.; Sabalpara, A.N. Trichoderma: A significant fungus for agriculture and environment. Afr. J. Agric. Res. 2016, 11, 1952–1965. [Google Scholar]
- Yoo, S.J.; Shin, D.J.; Won, H.Y.; Song, J.; Sang, M.K. Aspergillus terreus JF27 promotes the growth of tomato plants and induces resistance against Pseudomonas syringae pv. tomato. Mycobiology 2018, 46, 147–153. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Xie, J.; Jiang, D.; Fu, Y.; Li, G.; Lin, F. Antifungal substances produced by Penicillium oxalicum strain PY-1—Potential antibiotics against plant pathogenic fungi. World J. Microbiol. Biotechnol. 2008, 24, 909–915. [Google Scholar] [CrossRef]
- Waqas, M.; Khan, A.L.; Muhammad, H.; Shahzad, R.; Kang, S.M.; Kim, J.G.; Lee, I.J. Endophytic fungi promote plant growth and mitigate the adverse effects of stem rot: An example of Penicillium citrinum and Aspergillus terreus. J. Plant Interact. 2015, 10, 280–287. [Google Scholar] [CrossRef]
- Okoth, S.A.; Otadoh, J.A.; Ochanda, J.O. Improved seedling emergence and growth of maize and beans by Trichoderma harziunum. Trop. Subtrop. Agroecosyst. 2011, 13, 65–71. [Google Scholar]
- Banerjee, S.; Dutta, S. Plant growth promoting activities of a fungal strain Penicillium commune MCC 1720 and its effect on growth of black gram. Pharma Innov. 2019, 8, 121–127. [Google Scholar]
- Changas Junior, A.F.; Souza, M.C.; Martins, A.L.L.; Lima, C.A.; de Oliveira Moura, D.M.; Ferreira, A.L.L.; Lopes, M.B.; Chagas, L.F.B. Efficiency of Trichoderma asperellum as a promoter of vegetable growth and soybean productivity. Res. Soc. Dev. 2022, 11, e50711629200. [Google Scholar] [CrossRef]
- De Souza, R.R.; Moraes, M.P.; Paraginski, J.A.; Moreira, T.F.; Bittencourt, K.C.; Toebe, M. Effects of Trichoderma asperellum on germination indexes and seedling parameters of Lettuce cultivars. Curr. Microbiol. 2022, 79, 2–12. [Google Scholar] [CrossRef]
- Kumar, K.; Thakur, P.; Rathore, U.S.; Kumar, S.; Mishra, R.K.; Amaresan, N.; Pandey, S.; Mishra, M. Plant beneficial effects of Trichoderma spp. suppressing Fusarium wilt and enhancing growth in Tomato. Vegetos 2022, 35, 188–195. [Google Scholar] [CrossRef]
- Galeano, R.M.S.; Franco, D.G.; Chaves, P.O.; Giannesi, G.C.; Masui, D.C.; Ruller, R.; Corrêa, B.O.; da Silva Brasil, M.; Fonseca Zanoelo, F. Plant growth promoting potential of endophytic Aspergillus niger 9-p isolated from native forage grass in Pantanal of Nhecolândia region, Brazil. Rhizosphere 2021, 18, 100332. [Google Scholar] [CrossRef]
- Klich, M.A. Biogeography of Aspergillus species in soil and litter. Mycologia 2002, 94, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.N.; Verma, P.; Kumar, V.; Sangwan, P.; Mishra, S.; Panjiar, N.; Gupta, V.K.; Saxena, A.K. Biodiversity of the genus Penicillium in different habitats. In New and Future Developments in Microbial Biotechnology and Bioengineering, Penicillium System Properties and Applications; Gupta, V.K., Rodriguez-Couto, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–18. [Google Scholar]
- Nakkeeran, S.; Marimuthu, T.; Renukadevi, P.; Brindhadevi, S.; Jogaiah, S. Exploring the biogeographical diversity of Trichoderma for plant health. In Biocontrol Agents and Secondary Metabolites; Jogaiah, S., Ed.; Woodhead Publishing: Cambridge, UK, 2021; pp. 537–571. [Google Scholar]
- Cabral-Miramontes, J.P.; Olmedo-Monfil, V.; Lara-Banda, M.; Zúñiga-Romo, E.R.; Aréchiga-Carvajal, E.T. Promotion of plant growth in arid zones by selected Trichoderma spp. strains with adaptation plasticity to alkaline pH. Biology 2022, 11, 1206. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Penicillium and Talaromyces. In Fungi and Food Spoilage; Pitt, J.I., Hocking, A.D., Eds.; Springer: Cham, Switzerland, 2022; pp. 231–349. [Google Scholar]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- Pitt, J.I. The current role of Aspergillus and Penicillium in human and animal health. J. Med. Vet. Mycol. 1994, 32, 17–32. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Komon-Zelazowska, M.; Druzhinina, I.S. Fungal genus Hypocrea/Trichoderma: From barcodes to biodiversity. J. Zhejiang Univ. Sci. B 2008, 9, 753–763. [Google Scholar] [CrossRef] [Green Version]
- Nag, P.; Paul, S.; Shriti, S.; Das, S. Defence response in plants and animals against a common fungal pathogen, Fusarium oxysporum. Curr. Res. Microb. Sci. 2022, 3, 100135. [Google Scholar] [CrossRef] [PubMed]
- Allaga, H.; Zhumakayev, A.; Büchner, R.; Kocsubé, S.; Szűcs, A.; Vágvölgyi, C.; Kredics, L.; Hatvani, L. Members of the Trichoderma harzianum species complex with mushroom pathogenic potential. Agronomy 2021, 11, 2434. [Google Scholar] [CrossRef]
- Salinas, M.C.; Cavagnaro, P.F. In vivo and in vitro screening for resistance against Penicillium allii in garlic accessions. Eur. J. Plant Pathol. 2020, 156, 173–187. [Google Scholar] [CrossRef]
- Louw, J.P.; Korsten, L. Pathogenicity and host susceptibility of Penicillium spp. on citrus. Plant Dis. 2015, 99, 21–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louw, J.P.; Korsten, L. Pathogenic Penicillium spp. on apples and pears. Plant Dis. 2014, 98, 590–598. [Google Scholar] [CrossRef] [Green Version]
- Dugan, F.M.; Lupien, S.L.; Vahling-Armstrong, C.; Chastagner, G.A.; Schroeder, B.K. Host range of Penicillium species causing blue mold of bulbs crops in Washington State and Idaho. Crop Prot. 2017, 96, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.; Wang, X.; Chen, T.; Wang, S.; Chen, X.; Song, Z.; Shi, X.; Laborda, P. First report of Aspergillus flavus causing fruit rot on kiwifruit in China. Plant Dis. 2022, 106, 1990. [Google Scholar] [CrossRef] [PubMed]
- Khizar, M.; Haroon, U.; Ali, M.; Arif, S.; Shah, I.H.; Chaudhary, H.J.; Munis, M.F.H. Aspergillus tubigensis causes leaf spot of cotton (Gossypium hirsutum L.) in Pakistan. Phyton Int. J. Exp. Bot. 2020, 89, 103–109. [Google Scholar]
- El-Baky, N.A.; Abdel Rahman, R.A.; Sharaf, M.M.; Amara, A.A.A.F. The Development of a phytopathogenic fungi control trial: Aspergillus flavus and Aspergillus niger infection in jojoba tissue culture as a model. Sci. World J. 2021, 2021, 6639850. [Google Scholar] [CrossRef]
- Jarvis, W.R.; Traquair, J.A. Bunch rot of grapes caused by Aspergillus aculeatus. Plant Dis. 1984, 68, 718–719. [Google Scholar] [CrossRef]
- Çakar, G.; Tozlu, E. The biological control of Fusarium oxysporum, the causal agent of potato rot. Gesunde Pflanz. 2022, 74, 305–315. [Google Scholar] [CrossRef]
- Attia, M.S.; Abdelaziz, A.M.; Al-Askar, A.A.; Arishi, A.A.; Abdelhakim, A.M.; Hashem, A.H. Plant growth-promoting fungi as biocontrol tool against Fusarium wilt disease of tomato plant. J. Fungi 2022, 8, 775. [Google Scholar] [CrossRef] [PubMed]
- Ismaila, A.A.; Ahmad, K.; Siddique, Y.; Wahab, M.A.A.; Kutawa, A.B.; Abdullahi, A.; Zobir, S.A.M.; Abdu, A.; Abdullah, S.N.A. Fusarium wilt of banana: Current update and sustainable disease control using classical and essential oils approaches. Hortic. Plant J. 2022, in press.
- Soleha, S.; Muslim, A.; Suwandi, S.; Kadir, S.; Pratama, R. The identification and pathogenicity of Fusarium oxysporum causing acacia seedling wilt disease. J. For. Res. 2022, 33, 711–719. [Google Scholar] [CrossRef]
- Mahadeo, K.; Palama, T.L.; Côme, B.; Kodja, H. Vanilla: Culture, reproduction, phytochemistry, curing, pest, and diseases. In Orchids Phytochemistry, Biology and Horticulture; Reference Series in Phytochemistry; Mérillon, J.M., Kodja, H., Eds.; Springer: Cham, Switzerland, 2022; pp. 329–340. [Google Scholar]
- Costa, M.M.; Saleh, A.A.; Melo, M.P.; Guimarães, E.A.; Esele, J.P.; Zeller, K.A.; Summerell, B.A.; Pfenning, L.H.; Leslie, J.F. Fusarium mirum sp. nov, intertwining Fusarium madaense and Fusarium andiyazi, pathogens of tropical grasses. Fungal Biol. 2022, 126, 250–266. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.O. Effects of Fusarium nivale and F. culmorum on the establishment of four species of pasture grass. Ann. Appl. Biol. 1979, 91, 243–250. [Google Scholar] [CrossRef]
- Aly, A.H.; Debbab, A.; Proksch, P. Fungal endophytes: Unique plant inhabitants with great promises. Appl. Microbiol. Biotechnol. 2011, 90, 1829–1845. [Google Scholar] [CrossRef]
- Baron, N.C.; Rigobelo, E.C. Endophytic fungi: A tool for plant growth promotion and sustainable agriculture. Mycology 2022, 13, 39–55. [Google Scholar] [CrossRef]
- Li, X.; Han, S.; Wang, G.; Liu, X.; Amombo, E.; Xie, Y.; Fu, J. The fungus Aspergillus aculeatus enhances salt-stress tolerance, metabolite accumulation, and improves forage quality in perennial ryegrass. Front. Microbiol. 2017, 8, 1664. [Google Scholar] [CrossRef]
- Islam, S.; Akanda, A.M.; Prova, A.; Sultana, F.; Hossain, M.M. Growth promotion effect of Fusarium spp. PPF1 from bermudagrass (Cynodon dactylon) rhizosphere on Indian spinach (Basella alba) seedlings are linked to root colonization. Arch. Phytopathol. Plant Prot. 2014, 47, 2319–2331. [Google Scholar] [CrossRef]
- Singh, L.P.; Gill, S.S.; Tuteja, N. Unraveling the role of fungal symbionts in plant abiotic stress tolerance. Plant Signal. Behav. 2011, 6, 175–191. [Google Scholar] [CrossRef] [Green Version]
- Redman, R.S.; Dunigan, D.D.; Rodriguez, R.J. Fungal symbiosis: From mutualism to parasitism, who controls the outcome, host or invader? New Phytol. 2001, 151, 705–716. [Google Scholar] [CrossRef] [Green Version]
- Hirsch, A.M. Plant-microbe symbioses: A continuum from commensalism to parasitism. Symbiosis 2004, 37, 345–363. [Google Scholar]
- Newton, A.C.; Fitt, B.D.L.; Atkins, S.D.; Walters, D.R.; Daniell, T.J. Pathogenesis, parasitism, and mutualism in the trophic space of microbe-plant interactions. Trends Microbiol. 2010, 18, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, D.Y.; Crouch, J.A. Bacterial/fungal interactions: From pathogens to mutualistic endosymbionts. Annu. Rev. Phytopathol. 2009, 47, 63–82. [Google Scholar] [CrossRef] [Green Version]
- Burdon, J.J. Fungal pathogens as selective forces in plant populations and communities. Aust. J. Ecol. 1991, 16, 423–432. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttila, A.M.; Company, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [Green Version]
- Casadevall, A. Determinants of virulence in the pathogenic fungi. Fungal Biol. Rev. 2007, 21, 130–132. [Google Scholar] [CrossRef] [Green Version]
- Stergiopoulos, I.; Gordon, T.R. Cryptic fungal infections: The hidden agenda of plant pathogens. Front. Plant Sci. 2014, 5, 506. [Google Scholar] [CrossRef] [Green Version]
- Köller, W. The plant cuticle: A barrier to be overcome by fungal plant pathogens. In Fungal Spore and Disease Initiation in Plants and Animals; Cole, G.T., Hoch, H.C., Eds.; Plenum Press: New York, NY, USA, 1991; pp. 219–246. [Google Scholar]
- Ueda, H.; Kurose, D.; Kugimiya, S.; Kugimiya, S.; Mitsuhara, I.; Yoshida, S.; Tabata, J.; Ken Suzuki, K.; Kitamoto, H. Disease severity enhancement by an esterase from non-phytopathogenic yeast Pseudozyma antarctica and its potential as adjuvant for biocontrol agents. Sci. Rep. 2018, 8, 16455. [Google Scholar] [CrossRef]
- Mendgen, K.; Hahn, M.; Deising, H. Morphogenesis and mechanisms of penetration by plant pathogenic fungi. Annu. Rev. Phytopathol. 1996, 34, 367–386. [Google Scholar] [CrossRef] [Green Version]
- Deising, H.B.; Werner, S.; Wernitz, M. The role of fungal appressoria in plant infection. Microbes Infect. 2000, 2, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Chethana, K.W.T.; Jayawardena, R.S.; Chen, Y.J.; Konta, S.; Tibpromma, S.; Phukhamsakda, C.; Abeywickrama, P.D.; Samarakoon, M.C.; Senwanna, C.; Mapook, A.; et al. Appressorial interactions with host and their evolution. Fungal Divers. 2021, 110, 75–107. [Google Scholar] [CrossRef]
- Leung, T.L.F.; Poulin, R. Parasitism, commensalism, and mutualism: Exploring the many shades of symbioses. Vie Milieu/Life Environ. 2008, 58, 107–115. [Google Scholar]
- Mathis, K.A.; Bronstein, J.L. Our current understanding of commensalism. Annu. Rev. Ecol. Evol. Syst. 2020, 14, 167–189. [Google Scholar] [CrossRef]
- Rai, M.; Agarkar, G. Plant–fungal interactions: What triggers the fungi to switch among lifestyles? Crit. Rev. Microbiol. 2016, 42, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Creamer, R.; Baucom, D. Fungal endophytes of locoweeds: A commensal relationship? J. Plant Physiol. Pathol. 2013, 1, 2. [Google Scholar]
- Brundrett, M.C. Coevolution of roots and mycorrhizas of land plants. New Phytol. 2002, 154, 275–304. [Google Scholar] [CrossRef]
- Gross, M. The success story of plants and fungi. Curr. Biol. 2019, 29, 183–185. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, R.; Redman, R. More than 400 million years of evolution and some plants still can’t make it on their own: Plant stress tolerance via fungal symbiosis. J. Exp. Bot. 2008, 59, 1109–1114. [Google Scholar] [CrossRef]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 10, 763–775. [Google Scholar] [CrossRef]
- Francis, R.; Read, D.J. Mutualism and antagonism in the mycorrhizal symbiosis, with special reference to impacts on plant community structure. Can. J. Bot. 1995, 73, S1301–S1309. [Google Scholar] [CrossRef]
- Kogel, K.-H.; Franken, P.; Hückelhoven, R. Endophyte or parasite—What decides? Curr. Opin. Plant Biol. 2006, 9, 358–363. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, Y.-N.; Shu, B.; Wu, Q.-S. Deciphering molecular mechanisms regarding enhanced drought tolerance in plants by arbuscular mycorrhizal fungi. Sci. Hortic. 2023, 308, 111591. [Google Scholar] [CrossRef]
- Schulz, B.; Guske, S.; Dammann, U.; Boyle, C. Endophyte-host interactions II. Defining symbiosis of the endophyte-host interaction. Symbiosis 1998, 25, 213–227. [Google Scholar]
- Padhi, L.; Mohanta, Y.K.; Panda, S.K. Endophytic fungi with great promises: A review. J. Adv. Pharm. Educ. Res. 2013, 3, 152–171. [Google Scholar]
- Brundrett, M.C. Understanding the roles of multifunctional mycorrhizal and endophytic fungi. In Microbial Root Endophytes: Soil Biology; Schulz, B.J.E., Boyle, C.J.C., Sieber, T.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 9, pp. 281–285. [Google Scholar]
- Gao, F.K.; Dai, C.C.; Liu, X.Z. Mechanisms of fungal endophytes in plant protection against pathogens. Afr. J. Microbiol. Res. 2010, 4, 1346–1351. [Google Scholar]
- Saikkonen, K.; Wäli, P.; Helander, M.; Faeth, S.H. Evolution of endophyte–plant symbioses. Trends Plant Sci. 2004, 9, 275–280. [Google Scholar] [CrossRef]
- Pattnaik, S.S.; Busi, S. Rhizospheric Fungi: Diversity and Potential Biotechnological Applications. In Recent Advancement in White Biotechnology through Fungi: Fungal Biology; Yadav, A., Mishra, S., Singh, S., Gupta, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 63–65. [Google Scholar]
- Murali, M.; Naziya, B.; Ansari, M.A.; Alomary, M.N.; AlYahya, S.; Almatroudi, A.; Thriveni, M.C.; Gowtham, H.G.; Singh, S.B.; Aiyaz, M.; et al. Bioprospecting of rhizosphere-resident fungi: Their role and importance in sustainable agriculture. J. Fungi 2021, 7, 314. [Google Scholar] [CrossRef]
- Lombardi, N.; Caira, S.; Troise, A.D.; Scaloni, A.; Vitaglione, P.; Vinale, F.; Marra, R.; Salzano, A.M.; Lorito, M.; Woo, S.L. Trichoderma applications on strawberry plants modulate the physiological processes positively affecting fruit production and quality. Front Microbiol. 2020, 11, 1364. [Google Scholar] [CrossRef]
- Jin, F.; Hu, Q.; Zhao, Y.; Lin, X.; Zhang, J.; Zhang, J. Enhancing quinoa growth under severe saline-alkali stress by phosphate solubilizing microorganism Penicillium funicuiosum P1. PLoS ONE 2022, 17, e0273459. [Google Scholar] [CrossRef]
- Javed, A.; Shah, A.H.; Hussain, A.; Shinwari, Z.K.; Khan, S.A.; Khan, W.; Jan, S.A. Potential of endophytic fungus Aspergillus terreus as potent plant growth promoter. Pak. J. Bot. 2020, 52, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shao, Y.; Li, L.; Zhao, L.; Zhang, M.; Dong, C. The plant growth-promoting endophytic Fusarium oxysporum GG22 enhances Rehmannia glutinosa secondary metabolites accumulation. Ind. Crops Prod. 2022, 182, 114881. [Google Scholar] [CrossRef]
- Zhang, K.; Bonito, G.; Hsu, C.M.; Hameed, K.; Vilgalys, R.; Liao, H.L. Mortierella elongata increases plant biomass among non-leguminous crop species. Agronomy 2020, 10, 754. [Google Scholar] [CrossRef]
- Elsharkawy, M.M.; Suga, H.; Shimizu, M. Systemic resistance induced by Phoma sp. GS8-3 and nanosilica against cucumber mosaic virus. Environ. Sci. Pollut. Res. 2020, 27, 19029–19037. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, L.; Wang, X.; Zhu, P.; Wu, H.; Qi, S. Plant growth-promoting endophyte Piriformospora indica alleviates salinity stress in Medicago truncatula. Plant Physiol. Biochem. 2017, 119, 211–223. [Google Scholar] [CrossRef] [PubMed]
- El-Maraghy, S.S.; Tohamy, A.T.; Hussein, K.A. Plant protection properties of the plant growth-promoting fungi (PGPF): Mechanisms and potentiality. Curr. Res. Environ. Appl. Mycol. 2021, 11, 391–415. [Google Scholar] [CrossRef]
- Devi, R.; Kaur, T.; Kour, D.; Rana, K.L.; Yadav, A.; Yadav, A.N. Beneficial fungal communities from different habitats and their roles in plant growth promotion and soil health. Microb. Biosyst. 2020, 5, 21–47. [Google Scholar] [CrossRef]
- Da Silva, J.M.; Montaldo, Y.C.; de Almeida, A.C.P.S.; Dalbon, V.A.; Acevedo, J.P.M.; dos Santos, T.M.C.; de Andrade Lima, G.S. Rhizospheric fungi to plant growth promotion: A review. J. Agric. Stud. 2021, 9, 411–425. [Google Scholar] [CrossRef]
- Poveda, J.; Eugui, D.; Abril-Urías, P.; Velasco, P. Endophytic fungi as direct plant growth promoters for sustainable agricultural production. Symbiosis 2021, 85, 1–19. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Islam, S. Plant growth-promoting fungi (PGPF): Phytostimulation and induced systemic resistance. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Singh, D., Singh, H., Prabha, R., Eds.; Springer: Singapore, 2017; pp. 135–140. [Google Scholar]
- Sharma, S.; Kour, D.; Rana, K.L.; Dhiman, A.; Thakur, S.; Thakur, P.; Thakur, S.; Thakur, N.; Sudheer, S.; Yadav, N.; et al. Trichoderma: Biodiversity, Ecological Significances, and Industrial Applications. In Recent Advancement in White Biotechnology through Fungi: Fungal Biology; Yadav, A., Mishra, S., Singh, S., Gupta, A., Eds.; Springer: Cham, Switzerland, 2019; pp. 85–88. [Google Scholar]
- Morán-Diez, M.E.; Trushina, N.; Lamdan, N.L.; Rosenfelder, L.; Mukherjee, P.K.; Kenerley, C.M.; Horwitz, B.A. Host-specific transcriptomic pattern of Trichoderma virens during interaction with maize or tomato roots. BMC Genom. 2015, 16, 8. [Google Scholar] [CrossRef] [Green Version]
- Brotman, Y.; Kapuganti, J.G.; Viterbo, A. Trichoderma. Curr. Biol. 2010, 20, R390–R391. [Google Scholar] [CrossRef] [Green Version]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Hermosa, R.; Rubio, M.B.; Cardoza, R.E.; Nicolás, C.; Monte, E.; Gutiérrez, S. The contribution of Trichoderma to balancing the costs of plant growth and defense. Int. Microbiol. 2013, 16, 69–80. [Google Scholar]
- Stewart, A.; Hill, R. Applications of Trichoderma in plant growth promotion. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 415–428. [Google Scholar]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma–plant–pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The current status of its application in agriculture for the biocontrol of fungal phytopathogens and stimulation of plant growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Maisarah Nur Sarbani, M.; Yahaya, N. Advanced development of bio-fertilizer formulations using microorganisms as inoculant for sustainable agriculture and environment—A review. Malays. J. Sci. Health Technol. 2022, 8, 92–101. [Google Scholar] [CrossRef]
- Altaf, M.M.; Imran, M.; Abulreesh, H.H.; Khan, M.S.A.; Ahmad, I. Diversity and applications of Penicillium spp. in plant-growth promotion. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Rodriguez-Couto, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 261–276. [Google Scholar]
- Wakelin, S.A.; Warren, R.A.; Ryder, M.H. Effect of soil properties on growth promotion of wheat by Penicillium radicum. Aust. J. Soil. Res. 2004, 42, 897–904. [Google Scholar] [CrossRef]
- Gujar, P.D.; Bhavsar, K.P.; Khire, J.M. Effect of phytase from Aspergillus niger on plant growth and mineral assimilation in wheat (Triticuma estivum Linn.) and its potential for use as a soil amendment. J. Sci. Food Agric. 2013, 93, 2242–2247. [Google Scholar] [CrossRef]
- Mondal, G.; Dureja, P.; Sen, B. Fungal metabolites from Aspergillus niger AN27 related to plant growth promotion. Indian J. Exp. Biol. 2000, 38, 84–87. [Google Scholar]
- Hung, R.; Rutgers, S.L. Chapter 17—Applications of Aspergillus in plant growth promotion. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 223–227. [Google Scholar]
- Horinouchi, H.; Muslim, A.; Hyakumachi, M. Biocontrol of Fusarium wilt of spinach by the plant growth promoting fungus Fusarium equiseti GF183. J. Plant Pathol. 2010, 92, 251–256. [Google Scholar]
- Šišić, A.; Baćanović, J.; Finckh, M.R. Endophytic Fusarium equiseti stimulates plant growth and reduces root rot disease of pea (Pisum sativum L.) caused by Fusarium avenaceum and Peyronellaea pinodella. Eur. J. Plant Pathol. 2017, 148, 271–282. [Google Scholar] [CrossRef]
- Varma, A.; Bakshi, M.; Lou, B.; Hartmann, A.; Oelmueller, R. Piriformospora indica: A novel plant growth-promoting mycorrhizal fungus. Agric. Res. 2012, 1, 117–131. [Google Scholar] [CrossRef]
- Elsharkawy, M.M. Plant growth-promoting Phoma spp. In Phoma: Diversity, Taxonomy, Bioactivities, and Nanotechnology; Rai, M., Zimowska, B., Kovics, G.J., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 301–309. [Google Scholar]
- Singh, A.; Sharma, J.; Rexer, K.-H.; Varma, A. Plant productivity determinants beyond minerals, water and light: Piriformospora indica-A revolutionary plant growth promoting fungus. Curr. Sci. 2000, 79, 1548–1554. [Google Scholar]
- Shivanna, M.B.; Meera, M.S.; Hyakumachi, M. Role of root colonization ability of plant growth promoting fungi in the suppression of take-all and common root rot of wheat. Crop Prot. 1996, 15, 497–504. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Kubota, M.; Koyama, H.; Hyakumachi, M. Systemic resistance to bacterial leaf speck pathogen in Arabidopsis thaliana induced by the culture filtrate of a plant growth-promoting fungus (PGPF) Phoma sp. GS8-1. J. Gen. Plant Pathol. 2008, 74, 213–221. [Google Scholar] [CrossRef]
- Kellogg, E.A. Relationships of cereal crops and other grasses. Proc. Natl. Acad. Sci. USA 1998, 95, 2005–2010. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.; Patel, A.N.; Saini, M.K.; Deep, S. Field demonstration of Trichoderma harzianum as a plant growth promoter in wheat (Triticum aestivum L.). J. Agric. Sci. 2012, 4, 65. [Google Scholar] [CrossRef]
- Cuevas, V.C. Soil inoculation with Trichoderma pseudokoningii Rifai enhances yield of rice. Philipp. J. Sci. 2006, 135, 31–37. [Google Scholar]
- Whitelaw, M.A.; Harden, T.J.; Bender, G.L. Plant growth promotion of wheat inoculated with Penicillium radicum sp. nov. Aust. J. Soil. Res. 1997, 35, 291–300. [Google Scholar] [CrossRef]
- Wakelin, S.A.; Ryder, M.H. Plant growth-promoting inoculants in Australian agriculture. Crop Manag. 2004, 3, 1–5. [Google Scholar] [CrossRef]
- Murali, M.; Amruthesh, K.N. Plant growth-promoting fungus Penicillium oxalicum enhances plant growth and induces resistance Oliin pearl millet against downy mildew disease. J. Phytol. 2015, 163, 743–754. [Google Scholar]
- Gong, M.; Du, P.; Liu, X.; Zhu, C. Transformation of inorganic P fractions of soil and plant growth promotion by phosphate-solubilizing ability of Penicillium oxalicum I1. J. Microbiol. 2014, 52, 1012–1019. [Google Scholar] [CrossRef]
- Mehmood, A.; Hussain, A.; Irshad, M.; Hamayun, M.; Iqbal, A.; Khan, N. In vitro production of IAA by endophytic fungus Aspergillus awamori and its growth promoting activities in Zea mays. Symbiosis 2019, 77, 225–235. [Google Scholar] [CrossRef]
- Srivastava, R.; Mehta, C.M.; Sharma, A.K. Fusarium pallidoroseum—A new biofertilizer responsible for enhancing plant growth in different crops. Int. J. Microbiol. Res. 2011, 2, 192–199. [Google Scholar]
- Baron, N.C.; de Souza, P.A.; Rigobelo, E.C. Purpureocillium lilacinum and Metarhizium marquandii as plant growth-promoting fungi. PeerJ 2020, 8, e9005. [Google Scholar] [CrossRef]
- Kedar, A.; Rathod, D.; Yadav, A. Endophytic Phoma sp. isolated from medicinal plants promote the growth of Zea mays. Nusant. Biosci. 2014, 6, 132–139. [Google Scholar] [CrossRef]
- Clifford, H.T.; Bostock, P.D. Etymological Dictionary of Grasses; Springer: Berlin/Heidelberg, Germany, 2007; p. 320. [Google Scholar]
- Soreng, R.J.; Peterson, P.M.; Romaschenko, K.; Davidse, G.; Zuloaga, F.O.; Judziewicz, E.J.; Morrone, O. A worldwide phylogenetic classification of the Poaceae (Gramineae). J. Syst. Evol. 2015, 53, 117–137. [Google Scholar] [CrossRef]
- Soreng, R.J.; Peterson, P.M.; Zuloaga, F.O.; Romaschenko, K.; Clark, L.G.; Teisher, J.K.; Gillespie, L.J.; Barberá, P.; Welker, C.A.D.; Kellogg, E.A.; et al. A worldwide phylogenetic classification of the Poaceae (Gramineae) III: An update. J. Syst. Evol. 2022, 60, 476–521. [Google Scholar] [CrossRef]
- Robson, M.J.; Ryle, G.J.A.; Woledge, J. The grass plant—Its form and function. In The Grass Crop; Jones, M.B., Lazenby, A., Eds.; Springer: Dordrecht, The Netherlands, 1988; pp. 25–30. [Google Scholar]
- Vercoe, J.E. Pastures for prosperity. Trop. Grassl. 1996, 30, 58–72. [Google Scholar]
- Hernández, D.; Carballo, M.; Reyes, F. Reflexiones sobre el uso de los pastos en la producción sostenible de leche y carne de res en el trópico. Pas. Forr. 2000, 23, 1–16. [Google Scholar]
- Chirino-Valle, I.; Kandula, D.; Littlejohn, C.; Hill, R.; Walker, M.; Shields, M.; Cummings, N.; Hettiarachchi, D.; Wratten, S. Potential of the beneficial fungus Trichoderma to enhance ecosystem-service provision in the biofuel grass Miscanthus × giganteus in agriculture. Sci. Rep. 2016, 6, 25109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, J.O.; Corrall, A.J.; Terry, R.A. Grass species and varieties. In Relationships between Stage of Growth, Yield and Forage Quality; GRI Technical Report No. 8; Grassland Research Institute: Hurley, UK, 1971; p. 81. [Google Scholar]
- Pascual, I.; Ortiz, A.; Ramírez de la Rivera, J.; Figueredo, A. Predicción del rendimiento y la calidad de tres gramíneas en el valle del Cauto. Rev. Cuba. Cienc. Inform. 2017, 11, 144–158. [Google Scholar]
- Fonte, S.J.; Nesper, M.; Hegglin, D.; Velásquez, J.E.; Ramirez, B.; Rao, I.M.; Bernasconi, S.M.; Bünemann, E.K.; Frossard, E.; Oberson, A. Pasture degradation impacts soil phosphorus storage via changes to aggregate-associated soil organic matter in highly weathered tropical soils. Soil Biol. Biochem. 2014, 68, 150–157. [Google Scholar] [CrossRef]
- Fisher, M.J.; Rao, I.M.; Thomas, R.J. Nutrient cycling in tropical pastures, with special reference the neotropical savannas. In International Grassland Congress; Winnipeg Association Management Centre: Saskatoon, SK, Canada, 1997; pp. 371–382. [Google Scholar]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Thivakaran, A.G. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2013, 2, 587. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.P.A.; Boaretto, A.E.; Trivelin, P.C.O.; Oliveira, W.S.; de Corsi, M. Liming and fertilization to restore degraded Brachiaria decumbens pastures grown on an entisol. Sci. Agríc. 2003, 60, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Zaman, M.; Kurepin, L.V.; Catto, W.; Pharis, R.P. Evaluating the use of plant hormones and biostimulators in forage pastures to enhance shoot dry biomass production by perennial ryegrass (Lolium perenne L.). J. Sci. Food Agric. 2015, 96, 715–726. [Google Scholar] [CrossRef]
- Pedreira, B.C.; Barbosa, P.L.; Pereira, L.E.T.; Mombach, M.A.; Domiciano, L.F.; Pereira, D.H.; Ferreira, A. Tiller density and tillering on Brachiaria brizantha cv. Marandu pastures inoculated with Azospirillum brasilense. Arq. Bras. Med. Vet. Zootec. 2017, 69, 1039–1046. [Google Scholar] [CrossRef] [Green Version]
- Leite, R.C.; Santos, A.C.; Santos, J.G.D.; Leite, C.R.; Oliveira, L.B.T.; Hungria, M. Mitigation of mombasa Grass (Megathyrsus maximus) dependence on nitrogen fertilization as a function of inoculation with Azospirillum brasilense. Rev. Bras. Ciênc. Solo 2019, 43, 180–234. [Google Scholar] [CrossRef] [Green Version]
- Müller, C.B.; Krauss, J. Symbiosis between grasses and asexual fungal endophytes. Curr. Opin. Plant Biol. 2005, 8, 450–456. [Google Scholar] [CrossRef]
- Kivlin, S.N.; Mann, M.A.; Lynn, J.S.; Kazenel, M.R.; Taylor, D.L.; Rudgers, J.A. Grass species identity shapes communities of root and leaf fungi more than elevation. ISME Commun. 2022, 2, 25. [Google Scholar] [CrossRef]
- Scott, B.; Schardl, C.L. Fungal symbionts of grasses: Evolutionary insights and agricultural potential. Trends Microbiol. 1993, 1, 196–200. [Google Scholar] [CrossRef]
- Ranelli, L.B.; Hendricks, W.Q.; Lynn, J.S.; Kivlin, S.N.; Rudgers, J.A. Biotic and abiotic predictors of fungal colonization in grasses of the Colorado Rockies. Divers. Distrib. 2015, 21, 962–976. [Google Scholar] [CrossRef]
- He, Y.L.; Chen, T.X.; Zhang, H.J.; White, J.F.; Li, C.J. Fungal endophytes help grasses to tolerate sap-sucking herbivores through a hormone-signaling system. J. Plant Growth Regul. 2022, 41, 2122–2137. [Google Scholar] [CrossRef]
- Clay, K.; Schardl, C. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am. Nat. 2002, 160, S99–S127. [Google Scholar] [CrossRef]
- Marquez, S.S.; Bills, G.F.; Herrero, N.; Zabalgogeazcoa, I. Non-systemic fungal endophytes of grasses. Fungal Ecol. 2012, 5, 289–297. [Google Scholar] [CrossRef]
- Vera, M.; Zuern, S.; Henríquez-Valencia, C.; Loncoman, C.; Canales, J.; Waller, F.; Basoalto, E.; Garnica, S. Exploring interactions between Beauveria and Metarhizium strains through co-inoculation and responses of perennial ryegrass in a one-year trial. PeerJ 2022, 10, e12924. [Google Scholar] [CrossRef]
- He, W.-X.; Wu, Q.-S.; Hashem, A.; Abd Allah, E.F.; Muthuramalingam, P.; Al-Arjani, A.-B.F.; Zou, Y.-N. Effects of symbiotic fungi on sugars and soil fertility and structure-mediated changes in plant growth of Vicia villosa. Agriculture 2022, 12, 1523. [Google Scholar] [CrossRef]
- Xie, M.M.; Chen, S.M.; Zou, Y.N. Effects of Rhizophagus intraradices and Rhizobium trifolii on growth and N assimilation of white clover. Plant Growth Regul. 2021, 93, 311–318. [Google Scholar] [CrossRef]
- Cunningham, D.M. Evaluation of the Potential Use of Antagonistic Microbes on Grass Species, Turf and Pasture, for Disease Control and Growth Stimulation. Ph.D. Thesis, University of Natal, Pietermaritzburg, South Africa, 2003. [Google Scholar]
- Westermann Leigue, R. Respuesta del Pasto Brachiaria híbrido cv. Mulato a la Inoculación con los Hongos Benéficos Trichoderma harzianum y Micorrizas; Escuela Agrícola Panamericana de Zamorano: Tegucigalpa, Honduras, 2004. [Google Scholar]
- Bécquer-Granados, C.J.; Ávila-Cordoví, U.; Nápoles-Gómez, J.A.; Galdo-Rodríguez, Y.; Hernández-Obregón, M.; Muir-Rodríguez, I.; Álvarez-Figueroa, O.; Medinilla-Nápoles, F. Productivity of Tifton 85 bermudagrass, inoculated with Bradyrhizobium sp. and Trichoderma harzianum, subject to agricultural drought stress. Pas. Forr. 2018, 41, 182–187. [Google Scholar]
- Núñez Serracín, M.A. Efecto de Azospirillum sp., Trichoderma harzianum y Micorrizas en la Producción de pasto Marandú (Brachiaria brizantha) y pasto Guinea (Panicum maximum); Escuela Agrícola Panamericana de Zamorano: Tegucigalpa, Honduras, 2018. [Google Scholar]
- Kandula, D.R.W.; Stewart, A.; McDermid, J.; Gale, D.; Swaminathan, J. Bioinoculant formulations for enhanced seedling emergence and pasture growth. In Seeds for Futures, Agronomy Society of New Zealand Special Publication No. 13; McGill, R., Rowarth, J.S., Eds.; Grassland Research and Practice Series No. 14: Palmerston North, New Zealand, 2008; pp. 147–148. [Google Scholar]
- Vazquez, M.M.; Cesar, S.; Azcon, R.; Barea, J.M. Interactions between arbuscular mycorrhizal fungi and other microbial inoculants (Azospirillum, Pseudomonas, Trichoderma) and their effects on microbial population and enzyme activities in the rhizosphere of maize plants. Appl. Soil Ecol. 2000, 15, 261–272. [Google Scholar] [CrossRef]
- Osorio, N.W.; Moreno, J.; León, J.D. Tree seedling growth promotion by dual inoculation with Rhizoglomus fasciculatum (Thaxt.) Sieverding, Silva & Oehl and Mortierella sp., rhizosphere fungi for reforestation purposes, to promote plant P uptake and growth at the nursery state. Acta Agron. 2016, 65, 239–247. [Google Scholar]
- Anthony, M.A.; Crowther, T.W.; van der Linde, S.; Suz, L.M.; Bidartondo, M.I.; Cox, F.; Schaub, M.; Rautio, P.; Ferretti, M.; Vesterdal, L.; et al. Forest tree growth is linked to mycorrhizal fungal composition and function across Europe. ISME J. 2022, 16, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Acurio-Vásconez, R.D.; Imbaquingo-España, C.K. Aislamiento, caracterización y evaluación de Trichoderma spp. como promotor de crecimiento vegetal en pasturas de raygrass (Lolium perenne) y trébol blanco (Trifolium repens). Granja 2017, 25, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Umar, A.; Hampton, J.G.; Kandula, D.R.W.; Rolston, M.P.; Chng, S. The impacts of take-all, drought and their interaction on Bromus wildenowii seed yield and the alleviation of these stresses by Trichoderma atroviride. Biocontrol Sci. Technol. 2021, 31, 976–989. [Google Scholar] [CrossRef]
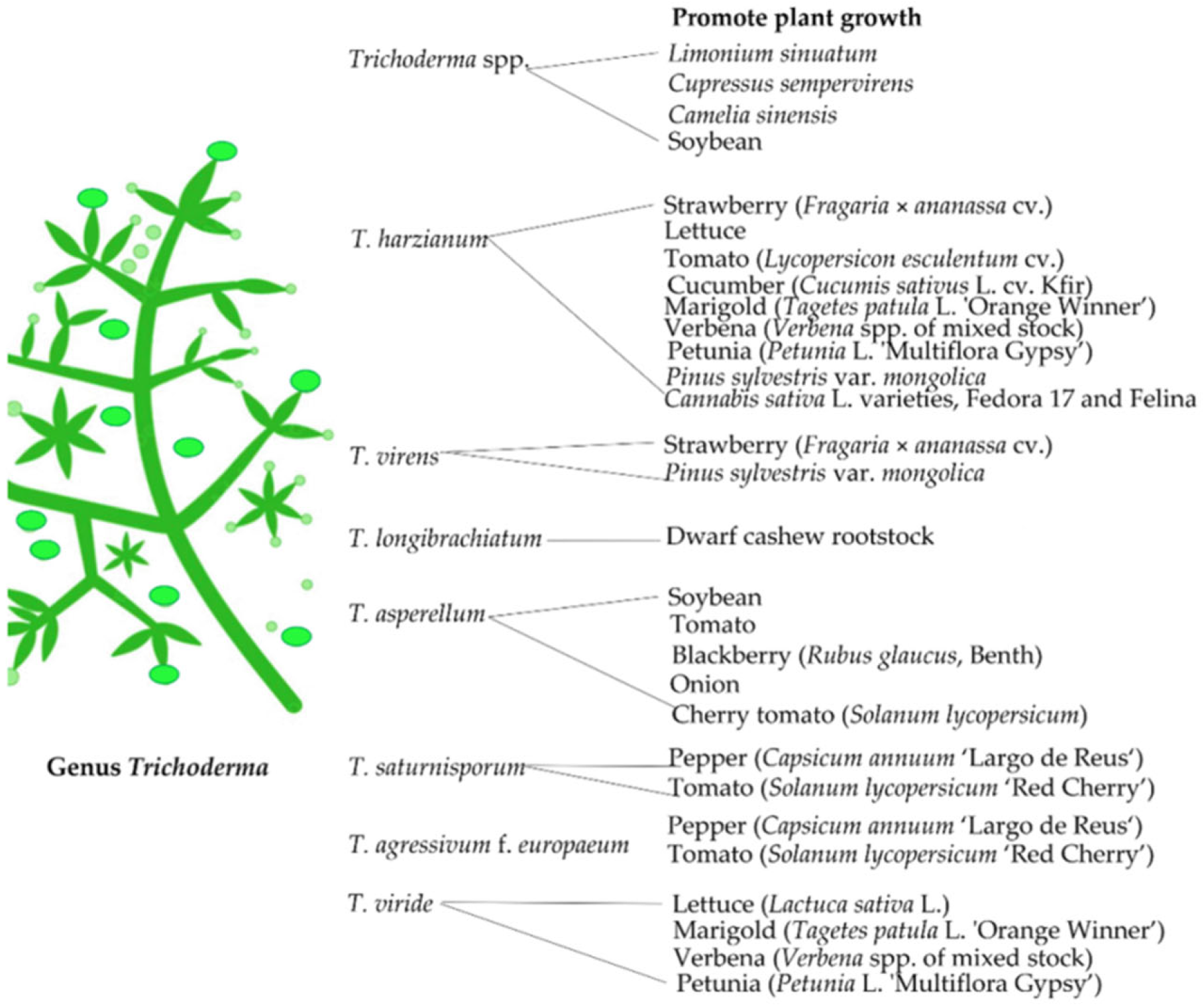
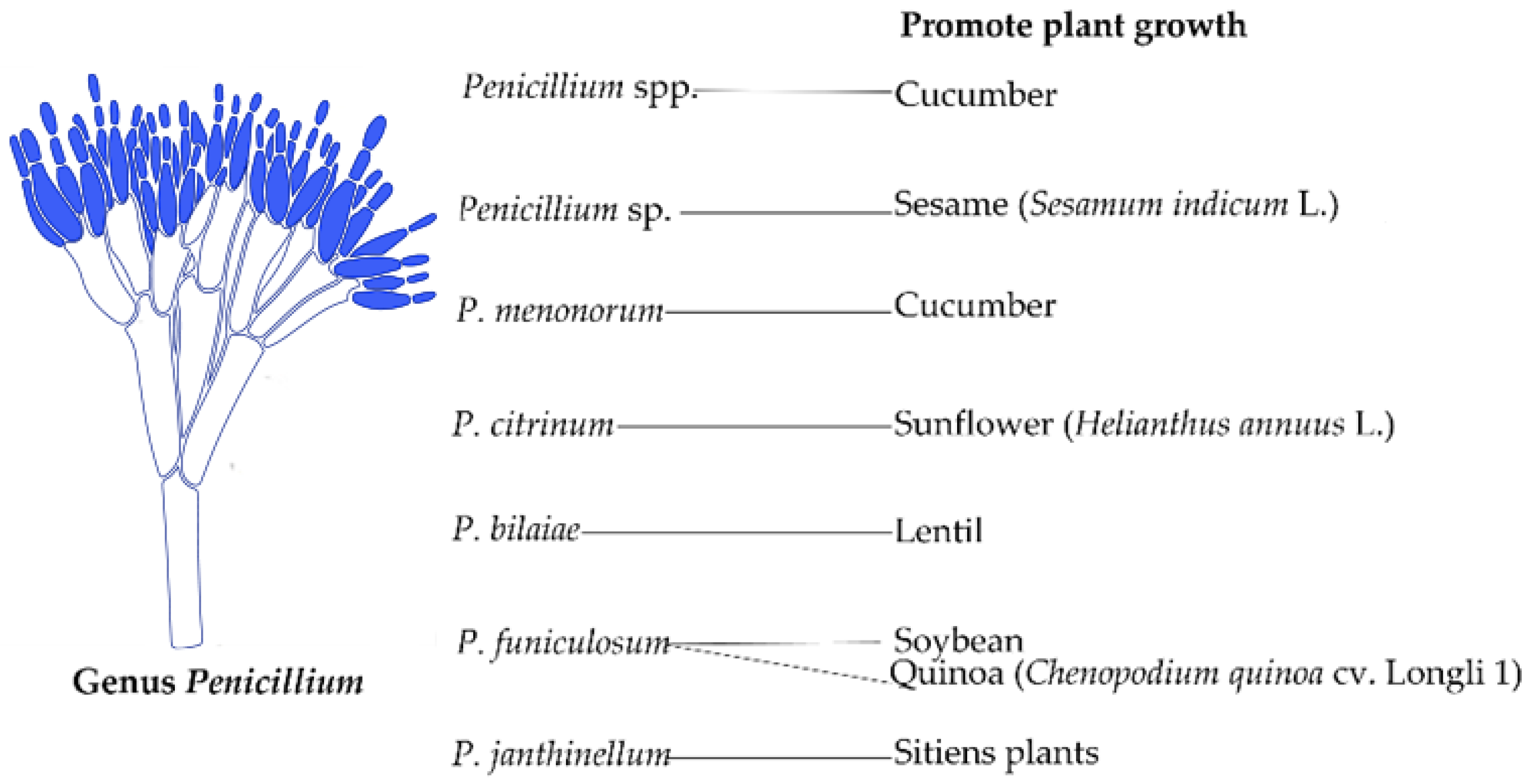
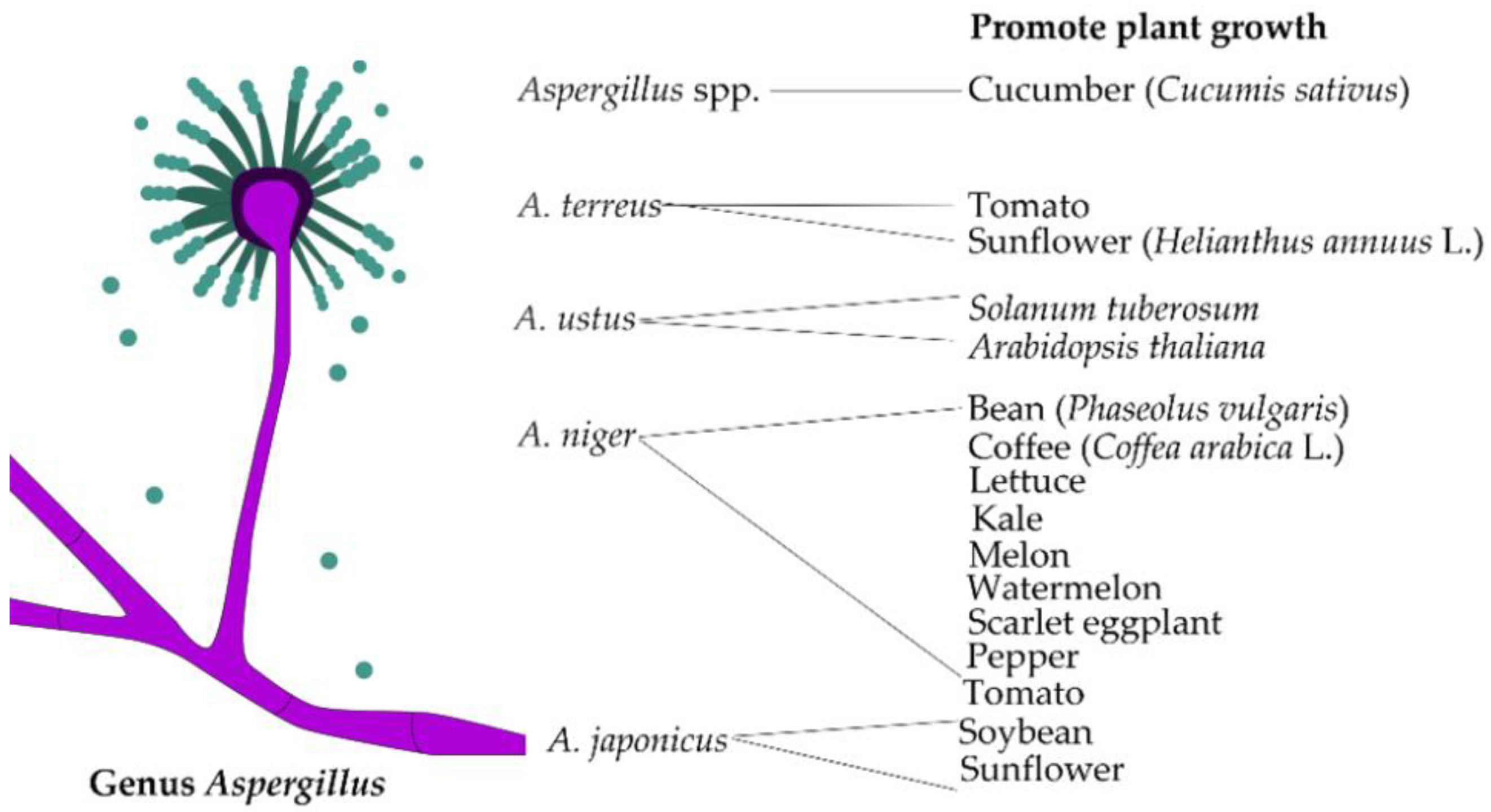
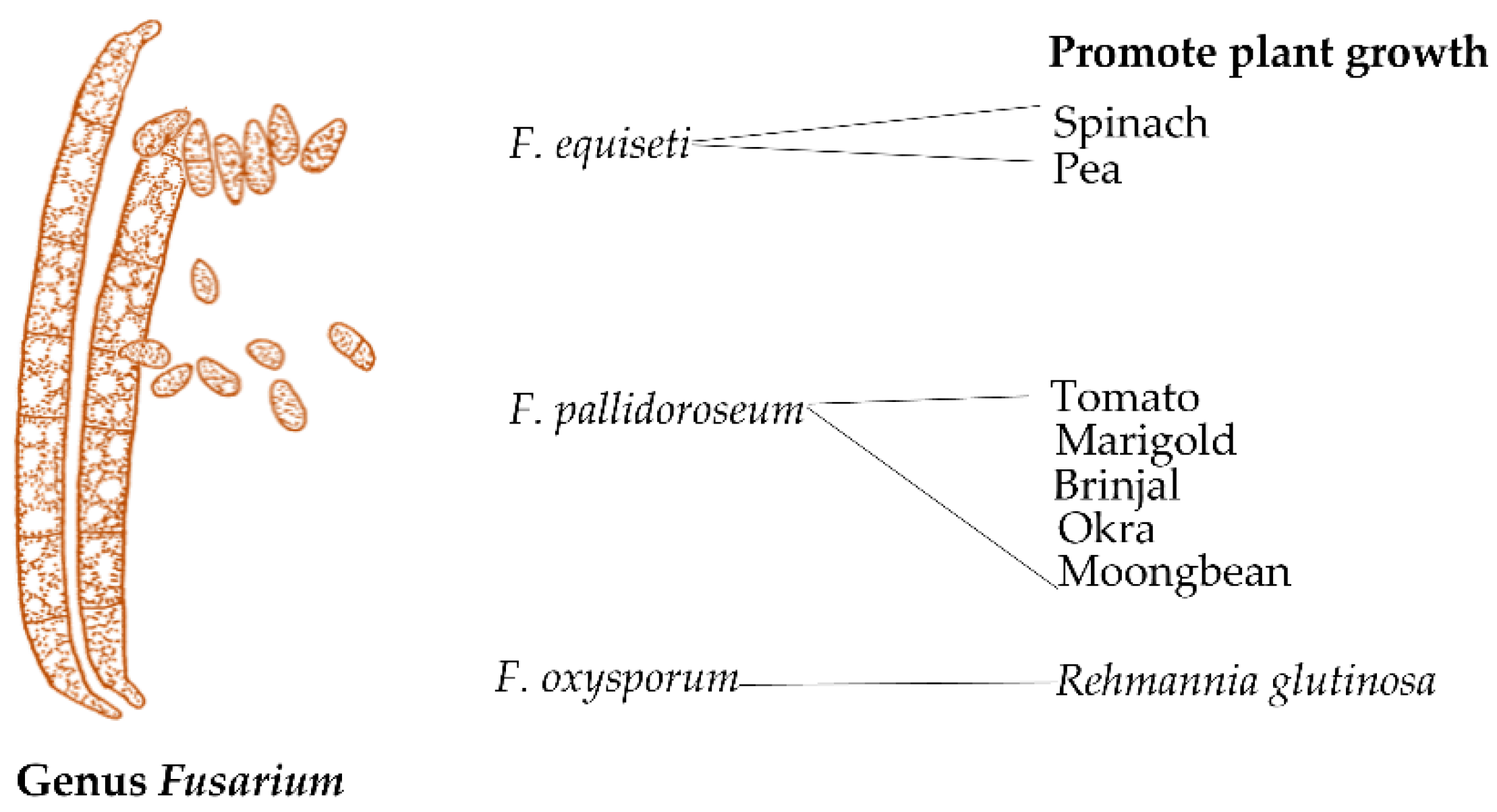

| Fungal Strains | Grasses | Benefit | References |
|---|---|---|---|
| T. harzianum strain Th3 | Wheat (Triticum aestivum L.) | Trichoderma inoculation significantly increased wheat yield | [126] |
| T. pseudokoningii Rifai | Rice | Trichoderma inoculation increased the availability of phosphorus and zinc, as well as increased crop yield | [127] |
| P. radicum (sp. nov.) | Wheat (Triticum aestivum L.) | Penicillium inoculation increased wheat yield in the greenhouse (9%) and in the field (14%), and increased phosphorus uptake (10%) | [128,129] |
| P. oxalicum | Pearl millet (Pennisetum glaucum) | P. oxalicum inoculation increased plant growth and nitrogen, potassium, and phosphorus uptake compared to controls under greenhouse conditions | [130] |
| P. oxalicum I1 | Maize | Inoculation with the filamentous fungus increased the corn yield by 14.47% compared to the control | [131] |
| A. awamori strain Wl1 | Maize (Zea mays) | Aspergillus inoculation promoted the growth of maize plants | [132] |
| F. pallidoroseum | Maize and wheat | Fusarium inoculation improved shoot dry weight and shoot length in all plants | [133] |
| Purpureocillium lilacinum, P. lavendulum, and Metarhizium marquandii | Maize | The inoculation of the fungal strains improved the growth of the plants, and some strains increased the availability of phosphorus and nitrogen | [134] |
| Mortierella elongata | Maize (Zea mays) | Mortierella inoculation increased height, leaf area, and plant dry weight of Zea mays | [96] |
| Phoma sp. strains GS6-1 and GS7-4 | Wheat | The inoculation of the fungal strains promoted plant growth and suppressed root rot caused by phytopathogens (Gaeumannomyces graminis var. tritici and Cochliobolus sativus) | [123] |
| Phoma sp. | Maize (Zea mays) | Phoma inoculation promoted plant growth | [135] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argumedo-Delira, R.; Gómez-Martínez, M.J.; Mora-Delgado, J. Plant Growth Promoting Filamentous Fungi and Their Application in the Fertilization of Pastures for Animal Consumption. Agronomy 2022, 12, 3033. https://doi.org/10.3390/agronomy12123033
Argumedo-Delira R, Gómez-Martínez MJ, Mora-Delgado J. Plant Growth Promoting Filamentous Fungi and Their Application in the Fertilization of Pastures for Animal Consumption. Agronomy. 2022; 12(12):3033. https://doi.org/10.3390/agronomy12123033
Chicago/Turabian StyleArgumedo-Delira, Rosalba, Mario J. Gómez-Martínez, and Jairo Mora-Delgado. 2022. "Plant Growth Promoting Filamentous Fungi and Their Application in the Fertilization of Pastures for Animal Consumption" Agronomy 12, no. 12: 3033. https://doi.org/10.3390/agronomy12123033
APA StyleArgumedo-Delira, R., Gómez-Martínez, M. J., & Mora-Delgado, J. (2022). Plant Growth Promoting Filamentous Fungi and Their Application in the Fertilization of Pastures for Animal Consumption. Agronomy, 12(12), 3033. https://doi.org/10.3390/agronomy12123033









