Mechanochemical Preparation of a Novel Slow-Release Fertilizer Based on K2SO4-kaolinite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Mechanochemical Process
- In the first one, the time of milling was kept constant with various milling speeds.
- In the second one, the milling speed was fixed with different milling times.
2.2. Characterization
3. Results and Discussion
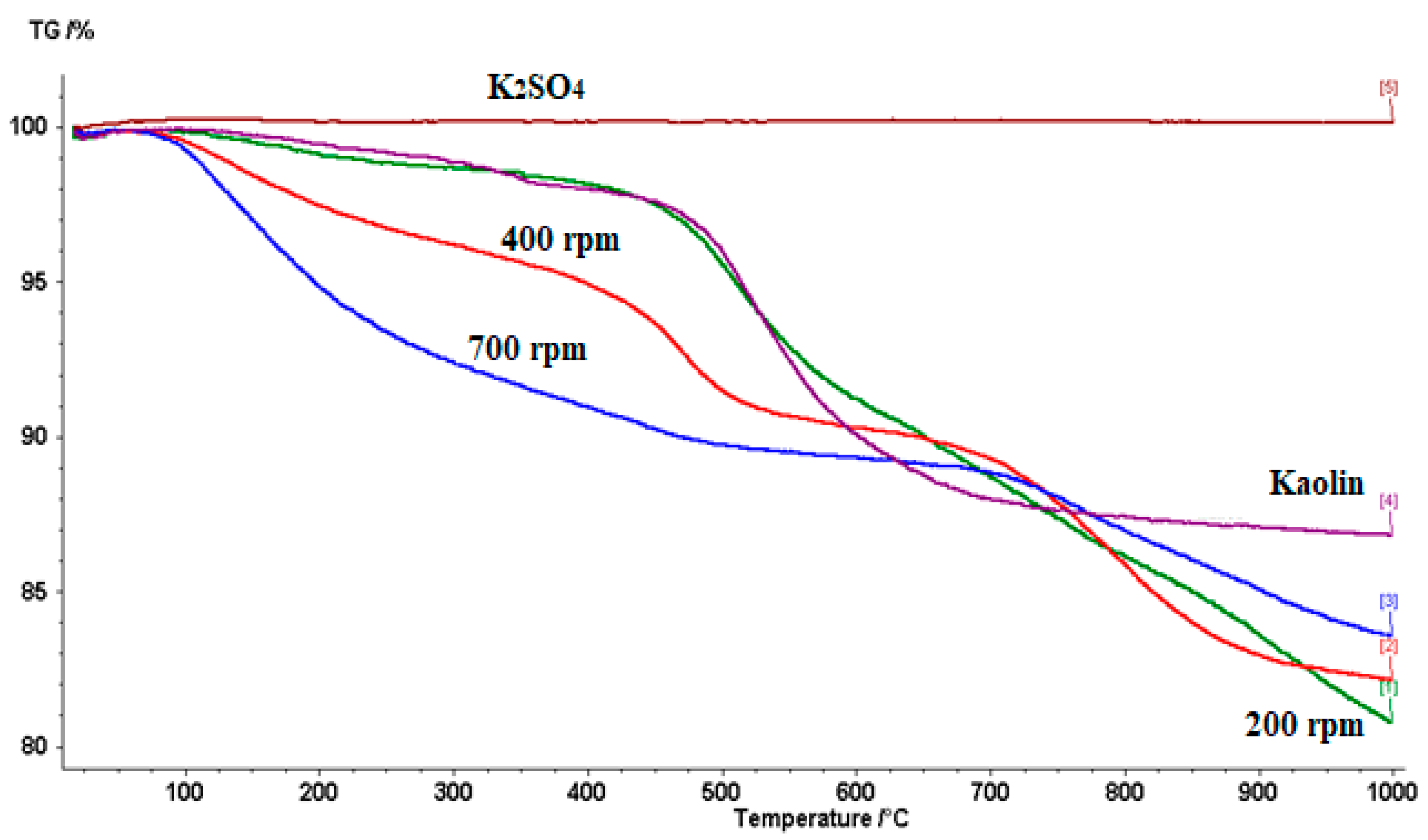
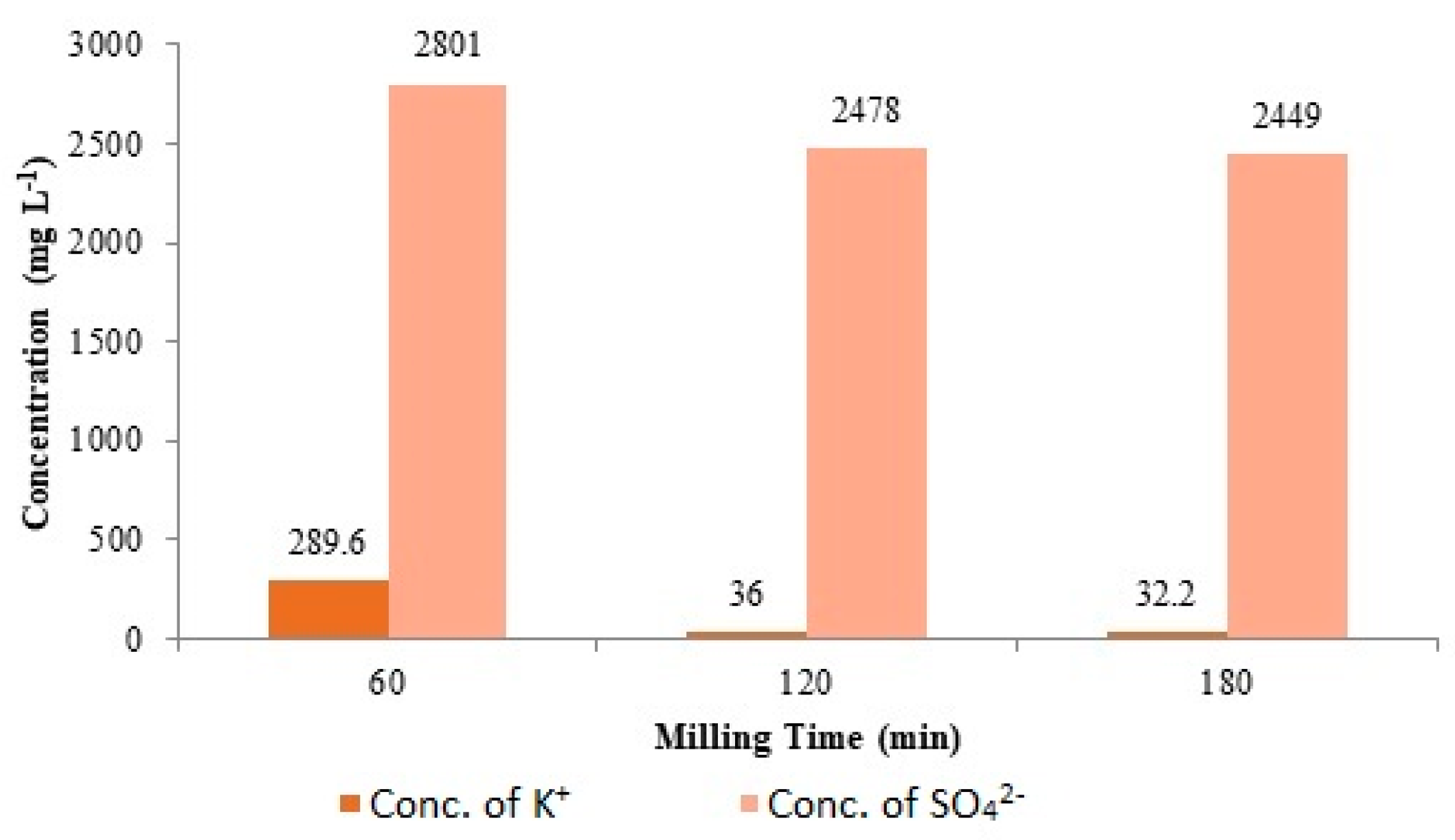
3.1. Milling Time Effect on Mechanochemical Synthesis of kaolinite-K2SO4 SRFs
3.2. Rotating Milling Speed Effect on the Mechanochemical Synthesis of the kaolinite-K2SO4 SRFs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pang, W.; Hou, D.; Wang, H.; Sai, S.; Wang, B.; Ke, J.; Wu, G.; Li, Q.; Holtzapple, M.T. Preparation of Microcapsules of Slow-Release NPK Compound Fertilizer and the Release Characteristics. J. Braz. Chem. Soc. 2018, 29, 2397–2404. [Google Scholar] [CrossRef]
- Araújo, B.R.; Romao, L.P.C.; Doumer, M.E.; Mangrich, A.S. Evaluation of the Interactions between Chitosan and Humics in Media for the Controlled Release of Nitrogen Fertilizer. J. Environ. Manag. 2017, 190, 122–131. [Google Scholar] [CrossRef] [PubMed]
- AlShamaileh, E.M.; Al-Rawajfeh, A.E.; Alrbaihat, M.R. Solid-State Mechanochemical Synthesis of Kaolinite-Urea Complexes for Application as Slow Release Fertilizer. J. Ecol. Eng. 2019, 20, 267–276. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, M.Â.; Santos, H.; Ruivo, S.; Arrobas, M. Slow-Release N Fertilisers Are Not an Alternative to Urea for Fertilisation of Autumn-Grown Tall Cabbage. Eur. J. Agron. 2010, 32, 137–143. [Google Scholar] [CrossRef] [Green Version]
- Madzokere, T.C.; Murombo, L.T.; Chiririwa, H. Nano-Based Slow Releasing Fertilizers for Enhanced Agricultural Productivity. Mater. Today Proc. 2020, 45, 3709–3715. [Google Scholar] [CrossRef]
- Al-rawajfeh, A.E.; Alrbaihat, M.R.; Ehab, M. Effects of Milling Time and Speed on Nutrient. Jordan J. Chem. 2020, 15, 51–59. [Google Scholar] [CrossRef]
- Azeem, B.; Kushaari, K.; Man, Z.B.; Basit, A.; Thanh, T.H. Review on Materials & Methods to Produce Controlled Release Coated Urea Fertilizer. J. Control. Release 2014, 181, 11–21. [Google Scholar] [CrossRef]
- Alrbaihat, M.R. Agricultural Nano Fertilizers: Macronutrient; Springer Nature: Singapore, 2023; ISBN 9789811973581. [Google Scholar]
- Mebdoua, S.; Ounane, G. Evaluation of Pesticide Residues in Wheat Grains and Its Products from Algeria. Food Addit. Contam. Part B 2019, 12, 289–295. [Google Scholar] [CrossRef]
- Mikkelsen, R.L.; Roberts, T.L. Inputs: Potassium Sources for Agricultural Systems. Improv. Potassium Recomm. Agric. Crop. 2021, 47–74. [Google Scholar] [CrossRef]
- Li, X.; Li, F.; Cui, Z.; Rengel, Z. Decomposition of Maize Straw in Saline Soil. Biol. Fertil. Soils 2006, 42, 366–370. [Google Scholar] [CrossRef]
- Çengel, M.; Göçmez, S.; Okur, N. Influence of Salinity on Microbial Respiration and Enzyme Activity of Soils. Acta Hortic. 2002, 573, 189–194. [Google Scholar] [CrossRef]
- Alrbaihat, M.R.; Al-rawajfeh, A.E.; Alshamaileh, E. A Mechanochemical Preparation, Properties and Kinetic Study of Kaolin–N, P Fertilizers for Agricultural Applications **. J. Mech. Behav. Mater. 2021, 30, 265–271. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, Q.; Li, X.; He, X.; Song, S. Mechanochemical Approaches to Synthesize Layered Double Hydroxides: A Review. Appl. Clay Sci. 2016, 119, 185–192. [Google Scholar] [CrossRef]
- Rudmin, M.; Abdullayev, E.; Ruban, A.; Buyakov, A.; Soktoev, B. Mechanochemical Preparation of Slow Release Fertilizer Based on Glauconite-Urea Complexes. Minerals 2019, 9, 507. [Google Scholar] [CrossRef] [Green Version]
- Fu, J.; Wang, C.; Chen, X.; Huang, Z.; Chen, D. Classification Research and Types of Slow Controlled Release Fertilizers (SRFs) Used—A Review. Commun. Soil Sci. Plant Anal. 2018, 49, 2219–2230. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Shawabkeh, R.; Al-Harahsheh, A.; Tarawneh, K.; Batiha, M.M. Surface Modification and Characterization of Jordanian Kaolinite: Application for Lead Removal from Aqueous Solutions. Appl. Surf. Sci. 2009, 255, 8098–8103. [Google Scholar] [CrossRef]
- Al-Rawajfeh, A.E.; AlShamaileh, E.M.; Alrbaihat, M.R. Clean and Efficient Synthesis Using Mechanochemistry: Preparation of Kaolinite–KH 2 PO 4 and Kaolinite–(NH 4) 2 HPO 4 Complexes as Slow Released Fertilizer. J. Ind. Eng. Chem. 2019, 73, 336–343. [Google Scholar] [CrossRef]
- AlShamaileh, E.; Al-Rawajfeh, A.E.; Alrbaihat, M. Mechanochemical Synthesis of Slow-Release Fertilizers: A Review. Open Agric. J. 2018, 12, 11–19. [Google Scholar] [CrossRef]
- Friščić, T.; Mottillo, C.; Titi, H.M. Mechanochemistry for Synthesis. Angew. Chem. 2020, 132, 1030–1041. [Google Scholar] [CrossRef]
- Bose, A.; Mal, P. Mechanochemistry of Supramolecules. Beilstein J. Org. Chem. 2019, 15, 881–900. [Google Scholar] [CrossRef]
- Cavallaro, G.; Lazzara, G.; Milioto, S.; Parisi, F.; Evtugyn, V.; Rozhina, E.; Fakhrullin, R. Nanohydrogel Formation within the Halloysite Lumen for Triggered and Sustained Release. ACS Appl. Mater. Interfaces 2018, 10, 8265–8273. [Google Scholar] [CrossRef] [PubMed]
- Makó, É.; Kristóf, J.; Horváth, E.; Vágvölgyi, V. Kaolinite–Urea Complexes Obtained by Mechanochemical and Aqueous Suspension Techniques—A Comparative Study. J. Colloid Interface Sci. 2009, 330, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Alali, J.M. Beneficiation of White Kaolinitic Sandstone to Produce Kaolin Concentrate from Wadi Siq-Rakyia Area in Wadi Araba, Jordan. Open J. Geol. 2020, 10, 829–850. [Google Scholar] [CrossRef]
- Rashidzadeh, A.; Olad, A. Slow-Released NPK Fertilizer Encapsulated by NaAlg-g-Poly(AA-Co-AAm)/MMT Superabsorbent Nanocomposite. Carbohydr. Polym. 2014, 114, 269–278. [Google Scholar] [CrossRef]
- Said, A.; Zhang, Q.; Qu, J.; Liu, Y.; Lei, Z.; Hu, H.; Xu, Z. Mechanochemical Activation of Phlogopite to Directly Produce Slow-Release Potassium Fertilizer. Appl. Clay Sci. 2018, 165, 77–81. [Google Scholar] [CrossRef]
- Gwenzi, W.; Nyambishi, T.J.; Chaukura, N.; Mapope, N. Synthesis and Nutrient Release Patterns of a Biochar-Based N–P–K Slow-Release Fertilizer. Int. J. Environ. Sci. Technol. 2018, 15, 405–414. [Google Scholar] [CrossRef]
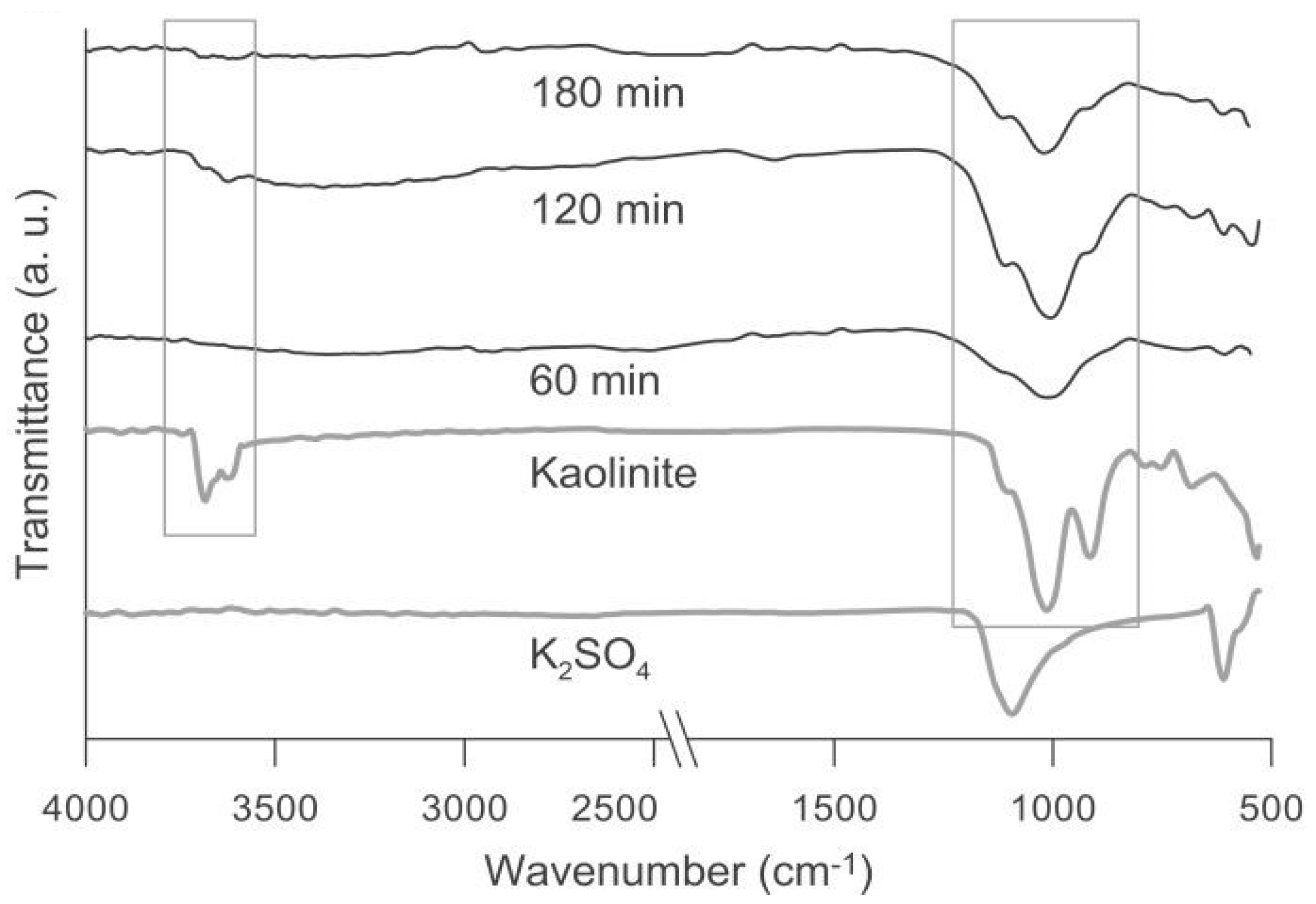
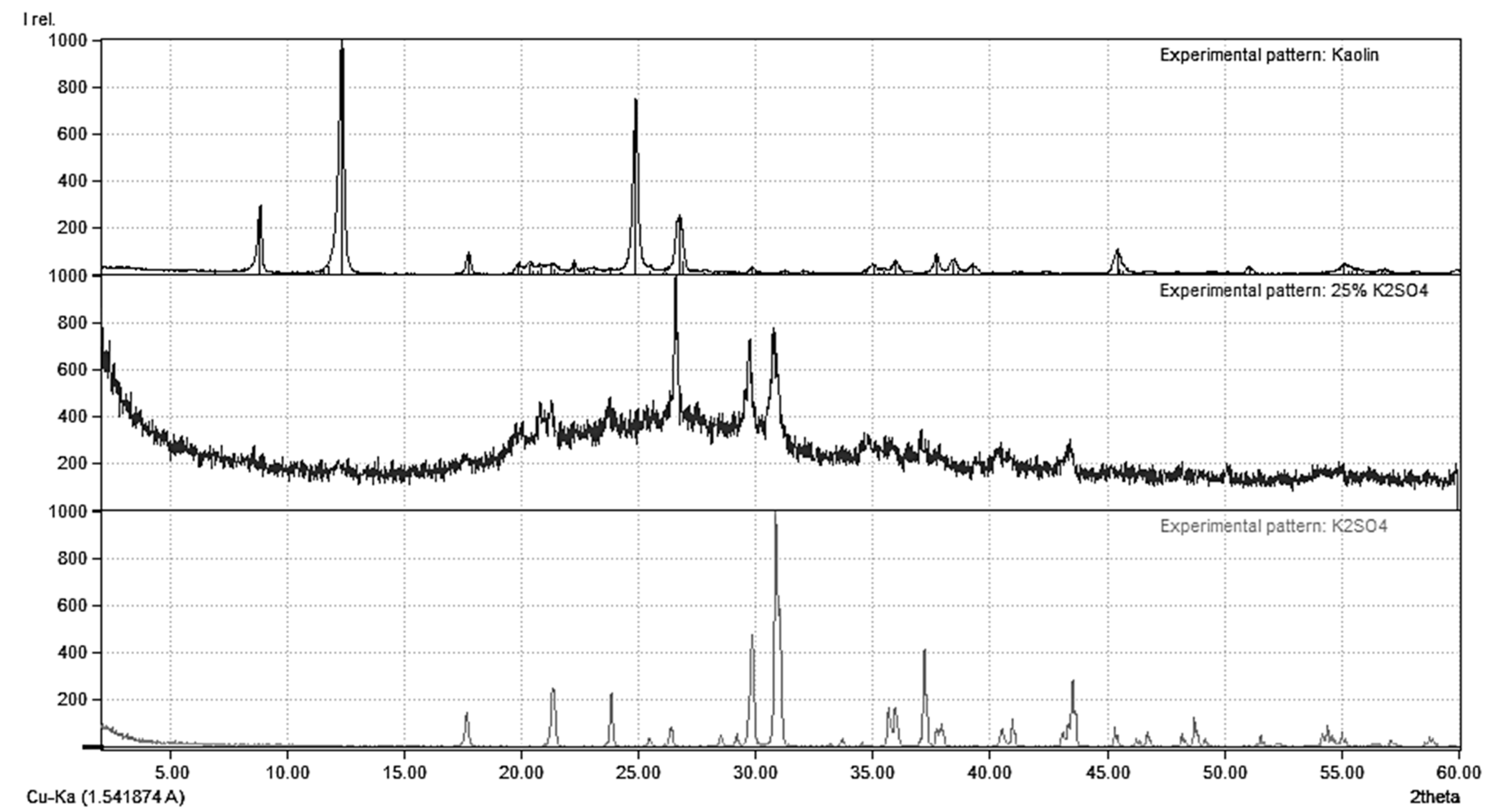
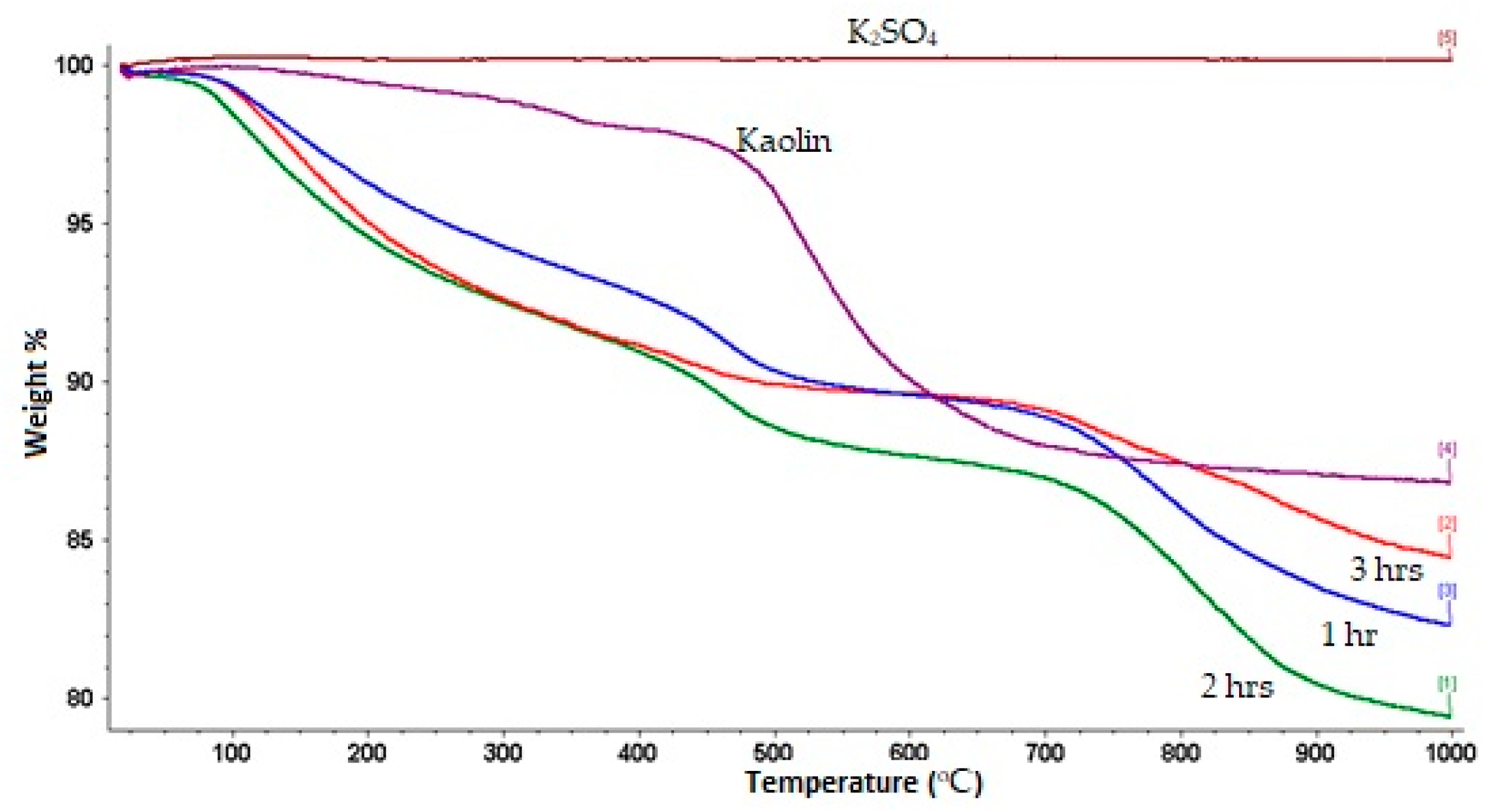

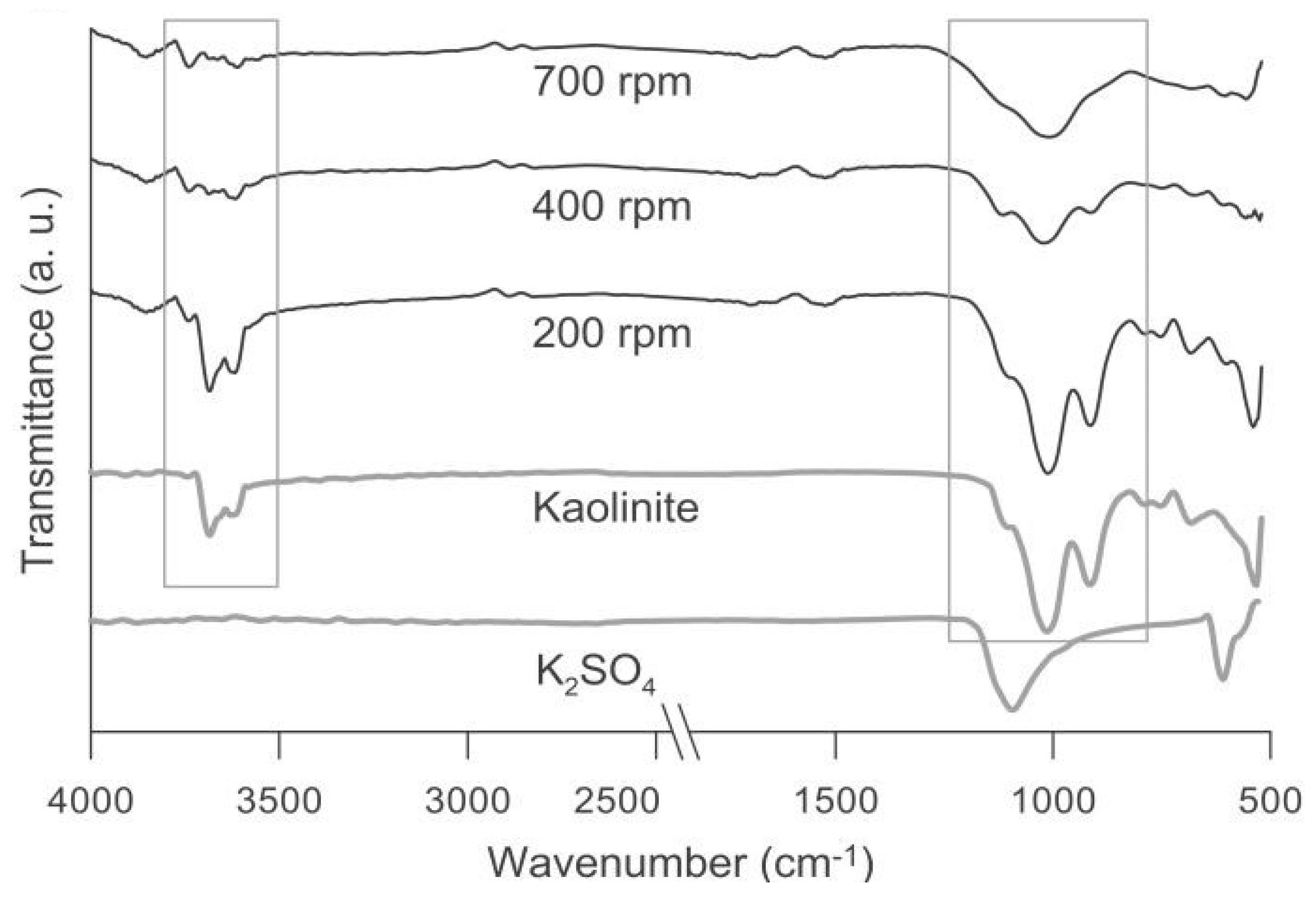
| Series | Sample (kaolinite-K2SO4) Weight Ratio | Milling Speed rpm | Milling Time min |
|---|---|---|---|
| Exp. 1 | 3:1 | 700 | 60, 120, 180 |
| Exp. 2 | 3:1 | 200, 400, 700 | 120 |
| Milling Time (min) | Conc. of K+ (mg L−1) | Conc. of SO42− (mg L−1) |
|---|---|---|
| 60 | 289.6 | 2801.0 |
| 120 | 36.0 | 2478.0 |
| Milling Speed (rpm) | Conc. of K+ (mg L−1) | Conc. of SO42− (mg L−1) |
|---|---|---|
| 200 | 450.8 | 4344.1 |
| 400 | 315.2 | 3110.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlShamaileh, E.; Alrbaihat, M.; Moosa, I.; Abu-Afifeh, Q.; Al-Fayyad, H.; Hamadneh, I.; Al-Rawajfeh, A. Mechanochemical Preparation of a Novel Slow-Release Fertilizer Based on K2SO4-kaolinite. Agronomy 2022, 12, 3016. https://doi.org/10.3390/agronomy12123016
AlShamaileh E, Alrbaihat M, Moosa I, Abu-Afifeh Q, Al-Fayyad H, Hamadneh I, Al-Rawajfeh A. Mechanochemical Preparation of a Novel Slow-Release Fertilizer Based on K2SO4-kaolinite. Agronomy. 2022; 12(12):3016. https://doi.org/10.3390/agronomy12123016
Chicago/Turabian StyleAlShamaileh, Ehab, Mohammad Alrbaihat, Iessa Moosa, Qusay Abu-Afifeh, Hebah Al-Fayyad, Imad Hamadneh, and Aiman Al-Rawajfeh. 2022. "Mechanochemical Preparation of a Novel Slow-Release Fertilizer Based on K2SO4-kaolinite" Agronomy 12, no. 12: 3016. https://doi.org/10.3390/agronomy12123016
APA StyleAlShamaileh, E., Alrbaihat, M., Moosa, I., Abu-Afifeh, Q., Al-Fayyad, H., Hamadneh, I., & Al-Rawajfeh, A. (2022). Mechanochemical Preparation of a Novel Slow-Release Fertilizer Based on K2SO4-kaolinite. Agronomy, 12(12), 3016. https://doi.org/10.3390/agronomy12123016







