Fluopsin C: A Review of the Antimicrobial Activity against Phytopathogens
Abstract
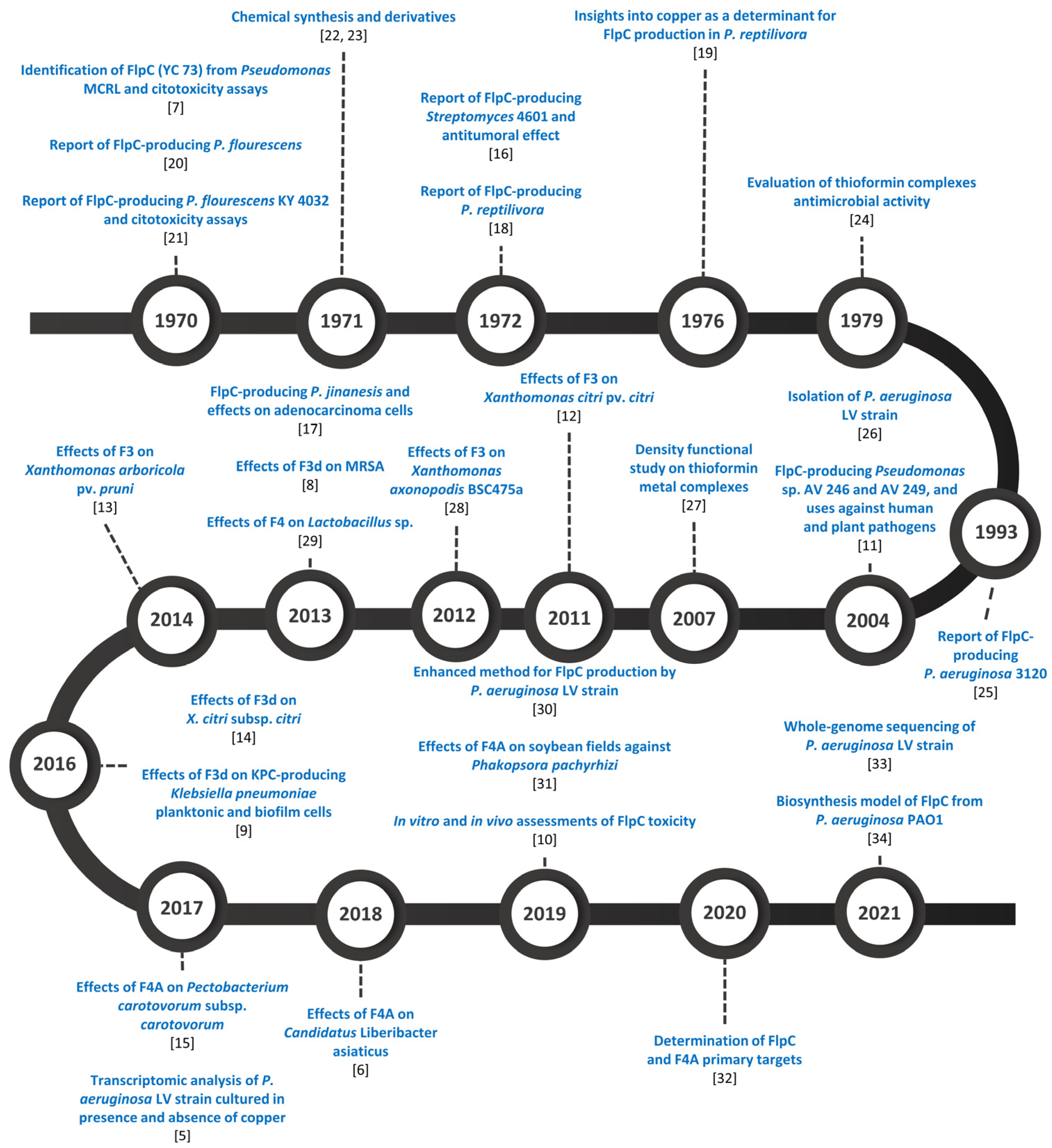
:1. Introduction
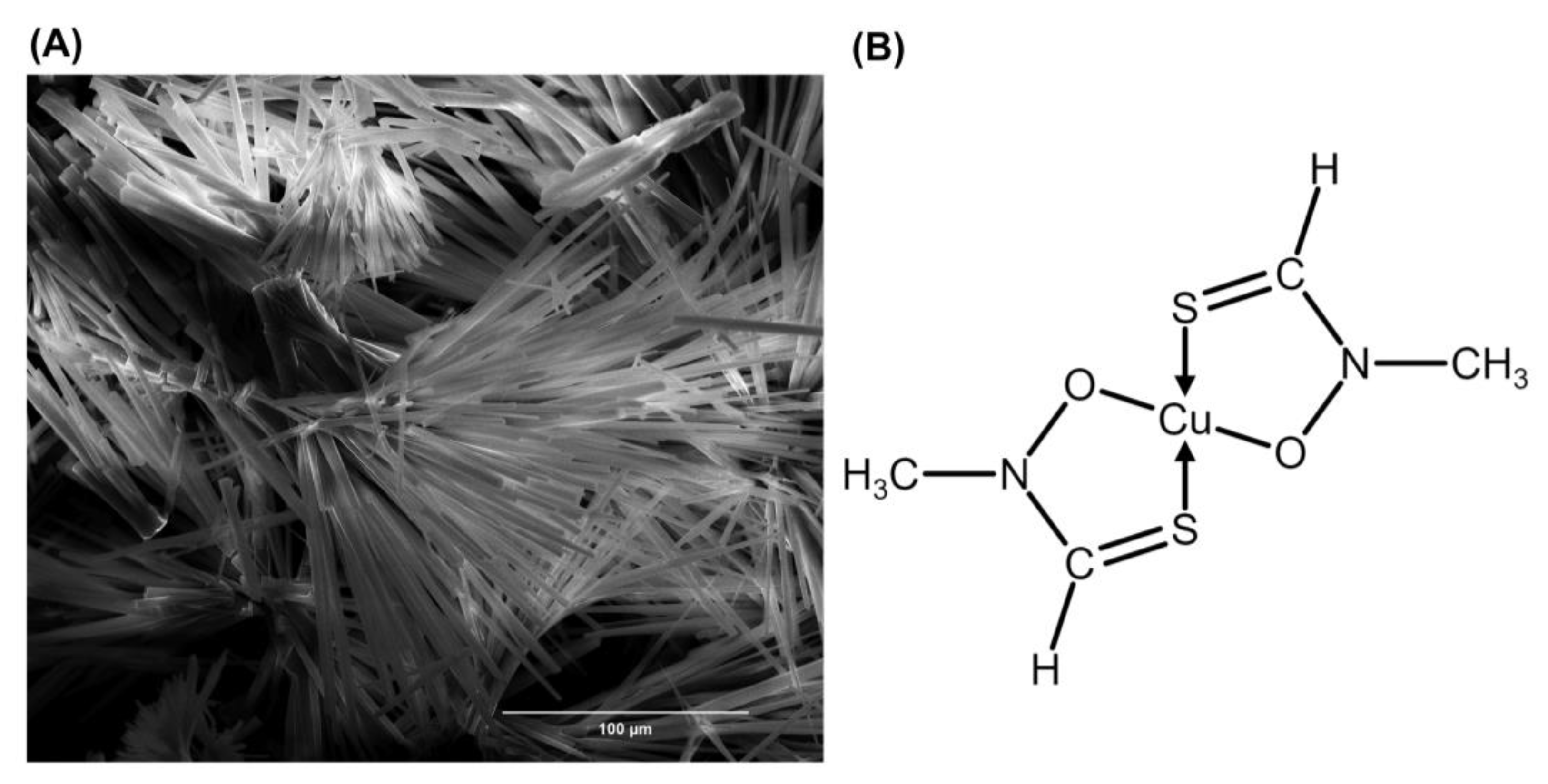
2. Fluopsin C
3. Fluopsin C-Producing Microorganisms
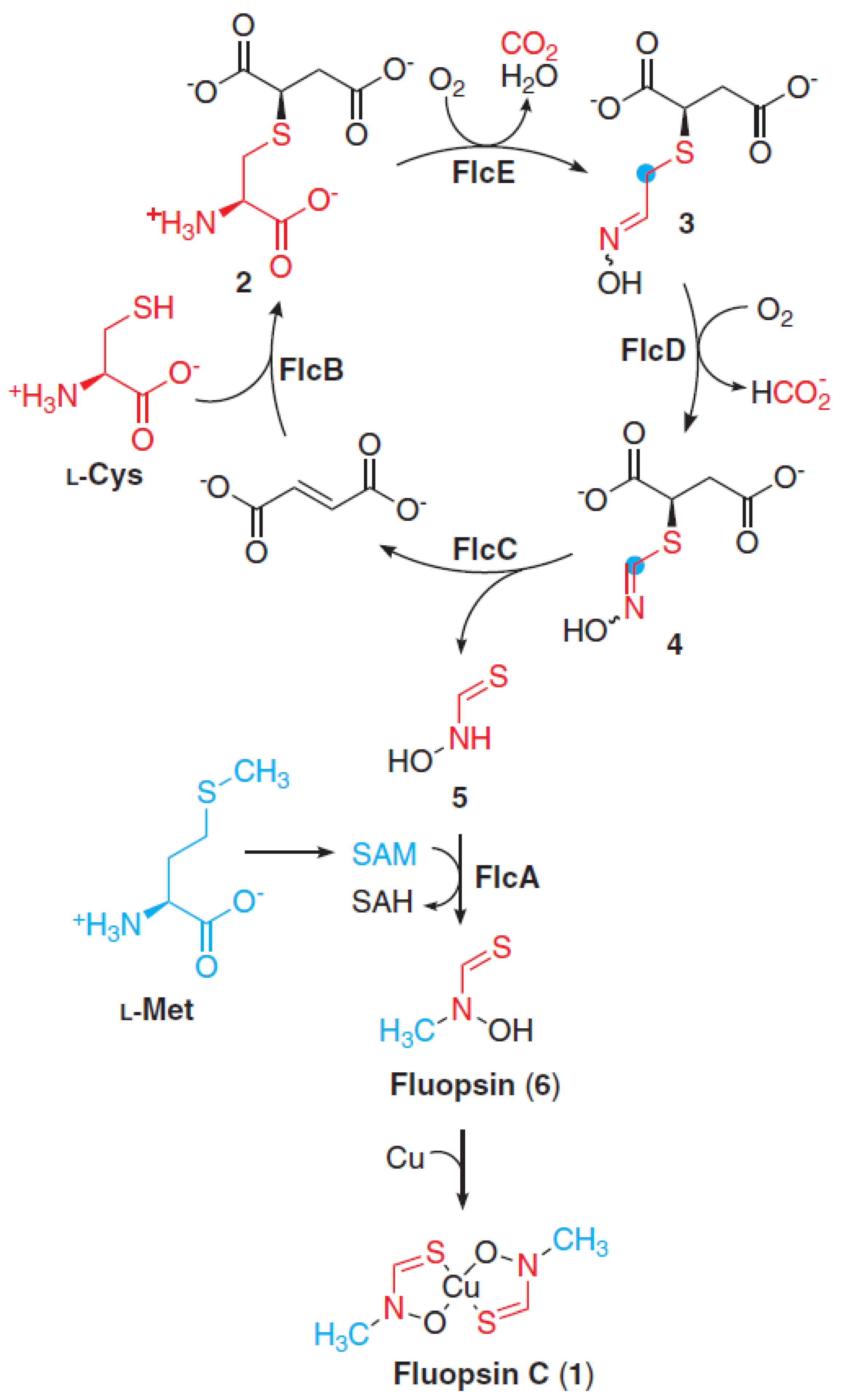
Molecular Insights on Fluopsin C Biosynthesis
4. Fluopsin C In Vitro and In Vivo Inhibitory Effects on Phytopathogens
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Martins, P.M.M.; Merfa, M.V.; Takita, M.A.; De Souza, A.A. Persistence in Phytopathogenic Bacteria: Do we Know Enough? Front. Microbiol. 2018, 9, 1099. [Google Scholar] [CrossRef] [PubMed]
- Hough, R.L. A World View of Pesticides. Nat. Geosci. 2021, 14, 183–184. [Google Scholar] [CrossRef]
- Gokulan, K.; Khare, S.; Cerniglia, C. Metabolic Pathways: Production of Secondary Metabolites of Bacteria. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 2, pp. 561–569. ISBN 978-0-19-969739-7. [Google Scholar]
- Patrick, G.L. An Introduction to Medical Chemistry, 5th ed.; Oxford University Press: Oxford, UK, 2013; pp. 199–213. ISBN 978-0-19-969739-7. [Google Scholar]
- Gionco, B.; Tavares, E.R.; Oliveira, A.G.; Yamada-Ogatta, S.F.; Carmo, A.O.; Pereira, U.P.; Chideroli, R.T.; Simionato, A.S.; Navarro, M.O.P.; Chryssafidis, A.L.; et al. New Insights about Antibiotic Production by Pseudomonas aeruginosa: A Gene Expression Analysis. Front. Chem. 2017, 5, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pistori, J.F.; Simionato, A.S.; Navarro, M.O.P.; Andreata, M.F.L.; Santos, I.M.O.; Meneguim, L.; Leite, R.P., Jr.; Oliveira, A.G.; Andrade, G. Low-molecular-weight Metabolites Produced by Pseudomonas aeruginosa as an Alternative to Control Huanglongbing in Citrus sinensis cv. Valencia. Trop. Plant Pathol. 2018, 43, 289–296. [Google Scholar] [CrossRef]
- Egawa, Y.; Umino, K.; Awataguchi, S.; Kawano, Y.; Okuda, T. Antibiotic YC 73 of Pseudomonas origin. I. Production, Isolation and Properties. J. Antibiot. 1970, 23, 267–270. [Google Scholar] [CrossRef] [Green Version]
- Cardozo, V.F.; Oliveira, A.G.; Nishio, E.K.; Perugini, M.R.; Andrade, C.G.; Silveira, W.D.; Durán, N.; Andrade, G.; Kobayashi, R.K.; Nakazato, G. Antibacterial Activity of Extracellular Compounds Produced by a Pseudomonas Strain against Methicillin-resistant Staphylococcus aureus (MRSA) strains. Ann. Clin. Microbiol. Antimicrob. 2013, 12, 12. [Google Scholar] [CrossRef] [Green Version]
- Kerbauy, G.; Vivan, A.C.P.; Simões, G.C.; Simionato, A.S.; Pelisson, M.; Vespero, E.C.; Costa, S.F.; Andrade, C.G.T.J.; Barbieri, D.M.; Mello, J.C.P.; et al. Effect of a Metalloantibiotic Produced by Pseudomonas aeruginosa on Klebsiella pneumoniae Carbapenemase (KPC)-producing K. pneumoniae. Curr. Pharm. Biotechnol. 2016, 17, 389–397. [Google Scholar] [CrossRef]
- Navarro, M.O.P.; Simionato, A.S.; Pérez, J.C.B.; Barazetti, A.R.; Emiliano, J.; Niekawa, E.T.G.; Andreata, M.F.L.; Modolon, F.; Dealis, M.L.; Araújo, E.J.A.; et al. Fluopsin C for Treating Multidrug-resistant Infections: In vitro Activity against Clinically Important Strains and in vivo Efficacy Against Carbapenemase-producing Klebsiella pneumoniae. Front. Microbiol. 2019, 10, 2431. [Google Scholar] [CrossRef] [Green Version]
- Hedman, R.; Levenfors, J.; Stanislaw, P.; Welch, C.; Daugherty, S. Use of a Known Substance for the Treatment of Cancer and Bacterial and Fungal Infections. WO 2004050095 A1. 2004. Available online: https://patents.google.com/patent/WO2004050095A1/en (accessed on 21 October 2022).
- Oliveira, A.G.; Murate, L.S.; Spago, F.R.; Lopes, L.P.; Beranger, J.P.O.; Martin, J.A.B.S.; Nogueira, M.A.; Mello, J.C.P.; Andrade, C.G.T.J.; Andrade, G. Evaluation of the Antibiotic Activity of Extracellular Compounds Produced by the Pseudomonas Strain Against the Xanthomonas citri pv. citri 306 Strain. Biol. Control 2011, 56, 125–131. [Google Scholar] [CrossRef]
- Vasconcellos, F.C.S.; Oliveira, A.G.; Lopes-Santos, L.; Beranger, J.P.O.; Cely, M.V.T.; Simionato, A.S.; Pistori, J.F.; Spago, F.R.; Mello, J.C.P.; San Martin, J.A.B.; et al. Evaluation of Antibiotic Activity Produced by Pseudomonas aeruginosa LV Strain Against Xanthomonas arboricola pv. pruni. Agric. Sci. 2014, 5, 71–76. [Google Scholar] [CrossRef]
- Oliveira, A.G.; Spago, F.R.; Simionato, A.S.; Navarro, M.O.P.; Silva, C.S.; Barazetti, A.R.; Cely, M.V.T.; Tischer, C.A.; San Martin, J.A.B.; Andrade, C.G.T.J.; et al. Bioactive Organocopper Compound from Pseudomonas aeruginosa Inhibits the Growth of Xanthomonas citri subsp. citri. Front. Microbiol. 2016, 7, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munhoz, L.D.; Fonteque, J.P.; Santos, I.M.O.; Navarro, M.O.P.; Simionato, A.S.; Goya, E.T.; Rezende, M.I.; Balbi-Peña, M.I.; Oliveira, A.G.; Andrade, G. Control of Bacterial Stem Rot on Tomato by Extracellular Bioactive Compounds Produced by Pseudomonas aeruginosa LV strain. Cogent Food Agric. 2017, 3, 1282592. [Google Scholar] [CrossRef]
- Miyamura, S.; Ogasawara, N.; Otsuka, H.; Niwayama, S.; Tanaka, H.; Take, T.; Uchiyama, T.; Nakazawa, H.; Abe, K.; Koizumi, K. An Antitumor Antibiotic, no. 4601 from Streptomyces, Identical with YC 73 of Pseudomonas origin. J. Antibiot. 1972, 25, 369–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, L.; Jiang, C.; Cui, M.; Lu, R.; Liu, S.; Zheng, B.; Li, L.; Li, X. Fluopsin C Induces Oncosis of Human Breast Adenocarcinoma Cells. Acta Pharmacol. Sin. 2013, 34, 1093–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Rio, L.A.; Gorgé, J.L.; Olivares, J.; Mayor, F. Antibiotics from Pseudomonas reptilivora II. Isolation, Purification, and Properties. Antimicrob. Agents Chemother. 1972, 2, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Molina, E.; Del Rio, L.A.; Olivares, J. Copper and Iron as Determinant Factors of Antibiotic Production by Pseudomonas reptilivora. J. Appl. Bacteriol. 1976, 41, 69–74. [Google Scholar] [CrossRef]
- Shirahata, K.; Deguchi, T.; Hayashi, T.; Matsubara, I.; Suzuki, T. The Structures of Fluopsins C and F. J. Antibiot. 1970, 23, 546–550. [Google Scholar] [CrossRef] [Green Version]
- Itoh, S.; Inuzuka, K.; Suzuki, T. New Antibiotics Produced by Bacteria Grown on n-paraffin (Mixture of C12, C13 and C14 Fractions). J. Antibiot. 1970, 23, 542–545. [Google Scholar] [CrossRef] [Green Version]
- Egawa, Y.; Umino, K.; Ito, Y.; Okuda, T. Antibiotic YC 73 of Pseudomonas origin. II. Structure and Synthesis of Thioformin and its Cupric Complex (YC 73). J. Antibiot. 1971, 24, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Shirahata, K.; Hayashi, T.; Matsubara, I. Syntheses of Fluopsins. J. Antibiot. 1971, 24, 140–141. [Google Scholar] [CrossRef]
- Bell, S.J.; Friedman, S.A.; Leong, J. Antibiotic Action of N -Methylthioformohydroxamate Metal Complexes. Antimicrob. Agents Chemother. 1979, 15, 384–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, H.R.; Chun, H.K.; Kho, Y.H.; Sung, N.K. Production, Isolation and Characterization of the Antibiotic from Pseudomonas aeruginosa 3120. Appl. Biol. Chem. 1993, 36, 428–433. [Google Scholar]
- Rampazo, L.G.L. Evaluation of Biological Agents and their Products on the Incidence of Citrus Canker Foliar Lesions. Master’s Thesis, State University of Londrina, Londrina, Brazil, 2004. [Google Scholar]
- Kakkar, R.; Dua, A.; Gahlot, P. Metal Ion Complexes of Thioformin: A Density Functional Study. Polyhedron 2007, 26, 5301–5308. [Google Scholar] [CrossRef]
- Lopes, L.P.; Oliveira, A.G.; Beranger, J.P.O.; Góis, C.G.; Vasconcellos, F.C.S.; Martin, J.A.B.S.; Andrade, C.G.T.J.; Mello, J.C.P.; Andrade, G. Activity of Extracellular Compounds of Pseudomonas sp. against Xanthomonas axonopodis in vitro and Bacterial Leaf Blight in Eucalyptus. Trop. Plant Pathol. 2012, 37, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Góis, C.G.M.; Lopes-Santos, L.; Beranger, J.P.O.; Oliveira, A.G.; Spago, F.R.; Andrade, G. The Control of Lactobacillus sp. by Extracellular Compound Produced by Pseudomonas aeruginosa in the Fermentation Process of Fuel Ethanol Industry in Brazil. J. Sustain. Bioenergy Syst. 2013, 3, 194–201. [Google Scholar] [CrossRef] [Green Version]
- Bedoya, J.C.; Dealis, M.L.; Silva, C.S.; Niekawa, E.T.G.; Navarro, M.O.P.; Simionato, A.S.; Modolon, F.; Chryssafidis, A.L.; Andrade, G. Enhanced Production of Target Bioactive Metabolites Produced by Pseudomonas aeruginosa LV Strain. Biocatal. Agric. Biotechnol. 2019, 17, 545–556. [Google Scholar] [CrossRef]
- Barazetti, A.R.; Simionato, A.S.; Navarro, M.O.P.; Dealis, M.L.; Matos, J.M.S.; Modolon, F.; Andreata, M.F.L.; Liuti, G.; Andrade, G. Evaluation of Bioproducts and Mycorrhizal Inoculation in Asian Soybean Rust Control, Nutrient Leaf Contents and Yield Under Field Conditions. In Microbial Probiotics for Agricultural Systems, 1st ed.; Zúñiga-Dávila, D., González-Andrés, F., Ormeño-Orrillo, E., Eds.; Springer: Cham, Switzerland, 2019; Volume 1, pp. 193–204. ISBN 978-3-030-17597-9. [Google Scholar]
- Navarro, M.O.P.; Dilarri, G.; Simionato, A.S.; Grzegorczyk, K.; Dealis, M.L.; Cano, B.G.; Barazetti, A.R.; Afonso, L.; Chryssafidis, A.L.; Ferreira, H.; et al. Determining the Targets of Fluopsin C Action on Gram-negative and Gram-positive Bacteria. Front. Microbiol. 2020, 11, 1076. [Google Scholar] [CrossRef]
- Simionato, A.S.; Cano, B.G.; Navarro, M.O.P.; Tavares, E.R.; Ribeiro, R.A.; Hungria, M.; Yamauchi, L.M.; Yamada-Ogatta, S.F.; Andrade, G. Whole-genome Sequence of Bioactive Compound-producing Pseudomonas aeruginosa Strain LV. Microbiol. Resour. Announc. 2021, 10, e01120-20. [Google Scholar] [CrossRef]
- Patteson, J.B.; Putz, A.T.; Tao, L.; Simke, W.C.; Bryant, L.H.; Britt, R.D.; Li, B. Biosynthesis of Fluopsin C, a Copper-containing Antibiotic from Pseudomonas aeruginosa. Science 2021, 374, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Leong, J.; Bell, S.J. Coordination Isomers of Antibiotic Thiohydroxamate-metal Complexes. Geometrical Isomers of tris(N-methylthioformohydroxamato)rhodium(III) and bis(N-methylthioformohydroxamato)platinum(II). Inorg. Chem. 1978, 17, 1886–1892. [Google Scholar] [CrossRef]
- Ito, Y.; Umino, K.; Sekiguchi, T.; Miyagishima, T.; Egawa, Y. Antibiotic YC 73 of Pseudomonas origin. III. Synthesis of Thioformin Analogues. J. Antibiot. 1971, 24, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Miyagishima, T.; Yamaguchi, T.; Umino, K. Further Studies on Synthesis and Antimicrobial Activity of Thioformin Analogues. Chem. Pharm. Bull. 1974, 22, 2283–2287. [Google Scholar] [CrossRef] [Green Version]
- Quintana, J.; Novoa-Aponte, L.; Argüello, J.M. Copper Homeostasis Networks in the Bacterium Pseudomonas aeruginosa. J. Biol. Chem. 2017, 292, 15691–15704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Du, X.; Lu, Z.J.; Wu, D.; Zhao, Y.; Ren, B.; Huang, J.; Huang, X.; Xu, Y.; Xu, Y. Regulatory Feedback Loop of two phz Gene Clusters through 5′-untranslated Regions in Pseudomonas sp. M18. PLoS ONE 2011, 6, e19413. [Google Scholar] [CrossRef] [PubMed]
- Teitzel, G.M.; Geddie, A.; De Long, S.K.; Kirisits, M.J.; Whiteley, M.; Parsek, M.R. Survival and Growth in the Presence of Elevated Copper: Transcriptional Profiling of Copper-stressed Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 7242–7256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaden, J.T.; Lory, S.; Gardner, T.S. Quorum-sensing Regulation of a Copper Toxicity System in Pseudomonas aeruginosa. J. Bacteriol. 2010, 192, 2557–2568. [Google Scholar] [CrossRef] [PubMed]
| Bacteria | Reference |
|---|---|
| Pseudomonas MCRL 10107 | [7] |
| Pseudomonas fluorescens | [20] |
| Pseudomonas fluorescens KY 4032 | [21] |
| Pseudomonas reptilivora | [18] |
| Pseudomonas aeruginosa 3120 | [25] |
| Pseudomonas aeruginosa LV | [26] |
| Pseudomonas sp. AV 246 (DSM 15085) | [11] |
| Pseudomonas sp. AV 249 (DSM 15086) | [11] |
| Pseudomonas jinanesis | [17] |
| Pseudomonas aeruginosa PAO1 | [34] |
| Streptomyces 4601 | [16] |
| Fluopsin | Complexed Ion | Morphology | Minimum Inhibitory Concentration | Reference | |
|---|---|---|---|---|---|
| Staphylococcus aureus FDA 209P (μg·mL−1) | Bacillus subtilis PCI-219 (μg·mL−1) | ||||
| C | Cu2+ | Dark-green needles | 0.09 | 0.09 | [7] |
| CA | Ca2+ | Colorless needles | 0.39 | 0.78 | [22] |
| F | Fe3+ | Black needles | 0.39 | 3.12 | |
| NI | Ni2+ | Dark red needles | 0.39 | 0.39 | |
| Z | Zn2+ | Colorless prisms | 0.78 | 1.56 | |
| S | Sn2+ | Pale brown powder | 0.78 | 1.56 | |
| N | Na2+ | Colorless needles | 0.39 | 0.78 | |
| S. aureus FDA 209P (μM) | Escherichia coli NIHJ (μM) | [24,35] | |||
| PD | Pd2+ | Red crystals | 0.50 | 5.00 | |
| PT | Pt2+ | Yellow slurry | NE * at 150.00 | NE at 150.00 | |
| CO | Co3+ | Brown crystals | 5.00 | 75.00 | |
| CR | Cr3+ | Green solids | 25.00 | 75.00 | |
| RH | Rh3+ | - | 150.00 | NE at 250.00 | |
| Microorganisms | Minimum Inhibitory Concentration (μg·mL−1) | Reference |
|---|---|---|
| Gram-negative | ||
| Pseudomonas syringae var. syringae | 2.00–3.00 | [11] |
| Xanthomonas citri pv. citri | 19.53 (F3) | [12] |
| Xanthomonas axonopodis BSC475a | 100.00 (F3) | [28] |
| Xanthomonas arboricola pv. pruni | 50.00 (F3) | [13] |
| Xanthomonas citri pv. citri | 0.12; 6.25 (F3d) | [14] |
| Pectobacterium carotovorum subsp. carotovorum | 7.81 (F4A) | [15] |
| Fungi | ||
| Bipolaris sorokiniana | 0.40–0.60 | [11] |
| Fusarium culmorum | 5.00–6.00 | |
| Fusarium oxysporum | 4.00–5.00 | |
| Heterobasidion annosum | 0.10–0.20 | |
| Sclerotinia sclerotiorum | 6.00–7.00 | |
| Oomycete | ||
| Pythium ultimum | 0.10–0.20 | [11] |
| Bacterial Model * | Ultrastructural Effects | |
|---|---|---|
| SEM | TEM | |
| Xanthomonas citri subsp. citri 306 | Disruption of cytoplasmatic membrane; shrunken and rough cells. | Modified cellular morphology; elongated cells; indistinction between the cytoplasmatic membrane and cell wall. |
| Klebsiella pneumoniae KPC19 | Cell wall and membranes disrupted. | Alterations in the cell wall, cytoplasmatic membrane, and cytoplasm. |
| Enterococcus faecalis ATCC 6569 | Absence of marked differences; reduction in the number of cells. | Absence of marked differences. |
| Staphylococcus aureus MRSA N315 | Alterations in the cell wall and cytoplasmatic membrane. | Cell wall loss; heterogeneous and electron-dense cytoplasm. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afonso, L.; Andreata, M.F.d.L.; Chryssafidis, A.L.; Alarcon, S.F.; das Neves, A.P.; da Silva, J.V.F.R.; Gonçalves, G.d.S.; Abussafi, L.D.d.S.; Simionato, A.S.; Cely, M.V.T.; et al. Fluopsin C: A Review of the Antimicrobial Activity against Phytopathogens. Agronomy 2022, 12, 2997. https://doi.org/10.3390/agronomy12122997
Afonso L, Andreata MFdL, Chryssafidis AL, Alarcon SF, das Neves AP, da Silva JVFR, Gonçalves GdS, Abussafi LDdS, Simionato AS, Cely MVT, et al. Fluopsin C: A Review of the Antimicrobial Activity against Phytopathogens. Agronomy. 2022; 12(12):2997. https://doi.org/10.3390/agronomy12122997
Chicago/Turabian StyleAfonso, Leandro, Matheus Felipe de Lima Andreata, Andreas Lazaros Chryssafidis, Stefani Fabiola Alarcon, Ana Paula das Neves, João Vittor Frossard Rodrigues da Silva, Gilmar da Silva Gonçalves, Leonardo Dib de Sousa Abussafi, Ane Stefano Simionato, Martha Viviana Torres Cely, and et al. 2022. "Fluopsin C: A Review of the Antimicrobial Activity against Phytopathogens" Agronomy 12, no. 12: 2997. https://doi.org/10.3390/agronomy12122997
APA StyleAfonso, L., Andreata, M. F. d. L., Chryssafidis, A. L., Alarcon, S. F., das Neves, A. P., da Silva, J. V. F. R., Gonçalves, G. d. S., Abussafi, L. D. d. S., Simionato, A. S., Cely, M. V. T., & Andrade, G. (2022). Fluopsin C: A Review of the Antimicrobial Activity against Phytopathogens. Agronomy, 12(12), 2997. https://doi.org/10.3390/agronomy12122997






