Abstract
Durum wheat grain can accumulate mycotoxins because it is highly sensitive to infections caused by pathogens of the genera Fusarium and Alternaria. Reduced fungicide use increases the demand for biological methods of pathogen control. The aim of the experiment was to evaluate the efficacy of Debaryomyces hansenii (Dh) yeast in reducing the content of secondary fungal metabolites present in the spikes of five durum wheat cultivars grown in southern and northern Poland. A total of 27 Fusarium metabolites and nine metabolites produced by other fungi were identified in the grain. The application of the Dh yeast strain decreased deoxynivalenol concentration in all samples relative to control treatments (by 14–100%) and treatments inoculated with F. graminearum (by 23–100%). In northern Poland, the biological treatment also led to a considerable reduction in the content of culmorin (by 83.2–100%) and enniatins A1 and B (by 9.5–65.3% and 6.7–70%, respectively) in the grain. An analysis of multiple fungal metabolites is a highly useful tool for determining grain quality and its suitability for consumption. When applied in the flowering stage, yeasts can partly complete fungicides in reducing Fusarium head blight.
1. Introduction
Progressing climate change creates increasingly favorable conditions for the cultivation of durum wheat (Triticum durum (Desf.)) in Poland. This species is tolerant to drought, and it is grown mainly in warmer European regions [1,2]. In recent years, the area under durum wheat in Poland has increased to more than 2000 hectares, and this crop is produced in all Polish regions [2]. However, the introduction of durum wheat to new areas is accompanied by the risk of the emergence of virulent and polyphagous pathogens that infect spikes [3,4]. Species of the genera Fusarium and Alternaria, the causative agents of Fusarium head blight (FHB) as well as sooty head molds and black point, respectively, produce numerous mycotoxins and pose the greatest threat to the quality of T. durum grain [3,5].
Species of the genus Fusarium are particularly dangerous for cereal crops. More than 20 Fusarium species and subspecies infect plants between seedling development and harvest, and they are difficult to control [6]. The effect of mixed Fusarium mycotoxicoses on human and animal health has rarely been studied. Increased or reduced toxicity of multiple deoxynivalenol-family mycotoxins (DON, NIV, and their acetyl derivatives of 3-acetyldeoxynivalenol (3-ADON) and 15-acetyldeoxynivalenol (15-ADON), deoxynivalenol-3-glucoside (D3G), and fusarenon X (FX)) against human gastric epithelial (GES-1) cells has been demonstrated [7]. In turn, Gao et al. [8] reported an increase in the cytotoxicity of aflatoxin M1 against human cell lines in the presence of ZEA. The combination of CUL (10 mg/kg of feed) and DON (25 mg/kg of feed) in feed increased mortality and decreased the weight of Heliothis zea larvae in comparison with larvae exposed to pure DON [9]. It should also be noted that Fusarium metabolites play an important role in plant infections caused by pathogens. For example, polyketides (such as aflatoxins and fumonisins), terpenes (such as T-2 toxin and DON), indole terpenes (such as paxilline and lolitrems), non-ribosomal peptides (such as sirodesmin, enniatins, and beauvericins), alkaloids (peramine) and siderophores (ferricrocin) often play a role in triggering infection symptoms in plants [10].
Sooty head molds manifest on durum wheat spikes in the grain ripening stage [11], whereas symptoms of black point are noted in threshed grain [12]. These diseases are most often caused by Alternaria species [12]. Alternaria alternata produces around 70 secondary metabolites, some of which are classified as mycotoxins that pose a potential threat to humans and animals [13]. Regulatory limit values for these compounds in foodstuffs and feedstuffs have not been introduced to date. Tenuazonic acid (TeA) is highly toxic for female mice at a dose of 81 mg/kg body weight, and altenuene (ALT) poses a similar threat when administered at 50 mg/g [14]. Alternariol (AOH) and alternariol monomethyl ether exerted mutagenic effects in cultured mammalian cells [15]. Depending on their concentrations, binary mixtures of AOH with altertoxin I and II exerted both antagonistic and synergistic effects on the activity of the CYP 1 enzyme in estrogen-sensitive MCF-7 mammary cancer cells [16]. Altertoxin II has been described as a compound with the strongest mutagenic properties [17].
The new approach to cereal protection against pathogens proposed under the European Green Deal advocates for the replacement of environmentally-toxic fungicides (including triazole fungicides) with environmentally-friendly biological methods [18,19]. New biological methods for reducing mycotoxin content in cereal grain have to be developed and tested. Yeasts widely colonize wheat grain, and they can be applied to crops to inhibit the development of plant pathogens [20,21]. Yeasts can differ in their mechanism of action. For example, Aureobasidium pullulans yeast compete for nutrients with F. graminearum by producing fusigen, which plays an important role in chelating and capturing iron [22]. Numerous studies have demonstrated that yeasts can biotransform or biodegrade toxins and convert them into non-toxic compounds or compounds with reduced toxicity [23,24]. However, the effectiveness of biological yeast treatments in reducing the content of secondary metabolites of Alternaria species in cereal grain has not been investigated to date [23,25].
The aims of this study were as follows: (1) to evaluate the efficacy of a biological treatment involving a suspension of Debaryomyces hansenii (Zopf) Lodder & Kreger-van Rij yeast in reducing the content of secondary fungal metabolites, mainly those produced by Fusarium spp. and Alternaria spp., in the grain of several spring durum wheat cultivars grown in two locations in Poland; (2) to analyze the influence of the biological treatment on plant health; (3) to assess grain colonization by pathogens, and (4) to determine the correlations between mycotoxin concentrations in grain, plant health, and selected yield parameters.
2. Materials and Methods
2.1. Location, Soil, Climate, and Experimental Design
Field-plot experiments were conducted in Bałcyny in north-eastern Poland (53°36′ N, 19°51′ E) and in Niedrzwica Kościelna in south-eastern Poland (51°05′ N 22°21′ E) (Figure 1). The study sites did not differ considerably in mean monthly temperature, total precipitation, or soil type (soil suitable for wheat cultivation) (Table 1). In both locations, considerable fluctuations in temperature and rainfall distribution were noted during the growing season of spring durum wheat cultivars. Spring temperatures were higher in southern than in northern Poland. Total precipitation did not exceed 13.3 mm during wheat flowering and ripening (in the last ten days of May and the first twenty days of June).
Figure 1.
The location of the two localities where the experiments were conducted.

Table 1.
Weather conditions in vegetation period in 2018.
The field experiments had a split-plot design with two replications. The harvested plot area was 9 m2. The experimental factors were location (Bałcyny, Niedrzwica Kościelna), cultivar (Floradur, IS Duranegra, Durasol, IS Duragold, Tamadur) (Table 2), biological control and plant inoculation (control- without biocontrol and pathogen, inoculation with Fusarium graminearum (Fg), biocontrol with Debaryomyces hansenii (Dh), and biocontrol with D. hansenii + inoculation with F. graminearum (Dh/Fg)).

Table 2.
Characteristics of spring durum wheat cultivars grown in two locations.
In Niedrzwica Kościelna, durum wheat was sown in the last ten days of March, and in Bałcyny—in the first ten days of April at 220–240 kg/ha. Before sowing, the plots were fertilized with nitrogen at 40–70 kg N/ha (34% ammonium nitrate), phosphorus at 25–60 kg P2O5/ha (40% superphosphate), and potassium at 80–100 kg K2O/ha (60% potash salt). During the growing season, nitrogen was applied twice at 25–60 and 40 kg N/ha (34% ammonium nitrate) in the tillering stage. Wheat plants were sprayed with the Fury® 100 EW insecticide (FMC Chemical, Belgium) at 0.1 L/ha in the tillering stage to control cereal leaf beetle infestations. Mustang™ 306 SE herbicide (Dow AgroSciences, Poland) was applied at 0.6 L/ha in the tillering stage. Plant height was controlled with the Moddus 250 EC growth regulator (Syngenta, Poland) according to the manufacturer’s instructions. The Tilt Turbo 575 EC systemic fungicide (Syngenta, Poland) was applied at 1 L/ha in the stem elongation stage (BBCH 31) to control the powdery mildew of cereals and grasses [26].
2.2. Grain Sampling in—Durum Wheat Cultivars
Grain for mycotoxin analyses was sampled in the fully ripe stage (BBCH 92). A total of 40 samples were collected from five durum wheat cultivars (Table 2). Durasol is a late flowering cultivar that is characterized by the highest susceptibility to FHB and lowest yields. IS Duragold and IS Duranegra are early flowering and relatively tall cultivars that are most resistant to FHB.
2.3. Biological Treatment with Debaryomyces hansenii in the Field
The biological treatment involved a cell suspension of the D. hansenii 2 yeast strain (GenBank accession number KX444669) isolated from apple cv. Antonówka. The strain was cultured on yeast extract peptone dextrose in a bioreactor (30 L, A&A Biotechnology, Gdańsk, Poland), and 120 g of yeast biomass, after centrifuging the cells at 4000 rpm for 5 min, was introduced to a backpack pressure sprayer (Marolex Titan 12, Dziekanów Leśny, Poland) and diluted in 12 dm3 of water with the addition of 1 cm3 of Tween® 80 (Merck, Wrocław, Poland). In each location, the first biological treatment (130 cm3/m2, cell suspension density 106 per 1 cm3) was applied at the beginning of flowering (BBCH 61), and the second treatment was performed after seven days in the full flowering stage (BBCH 67).
2.4. Spike Inoculation with Fusarium graminearum
In selected plots (control plots and plots with biological control), spikes were inoculated with the virulent F. graminearum pathogen (15-ADON genotype, GenBank accession number MZ827461) 24 h after the second biological treatment. The applied fungal strain had been isolated from durum wheat grain. The strain’s identity and genotype (15-ADON) were confirmed by PCR according to a previously described procedure [27]. Fungal spores were rinsed from 14-day colonies cultured on potato dextrose agar (PDA, Merck, Poland) with the use of sterile water. The obtained spore suspension had a density of 1 × 105/cm3. Directly before spike inoculation, the 1 dm3 suspension was diluted with water to a volume of 10 dm3 with the addition of 1 cm3 Tween® 80. Spikes were inoculated with a backpack pressure sprayer (Marolex Titan 12, Poland).
2.5. Mycotoxin Analysis
For the analysis of Fusarium mycotoxins, grain subsamples of 150 g were obtained with a riffle divider and ground in a mill (Cyclotec™ 1093; Foss Tecator, Sweden; 1 mm mesh size). Fusarium mycotoxins were extracted over a period of 90 min on a rotary shaker with a mixture of acetonitrile/water/acetic acid (79/20/1), applied at 20 mL per 5 g of grain. Fusarium mycotoxins were identified and quantified according to the method described by Sulyok et al. [28] with the use of the QTrap5500 LC-MS/MS System (Applied Biosystems, Foster City, CA, USA) equipped with a TurboIon Spray electrospray ionization (ESI) source and the 1290 Series UHPLC System (Agilent Technologies, Waldbronn, Germany).
2.6. Evaluation of Spike Health and Selected Yield Parameters in the Field
Spike health was assessed on 100 infected organs in the ripening stage (BBCH 83), according to the guidelines of the European and Mediterranean Plant Protection Organization [29]. The number of spikes with symptoms of sooty head molds and FHB was determined, and infection severity was expressed as the percentage of affected spike area. Plant height and spike density (number of spikelets per 10 cm of spike rachis length) were measured. Crop yield per m2 of plot area was estimated.
2.7. Mycobiota on Durum Wheat Grain
Fungal counts on wheat kernels were determined at harvest according to a previously described method [27]. Grain samples of 10 g each were placed in 250 cm3 flasks containing 90 cm3 of sterile water with the addition of Tween® 80, and the flasks were shaken on the Elpin Plus 358 S laboratory shaker (Elpin Plus, Poland, 180 rpm) for 30 min. 0.1 cm3 of the fungal suspension from the second dilution was transferred to Petri plates and cultured on Martin agar. The number of colony-forming units (CFU) was calculated according to the formula given by Wachowska et al. [27].
2.8. Identification of Fusarium and Alternaria pathogens
After 7 days of incubation at a temperature of 24 °C, the emerging fungal colonies of the genus Alternaria were transferred to sterile plates with PDA, and the emerging fungal colonies of the genus Fusarium were transferred to Spezieller Nährstoffarmer Agar (SNA, Merck, Poland) with the addition of kanamycin (A&A Biotechnology, Poland) and streptomycin (Sigma, Poland). The fungal isolates were identified under a light microscope (Nikon E200, Japan) [30,31]. Single-spore cultures were incubated on a liquid medium with the following composition: beef extract—1 g, soy peptone—5 g, NaCl—5 g, glucose—1 g, yeast extract—7 g in 1 dm3 of water. The biomass of fungal isolates was dried on blotting paper with the use of a vacuum pump (Rocker, Arlab, Poland), and it was transferred to Eppendorf tubes. After short-term storage at a temperature of −20 °C, the isolates were subjected to molecular analysis.
2.9. DNA Extraction and the Identification of Molecular Species and Chemotype
The taxonomic identity of Fusarium and Alternaria pathogens was confirmed with the use of a molecular technique. Genomic DNA of selected isolates was extracted with the Bead Baet MicroAX Gravity kit (A&A Biotechnology, Poland) according to the manufacturer’s instructions. The taxonomic identity of the reference isolates belonging to the Fusarium graminearum species complex (FGSC) was confirmed by amplifying DNA fragments (280 bp) of F. culmorum (amplicon 570), F. avenaceum (amplicon 220), and F. sporotrichioides (amplicon 332) [32]. The thermal profiles in the PCR assay were described previously by Duba et al. [32]. The amplification products were sequenced by Genomed S.A. in Warsaw (http://genomed.pl, accessed on 3 January 2023). Fungal species were identified based on their similarity to the sequences deposited in the NCBI database with the use of the BLAST tool.
2.10. Statistical Analysis
The results obtained from the field experiment (incidence of FHB and Sooty black mold, severity of FHB and Sooty black mold, occurrence of yeasts, Cladosporium spp., Alternaria spp. Fusarium spp. Epicoccum spp., and Drechslera spp. and yield) were processed by analysis of variance (ANOVA) with the Student–Newman–Keuls (SNK, p < 0.01) multiple range tests to determine the significance of differences between means, as well as Pearson’s correlation analysis. Statistical analyses were performed in Statistica 13 software [33].
3. Results
3.1. Fusarium Metabolites/Mycotoxins
Deoxynivalenol and D3G were identified in 25 out of the 40 analyzed grain samples (Supplementary Table S1). The content of DON was particularly high in the control grain samples of cvs. Floradur and Tamadur from Niedrzwica Kościelna at 2081 and 1113 µg/kg, respectively (Supplementary Table S1, Figure 2). In most cases, spike inoculation with F. graminearum increased DON levels in grain by up to 1519 µg/kg (in cv. Tamadur from Niedrzwica Kościelna).
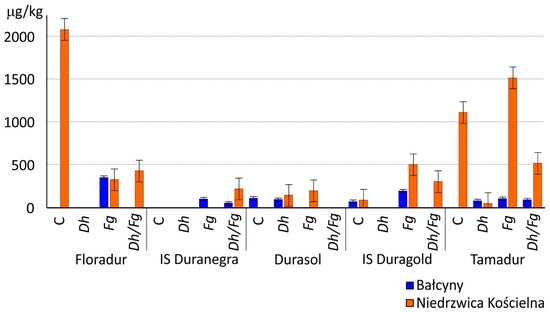
Figure 2.
The levels of the DON (µg/kg) (including the error bars) in the grain of five T. durum cultivars. C—control- without biocontrol and pathogen, Dh—biocontrol with Debaryomyces hansenii, Fg—inoculation with Fusarium graminearum, Dh/Fg—biocontrol with D. hansenii + inoculation with F. graminearum.
Nivalenol and NIV3G were detected in all grain samples from Niedrzwica Kościelna (11.9–997.6 µg/kg) and in three samples from Bałcyny (11.5–25.7 µg/kg) (Supplementary Table S1). T-2 and HT-2 toxins were identified in nine samples from Niedrzwica Kościelna (2.4–108.8 µg/kg) and in one sample from Bałcyny. Culmorin (CUL), 15-hydroxyculmorin, and 5-hydroxyculmorin, were detected in 34 grain samples. Culmorin concentration was particularly high in the grain of cvs. Floradur and Tamadur from Niedrzwica Kościelna at 2941.2 and 2887.8 µg/kg, respectively. Zearalenone (ZEA) was identified in 21 grain samples (0.9–65.1 µg/kg). Apicidin (API) was detected in all grain samples from southern Poland (3.1–457.5 µg/kg) and in 11 samples from northern Poland (0.9–66.2 µg/kg), whereas all samples were contaminated with aurofusarin (AURO) (55.3–3236.7 µg/kg). In most cases, spike inoculation with F. graminearum increased AURO concentration in grain. Enniatins (ENNs) A1, B, B1 and B2 were found in all tested samples at a concentration of 1.7 (ENN B2) to 2085.6 (ENN B1) µg/kg. Enniatin B3 was present in most samples in small concentrations (0.01- 1.9 µg/kg). Two cyclic dipeptides, cyclo(L-Pro-L-Tyr) and cyclo (L-Pro-L-Val), and tryptophol were detected in all samples (respectively at concentrations 21.7 to 58.7, 2.6 to 14.4, and 24.1 to 150.2 µg/kg) whereas W493, equisetin and chrysogin were present in most samples. Fungerin, antibiotic Y, chlamydospordiol, and chlamydosporol were identified in a small number of grain samples.
3.2. Alternaria and Penicillium Metabolites
Infectopyron was detected in all samples, and its concentration was clearly higher in grain from Niedrzwica Kościelna (1043.0–2466.4 µg/kg) than Bałcyny (487.3–816.6 µg/kg) (Supplementary Table S1). Altersetin was present in all samples, and its content was not correlated with the geographic origin of the grain. Tenuazonic acid was identified in eight samples, but its concentration was generally low. Tentoxin and alternariol were found mainly in the samples from Niedrzwica Kościelna. Asperphenamate was detected mainly in the grain from Bałcyny, and citreorosein and curvularin—in the samples from Niedrzwica Kościelna. Only four samples contained abscisic acid.
3.3. The Efficacy of the Biological Treatment in Reducing the Concentrations of the Secondary Metabolites of Fusarium spp.
The D. hansenii (Dh) yeast strain decreased DON levels in all grain samples from both experimental sites relative to control grain or grain samples from treatments inoculated with F. graminearum (Fg) (Figure 2, Supplementary Table S1). In samples where DON content exceeded 1250 mg per kg of grain, the effectiveness of the biological treatment was estimated at 100% for cv. Floradur and 95.3% for cv. Tamadur (Figure 2). The biological treatment reduced DON levels in nearly all samples from treatments where biocontrol was combined with spike inoculation with F. graminearum (Dh/Fg). In Bałcyny, the biological treatment also induced a clear decrease in CUL, ENN A1, and ENN B concentrations in the grain of cvs. Floradur, IS Duranegra, Durasol, and IS Duragold (Figure 3, Supplementary Table S1).
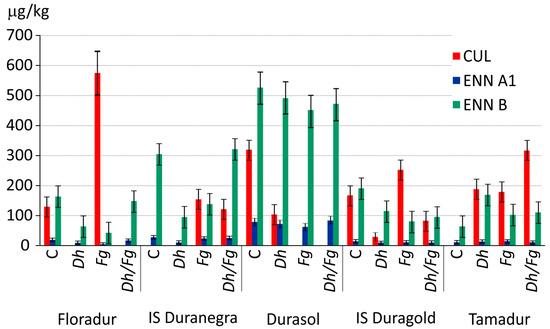
Figure 3.
The levels of the CUL, ENN A1, and ENN B (µg/kg) (including the error bars) in the grain of five T. durum cultivars. C—control- without biocontrol and pathogen, Dh—biocontrol with Debaryomyces hansenii, Fg—inoculation with Fusarium graminearum, Dh/Fg—biocontrol with D. hansenii + inoculation with F. graminearum.
3.4. Health Status of Durum Wheat Spikes
Symptoms of FHB and sooty head molds were observed on wheat spikes in both study sites (Table 3). On average, the biological treatment induced a non-significant decrease in the incidence and severity of FHB (by 45.6% and 41.1%, respectively). Symptoms of sooty head molds were significantly more prevalent in Bałcyny than in Niedrzwica Kościelna. In Bałcyny, cv. Floradur was more severely infected with sooty head molds than the remaining cultivars (Table 3 and Table 4).

Table 3.
The health status of spring durum wheat cultivars and mycobiota on grain.

Table 4.
The F-values for experiment treatments and interactions obtained in the three-way ANOVA.
3.5. Mycobiota Composition
Durum wheat grain was colonized predominantly by yeasts, and their abundance reached 0.63 to 88 × 102 CFU per gram of grain (Table 3). The average yeast abundance was significantly higher (more than three times) on the grain grown in Bałcyny than Niedrzwica Kościelna. The biological treatment significantly increased the average yeast density on grain. Fungi of the genus Cladosporium were significantly more often noted in Bałcyny than in Niedrzwica Kościelna. Alternaria and Fusarium species isolates were more prevalent in Niedrzwica Kościelna (Table 3, Figure 4). The application of D. hasenii (Dh) induced a greater decrease in the counts of Fusarium species isolates colonizing wheat cv. Durasol in Bałcyny than grain inoculated with F. graminearum (Fg). Fungi of the genera Epicoccum and Drechslera were identified sporadically, mainly in the grain from Bałcyny.
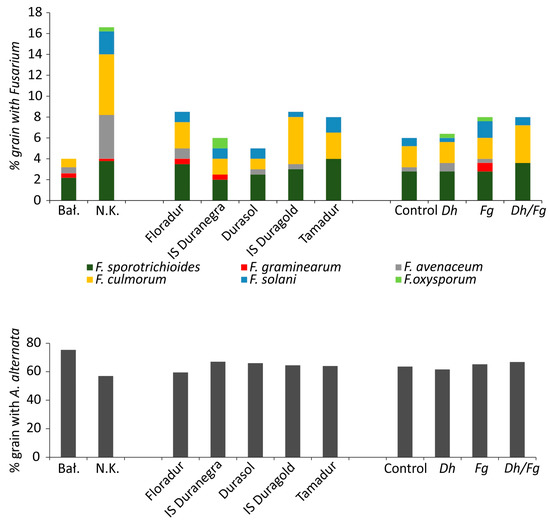
Figure 4.
Percentage of kernels colonized by Fusarium and Alternaria spp. species across locations, cultivars, and protective treatments. Bał—Bałcyny, N.K.—Niedrzwica Kościelna, Fg—inoculation with Fusarium graminearum, Dh—biocontrol with Debaryomyces hansenii, Dh/Fg—biocontrol with D. hansenii + inoculation with F. graminearum.
3.6. Identification of Fusarium and Alternaria Species
Colonies of Fusarium species emerged from 4% of the kernels sampled from Bałcyny and 16.6% of the kernels sampled from Niedrzwica Kościelna (Figure 4). Six Fusarium species were identified in Niedrzwica Kościelna (F. avenaceum—4.2%, F. culmorum—5.8%, F. graminearum—0.2%, F. oxysporum—0.4%, F. solani—2.2 %, and F. sporotrichioides—3.8%), whereas four Fusarium species were detected in Bałcyny (F. avenaceum—0.6%, F. culmorum—0.2%, F. graminearum—0.4%, and F. sporotrichioides—2.2%). Alternaria alternata was isolated from 75.4% of the kernels in Bałcyny and from 57% of the kernels in Niedrzwica Kościelna. Neither wheat cultivar nor protective treatment were correlated with A. alternata counts (CFU) on kernels cultured on PDA.
3.7. Pearson’s Correlation Analysis
An analysis of the simple correlations between yield, spike health, grain colonization by fungi, and the content of secondary fungal metabolites revealed significant negative correlations (−0.343 and −0.568) between yield vs. severity of sooty head molds and Cladosporium counts on grain, respectively, significant positive correlations with the concentrations of TEN (0.546) and INFE (0.616), and weak positive correlations with the content of NIV, NIV3G, ENNB, ENNB2, W493, AOH, CTO, CUR, and Trypt (Supplementary Table S2, Figure 5). In many cases, strong positive correlations were noted between the co-existence of different mycotoxins. For example, CUL concentration was bound by a strong positive correlation with the content of DON (0.883), D3G (0.897), 15HCUL (0.821), 5HCUL (0.801), ZEA (0.706), and W493 (0.653). Interestingly, AsP concentration was negatively correlated with the content of most of the analyzed mycotoxins.
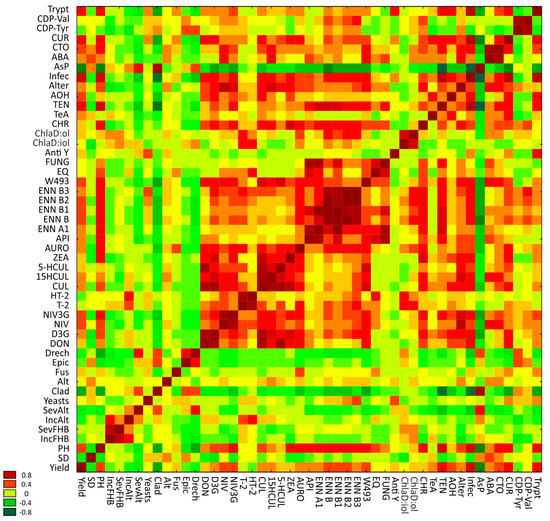
Figure 5.
The correlation plot for yield, spike health, spike density, plant height, grain colonization by fungi, and the content of fungal metabolites presented in the form of heat map. SD—spike density, PH—plant height, IncFHB—incidence of FHB, SevFHB—severity of FHB, IncAlt—incidence of sooty head molds, SevAlt—severity of sooty head molds, Clad—Cladosporium spp., Alt—Alternaria spp., Fus—Fusarium spp., Epic—Epiccocum spp., Drech—Drechslera spp., DON—deoxynivalenol, D3G—DON-3-glucoside, NIV—nivalenol, NIV3G—nivalenol glucoside, CUL—culmorin, 15-HCUL—15-hydroxyculmorin, 5-HCUL—5-hydroxyculmorin, ZEA—zearalenone, AURO—aurofusarin, ENN A1—enniatin A1, ENN B—enniatin B, ENN B1—enniatin B1, ENN B2—enniatin B2, ENN B3—enniatin B3, EQ—equisetin, FUNG—fungerin, Anti Y—antibiotic Y, ChlaD:iol—chlamydospordiol, ChlaD:ol—chlamydosporol, CHR—chrysogin, TeA—tenuazonic acid, TEN—tentoxin, AOH—tentoxin, ALT—altersetin, INFE—infectopyron, AsP—asperphenamate, ABA—abscisic acid, CTO—citreorosein, CUR—curvularin, CDP-Tyr—cyclo(L-Pro-L-Tyr), CDP-Val—cyclo(L-Pro-L-Val), Trypt—tryptophol.
4. Discussion
The article presents the results of field experiments investigating the content of 27 secondary metabolites produced by fungi of the genus Fusarium and nine secondary metabolites produced by other fungi colonizing the grain of several durum wheat cultivars grown in two locations in Poland. Wheat spikes were inoculated with F. graminearum and protected with two treatments involving a suspension of D. hansenii yeasts. The content of type A and B trichothecenes was clearly correlated with location. These toxins were more prevalent and were detected in higher concentrations in southern than in northern Poland. The above can be attributed mainly to higher precipitation in southern Poland in the last 20 days of June during flowering and kernel development. Numerous studies conducted in Poland [3,34,35] and in other European regions [36] also demonstrated that humidity levels during durum wheat flowering significantly affected the incidence of Fusarium infections. In a study by Lenc [34], the incidence of Fusarium infections was highest during a particularly wet period in the first ten days of June 2007. Gorczyca et al. [3] analyzed the content of DON in the grain of three durum wheat cultivars and observed more than a 10-fold increase in DON levels in 2013 which was characterized by abundant precipitation in June. A long-term study conducted by Landschoot et al. [36] revealed a strong positive correlation between air humidity in June and DON levels in the grain of nearly 100 wheat genotypes.
Trichothecene concentrations in non-inoculated grain exceeded the maximum limits only in three cases: in Niedrzwica Kościelna—for DON, and in Bałcyny—for the sum of T-2 and HT-2. In a study of the Polish durum wheat cultivar Komnata, DON concentration was determined at 3860 µg/kg in 2012 and 8460 µg/kg in 2013, whereas the content of HT-2 reached 148 µg/kg in 2013 [35]. In the cited study, the content of the analyzed toxins was much lower in the grain of T. aestivum ssp. aestivum, T. aestivum ssp. spelta, and T. monococcum in the same period. Shah et al. [37] and Covarelli et al. [38] observed that durum wheat was more susceptible to Fusarium infections than common wheat. In the current study, German and Slovakian genotypes were less sensitive to Fusarium spp. than the Polish cultivar Komnata, which was analyzed in our previous study. Wheat plants have developed several types of resistance to Fusarium pathogens through physiological and biochemical mechanisms [32,39]. For instance, the Fhb1 gene encodes the glycosyltransferase enzyme that converts DON to DON-3-O-glucoside in plant tissues [40].
In the present study, CUL levels were generally higher than DON levels, and a positive correlation between CUL and DON was also noted, which is consistent with the findings of other authors [41,42]. Culmorin is a tricyclic sesquiterpene diol derived from terpene synthase Clm1, which converts farnesyl diphosphate (FPP) to longiborneol [43], whereas Clm2-encoded cytochrome P450 catalyzes the hydroxylation of longiborneol to CUL [44]. Interestingly, Wipfler et al. [44] reported that CUL did not exert phytotoxic effects when applied independently to wheat seedlings, but it was highly phytotoxic when applied in combination with DON, 3-ADON, 15-ADON, and NX-3, but not with NIV. Recent research has shown that CUL can suppress the activity of uridine diphosphate glucosyltransferases (UGTs, found in all living organisms) that catalyze the glycosylation of DON into the less toxic D3G [45].
Only several grain samples contained low concentrations (up to 59.33 µg/kg) of several Fusarium mycotoxins (fungerin, chlamydospordiol, chlamydosporol, and antibiotic Y) that are very rarely described in the literature. Chlamydospordiol and chlamydosporol were first described by Solfrizzo et al. [46] as metabolites of F. chlamydosporum and F. tricinctum. In the work of Ariantari et al. [47], chlamydospordiol did not exert cytotoxic or antibacterial effects. Fungerin, an alkaloid that was first isolated from silvergrass and classified as a metabolite of an unknown Fusarium species, contains an imidazole moiety and has fungistatic properties [48,49]. In recent years, FUNG was frequently detected in oats [50] and feed [51].
This study revealed a negative correlation between AsP and most other mycotoxins. Asperphenamate was identified in small concentrations (0.33–2.02 µg/kg), mainly in grain samples from northern Poland. This compound had been previously detected in cereal grain [52]. Asperphenamate is a linear amino acid (AA) ester composed of N-benzoylphenylalanine and N-benzoylphenylalaninol [52]. Asperphenamate was first isolated from Aspergillus flavipes in 1977 [53]. Research studies conducted in the following decades revealed that AsP is produced by many species of the genus Aspergillus [54] as well as fungi of the genus Penicillium [52]. Penicillium astrolabium synthesizes many asperphenamate analogs, and due to the high plasticity of nonribosomal peptide synthetase (NRPS), the structure of these analogs is determined by the availability of amino acids in the substrate [52]. Asperphenamate has also attracted considerable interest due to its anticarcinogenic properties and the possibility of synthetic modification [55].
In the current experiment, most of the mycotoxins isolated from disinfected grain were produced by fungal species of the genera Alternaria and Fusarium. These fungi were also predominant on durum wheat grain grown in other Polish regions [3], in European countries [4], and in Argentina [11]. This study also demonstrated that Fusarium species (mainly F. culmorum and F. avenaceum) exerted greater pressure in southern Poland, whereas durum wheat grown in northern Poland was more susceptible to A. alternata infections.
The applicability of yeast strains for controlling plant pathogens has been studied extensively, but none of the registered commercial yeast preparations have been designed to reduce the severity of FHB [56]. In Europe, only Cerall® (Koppert) and Polyversum® (Biopreparáty/De Sangosse) commercial preparations containing Pseudomonas chlororaphis and Pythium oligandrum, respectively, target FHB [57]. Several yeast species had been previously isolated from cereal tissues and tested as biocontrol agents against FHB. In the work of Khan et al. [57], the Cryptococcus flavenses OH182.9 isolate inhibited FHB symptoms by 56%. In turn, Comby et al. [58] and Wachowska and Głowacka [59] demonstrated that Aureobasidium pullulans inhibited the development of F. culmorum. In a study by Rojas et al. [60] Anthracocystis flocculosa F63P (syn.: Pseudozyma flocculosa) significantly reduced FHB symptoms when applied at 2 dai and 3 dai before the pathogen.
The present study evaluated the efficacy of the D. hansenii strain isolated from the surface of apples in inhibiting the development of toxin-producing pathogens colonizing durum wheat spikes. In our opinion, this strain can be safely used in crops because it is often detected in dairy products, and it was given the qualified presumption of safety (QPS) status [61] by the European Food Safety Authority [62]. Debaryomyces hansenii was also effective in protecting stone fruit against Monilinia fructigena and Monilinia fructicola [63], and common wheat against FHB [27]. Our previous study demonstrated that D. hansenii inhibits the development of Fusarium species by competing for food and producing compounds that limit the growth of Fusarium pathogens [27]. Similar observations were made by Huang et al. [64], who detected 71 volatile compounds, including six acids, 22 alcohols (including 2-phenylethanol, a well-known quorum sensing molecule), 15 aldehydes, three benzene derivatives, eight esters, three heterocyclic compounds, 12 ketones and two phenols, in cell-free supernatants of D. hansenii strains. In turn, McClelland et al. [65] found that D. hansenii DSMZ70238 can produce killer toxins with antifungal properties. Endophytic strains of D. hansenii can also produce indole acetic acid (IAA) and trigger protective mechanisms against Fusarium pathogens in tomato plants by increasing the concentrations of phenolic and salicylic acids in plant tissues [66]. However, abiotic factors such as air temperature and substrate salinity affect the synthesis of many active compounds, and this fact should be taken into consideration when D. hansenii biological treatments are applied to protect wheat [64,65].
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy13030721/s1. Table S1: Mycotoxins in durum wheat grain from two localizations in Poland; Table S2: The Pearson’s correlation coefficient for yield, spike density, plant height, spike health, grain colonization by fungi, and the content of fungal metabolites. The statistically significant values are marked in red. For all designations, see Figure 5.
Author Contributions
Conceptualization, U.W.; methodology, U.W. and M.S.; validation, U.W. and M.W.; formal analysis, U.W. and M.W.; investigation, U.W., M.S., E.S., W.G. and W.K.; data curation, U.W. and M.W.; writing—original draft preparation, U.W.; writing—review and editing, M.W. and E.S.; visualization, M.W. and U.W.; supervision, M.W. and R.K.; project administration, U.W. and D.G.; funding acquisition, D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Smart Growth Operational Program, project number POIR.01.01.01-00-0251/17 “Development and implementation of a complex technology for obtaining high-quality pasta products with the addition of Polish durum wheat”. The task number 30.690.012-500: Studies on improving durum wheat resistance to fungal diseases, especially to Fusarium. The beneficiaries of the project are Polskie Zakłady Zbożowe Lubella GMW Ltd. and the University of Warmia and Mazury in Olsztyn, Poland.
Data Availability Statement
All data are provided in tables and supplementary materials. Other raw data are available from Urszula Wachowska (urszula.wachowska@uwm.edu.pl).
Acknowledgments
The authors express their gratitude to A. Poprawska for language editing.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
15-ADON—15-acetyldeoxynivalenol; 15-HCUL—15-hydroxyculmorin; 3-ADON—3-acetyldeoxynivalenol; 5-HCUL—5-hydroxyculmorin; ABA—abscisic acid; AFM1—aflatoxin M1; ALT– altersetin; ANOVA—analysis of variance; Anti Y—antibiotic Y; AOH—alternariol; API—Apicidin; AsP- asperphenamate; AURO—aurofusarin; BBCH—Biologische Bundesanstalt, Bundessortenamt und Chemische Industrie; CFU—colony-forming unit; ChlaD:iol—chlamydospordiol; ChlaD:ol—chlamydosporol; CHR—chrysogin; CTO—citreorosein; CUL—culmorin; CUR—curvularin; CDP-Tyr—cyclo(L-Pro-L-Tyr); CDP-Val—cyclo(L-Pro-L-Val); D3G—deoxynivalenol-3-glucoside; DON—deoxynivalenol; ENNs—enniatins; EQ– equisetin; FHB—Fusarium head blight; FPP—farnesyl diphosphate; FUMs—fumonisins; FUNG—fungerin; FX—fusarenon X; IAA—indole acetic acid; INFE—infectopyron; LC-MS/MS—liquid chromatography with tandem mass spectrometry; MON—moniliformin; NIV—nivalenol; NIV3G—nivalenol-3-glucoside; PDA—potato dextrose agar; SNK—multiple Student–Newman–Keuls test; TeA—tenuazonic acid; TEN—tentoxin; Trypt—tryptophol; ZEA—zearalenone.
References
- Beccari, G.; Colasante, V.; Tini, F.; Senatore, M.T.; Prodi, A.; Sulyok, M.; Covarelli, L. Causal agents of Fusarium head blight of durum wheat (Triticum durum Desf.) in central Italy and their in vitro biosynthesis of secondary metabolites. Food Microbiol. 2018, 70, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Bobryk-Mamcarz, A.; Kiełtyka-Dadasiewicz, A.; Rachoń, L. Usefulness of hulled wheats grown in Polish environment for wholegrain pasta-making. Foods 2021, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, A.; Oleksy, A.; Gala-Czekaj, D.; Urbaniak, M.; Laskowska, M.; Waśkiewicz, A.; Stępień, Ł. Fusarium head blight incidence and mycotoxin accumulation in three durum wheat cultivars in relation to sowing date and density. Sci. Nat. Heidelb. 2017, 105, 2. [Google Scholar] [CrossRef] [PubMed]
- Solanki, M.K.; Abdelfattah, A.; Sadhasivam, S.; Zakin, V.; Wisniewski, M.; Droby, S.; Sionov, E. Analysis of stored wheat grain-associated microbiota reveals biocontrol activity among microorganisms against mycotoxigenic fungi. J. Fungi 2021, 7, 781. [Google Scholar] [CrossRef] [PubMed]
- Tittlemier, S.A.; Blagden, R.; Chan, J.; Gaba, D.; Mckendry, T.; Pleskach, K.; Roscoe, M. Fusarium and Alternaria mycotoxins present in Canadian wheat and durum harvest samples. Can. J. Plant Pathol. 2019, 41, 403–414. [Google Scholar] [CrossRef]
- Senatore, M.T.; Ward, T.J.; Cappelletti, E.; Beccari, G.; McCormick, S.P.; Busman, M.; Laraba, I.; O’Donnell, K.; Prodia, A. Species diversity and mycotoxin production by members of the Fusarium tricinctum species complex associated with Fusarium head blight of wheat and barley in Italy. Int. J. Food Microbiol. 2021, 358, 109298. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, S.; Tan, Y.; Liu, N.; Wu, A. Individual and combined cytotoxic effects of cooccurring deoxynivalenol family mycotoxins on human gastric epithelial cells. Toxins 2017, 9, 96. [Google Scholar] [CrossRef]
- Gao, Y.N.; Wang, J.Q.; Li, S.L.; Zhang, Y.D.; Zheng, N. Aflatoxin M1 cytotoxicity against human intestinal Caco-2 cells is enhanced in the presence of other mycotoxins. Food Chem. Toxicol. 2016, 96, 79–89. [Google Scholar] [CrossRef]
- Dowo, P.F.; Miller, J.D.; Greenhalgh, R. Toxicity and interactions of some Fusarium graminearum metabolites to caterpillars. Mycologia 1989, 81, 646–650. [Google Scholar]
- Perincherry, L.; Lalak-Kańczugowska, J.; Stępień, Ł. Fusarium-produced mycotoxins in plant-pathogen interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef]
- Cipollone, M.J.; Moya, P.; Martínez, I.; Saparrat, M.; Sisterna, M. Grain discoloration in different genotypes of durum wheat (Triticum durum L.) in Argentina: Associated mycobiota and peroxidase activity. J. Plant Prot. Res. 2020, 60, 14–20. [Google Scholar]
- Masiello, M.; Somma, S.; Susca, A.; Ghionna, V.; Logrieco, A.F.; Franzoni, M.; Ravaglia, S.; Meca, G.; Moretti, A. Molecular identification and mycotoxin production by Alternaria species occurring on durum wheat, showing black point symptoms. Toxins 2020, 12, 275. [Google Scholar] [CrossRef] [PubMed]
- Escrivá, L.; Oueslati, S.; Font, G.; Manyes, L. Alternaria mycotoxins in food and feed: An overview. J. Food Quality 2017, 1569748. [Google Scholar] [CrossRef]
- Pero, R.W.; Posner, H.; Blois, M.; Harvan, D.; Spalding, J.W. Toxicity of metabolites produced by the “Alternaria”. Environ. Health Perspect. 1973, 4, 87–94. [Google Scholar] [CrossRef]
- Brugger, E.; Wagner, J.; Schumacher, D.M.; Koch, K.; Podlech, J.; Metzler, M.; Lehmann, L. Mutagenicity of the mycotoxin alternariol in cultured mammalian cells. Toxicol. Lett. 2006, 164, 221–230. [Google Scholar] [CrossRef]
- Hohenbichler, J.; Aichinger, G.; Rychlik, M.; Del Favero, G.; Marko, D. Alternaria alternata toxins synergistically activate the aryl hydrocarbon receptor pathway in vitro. Biomolecules 2020, 10, 1018. [Google Scholar] [CrossRef]
- Fleck, S.C.; Burkhardt, B.; Pfeiffer, E.; Metzler, M. Alternaria toxins: Altertoxin II is a much stronger mutagen and DNA strand breaking mycotoxin than alternariol and its methyl ether in cultured mammalian cells. Toxicol. Lett. 2012, 214, 27–32. [Google Scholar] [CrossRef]
- EFSA. Conclusion on the peer review of the pesticide risk assessment of the active substance tebuconazole. EFSA J. 2014, 12, 3485. [Google Scholar]
- European Green Deal. The 2022 Work Programme of the European Commission. 2022. Available online: https://www.csreurope.org (accessed on 3 January 2023).
- Wachowska, U.; Stuper-Szablewska, K.; Perkowski, J. Yeasts isolated from wheat grain can suppress Fusarium head blight and decrease trichothecene concentrations in bread wheat and durum wheat grain. Pol. J. Environ. Stud. 2020, 29, 4345–4360. [Google Scholar] [CrossRef]
- Schisler, D.A.; Slininger, P.J.; Boehm, M.J.; Paul, P.A. Co-culture of yeast antagonists of Fusarium head blight and their effect on disease development in wheat. Plant Path. J. 2011, 10, 128–137. [Google Scholar] [CrossRef]
- Chi, Z.; Wang, X.X.; Ma, Z.C.; Buzdar, M.A.; Chi, Z.M. The unique role of siderophore in marine-derived Aureobasidium pullulans HN6.2. Biometals 2012, 25, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, I.; Audenaert, K.; De Gelder, L. Biodegradation of Mycotoxins: Tales from Known and Unexplored Worlds. Front. Microbiol. 2016, 7, 561. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xie, M.; Wei, D. Biological detoxification of mycotoxins: Current status and future advances. Int. J. Mol. Sci. 2022, 23, 1064. [Google Scholar] [CrossRef]
- Papp, L.A.; Horváth, E.; Peles, F.; Pócsi, I.; Miklós, I. Insight into Yeast–Mycotoxin Relations. Agriculture 2021, 11, 1291. [Google Scholar] [CrossRef]
- Meier, U. Phenological Growth Stages. In Phenology: An Integrative Environmental Science. Tasks for Vegetation Science; Schwartz, M.D., Ed.; Springer: Dordrecht, The Netherlands, 2003; Volume 39, pp. 269–283. [Google Scholar]
- Wachowska, U.; Sulyok, M.; Wiwart, M.; Suchowilska, E.; Kandler, W.; Krska, R. The application of antagonistic yeasts and bacteria: An assessment of in vivo and under field conditions pattern of Fusarium mycotoxins in winter wheat grain. Food Control 2022, 138, 109039. [Google Scholar] [CrossRef]
- Sulyok, M.; Stadler, D.; Steiner, D.; Krska, R. Validation of an LC-MS/MS-based dilute-and-shoot approach for the quantification of >500 mycotoxins and other secondary metabolites in food crops: Challenges and solutions. Anal. Bioanal. Chem. 2020, 412, 2607–2620. [Google Scholar] [CrossRef]
- PP1/026(4); Foliar and Ear Diseases on Cereals. EPPO Standards: Paris, France, 2012; Volume 42, pp. 419–425.
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual, 1st ed.; Blackwell Publishing Ltd.: Oxford, UK, 2006; p. 388. [Google Scholar]
- Woudenberg, J.H.C.; Groenewald, J.Z.; Binder, M.; Crous, P.W. Alternaria redefined. Stud. Mycol. 2013, 75, 171–212. [Google Scholar] [CrossRef]
- Duba, A.; Goriewa-Duba, K.; Wachowska, U. Trichothecene genotypes analysis of Fusarium isolates from di-, tetra-and hexaploid wheat. Agronomy 2019, 9, 698. [Google Scholar] [CrossRef]
- TIBCO Software Inc. Statistica (Data Analysis Software System), Version 13. 2017. Available online: http://statistica.io (accessed on 16 January 2023).
- Lenc, L. Fusarium head blight (FHB) and Fusarium populations in grain of winter wheat grown in different cultivation systems. J. Plant Prot. Res. 2015, 55, 94–109. [Google Scholar] [CrossRef]
- Rachoń, L.; Bobryk-Mamczarz, A.; Szumiło, G. Mycotoxin contamination of grain of selected winter wheat genotypes. Pol. J. Agron. 2016, 25, 13–18. [Google Scholar]
- Landschoot, S.; Waegeman, W.; Audenaert, K.; Vandepitte, J.; Baetens, J.M.; De Baets, B.; Haesaert, G. An empirical analysis of explanatory variables affecting Fusarium head blight infection and deoxynivalenol content in wheat. J. Plant Pathol. 2012, 94, 135–147. [Google Scholar]
- Shah, L.; Ali, A.; Yahya, M.; Zhu, Y.; Wang, S.; Si, H.; Rahman, H.; Ma, C. Integrated control of fusarium head blight and deoxynivalenol mycotoxin in wheat. Plant Pathol. 2018, 67, 532–548. [Google Scholar] [CrossRef]
- Covarelli, L.; Beccari, G.; Prodi, A.; Generotti, S.; Etruschi, F.; Juan, C.; Ferrer, E.; Mañes, J. Fusarium species, chemotype characterisation and trichothecene contamination of durum and soft wheat in an area of central Italy. J. Sci. Food Agric. 2015, 95, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Lionetti, V.; Giancaspro, A.; Fabri, E.; Giove, S.L.; Reem, N.; Zabotina, O.A.; Blanco, A.; Gadaleta, A.; Bellincampi, D. Cell wall traits as potential resources to improve resistance of durum wheat against Fusarium graminearum. BMC Plant Biol. 2015, 15, 1–15. [Google Scholar] [CrossRef]
- Lemmens, M.; Scholz, U.; Berthiller, F.; Dall’Asta, C.; Koutnik, A.; Schuhmacher, R.; Adam, G.; Buerstmayr, H.; Mesterházy, A.; Krska, R.; et al. The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for Fusarium head blight resistance in wheat. Mol. Plant-Microbe Interact. 2005, 18, 1318–1324. [Google Scholar] [CrossRef]
- Uhlig, S.; Eriksen, G.; Hofgaard, I.; Krska, R.; Beltrán, E.; Sulyok, M. Faces of a changing climate: Semi-quantitative multi-mycotoxin analysis of grain grown in exceptional climatic conditions in Norway. Toxins 2013, 5, 1682–1697. [Google Scholar] [CrossRef]
- Spanic, V.; Katanic, Z.; Sulyok, M.; Krska, R.; Puskas, K.; Vida, G.; Drezner, G.; Šarkanj, B. Multiple fungal metabolites including mycotoxins in naturally infected and Fusarium-inoculated wheat samples. Microorganisms 2020, 8, 578. [Google Scholar] [CrossRef]
- McCormick, S.P.; Alexander, N.J.; Harris, L.J. CLM1 of Fusarium graminearum encodes a longiborneol synthase required for culmorin production. Appl. Environ. Microbiol. 2010, 76, 136–141. [Google Scholar] [CrossRef]
- Wipfler, R.; McCormick, S.P.; Proctor, R.; Teresi, J.; Hao, G.; Ward, T.; Alexander, N.; Vaughan, M.M. Synergistic phytotoxic effects of culmorin and trichothecene mycotoxins. Toxins 2019, 11, 555. [Google Scholar] [CrossRef]
- Woelflingseder, L.; Warth, B.; Vierheilig, I.; Schwartz-Zimmermann, H.; Hametner, C.; Nagl, V.; Marko, D.; Novak, B.; Berthiller, F.; Adam, G.; et al. The Fusarium metabolite culmorin suppresses the in vitro glucuronidation of deoxynivalenol. Arch. Toxicol 2019, 93, 1729–1743. [Google Scholar] [CrossRef]
- Solfrizzo, M.; Visconti, A.; Savard, M.E.; Blackwell, B.A.; Nelson, P.E. Isolation and characterization of new chlamydosporol related metabolites of Fusarium chlamydosporum and Fusarium tricinctum. Mycopathologia 1994, 127, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Ariantari, N.P.; Frank, M.; Gao, Y.; Stuhldreier, F.; Kiffe-Delf, A.L.; Hartmann, R.; Höfert, S.P.; Janiak, C.; Wesselborg, S.; Müller, W.E.; et al. Fusaristatins D–F and (7S,8R)-(−)-chlamydospordiol from Fusarium sp. BZCB-CA, an endophyte of Bothriospermum chinense. Tetrahedron 2021, 85, 132065. [Google Scholar] [CrossRef]
- Kato, Y.; Koshino, H.; Uzawa, J.; Anzai, K. Fungerin, a New Antifungal Alkaloid from Fusarium sp. Biosci. Biotech. Bioch. 1996, 60, 2081–2083. [Google Scholar] [CrossRef]
- Li, M.; Yu, R.; Bai, X.; Wang, H.; Zhang, H. Fusarium: A treasure trove of bioactive secondary metabolites. Nat. Prod. Rep. 2020, 37, 1568–1588. [Google Scholar] [CrossRef]
- Ramos-Diaz, J.M.; Sulyok, M.; Jacobsen, S.E.; Jouppila, K.; Nathanail, A.V. Comparative study of mycotoxin occurrence in Andean and cereal grains cultivated in South America and North Europe. Food Control 2021, 130, 108260. [Google Scholar] [CrossRef]
- Kemboi, D.C.; Ochieng, P.E.; Antonissen, G.; Croubels, S.; Scippo, M.-L.; Okoth, S.; Kangethe, E.K.; Faas, J.; Doupovec, B.; Lindahl, J.F.; et al. Multi-mycotoxin occurrence in dairy cattle and poultry feeds and feed ingredients from machakos Town, Kenya. Toxins 2020, 12, 762. [Google Scholar] [CrossRef]
- Subko, K.; Wang, X.; Nielsen, F.H.; Isbrandt, T.; Gotfredsen, C.H.; Ramos Maria, C.; Mackenzie, T.; Vicente, F.; Genilloud, O.; Frisvad, J.C.; et al. Mass spectrometry guided discovery and design of novel asperphenamate analogs from Penicillium astrolabium Reveals an Extraordinary NRPS Flexibility. Front. Microbiol. 2021, 11, 618730. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.M.; Hufford, C.D.; Robertson, L.W. Two metabolites from Aspergillus flavipes. Lloydia 1977, 40, 146–151. [Google Scholar]
- Hou, X.M.; Zhang, Y.H.; Hai, Y.; Zheng, J.Y.; Gu, Y.C.; Wang, C.Y.; Shao, C.L. Aspersymmetide A, a new centrosymmetric cyclohexapeptide from the marine-derived fungus Aspergillus versicolor. Mar. Drugs 2017, 15, 363. [Google Scholar] [CrossRef]
- Yuan, L.; Li, Y.; Zou, C.; Wang, C.; Gao, J.; Miao, C.; Ma, E.; Sun, T. Synthesis and in vitro antitumor activity of asperphenamate derivatives as autophagy inducer. Bioorg. Med. Chem. Lett. 2012, 22, 2216–2220. [Google Scholar] [CrossRef]
- Freimoser, F.M.; Rueda-Mejia, M.P.; Tilocca, B.; Migheli, Q. Biocontrol yeasts: Mechanisms and applications. World J. Microb. Biot. 2019, 35, 154. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.I.; Schisler, D.A.; Boehm, M.J.; Slininger, P.J.; Bothast, R.J. Selection and evaluation of microorganisms for biocontrol of fusarium head blight of wheat incited by Gibberella zeae. Plant Dis. 2001, 85, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Comby, M.; Gacoin, M.; Robineau, M.; Rabenoelina, F.; Ptas, S.; Dupont, J.; Profizi, C.; Baillieul, F. Screening of wheat endophytes as biological control agents against Fusarium head blight using two different in vitro tests. Microbiol. Res. 2017, 202, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Wachowska, U.; Głowacka, K. Antagonistic interactions between Aureobasidium pullulans and Fusarium culmorum, a fungal pathogen of winter wheat. BioControl 2014, 59, 635–645. [Google Scholar] [CrossRef]
- Rojas, E.C.; Jensen, B.; Jørgensen, H.J.; Latz, M.A.; Esteban, P.; Ding, Y.; Collinge, D.B. Selection of fungal endophytes with biocontrol potential against Fusarium head blight in wheat. Biol. Control 2020, 144, 104222. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 12: Suitability of taxonomic units notified to EFSA until March 2020. EFSA J. 2020, 18, e06174. [Google Scholar]
- European Food Safety Authority (EFSA). Scientific opinion on the risks for animal and public health related to the presence of Alternaria toxins in feed and food. EFSA J. 2011, 9, 1–97. [Google Scholar]
- Grzegorczyk, M.; Żarowska, B.; Restuccia, C.; Cirvilleri, G. Postharvest biocontrol ability of killer yeasts against Monilinia fructigena and Monilinia fructicola on stone fruit. Food Microbiol. 2017, 61, 93–101. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, L.; Johansen, P.G.; Petersen, M.A.; Arneborg, N.; Jespersen, L. Debaryomyces hansenii strains isolated from Danish cheese brines act as biocontrol agents to inhibit germination and growth of contaminating molds. Front. Microbiol. 2021, 12, 662785. [Google Scholar] [CrossRef]
- McClelland, M.; Al-Qaysi, S.A.S.; Al-Haideri, H.; Thabit, Z.A.; Al-Kubaisy, W.H.A.A.-R.; Ibrahim, J.A.A.-R. Production, Characterization, and Antimicrobial Activity of Mycocin Produced by Debaryomyces hansenii DSMZ70238. Int. J. Microbiol. 2017, 2605382. [Google Scholar] [CrossRef]
- Ahmad, A.; Aslam, Z.; Naz, M.; Hussain, S.; Javed, T.; Aslam, S.; Raza, A.; Ali, H.M.; Siddiqui, M.H.; Salem, M.Z.M.; et al. Exogenous salicylic acid-induced drought stress tolerance in wheat (Triticum aestivum L.) grown under hydroponic culture. PLoS ONE 2021, 16, e0260556. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).