Evidence of Resistance to QoI Fungicides in Contemporary Populations of Mycosphaerella fijiensis, M. musicola and M. thailandica from Banana Plantations in Southeastern Brazil
Abstract
:1. Introduction
2. Materials and Methods
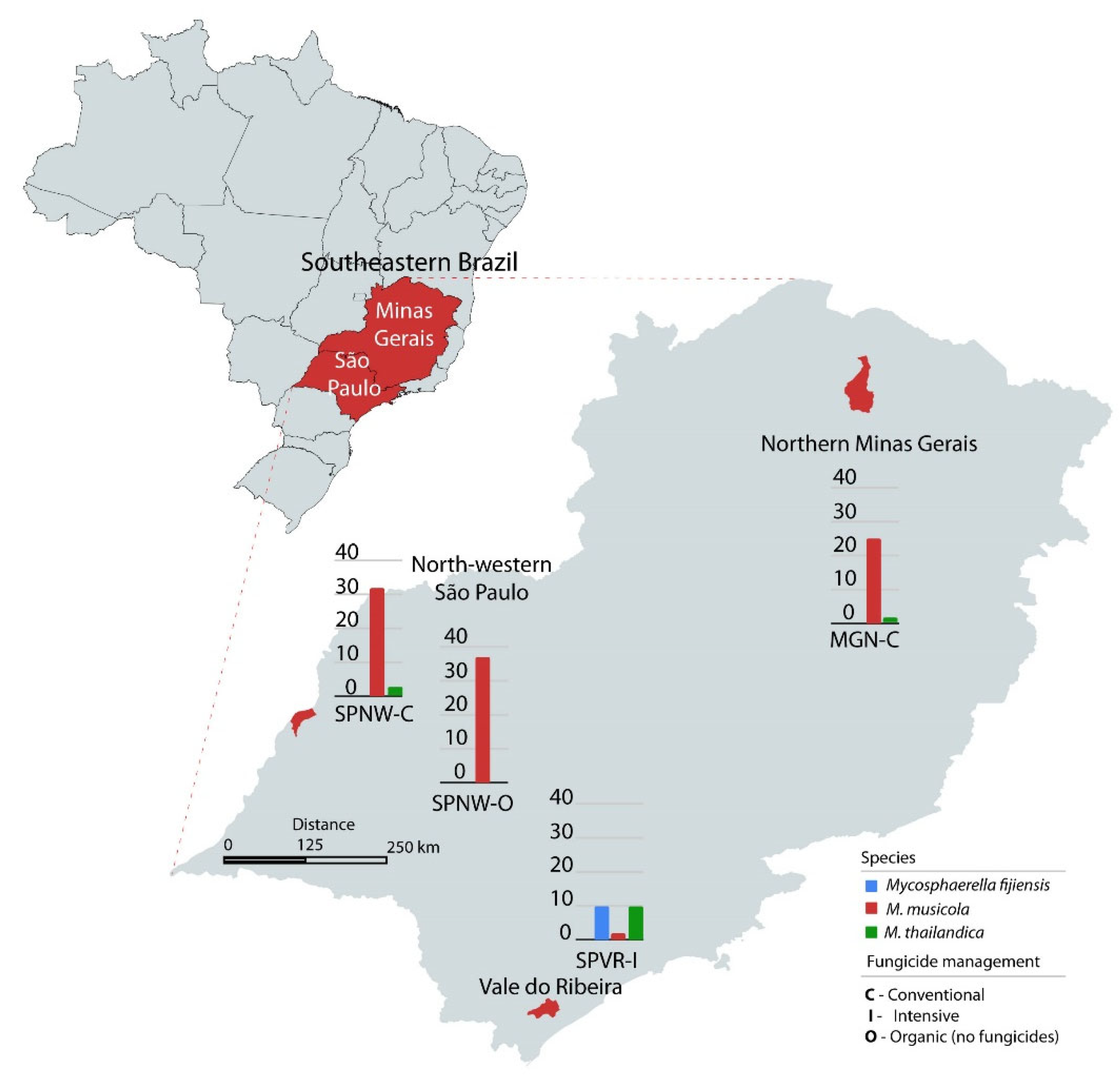
2.1. Yellow and Black Sigatoka Pathogens’ Population Sampling
2.2. Mycosphaerella Isolation
2.3. Molecular Identification of the Pathogens
2.4. QoI Qualitative Fungicide Sensitivity Assays at Discriminatory Dose
2.5. QoI Quantitative Fungicide Sensitivity Assays Based on EC50 Values
- RRMycosphaerella = T0 − T24, where:
- RRMycosphaerella = relative reduction of resazurin;
- T = Absorbance reading at 569 ηm;
- T0 = reading at time zero (immediately after adding resazurin to the fungal 10-day-old liquid culture in buffered PD broth);
- T24 = reading at time 24 (24 h after adding resazurin).
2.6. Analysis of Allelic Variation in Target Gene cyt b in Mycosphaerella Populations
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arzanlou, M.; Groenewald, J.Z.; Fullerton, R.A.; Abeln, E.C.A.; Carlier, J.; Zapater, M.-F.; Buddenhagen, I.W.; Viljoen, A.; Crous, P.W. Multiple Gene Genealogies and Phenotypic Characters Differentiate Several Novel Species of Mycosphaerella and Related Anamorphs on Banana. Persoonia Mol. Phylogeny Evol. Fungi 2008, 20, 19–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malimpensa, J.R. Caracterização da Resistência a Fungicidas Em Populações de Fungos Associados a Lesões de Sigatokas em Bananais do Vale do Ribeira (SP). Master’s Thesis, Instituto Biológico, São Paulo, Brazil, 2018. [Google Scholar]
- Brito, F.S.D.; Fraaije, B.; Miller, R.N.G. Sigatoka Disease Complex of Banana in Brazil: Management Practices and Future Directions. Outlooks Pest Manag. 2015, 26, 78–81. [Google Scholar] [CrossRef]
- Brito, F.S.D.; Santos, J.R.P.; Azevedo, V.C.R.; Peixouto, Y.S.; de Oliveira, S.A.; Ferreira, C.F.; Haddad, F.; Amorim, E.P.; Fraaije, B.; Miller, R.N.G. Genetic Diversity and Azole Fungicide Sensitivity in Pseudocercospora Musae Field Populations in Brazil. Front. Microbiol. 2020, 11, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, E.S.; da Moraes, W.S.; Kobori, R.T.; Penteado, L.A.; de Doenças, C. Cultivo da Bananeira; Manual Técnico; Coordenadoria de Assistência Técnica Integral (CATI): Campinas, Brazil, 2020; pp. 107–134. ISBN CDD.634.722. [Google Scholar]
- Rocha, F.S.; Catão, H.C.R.M.; Muniz, M.F.S. Aspectos Diagnósticos Entre Mycosphaerella spp. da Bananeira, Distribuição e Manejo No Brasil. Enciclopédia Biosf. 2012, 8, 64–84. [Google Scholar]
- Moraes, W.S.; Mendonça, J.C.; Fukuda, E.; Mendes, C.; Lima, J.D.; Santos, A.J. Dominância da Sigatoka Negra em Bananais do Vale do Ribeira. Fitopatol. Bras. 2005, 30, 193. [Google Scholar]
- Crous, P.W.; Groenewald, J.Z.; Pongpanich, K.; Himaman, W.; Arzanlou, M.; Wingfield, M.J. Cryptic Speciation and Host Specificity among Mycosphaerella spp. Occurring on Australian Acacia Species Grown as Exotics in the Tropics. Stud. Mycol. 2004, 50, 457–469. [Google Scholar]
- Burt, P.J.A. Windborne Dispersal of Sigatoka Leaf Spot Pathogens. Grana 1994, 33, 108–111. [Google Scholar] [CrossRef]
- Beltrán-García, M.J.; Prado, F.M.; Oliveira, M.S.; Ortiz-Mendoza, D.; Scalfo, A.C.; Pessoa, A., Jr.; Medeiros, M.H.G.; White, J.F.; Di Mascio, P. Singlet Molecular Oxygen Generation by Light-Activated DHN-Melanin of the Fungal Pathogen Mycosphaerella fijiensis in Black Sigatoka Disease of Bananas. PLoS ONE 2014, 9, e91616. [Google Scholar] [CrossRef] [Green Version]
- Gomes, L.I.S.; Douhan, G.W.; Lehner, M.S.; Bibiano, L.B.J.; Mizubuti, E.S.G. Yellow Sigatoka Epidemics Caused by a Panmictic Population of Mycosphaerella Musicola in Brazil. Plant Pathol. 2018, 67, 295–302. [Google Scholar] [CrossRef]
- Manzo-Sánchez, G.; Orozco-Santos, M.; Islas-Flores, I.; Martínez-Bolaños, L.; Guzmán-González, S.; Leopardi-Verde, C.L.; Canto-Canché, B. Genetic Variability of Pseudocercospora fijiensis, the Black Sigatoka Pathogen of Banana (Musa spp.) in Mexico. Plant Pathol. 2019, 68, 513–522. [Google Scholar] [CrossRef]
- Mendoza, M.J.; Ardales, E. Population Structure of the Banana Black Sigatoka Pathogen [Pseudocercospora fijiensis (M. Morelet) Deighton] in Luzon, Philippines. Philipp. Agric. Sci. 2019, 102, 211–219. [Google Scholar]
- Cañas-Gutiérrez, G.P.; Angarita-Velásquez, M.J.; Restrepo-Flórez, J.M.; Rodríguez, P.; Moreno, C.X.; Arango, R. Analysis of the CYP51 Gene and Encoded Protein in Propiconazole-Resistant Isolates of Mycosphaerella fijiensis: Characterisation of the CYP51 Gene in Field Isolates of M. Fijiensis. Pest Manag. Sci. 2009, 65, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Grice, K.; Vawdrey, L.B.; Stammler, G.C.B. Yellow Sigatoka (Mycosphaerella musicola) Populations Develop Resistance to QoI Fungicides in Australia. In Modern Fungicides and Antifungal Compounds; Dehne, H.W., Deising, H.B., Fraaije, B., Eds.; Deutsche Phytomedizinische Gesellschaft: Braunschweig, Germany, 2013; Volume 7, pp. 269–270. [Google Scholar]
- MAPA. Ministério da Agricultura Pecuária e Abastecimento—Brazil Agrofit—Sistemas de Agrotóxicos Fitossanitários, Coordenação Geral de Agrotóxicos e Afins. Available online: http://agrofit.agricultura.gov.br/agrofit_cons/principal_agrofit_cons. (accessed on 5 March 2021).
- Gasparotto, L.; Pereira, J.C.R.; Pereira, M.C.N.; Costa, M.M. Controle Químico da Sigatoka Negra da Bananeira. I—Trifloxistrobin, Propiconazole e Difenoconazole. Comun. Téc. Embrapa 2000, 7, 1. [Google Scholar]
- Rangel, A.; Penteado, L.A.C.; Tonet, R.M. Cultura da Banana. In Boletim Técnico, 2nd ed.; Coordenadoria de Assistência Técnica Integral (CATI): Campinas, Brazil, 2002. [Google Scholar]
- Moraes, W.S.; Nomura, E.S. Monitoramento da Sigatoka e Controle Químico. In Cultivo da Bananeira; Manual Técnico; Coordenadoria de Assistência Técnica Integral (CATI): Campinas, Brazil, 2020; pp. 135–144. ISBN CDD.634.722. [Google Scholar]
- Moraes, W.S.; Lima, J.D.; Santos, A.J. Técnica de Avaliação da Eficiência de Fungicidas Protetor e Sistêmico para Controle da Sigatoka Negra em Bananeira. Pesqui. Tecnol. 2016, 13, 1–7. [Google Scholar]
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hall, A.A.; Hamer, M.; Parr-Dobrzanski, B. The Strobilurin Fungicides. Pest Manag. Sci. 2002, 58, 649–662. [Google Scholar] [CrossRef]
- Nofiani, R.; de Mattos-Shipley, K.; Lebe, K.E.; Han, L.-C.; Iqbal, Z.; Bailey, A.M.; Willis, C.L.; Simpson, T.J.; Cox, R.J. Strobilurin Biosynthesis in Basidiomycete Fungi. Nat. Commun. 2018, 9, 3940. [Google Scholar] [CrossRef]
- Fernández-Ortuño, D.; Torés, J.A.; de Vicente, A.; Pérez-García, A. Mechanisms of Resistance to QoI Fungicides in Phytopathogenic Fungi. Int. Microbiol. Off. J. Span. Soc. Microbiol. 2008, 11, 1–9. [Google Scholar]
- FRAC. Fungicide Resistance Action Committee Quinone ‘Outside’ Inhibitor (QoI) Working Group Meeting. Available online: https://www.frac.info/frac-teams/working-groups/qol-fungicides/recommendations-for-qoi (accessed on 4 September 2022).
- Sierotzki, H.; Parisi, S.; Steinfeld, U.; Tenzer, I.; Poirey, S.; Gisi, U. Mode of Resistance to Respiration Inhibitors at the Cytochrome Bc1 Enzyme Complex of Mycosphaerella fijiensis Field Isolates. Pest Manag. Sci. 2000, 56, 833–841. [Google Scholar] [CrossRef]
- FRAC. Fungicide Resistance Action Committee Banana WG Meeting—FRAC Recommendations for Bananas. Available online: https://www.frac.info/frac-teams/working-groups/banana-group/recommendations-for-bananas (accessed on 4 September 2022).
- Amil, A.F.; Heaney, S.P.; Stanger, C.; Shaw, M.W. Dynamics of QoI Sensitivity in Mycosphaerella fijiensis in Costa Rica during 2000 to 2003. Phytopathology 2007, 97, 1451–1457. [Google Scholar] [CrossRef] [Green Version]
- Garcia, S.A.L. Identification Validation and Use of EST-Derived Molecular Markers from the Genomes of Mycosphaerella fijiensis and Musa spp. Master’s Thesis, Federal University of Lavras (UFLA), Lavras, Minas Gerais, Brazil, 2009. [Google Scholar]
- Chin, K.M.; Wirz, M.; Laird, D. Sensitivity of Mycosphaerella fijiensis from Banana to Trifloxystrobin. Plant Dis. 2001, 85, 1264–1270. [Google Scholar] [CrossRef] [Green Version]
- Alfaro-Alvarado, F. Estudio Genético de la Resistencia a las Estrobilurinas en Aislamientos de Pseudocercospora fijiensis de Plantaciones de Banano en Costa Rica. Master’s Thesis, Universidad de Costa Rica, San José, Costa Rica, 2019. [Google Scholar]
- Gisi, U.; Sierotzki, H.; Cook, A.; McCaffery, A. Mechanisms Influencing the Evolution of Resistance to Qo Inhibitor Fungicides. Pest Manag. Sci. 2002, 58, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Ghini, R.; Hamada, E.; Gonçalves, R.R.V.; Gasparotto, L.; Pereira, J.C.R. Análise de Risco das Mudanças Climáticas Globais Sobre a Sigatoka-Negra da Bananeira No Brasil. Fitopatol. Bras. 2007, 32, 197–204. [Google Scholar] [CrossRef]
- Bendini, H.N.; da Moraes, W.S.; da Silva, S.H.M.G.; Tezuka, E.S.; Cruvinel, P.E. Análise de Risco da Ocorrência de SigatokaNegra Baseada Em Modelos Polinomiais: Um Estudo de Caso. Trop. Plant Pathol. 2013, 38, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Bendini, H.d.N. Processamento Digital de Imagens para Inferência de Risco de Doença Fúngica da Bananicultura. Master’s Thesis, Federal University of São Carlos (UFScar), São Carlos, Brazil, 2012. [Google Scholar]
- Arzanlou, M.; Abeln, E.C.A.; Kema, G.H.J.; Waalwijk, C.; Carlier, J.; de Vries, I.; Guzmán, M.; Crous, P.W. Molecular Diagnostics for the Sigatoka Disease Complex of Banana. Phytopathology 2007, 97, 1112–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols; Elsevier: Amsterdam, The Netherlands, 1990; pp. 315–322. ISBN 978-0-12-372180-8. [Google Scholar]
- Vicentini, S.N.; Casado, P.S.; de Carvalho, G.; Moreira, S.I.; Dorigan, A.F.; Silva, T.C.; Silva, A.G.; Custódio, A.A.; Gomes, A.C.S.; Nunes Maciel, J.L. Monitoring of Brazilian Wheat Blast Field Populations Reveals Resistance to QoI, DMI, and SDHI Fungicides. Plant Pathol. 2022, 71, 304–321. [Google Scholar] [CrossRef]
- Statistical Tools For High-Throughput Data Analysis (STHDA) Two Proportions Z-Test in R. Available online: https://sthda.com/english/wiki/two-proportions-z-test-in-r (accessed on 15 March 2022).
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Viena, Austria, 2022. [Google Scholar]
- Barua, P.; You, M.P.; Bayliss, K.; Lanoiselet, V.; Barbetti, M.J. A Rapid and Miniaturized System Using Alamar Blue to Assess Fungal Spore Viability: Implications for Biosecurity. Eur. J. Plant Pathol. 2017, 148, 139–150. [Google Scholar] [CrossRef]
- Cox, K.D.; Quello, K.; Deford, R.J.; Beckerman, J.L. A Rapid Method to Quantify Fungicide Sensitivity in the Brown Rot Pathogen Monilinia Fructicola. Plant Dis. 2009, 93, 328–331. [Google Scholar] [CrossRef]
- Vega, B.; Liberti, D.; Harmon, P.F.; Dewdney, M.M. A Rapid Resazurin-Based Microtiter Assay to Evaluate QoI Sensitivity for Alternaria Alternata Isolates and Their Molecular Characterization. Plant Dis. 2012, 96, 1262–1270. [Google Scholar] [CrossRef] [Green Version]
- Fraaije, B.A.; Bayon, C.; Atkins, S.; Cools, H.J.; Lucas, J.A.; Fraaije, M.W. Risk Assessment Studies on Succinate Dehydrogenase Inhibitors, the New Weapons in the Battle to Control Septoria Leaf Blotch in Wheat: SDHI Fungicides and Septoria Leaf Blotch Control. Mol. Plant Pathol. 2012, 13, 263–275. [Google Scholar] [CrossRef] [Green Version]
- Montoya, J.E.P.; David, L.E.V.; Brito, T.J.D.; Sánchez, D.A.C.; Beltrán, E.R.; Isaza, R.E.A. Utilización de Un Ensayo de Dilución en Microplatos para Medir La Actividad Antifúngica de Sustancias Contra Mycosphaerella fijiensis Morelet. Rev. Fac. Nac. Agron. Medellín 2006, 59, 3425–3433. [Google Scholar]
- Ma, Z.; Proffer, T.J.; Jacobs, J.L.; Sundin, G.W. Overexpression of the 14α-Demethylase Target Gene (CYP51) Mediates Fungicide Resistance in Blumeriella jaapii. Appl. Environ. Microbiol. 2006, 72, 2581–2585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knezevic, S.Z.; Streibig, J.C.; Ritz, C. Utilizing R Software Package for Dose-Response Studies: The Concept and Data Analysis. Weed Technol. 2007, 21, 840–848. [Google Scholar] [CrossRef]
- Mair, W.; Lopez-Ruiz, F.; Stammler, G.; Clark, W.; Burnett, F.; Hollomon, D.; Ishii, H.; Thind, T.S.; Brown, J.K.; Fraaije, B.; et al. Proposal for a Unified Nomenclature for Target-site Mutations Associated with Resistance to Fungicides. Pest Manag. Sci. 2016, 72, 1449–1459. [Google Scholar] [CrossRef] [PubMed]
- Standish, J.R.; Brenneman, T.B.; Bock, C.H.; Stevenson, K.L. Fungicide Resistance in Venturia Effusa, Cause of Pecan Scab: Current Status and Practical Implications. Phytopathology 2021, 111, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.I.S.; Bibiano, L.B.J.; da Silva, G.F.; Hanada, R.E.; Mizubuti, E.S.G. Baseline Sensitivity of Brazilian Mycosphaerella fijiensis Isolates to Protectant and Systemic Fungicides. Trop. Plant Pathol. 2014, 39, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Vicentini, S.N.C.; Moreira, S.I.; da Silva, A.G.; de Oliveira, T.Y.K.; Silva, T.C.; Assis Junior, F.G.; Krug, L.D.; de Paiva Custódio, A.A.; Leite Júnior, R.P.; Teodoro, P.E.; et al. Efflux Pumps and Multidrug-Resistance in Pyricularia oryzae Triticum Lineage. Agronomy 2022, 12, 2068. [Google Scholar] [CrossRef]
- Omrane, S.; Sghyer, H.; Audéon, C.; Lanen, C.; Duplaix, C.; Walker, A.; Fillinger, S. Fungicide Efflux and the MgMFS 1 Transporter Contribute to the Multidrug Resistance Phenotype in Z Ymoseptoria Tritici Field Isolates. Environ. Microbiol. 2015, 17, 2805–2823. [Google Scholar] [CrossRef]
- Hawkins, N.J.; Fraaije, B.A. Fitness Penalties in the Evolution of Fungicide Resistance. Annu. Rev. Phytopathol. 2018, 56, 339–360. [Google Scholar] [CrossRef]
- West, J.S.; Kimber, R.B.E. Innovations in Air Sampling to Detect Plant Pathogens. Ann. Appl. Biol. 2015, 166, 4–17. [Google Scholar] [CrossRef] [Green Version]
- West, J.S.; Canning, G.G.M.; Perryman, S.A.; King, K. Novel Technologies for the Detection of Fusarium Head Blight Disease and Airborne Inoculum. Trop. Plant Pathol. 2017, 42, 203–209. [Google Scholar] [CrossRef] [Green Version]
- West, J.S.; Atkins, S.D.; Emberlin, J.; Fitt, B.D.L. PCR to Predict Risk of Airborne Disease. Trends Microbiol. 2008, 16, 380–387. [Google Scholar] [CrossRef] [PubMed]
- King, K.; Kirikyali, N.; West, J.; Fraaije, B. Rapid LAMP Assays to Detect MgCYP51 and/or MgMFS1 Overexpressing Strains of Zymoseptoria Tritici in Leaf Samples. Mod. Fungic. Antifung. Compd. 2017, 8, 67–72. [Google Scholar]
- Vicentini, S.N.C.; Krug, L.D.; Ceresini, P.C.; Custódio, A.A.P.; Leite, R.P., Jr.; West, J.S.; Fraaije, B.A. Can Fungal Aerobiology Tools Help Tracking Fungicide Resistance Alleles and Block the Spread of Fungicide Resistance in the Agroecosystem? In Proceedings of the VI EpidemioBrasil: Brazilian Workshop of Plant Disease Epidemiology; Canale, M.C., Nesi, C.N., Bergamim Filho, A., Del Ponte, E., Amorim, L., May De Mio, L.L., Laranjeira, F., Eds.; Epagri: Chapecó, Brazil, 2022; pp. 25–26, ISBN 978-65-998614-0-6. [Google Scholar]





| a Target gene | Primer | Sequence (5′-3′) | Amplicon Size (bp) | Annealing Temperature (°C) |
|---|---|---|---|---|
| b Mf cyt b | cytb_exon1-1_Mf_F2036 | CGTCGCCGTAATGTGGTTC | 475 | 56 |
| cytb_exon1-1_Mf_R2511 | GCCGCAACCTTCTAATATTAG | |||
| cytb_exon2_Mf_F25 | CGTGCTTCTGATTCTATTAGGGG | 996 | 56 | |
| cytb_exon2_Mf_R1020 | GGCGACTACCAACACAAAT | |||
| b Mm cyt b | cytb_Mm_F1251 | GTTACCTTTGAAACTTCGGATC | 963 | 58.5 |
| cytb_Mm_R2213 | GACTCAACGTGTTTAGCCC | |||
| c Mt cyt b | cytb_Mt_F2 | GAAGCATTTAATTCAGTAGAAC | 400 | 58 |
| cytb_Mt_R2 | CAACTATATCTTGTCCTACTC |
| Species, Type of Management with Fungicides and Population | Average Number of Fungicide Sprays per Crop | Number of Isolates with Growth In Culture Medium Supplemented with 0 or 10 μg·mL−1 of the QoI Fungicides Azoxystrobin or Trifloxystrobin | ||
|---|---|---|---|---|
| μg·mL−1 | Resistant Isolates (Proportion) | |||
| 0 | 10 | |||
| Mycosphaerella musicola | ||||
| Organic field | ||||
| SPNW-O | 0 | 37 | 0 | 0.0000 |
| Conventional fields | ||||
| SPNW-C | 4 | 32 | 6 | 0.1875 |
| MGN-C | 5 | 25 | 1 | 0.0400 |
| Intensive field | ||||
| SPVR-I | >10 | 2 | 2 | 1.0000 |
| M. fijiensis | ||||
| Intensive field | ||||
| SPVR-I | >10 | 10 | 1 | 0.1000 |
| M. thailandica | ||||
| Conventional fields | ||||
| SPNW-C | 4 | 2 | 1 | 0.5000 |
| MGN-C | 5 | 1 | 1 | 1.0000 |
| Intensive field | ||||
| SPVR-I | >10 | 10 | 10 | 1.0000 |
| Total | 119 | 22 | 0.1849 | |
| Pearson’s Chi-Square Values below the Diagonal and p Values above the Diagonal | ||||
|---|---|---|---|---|
| Comparisons | SPNW-O | SPNW-C | MGN-C | SPVR-I |
| SPNW-O | - | 0.012 | 0.325 | 0.000 |
| SPNW-C | 6.29 | - | 0.307 | 0.008 |
| MGN-C | 0.97 | 1.04 | - | 0.000 |
| SPVR-I | 24.71 | 7.03 | 12.34 | - |
| Gene | Reference Sequence (GenBank NCBI) | Length of Amplicon Sequence (bp) | a Synonymous Mutations: Substitutions (s); Deletions (d); Insertions (i)/Position a | Haplo- types detected | Species | Isolates | N | b QoI resistance class | Coding region (bp) | Protein length (aa) | Non-Synonymous Mutations Detected /Position | Non- Synonymous a.a. Change/ Position | a.a Change |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mm cyt b | OP715652, OP715653, and OP715654 | 963 (from 1251 to 2213) | 0 | Hm1 | Mm | cMm populations | 87 | S | 936 | 312 | 0 | 0 | |
| OP715650, OP715651, and OP715649 | 0 | Hm2 | Mm | d ISC9, ISC55a, ISC64, ISC110, ISC116, ISC117, MG118 e JA1.37, e JA1.38c | 9 | R | f CCT:CGT/1599 | f G:A/143 | Mm: Grice et al. [15] | ||||
| NC037198.1 | 1173 (from 1172 to 2344) | 20 s: A:T/1267; T:A/1312; A:T/1324/T:A/1432; C:G/1450; T:A/1549; A:T/1675;T:A/1816; G:C/1837; A:T/1925; A:T/1957; T:A/1972; C:G/1975; G:C/2018; C:G/2092; T;A/2107; T:A/2156; A:T/2167; A:T/2173; C:G/2194 | Hm3 | P. mori | - | 1 | - | 1170 | 390 | 0 | 0 | ||
| LFZO01000638.1 | 0 | Hm1 | Mm | CBS116634 | 1 | - | 1173 | 391 | 0 | 0 |
| Gene | Reference Sequence (GenBank NCBI) | Length of Amplicon Sequence (bp) | a Synonymous Mutations: Substitutions (s); Deletions (d); Insertions (i)/Position a | Haplo- Types Detected | Species | Isolates | N | b QoI Resistance Class | Coding Region (bp) | Protein Length (aa) | Non-Synonymous Mutations Detected/Position | Non- Synonymous a.a. Change/ Position | Previous Report for a.a. Change |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mf cyt b exon-1 | OP734340 toOP734348 | 475 (2511-2036) | 9 i: GA(TA)3/2058 | Hf1-1 | Mf | c SPVR population | 9 | S | 475 | 151 | 0 | 0 | - |
| OP734339 | 0 | Hf1-2 | Mf | c JA2.24 | 1 | R | CCT:CGT/ 1599 | G:A/143 | Mf: Sierotzki et al. [25] | ||||
| NC044132 | 507 (from 2056 to 2562) | 2 d: 2525 to 2521. 4 s: A:T/2521; C:G/2515; A:T/2512; C:G/2509. 8 i: GA(TA)3/2058; 2 i: (TA)/2521,2522 | Hf1-3 | Mf | - | 1 | - | 507 | 169 | 0 | 0 | - | |
| AF343069 | 459 (2525-2565 | 0 | Hf1-2 | Mf | 184.97.1 | 1 | R | 459 (−2 gaps) | 153 | d CCT:CGT/ 1599 | d G:A/143 | Mf: Sierotzki et al. [25] | |
| AF343070 | 0 | Hf1-4 | Mf | 185.97.3 | 1 | S | 459 (−2 gaps) | 153 | 0 | 0 | - | ||
| Mf cyt b exon-2 | OP734349 toOP734358 | 996 (from 25 to 1020) | 0 | Hf2-1 | Mf | c SPVR population | 10 | S | 657 | 219 | 0 | 0 | - |
| … | |||||||||||||
| NC044132 | 0 | Hf2-2 | Mf | - | 1 | - | 0 | 0 | - | ||||
| AF343069 | 3 s: A:T/431; G:N/481; A:G/808 | Hf2-3 | Mf | 184.97.1 | 1 | R | 0 | 0 | - | ||||
| AF343070 | 3 s: A:T/431; G:N/481; A:G/808 | Hf2-4 | Mf | 185.97.3 | 1 | S | AAA:CAA/788 | L:F/68 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, T.Y.K.; Silva, T.C.; Moreira, S.I.; Christiano, F.S., Jr.; Gasparoto, M.C.G.; Fraaije, B.A.; Ceresini, P.C. Evidence of Resistance to QoI Fungicides in Contemporary Populations of Mycosphaerella fijiensis, M. musicola and M. thailandica from Banana Plantations in Southeastern Brazil. Agronomy 2022, 12, 2952. https://doi.org/10.3390/agronomy12122952
Oliveira TYK, Silva TC, Moreira SI, Christiano FS Jr., Gasparoto MCG, Fraaije BA, Ceresini PC. Evidence of Resistance to QoI Fungicides in Contemporary Populations of Mycosphaerella fijiensis, M. musicola and M. thailandica from Banana Plantations in Southeastern Brazil. Agronomy. 2022; 12(12):2952. https://doi.org/10.3390/agronomy12122952
Chicago/Turabian StyleOliveira, Tamiris Y. K., Tatiane C. Silva, Silvino I. Moreira, Felix S. Christiano, Jr., Maria C. G. Gasparoto, Bart A. Fraaije, and Paulo C. Ceresini. 2022. "Evidence of Resistance to QoI Fungicides in Contemporary Populations of Mycosphaerella fijiensis, M. musicola and M. thailandica from Banana Plantations in Southeastern Brazil" Agronomy 12, no. 12: 2952. https://doi.org/10.3390/agronomy12122952
APA StyleOliveira, T. Y. K., Silva, T. C., Moreira, S. I., Christiano, F. S., Jr., Gasparoto, M. C. G., Fraaije, B. A., & Ceresini, P. C. (2022). Evidence of Resistance to QoI Fungicides in Contemporary Populations of Mycosphaerella fijiensis, M. musicola and M. thailandica from Banana Plantations in Southeastern Brazil. Agronomy, 12(12), 2952. https://doi.org/10.3390/agronomy12122952








