Tissue-Specific Recovery Capability of Aroma Biosynthesis in ‘Golden Delicious’ Apple Fruit after Low Oxygen Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Apple Fruit Handling and Treatment
2.2. Determination of Firmness and Soluble Solid Content
2.3. Determination of Ethylene Production and Respiration Rates
2.4. Volatile Compound Analysis
2.5. Gene Expression Analysis
2.6. Statistical Analysis
3. Results
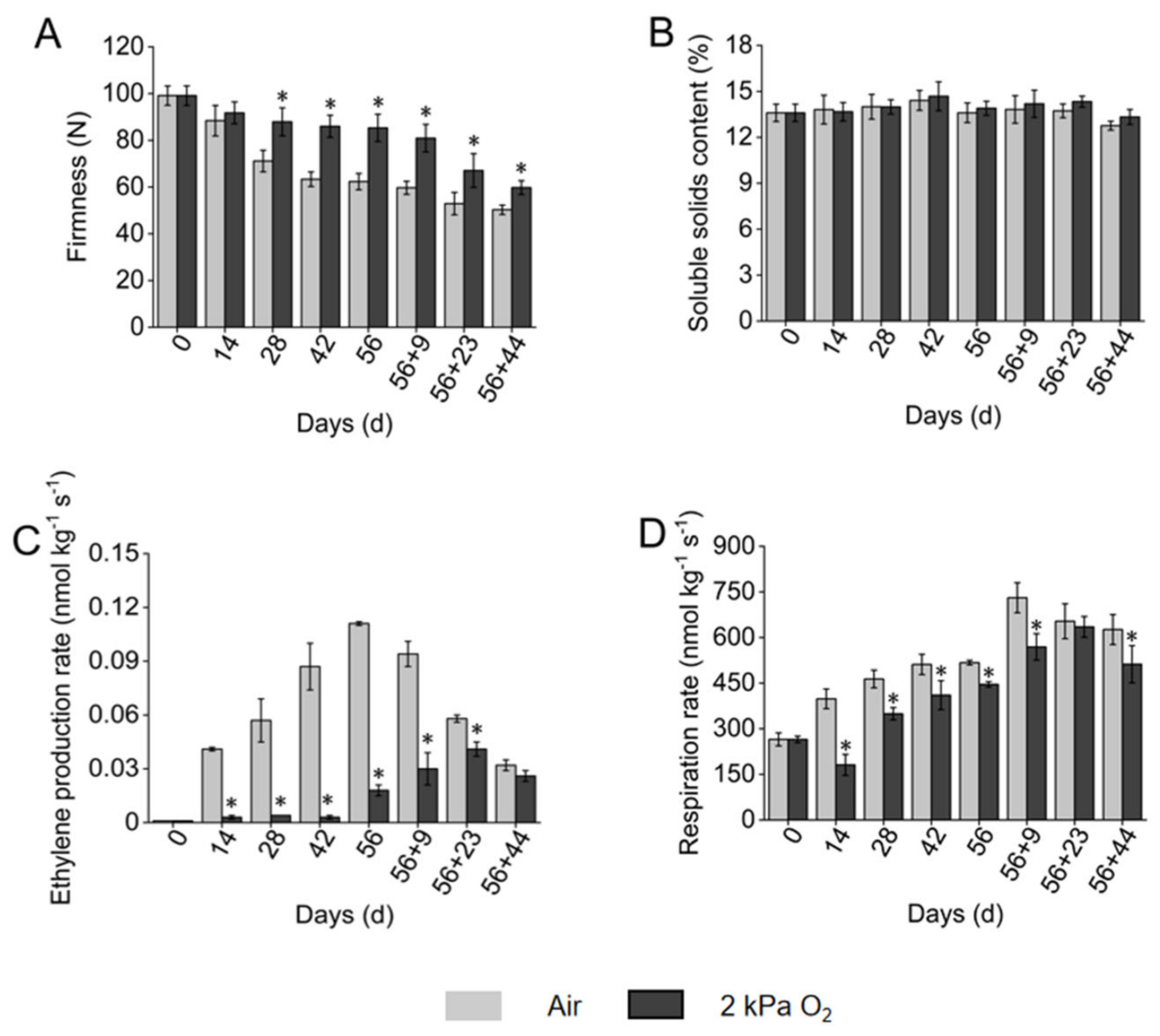
3.1. Firmness, Ethylene Production, Respiration Rate, and SSC
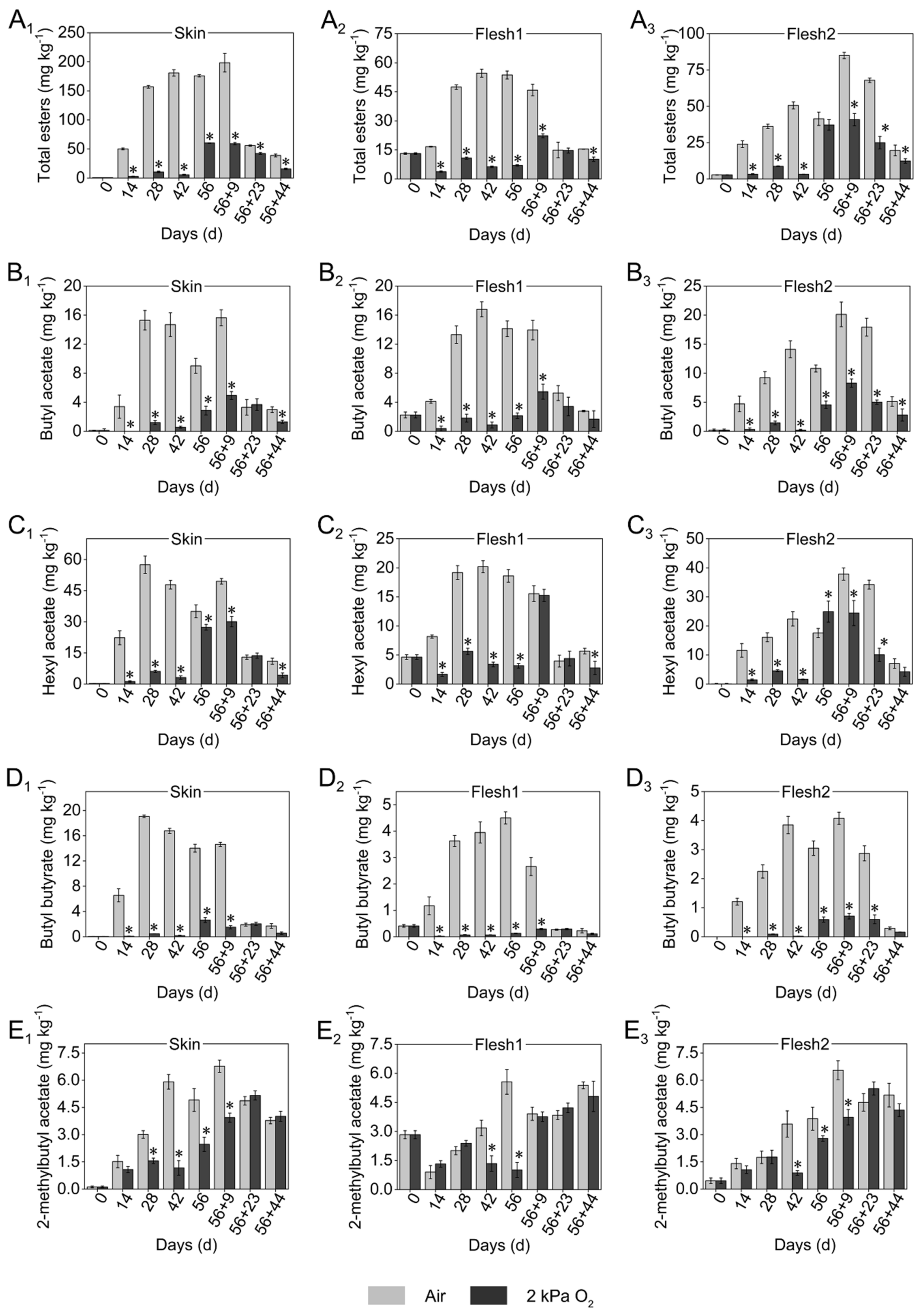
3.2. Emission of Key Esters
3.3. Emission of Key Alcohols and Aldehydes
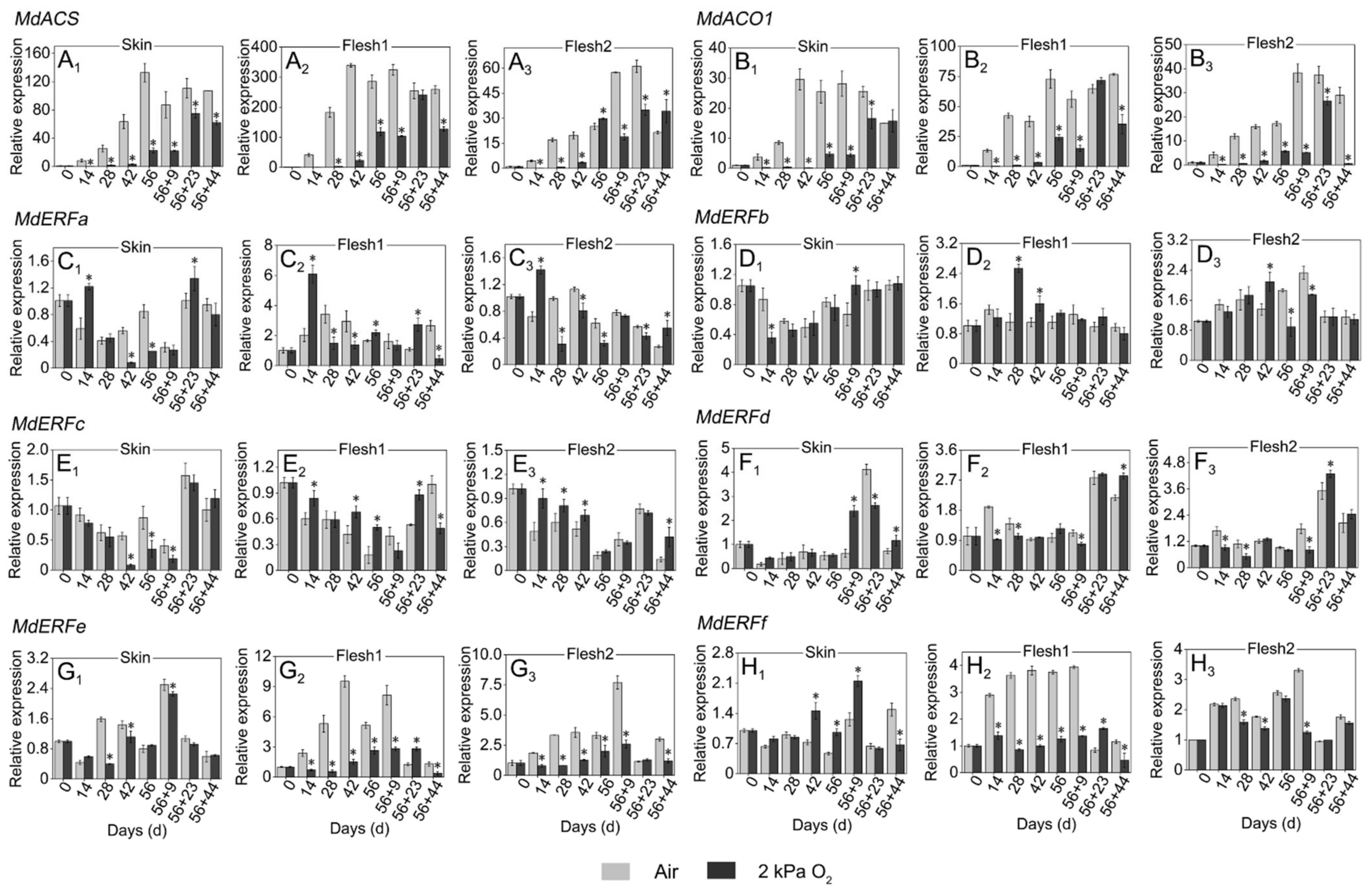
3.4. Expressions of Genes Involved in Fatty Acid Metabolism
3.5. Expressions of Genes Involved in Ethylene Production
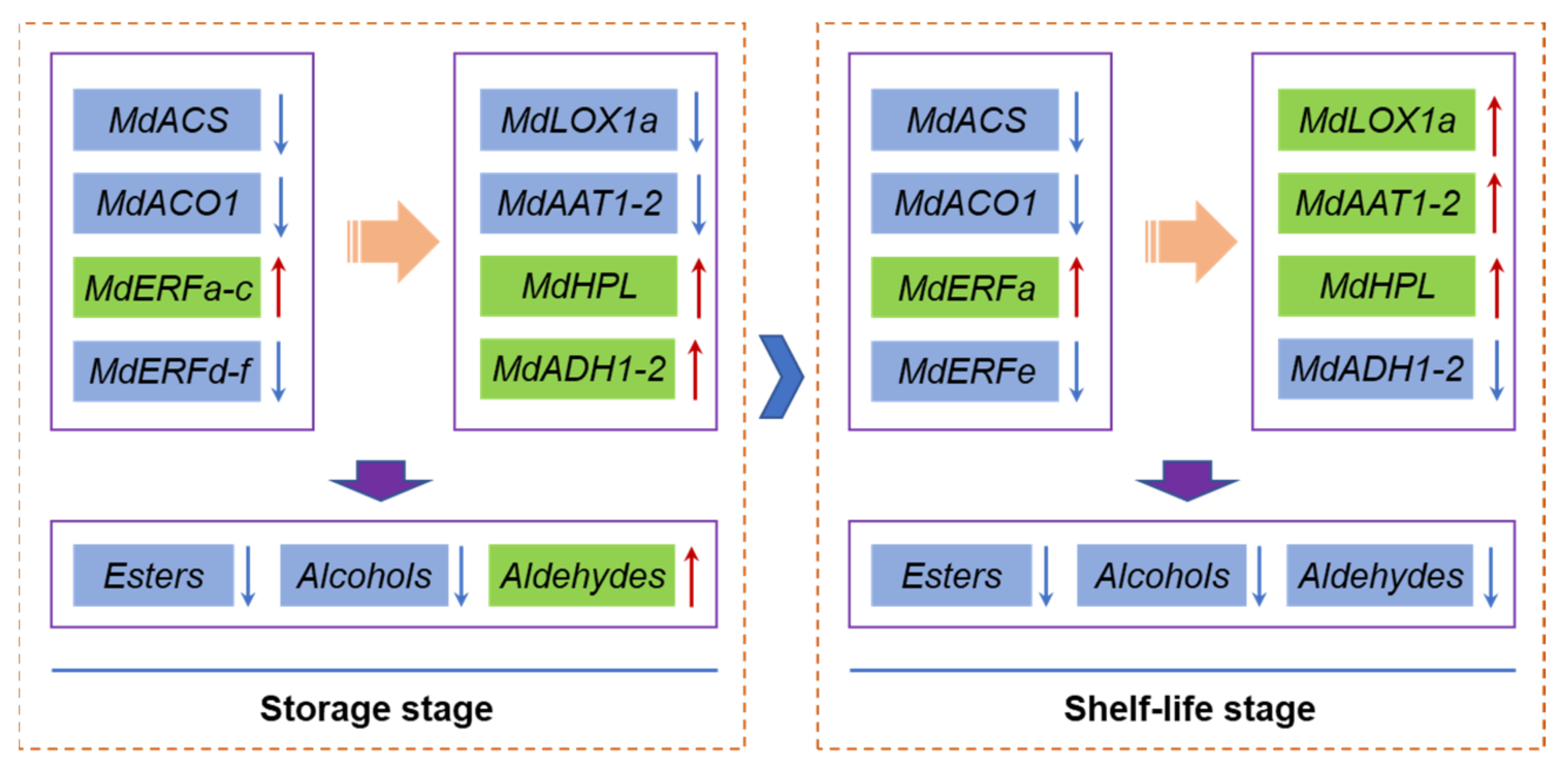
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Hadi, M.A.M.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in fruit aroma volatile research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef]
- Espino-Díaz, M.; Sepúlveda, D.R.; González-Aguilar, G.; Olivas, G.I. Biochemistry of apple aroma: A review. Food Technol. Biotech. 2016, 54, 375–394. [Google Scholar] [CrossRef]
- Yang, S.; Hao, N.; Meng, Z.; Li, Y.; Zhao, Z. Identification, comparison and classification of volatile compounds in peels of 40 apple cultivars by HS–SPME with GC-MS. Foods 2021, 10, 1051. [Google Scholar] [CrossRef] [PubMed]
- Altisent, R.; Echeverría, G.; Graell, J.; López, L.; Lara, I. Lipoxygenase activity is involved in the regeneration of volatile ester-synthesizing capacity after ultra-low oxygen storage of ‘Fuji’ apple. J. Agric. Food Chem. 2009, 57, 4305–4312. [Google Scholar] [CrossRef] [PubMed]
- Aprea, E.; Charles, M.; Endrizzi, I.; Laura Corollaro, M.; Betta, E.; Biasioli, F.; Gasperi, F. Sweet taste in apple: The role of sorbitol, individual sugars, organic acids and volatile compounds. Sci. Rep. -UK 2017, 7, 44950. [Google Scholar] [CrossRef] [PubMed]
- Both, V.; Thewes, F.R.; Brackmann, A.; de Freitas Ferreira, D.; Pavanello, E.P.; Wagner, R. Effect of low oxygen conditioning and ultra-low oxygen storage on the volatile profile, ethylene production and respiration rate of ‘Royal Gala’ apples. Sci. Hortic. 2016, 209, 156–164. [Google Scholar] [CrossRef]
- Thewes, F.R.; Anese, R.O.; Thewes, F.R.; Ludwig, V.; Klein, B.; Wagner, R.; Nora, F.R.; Rombaldi, C.V.; Brackmann, A. Dynamic controlled atmosphere (DCA) and 1-MCP: Impact on volatile esters synthesis and overall quality of ‘Galaxy’ apples. Food Packag. Shelf 2020, 26, 100563. [Google Scholar] [CrossRef]
- Mattheis, J.P.; Fan, X.; Argenta, L.C. Interactive responses of gala apple fruit volatile production to controlled atmosphere storage and chemical inhibition of ethylene action. J. Agric. Food Chem. 2005, 53, 4510–4516. [Google Scholar] [CrossRef]
- Thompson, A.K.; Prange, R.K.; Bancroft, R.; Puttongsiri, T. Controlled Atmosphere Storage of Fruits and Vegetables, 3rd ed.; CABI: Cambridge, MA, USA, 2018; pp. 11–12. ISBN 9781786393739. [Google Scholar]
- Brackmann, A.; Streif, J.; Bangerth, F. Relationship between a reduced aroma production and lipid metabolism of apples after long-term controlled-atmosphere storage. J. Am. Soc. Hortic. Sci. 2019, 118, 243–247. [Google Scholar] [CrossRef]
- Lau, O.L. Tolerance of three apple cultivars to ultra-low levels of oxygen. HortScience 1990, 25, 1412–1414. [Google Scholar] [CrossRef]
- Lopez, M.L.; Lavilla, M.T.; Recasens, I.; Graell, J.; Vendrell, M. Changes in aroma quality of ‘Golden Delicious’ apples after storage at different oxygen and carbon dioxide concentrations. J. Sci. Food Agric. 2000, 80, 311–324. [Google Scholar] [CrossRef]
- Kreuzwieser, J.; Rennenberg, H. Molecular and physiological responses of trees to waterlogging stress. Plant Cell Environ. 2014, 37, 2245–2259. [Google Scholar] [CrossRef]
- Ho, Q.T.; Verboven, P.; Verlinden, B.E.; Herremans, E.; Wevers, M.; Carmeliet, J.; Nicolaï, B.M. Athree-dimensional multiscale model for gas exchange in fruit. Plant Physiol. 2011, 155, 1158–1168. [Google Scholar] [CrossRef]
- Ho, Q.T.; Verboven, P.; Verlinden, B.E.; Schenk, A.; Delele, M.A.; Rolletschek, H.; Vercammen, J.; Nicolaï, B.M. Genotype effects on internal gas gradients in apple fruit. J. Exp. Bot. 2010, 61, 2745–2755. [Google Scholar] [CrossRef]
- De Anese, R.O.; Brackmann, A.; Thewes, F.R.; Schultz, E.E.; Ludwig, V.; Wendt, L.M.; Wagner, R.; Klein, B. Impact of dynamic controlled atmosphere storage and 1-methylcyclopropene treatment on quality and volatile organic compounds profile of ‘Galaxy’ apple. Food Packag. Shelf 2020, 23, 100443. [Google Scholar] [CrossRef]
- Liu, B.; Santo Domingo, M.; Mayobre, C.; Martín-Hernández, A.M.; Pujol, M.; Garcia-Mas, J. Knock-Out of CmNAC-NOR affects melon climacteric fruit ripening. Front. Plant Sci. 2022, 13, 878037. [Google Scholar] [CrossRef]
- Both, V.; Brackmann, A.; Thewes, F.R.; Ferreira, D.D.F.; Wagner, R. Effect of storage under extremely low oxygen on the volatile composition of ‘Royal Gala’ apples. Food Chem. 2014, 156, 50–57. [Google Scholar] [CrossRef]
- Chang, S.; Puryear, J.; Cairney, J. A simple and efficient method for isolating RNA from pine trees. Plant Mol. Biol. Rep. 1993, 11, 113–116. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Holland, D.; Larkov, O.; Bar-Ya’akov, I.; Bar, E.; Zax, A.; Brandeis, E.; Ravid, U.; Lewinsohn, E. Developmental and varietal differences in volatile ester formation and acetyl-CoA: Alcohol acetyl transferase activities in apple (Malus domestica Borkh.) fruit. J. Agric. Food Chem. 2005, 53, 7198–7203. [Google Scholar] [CrossRef]
- Cukrov, D.; Zermiani, M.; Brizzolara, S.; Cestaro, A.; Licausi, F.; Luchinat, C.; Santucci, C.; Tenori, L.; van Veen, H.; Zuccolo, A.; et al. Extreme hypoxic conditions induce selective molecular responses and metabolic reset in detached apple fruit. Front. Plant Sci. 2016, 7, 146. [Google Scholar] [CrossRef]
- Yahia, E.M. Modified and Controlled Atmospheres for the Storage, Transportation, and Packaging of Horticultural Commodities, 1st ed.; CRC Press: Boca Raton, FL, USA, 2009; pp. 8–13. ISBN 978-1-4200-6958-7. [Google Scholar]
- Saquet, A.A. Storability of ‘Jonagold’ apple fruit under extreme controlled atmosphere conditions. J. Agric. Sci. Tech. 2016, 6, 262–268. [Google Scholar] [CrossRef][Green Version]
- Suni, M.; Nyman, M.; Eriksson, N.A.; Björk, L.; Björck, I. Carbohydrate composition and content of organic acids in fresh and stored apples. J. Sci. Food Agric. 2000, 80, 1538–1544. [Google Scholar] [CrossRef]
- Defilippi, B.G.; Dandekar, A.M.; Kader, A.A. Relationship of ethylene biosynthesis to volatile production, related enzymes, and precursor availability in apple peel and flesh tissues. J. Agric. Food Chem. 2005, 53, 3133–3141. [Google Scholar] [CrossRef]
- Guadagni, D.G.; Bomben, J.L.; Hudson, J.S. Factors influencing the development of aroma in apple peels. J. Sci. Food Agric. 1971, 22, 110–115. [Google Scholar] [CrossRef]
- Plotto, A.; McDaniel, M.R.; Mattheis, J.P. Characterization of ‘Gala’ apple aroma and flavor: Differences between controlled atmosphere and air storage. J. Am. Soc. Hortic. Sci. 1999, 124, 416–423. [Google Scholar] [CrossRef]
- Ho, Q.T.; Verlinden, B.E.; Verboven, P.; Nicolaï, B.M. Gas diffusion properties at different positions in the pear. Postharvest Biol. Tec. 2006, 41, 113–120. [Google Scholar] [CrossRef]
- Schotsmans, W.; Verlinden, B.E.; Lammertyn, J.; Nicolaï, B.M. The relationship between gas transport properties and the histology of apple. J. Sci. Food Agric. 2004, 84, 1131–1140. [Google Scholar] [CrossRef]
- Saquet, A.A.; Streif, J.; Bangerth, F. Impaired aroma production of CA-stored ‘Jonagold’ apples as affected by adenine and pyridine nucleotide levels and fatty acid concentrations. J. Hortic. Sci. Biotech. 2003, 78, 695–705. [Google Scholar] [CrossRef]
- Villatoro, C.; Echeverría, G.; Graell, J.; López, M.L.; Lara, I. Long-term storage of Pink Lady apples modifies volatile-involved enzyme activities: Consequences on production of volatile esters. J. Agric. Food Chem. 2008, 56, 9166–9174. [Google Scholar] [CrossRef] [PubMed]
- Bangerth, F.K.; Song, J.; Streif, J. Physiological impacts of fruit ripening and storage conditions on aroma volatile formation in apple and strawberry fruit: A review. HortScience 2012, 47, 4–10. [Google Scholar] [CrossRef]
- Zhang, B.; Xi, W.; Wei, W.; Shen, J.; Ferguson, I.; Chen, K. Changes in aroma-related volatiles and gene expression during low temperature storage and subsequent shelf-life of peach fruit. Postharvest Biol. Tec. 2011, 60, 7–16. [Google Scholar] [CrossRef]
- Gorny, J.R.; Kader, A.A. Controlled-atmosphere suppression of ACC synthase and ACC oxidase in “Golden Delicious” apples during long-term cold storage. J. Am. Soc. Hortic. Sci. 1996, 121, 751–755. [Google Scholar] [CrossRef]
- Schmidt, S.F.P.; Schultz, E.E.; Ludwig, V.; Berghetti, M.R.P.; Thewes, F.R.; de Anese, R.O.; Both, V.; Brackmann, A. Volatile compounds and overall quality of ‘Braeburn’ apples after long-term storage: Interaction of innovative storage technologies and 1-MCP treatment. Sci. Hortic. 2020, 262, 109039. [Google Scholar] [CrossRef]
- Bui, L.T.; Giuntoli, B.; Kosmacz, M.; Parlanti, S.; Licausi, F. Constitutively expressed ERF-VII transcription factors redundantly activate the core anaerobic response in Arabidopsis thaliana. Plant Sci. 2015, 236, 37–43. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhang, D.; Mi, H.; Pristijono, P.; Ge, Y.; Lv, J.; Li, Y.; Liu, B. Tissue-Specific Recovery Capability of Aroma Biosynthesis in ‘Golden Delicious’ Apple Fruit after Low Oxygen Storage. Agronomy 2022, 12, 2794. https://doi.org/10.3390/agronomy12112794
Chen J, Zhang D, Mi H, Pristijono P, Ge Y, Lv J, Li Y, Liu B. Tissue-Specific Recovery Capability of Aroma Biosynthesis in ‘Golden Delicious’ Apple Fruit after Low Oxygen Storage. Agronomy. 2022; 12(11):2794. https://doi.org/10.3390/agronomy12112794
Chicago/Turabian StyleChen, Jingxin, Demei Zhang, Hongbo Mi, Penta Pristijono, Yonghong Ge, Jingyi Lv, Yushun Li, and Bin Liu. 2022. "Tissue-Specific Recovery Capability of Aroma Biosynthesis in ‘Golden Delicious’ Apple Fruit after Low Oxygen Storage" Agronomy 12, no. 11: 2794. https://doi.org/10.3390/agronomy12112794
APA StyleChen, J., Zhang, D., Mi, H., Pristijono, P., Ge, Y., Lv, J., Li, Y., & Liu, B. (2022). Tissue-Specific Recovery Capability of Aroma Biosynthesis in ‘Golden Delicious’ Apple Fruit after Low Oxygen Storage. Agronomy, 12(11), 2794. https://doi.org/10.3390/agronomy12112794






