Powdery Mildew Fungus Oidium lycopersici Infected-Tomato Plants Attracts the Non-Vector Greenhouse Whitefly, Trialeurodes vaporariorum, but Seems Impair Their Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants, Pathogens, and Insects
2.2. Inoculations and Plant Treatments
2.3. Behavioral Bioassays
2.4. Herbivore Fitness Performance
2.5. Statistical Analysis
3. Results
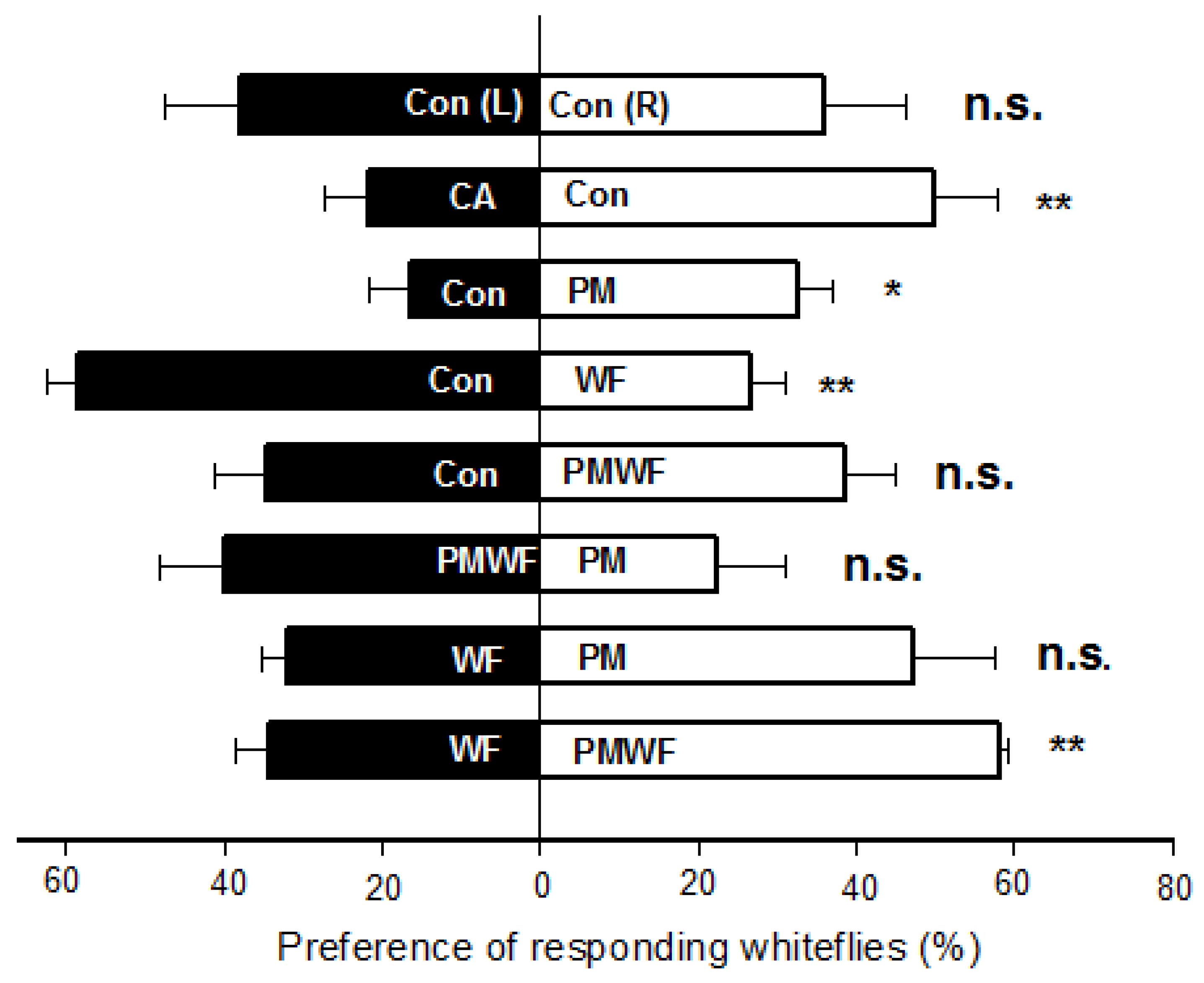
3.1. Behavioral Bioassays
3.2. Herbivore Fitness Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karban, R.; Baldwin, I.T. Induced Responses to Herbivory; University of Chicago Press: Chicago, IL, USA, 1997. [Google Scholar]
- Hammerschmidt, R.; Nicholson, R.L. A survey of plant defense responses to pathogens. In Induced Plant Defenses Against Pathogens and Herbivores; Agrawal, A.A., Tuzun, S., Bent, E., Eds.; APS Press: St. Paul, MN, USA, 1999; pp. 55–71. [Google Scholar]
- Qasim, M.; Lin, Y.; Dash, C.K.; Bamisile, B.S.; Ravindran, K.; Islam, S.U.; Ali, H.; Wang, F.; Wang, L. Temperature-dependent development of Asian citrus psyllid on various hosts, and mortality by two strains of Isaria. Microb. Pathog. 2018, 119, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Biere, A.; Bennett, A.E. Three-way interactions between plants, microbes and insects. Funct. Ecol. 2013, 27, 567–573. [Google Scholar] [CrossRef]
- Ratnadass, A.; Deguine, J.-P. Three-way interactions between crop plants, phytopathogenic fungi, and mirid bugs. A review. Agron. Sustain. Dev. 2020, 40, 46. [Google Scholar] [CrossRef]
- Qasim, M.; Islam, S.U.; Islam, W.; Noman, A.; Khan, K.A.; Hafeez, M.; Hussain, D.; Dash, C.K.; Bamisile, B.S.; Akutse, K.S.; et al. Characterization of mycotoxins from entomopathogenic fungi (Cordyceps fumosorosea) and their toxic effects to the development of asian citrus psyllid reared on healthy and diseased citrus plants. Toxicon 2020, 188, 39–47. [Google Scholar] [CrossRef]
- Lin, Y.; Qasim, M.; Hussain, M.; Akutse, K.S.; Avery, P.B.; Dash, C.K.; Wang, L. The herbivore-induced plant volatiles methyl salicylate and menthol positively affect growth and pathogenicity of entomopathogenic fungi. Sci. Rep. 2017, 7, 40494. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Hussain, M.; Avery, P.B.; Qasim, M.; Fang, D.; Wang, L. Volatiles from plants induced by multiple aphid attacks promote conidial performance of Lecanicillium lecanii. PLoS ONE 2016, 11, e0151844. [Google Scholar] [CrossRef] [PubMed]
- Ayres, P.G. Pests and Pathogens—Plant Responses to Foliar Attack; Bios Scientific Publishers: Oxford, UK, 1992. [Google Scholar]
- Wandeler, H.; Bacher, S. Insect-transmitted urediniospores of the rust Puccinia punctiformis cause systemic infections in established Cirsium arvense plants. Phytopathology 2006, 96, 813–818. [Google Scholar] [CrossRef][Green Version]
- Noman, A.; Aqeel, M.; Qasim, M.; Haider, I.; Lou, Y. Plant-insect-microbe interaction: A love triangle between enemies in ecosystem. Sci. Total Environ. 2020, 699, 134181. [Google Scholar] [CrossRef]
- Noman, A.; Aqeel, M.; Islam, W.; Khalid, N.; Akhtar, N.; Qasim, M.; Yasin, G.; Hashem, M.; Alamri, S.; Al-Zoubi, O.M. Insects–plants-pathogens: Toxicity, dependence and defense dynamics. Toxicon 2021, 197, 87–98. [Google Scholar] [CrossRef]
- Raman, A.; Suryanarayanan, T.S. Fungus–plant interaction influences plant-feeding insects. Fungal Ecol. 2017, 29, 123–132. [Google Scholar] [CrossRef]
- Moran, P.J.; Schultz, J.C. Ecological and chemical associations among late-season squash pests. Environ. Entomol. 1998, 27, 39–44. [Google Scholar] [CrossRef]
- Stephanie, K.; Andreas, K.; Teja, T. Interactions between the rust fungus Puccinia punctiformis and ectophagous and endophagous insects on creeping thistle. J. Appl. Ecol. 2001, 38, 548–556. [Google Scholar] [CrossRef]
- Desneux, N.; Mouttet, R.; Bearez, P.; Poncet, C. Indirect two-way interactions between aphids and a pathogen on roses. In Proceedings of the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on 927, Lisbon, Portugal, 22–27 August 2010; pp. 237–244. [Google Scholar]
- Li, Y.; Duan, T.; Li, Y. Research progress in the interactions of fungal pathogens and insect pests during host plant colonization. J. Plant Dis. Prot. 2021, 128, 633–647. [Google Scholar] [CrossRef]
- Rostás, M.; Simon, M.; Hilker, M. Ecological cross-effects of induced plant responses towards herbivores and phytopathogenic fungi. Basic Appl. Ecol. 2003, 4, 43–62. [Google Scholar] [CrossRef]
- Whipps, J.M.; Budge, S.P.; Fenlon, J.S. Characteristics and host range of tomato powdery mildew. Plant Physiol. 1998, 47, 36–48. [Google Scholar] [CrossRef]
- Whipps, J.M.; Budge, S.P. Effect of humidity on development of tomato powdery mildew (Oidium lycopersici) in the glasshouse. Eur. J. Plant Pathol. 2000, 106, 395–397. [Google Scholar] [CrossRef]
- Lindquist, R.K. Effect of greenhouse whitefly on yields of greenhouse tomatoes. J. Econ. Entomol. 1972, 65, 1406–1408. [Google Scholar] [CrossRef]
- Shavit, R.; Ofek-Lalzar, M.; Burdman, S.; Morin, S. Inoculation of tomato plants with rhizobacteria enhances the performance of the phloem-feeding insect Bemisia tabaci. Front. Plant Sci. 2013, 4, 306. [Google Scholar] [CrossRef]
- Isaacs, R.; Byrne, D.N.; Hendrix, D.L. Feeding rates and carbohydrate metabolism by Bemisia tabaci (Homoptera: Aleyrodidae) on different quality phloem saps. Physiol. Entomol. 1998, 23, 241–248. [Google Scholar]
- Pathak, P.K.; Heinrichs, E.A. Bromocresol green indicator for measuring feeding activity of Nilaparvata lugens on rice varieties. Philipp. Entomol. 1982, 5, 195–198. [Google Scholar]
- Fernandez-Conradi, P.; Jactel, H.; Robin, C.; Tack, A.J.M.; Castagneyrol, B. Fungi reduce preference and performance of insect herbivores on challenged plants. Ecology 2018, 99, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.S.; Bray, J.L.; Lehman, M.E.; Lentz-Ronning, A.J. Effects of host plant phenolic acids and nutrient status on oviposition and feeding of the cabbage white butterfly, Pieris rapae. BIOS 2014, 85, 95–101. [Google Scholar] [CrossRef]
- Hu, P.; Li, H.-L.; Zhang, H.-F.; Luo, Q.-W.; Guo, X.-R.; Wang, G.-P.; Li, W.-Z.; Yuan, G. Experience-based mediation of feeding and oviposition behaviors in the cotton bollworm: Helicoverpa armigera (Lepidoptera: Noctuidae). PLoS ONE 2018, 13, e0190401. [Google Scholar] [CrossRef] [PubMed]
- Dicke, M.; Baldwin, I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Moran, P.J. Plant-mediated interactions between insects and a fungal plant pathogen and the role of plant chemical responses to infection. Oecologia 1998, 115, 523–530. [Google Scholar] [CrossRef]
- Hatcher, P.E.; Paul, N.D.; Ayres, P.G.; Whittaker, J.B. The effect of a foliar disease (rust) on the development of Gastrophysa viridula (Coleoptera: Chrysomelidae). Ecol. Entomol. 1994, 19, 349–360. [Google Scholar] [CrossRef]
- Tofangsazi, N.; Hogg, B.N.; Portman, S.L.; Pratt, P.D. Tritrophic interactions between an invasive weed (Lepidium latifolium), an insect herbivore (Bagrada hilaris), and a plant pathogenic fungus (Albugo lepidii). Environ. Entomol. 2019, 48, 1317–1322. [Google Scholar] [CrossRef]
- Ajayi, O.; Dewar, A.M. The effect of barley yellow dwarf virus on honeydew production by the cereal aphids, Sitobion avenae and Metopolophium dirhodum. Ann. Appl. Biol. 1982, 100, 203–212. [Google Scholar] [CrossRef]
- Fiebig, M.; Poehling, H.M.; Borgemeister, C. Barley yellow dwarf virus, wheat, and Sitobion avenae: A case of trilateral interactions. Entomol. Exp. Et Appl. 2004, 110, 11–21. [Google Scholar] [CrossRef]
- Xin, Z.; Zhang, L.; Zhang, Z.; Chen, Z.; Sun, X. A tea hydroperoxide lyase gene, CsiHPL1, regulates tomato defense response against Prodenia litura (Fabricius) and Alternaria alternata f. sp. lycopersici by modulating green leaf volatiles (GLVs) release and jasmonic acid (JA) gene expression. Plant Mol. Biol. Rep. 2014, 32, 62–69. [Google Scholar] [CrossRef]
- Kang, Z.-W.; Liu, F.-H.; Tan, X.-L.; Zhang, Z.-F.; Zhu, J.-Y.; Tian, H.-G.; Liu, T.-X. Infection of powdery mildew reduces the fitness of grain aphids (Sitobion avenae) through restricted nutrition and induced defense response in wheat. Front. Plant Sci. 2018, 9, 778. [Google Scholar] [CrossRef] [PubMed]
- Cardoza, Y.J.; Teal, P.E.A.; Tumlinson, J.H. Effect of peanut plant fungal infection on oviposition preference by Spodoptera exigua and on host-searching behavior by Cotesia marginiventris. Environ. Entomol. 2003, 32, 970–976. [Google Scholar] [CrossRef]
- Wang, L.; Vidal, S. Host plant infection by a plant pathogen changes parasitoid host-searching behavior: A case study using whiteflies and Encarsia formosa. J. Insect Sci. 2006, 8, 51. [Google Scholar]
- Wang, L.; Vidal, S. Interaction between whiteflies, Encarsia formosa, and powdery mildew infections on tomatoes. Mittelungen aus der Biologischen Bundesanstalt für Land-und Forstwirtschaft 54. Deutsche Pflanzenschutztagung, Hamburg. In Proceedings of the The 54th German Plant Protection Conference, Hamburg, Germany, 20–23 September, 2004; Volume 396, p. 475. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qasim, M.; Akutse, K.S.; Hussain, D.; Al-Zoubi, O.M.; Mustafa, T.; Aguila, L.C.R.; Alamri, S.; Hashem, M.; Wang, L. Powdery Mildew Fungus Oidium lycopersici Infected-Tomato Plants Attracts the Non-Vector Greenhouse Whitefly, Trialeurodes vaporariorum, but Seems Impair Their Development. Agronomy 2022, 12, 2791. https://doi.org/10.3390/agronomy12112791
Qasim M, Akutse KS, Hussain D, Al-Zoubi OM, Mustafa T, Aguila LCR, Alamri S, Hashem M, Wang L. Powdery Mildew Fungus Oidium lycopersici Infected-Tomato Plants Attracts the Non-Vector Greenhouse Whitefly, Trialeurodes vaporariorum, but Seems Impair Their Development. Agronomy. 2022; 12(11):2791. https://doi.org/10.3390/agronomy12112791
Chicago/Turabian StyleQasim, Muhammad, Komivi Senyo Akutse, Dilbar Hussain, Omar Mahmoud Al-Zoubi, Tariq Mustafa, Luis Carlos Ramos Aguila, Saad Alamri, Mohamed Hashem, and Liande Wang. 2022. "Powdery Mildew Fungus Oidium lycopersici Infected-Tomato Plants Attracts the Non-Vector Greenhouse Whitefly, Trialeurodes vaporariorum, but Seems Impair Their Development" Agronomy 12, no. 11: 2791. https://doi.org/10.3390/agronomy12112791
APA StyleQasim, M., Akutse, K. S., Hussain, D., Al-Zoubi, O. M., Mustafa, T., Aguila, L. C. R., Alamri, S., Hashem, M., & Wang, L. (2022). Powdery Mildew Fungus Oidium lycopersici Infected-Tomato Plants Attracts the Non-Vector Greenhouse Whitefly, Trialeurodes vaporariorum, but Seems Impair Their Development. Agronomy, 12(11), 2791. https://doi.org/10.3390/agronomy12112791








