The Impact of Polymer on the Productivity and Photosynthesis of Soybean under Different Water Levels
Abstract
1. Introduction
2. Materials and Methods
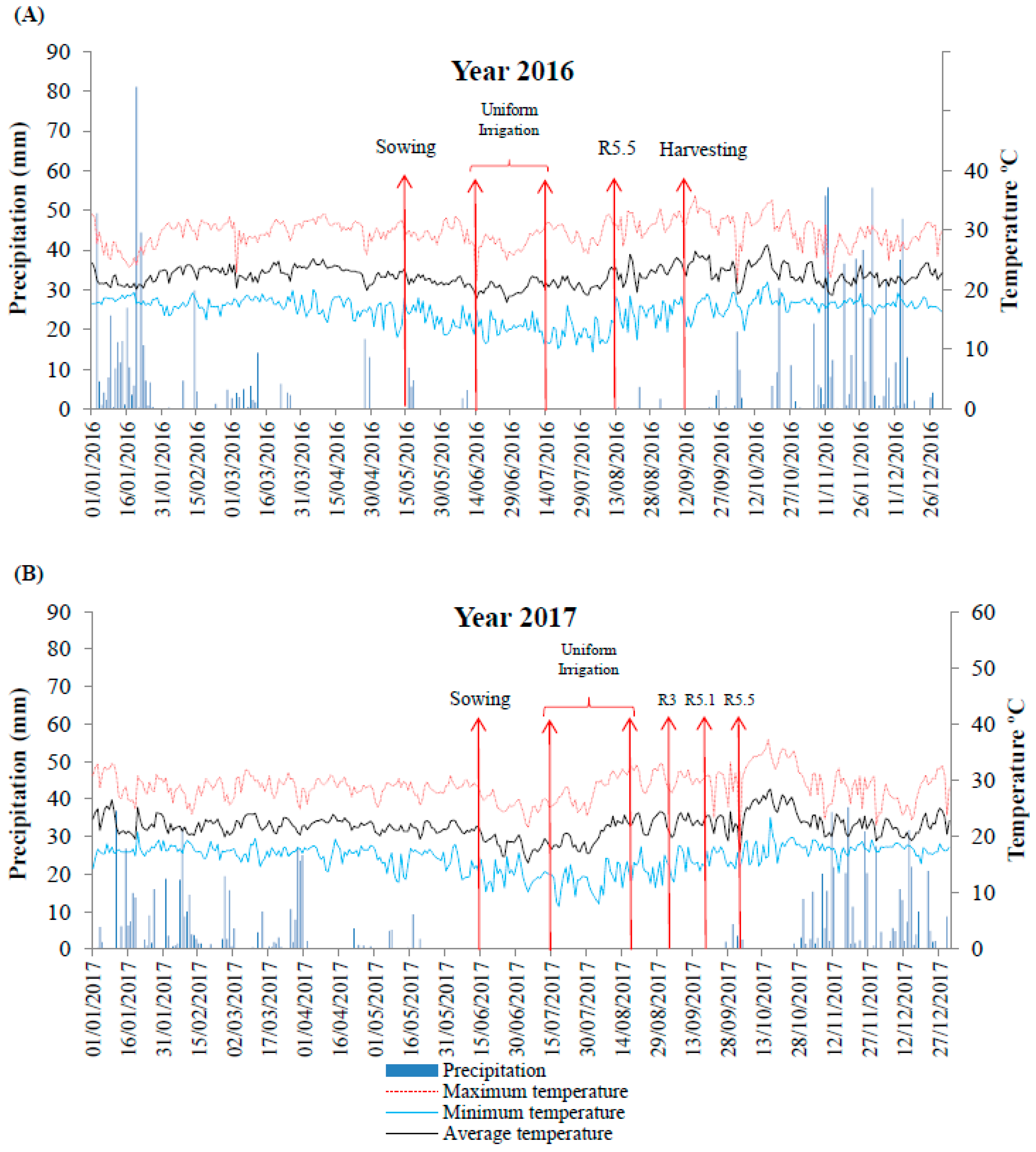
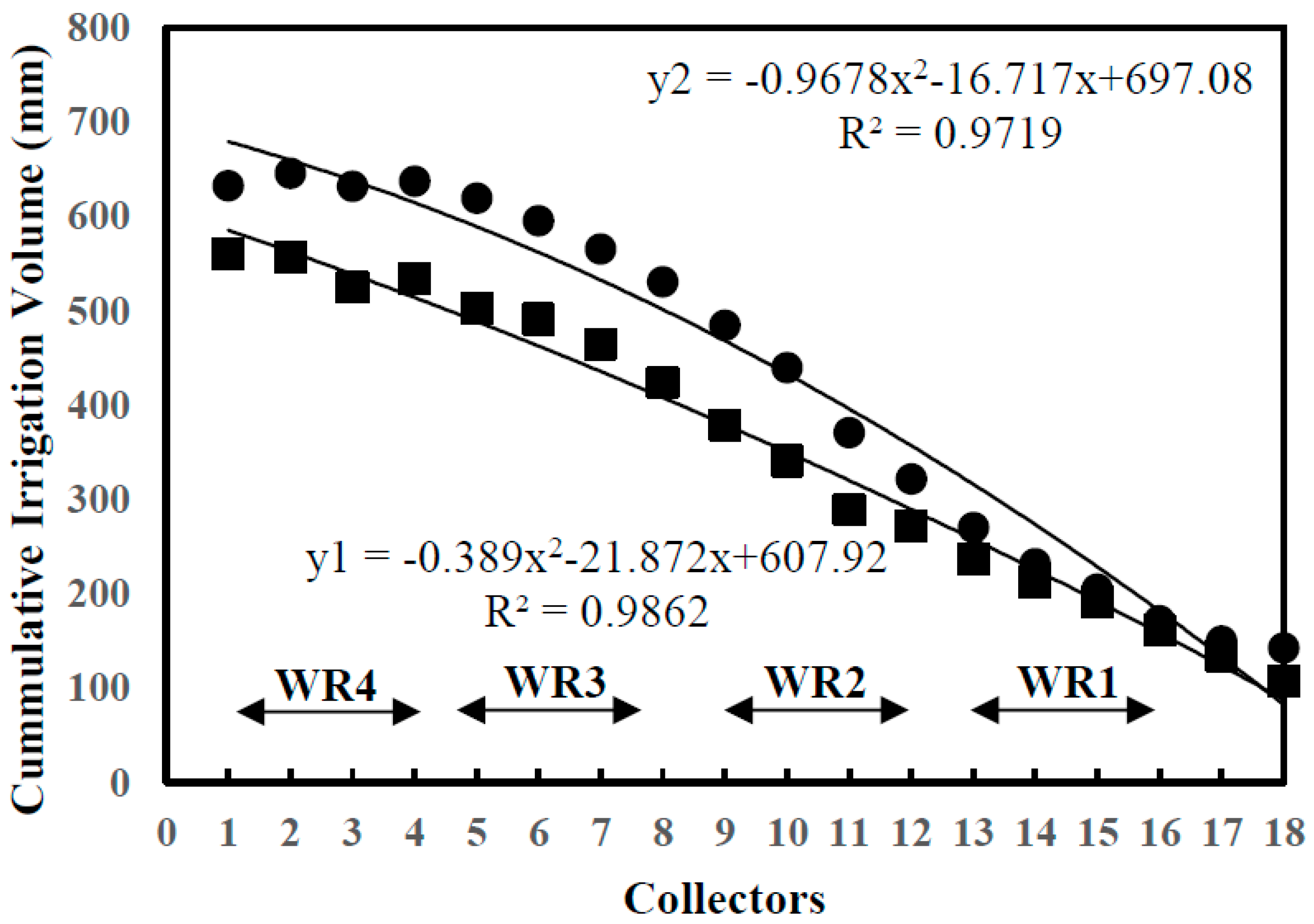
2.1. Experimental Design and Water Levels
2.2. Plant Analysis
2.3. Statistical Analysis
3. Results
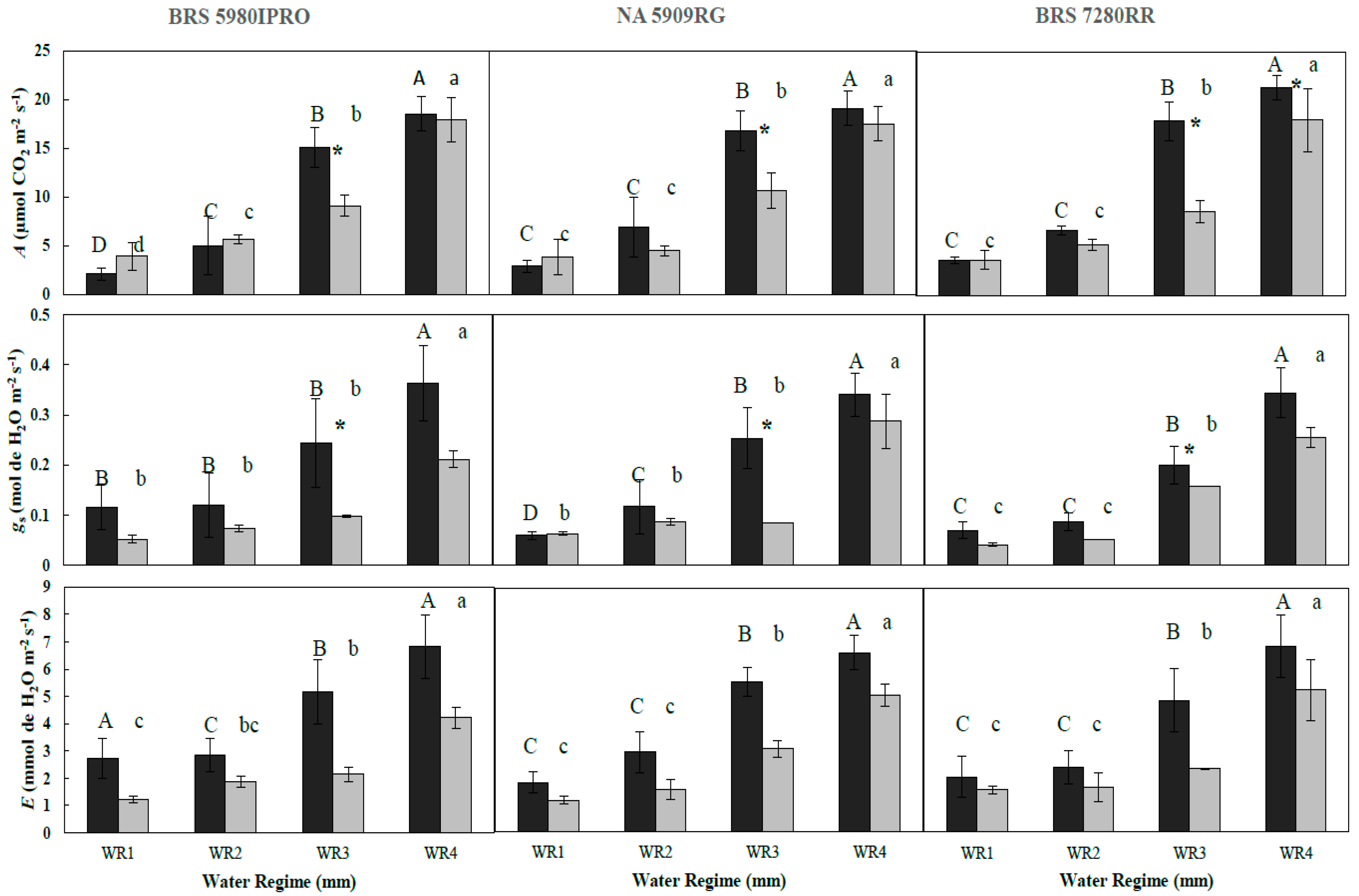
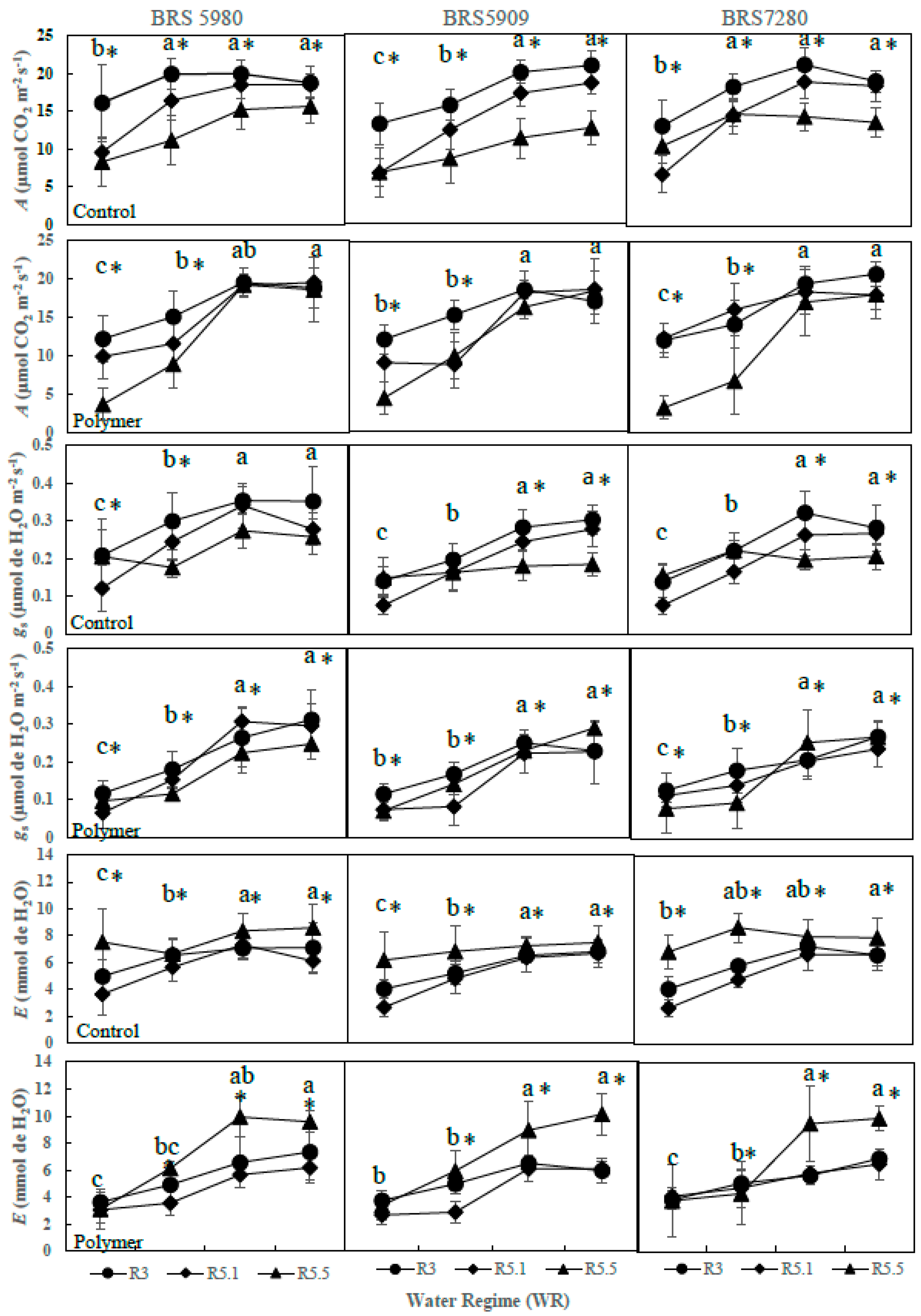
3.1. Effect of Water Regime and Polymer Supply on Photosynthetic Parameters (Year 1—2016)
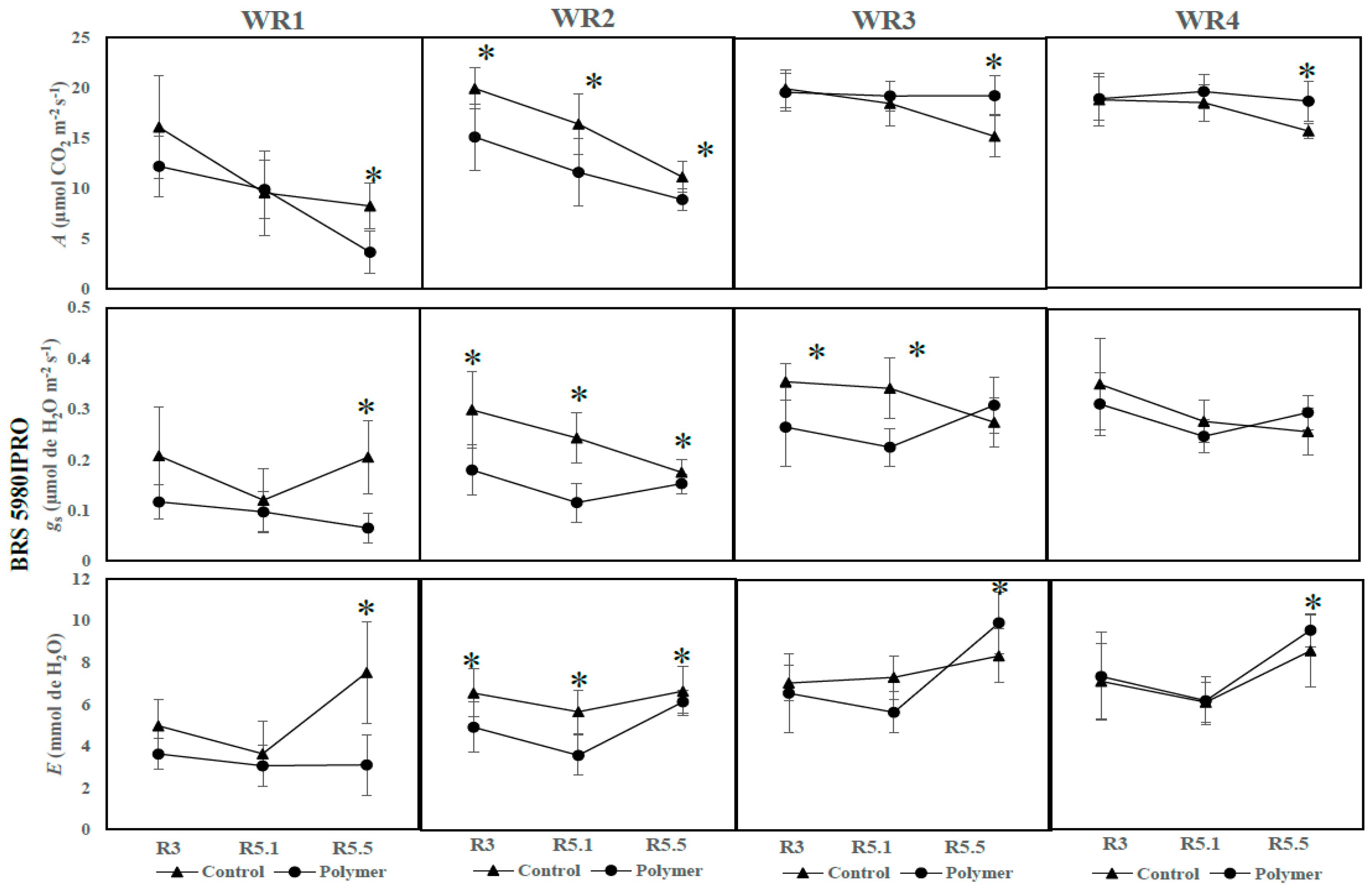
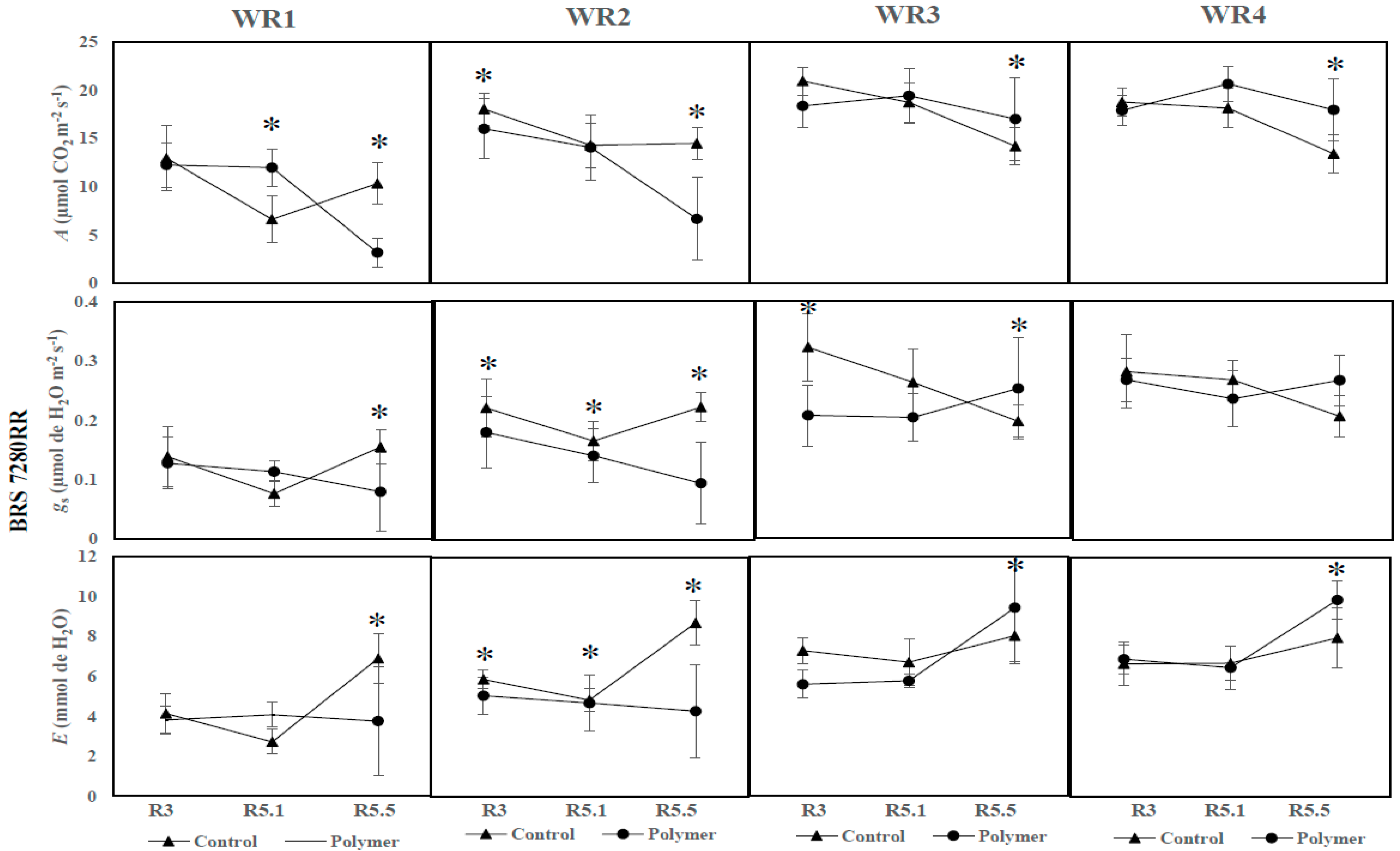
3.2. The Effects of Polymer Addition on Photosynthetic Parameters (Year 2—2017)
3.3. The Influence of Water Regime and Polymer on Physiological Parameters across Different Phenological Phases (Year 2—2017)
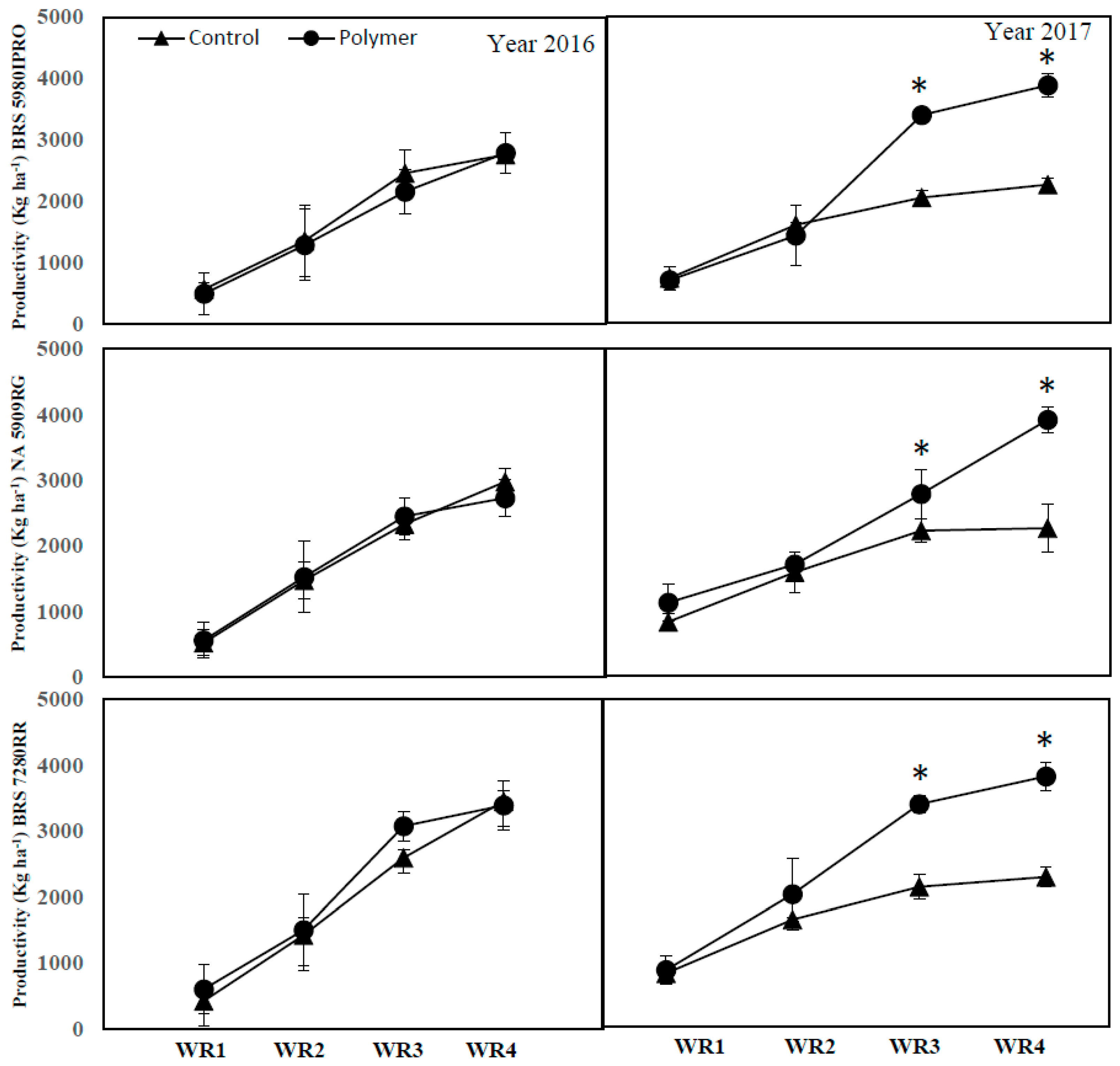
3.4. The Impact of Polymer and Water Regime on Soybean Productivity (Years 1 and 2)
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPEA—Instituto de Pesquisa Econômica Aplicada; IBGE—Instituto Brasileiro de Geografia e Estatística. Relatório Econômico; IPEA: Brasília, Brazil; IBGE: Rio de Janeiro, Brazil, 2021. [Google Scholar]
- Cattelan, A.J.; Dall’Agnol, A. The rapid soybean growth in Brazil. Oilseeds Fats Crops Lipids 2018, 25, D102. [Google Scholar] [CrossRef]
- Sarto, M.V.M.; Sarto, J.R.W.; Rampim, L.; Rosset, J.S.; Bassegio, D.; Costa, P.F.; Inagaki, A.M. Wheat phenology and yield under drought: A review. Aust. J. Crop Res. 2017, 11, 941–946. [Google Scholar] [CrossRef]
- Cunha, A.P.M.A.; Zeri, M.; Leal, K.D.; Costa, L.; Cuartas, L.A.; Marengo, J.A.; Tomasella, J.; Vieira, R.M.; Barbosa, A.A.; Cunningham, C.; et al. Extreme drought events over Brazil from 2011 to 2019. Atmosphere 2019, 10, 642. [Google Scholar] [CrossRef]
- Muller, B.; Pantin, F.; Génard, M.; Turc, O.; Freixes, S.; Piques, M.; Gibon, Y. Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. J. Exp. Bot. 2011, 62, 1715–1729. [Google Scholar] [CrossRef]
- Oliveira, G.L.P. The geopolitics of Brazilian soybeans. J. Peasant Stud. 2016, 46, 348–372. [Google Scholar] [CrossRef]
- Board, J.E.; Kahlon, C.S. Soybean yield formation: What controls it and whatcan be improved? In Soybean Physiology and Bio-chemistry; El-Shemy, H.A., Ed.; InTech: London, UK, 2011; pp. 1–36. [Google Scholar]
- Niinemets, Ü.; García-Plazaola, J.I.; Tosens, T. Photosynthesis during leaf development and ageing. In Terrestrial Photosynthesis in a Changins Environment: A Molecular, Physiological and Ecological Approach; Flexas, J., Loreto, F., Medrano, H., Eds.; Cambridge University Press: Cambridge, UK, 2012; pp. 353–372. [Google Scholar]
- Feller, U. Drought stress and carbon assimilation in a warming climate: Reversible and irreversible impacts. J. Plant Physiol. 2016, 203, 84–94. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Arolu, F.; Chukwu, S.C.; Salisu, M.A.; Fagbohun, I.K.; Muftaudeen, T.K.; Swaray, S.; Haliru, B.S. Superabsorbent Polymer Hydrogels for Sustainable Agriculture: A Review. Horticulturae 2022, 8, 605. [Google Scholar] [CrossRef]
- Bodner, G.; Nakhforoosh, A.; Kaul, H.P. Management of crop water under drought: A review. Agron. Sustain. Dev. 2015, 35, 401–442. [Google Scholar] [CrossRef]
- Felippe, D.; Navroski, M.C.; Sampietro, J.A.; Frigotto, T.; Albuquerque, J.A.; Mota, C.S.; Pereira, M.O. Efeito do hidrogel no crescimento de mudas de Eucalyptus benthamii submetidas a diferentes frequências de irrigação. Floresta 2016, 46, 215–225. [Google Scholar] [CrossRef]
- Jamnická, G.; Ditmarová, Ľ.; Kurjak, D.; Kmeť, J.; Pšidová, E.; Macková, M.; Gömöry, D.; Střelcová, K. The soil hydrogel improved photosynthetic performance of beech seedlings treated under drought. Plant Soil Environ. 2013, 59, 446–451. [Google Scholar] [CrossRef]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Survey Laboratory Methods Manual. Soil Survey Investigations Report No. 42, Version 5.0; Burt, R., Soil Survey Staff, Eds.; Natural Resources Conservation Service, US Department of Agriculture: Washington, DC, USA, 2014.
- Hanks, R.J.; Keller, J.; Rasmussen, V.P.; Wilson, G.D. Line source sprinkler for continuous variable irrigation-crop production studies. Soil Sci. Soc. Am. J. 1976, 40, 426–429. [Google Scholar] [CrossRef]
- EMBRAPA—Empresa Brasileira de Pesquisa Agropecuária. Monitoramento de Irrigação no Cerrado. 2016. Available online: http://hidro.cpac.embrapa.br (accessed on 20 May 2016).
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Aeta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence: A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo. Ann. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Yu, J.Y.; Shi, J.G.; Ma, X.; Dang, P.F.; Yan, Y.L.; Mamedov, A.I.; Shainberg, I.; Levy, G.J. Superabsorbent Polymer Properties and Concentration Effects on Water Retention under drying conditions. Soil Sci. Soc. Am. J. 2017, 81, 889–901. [Google Scholar] [CrossRef]
- Abdallah, A.M. The effect of hydrogel particle size on water retention properties and availability under water stress. Int. Soil Water Conserv. Res. 2019, 7, 275–285. [Google Scholar] [CrossRef]
- Malekian, A.; Valizadeh, E.; Dastoori, M.; Samadi, S.; Bayat, V. Soil water retention and maize (Zea mays L.) growth as affected by different amounts of Pumice. Aust. J. Plant Sci. 2012, 6, 450–454. [Google Scholar]
- Milani, P.; França, D.; Balieiro, A.G.; Faez, R. Polymers and its applications in agriculture. Polímeros 2017, 27, 256–266. [Google Scholar] [CrossRef]
- Brodribb, T.J.; McAdam, S.A.M. Evolution of the Stomatal Regulation of Plant Water Content. Plant Physiol. 2017, 174, 639–649. [Google Scholar] [CrossRef]
- Takagi, D.; Ihara, H.; Takumi, S.; Miyake, C. Growth Light Environment Changes the Sensitivity of Photosystem I Photoinhibition Depending on Common Wheat Cultivars. Front. Plant Sci. 2019, 10, 686. [Google Scholar] [CrossRef]
- Rehman, A.; Ahmad, R.; Safdar, M. Effect of hydrogel on the performance of aerobic rice sown under different techniques. Plant Soil Environ. 2011, 57, 321–325. [Google Scholar] [CrossRef]
- Tan, Q.; Liu, Y.; Dai, L.; Pan, T. Shortened key growth periods of soybean observed in China under climate change. Sci. Rep. 2021, 11, 8197. [Google Scholar] [CrossRef]
- Board, J.E.; Tan, Q. Assimilatory capacity effects on soybean yield components and pod number. Crop Sci. 1995, 35, 846–851. [Google Scholar] [CrossRef]
- Blum, A. Drought resistance, water-use efficiency, and yield potential—Are they compatible, dissonant, or mutually exclusive? Aust. J. Agric. Res. 2005, 56, 1159–1168. [Google Scholar] [CrossRef]
- Massonnet, C.; Costes, E.; Rambal, S.; Dreyer, E.; Regnard, J.L. Stomatal regulation of photosynthesis in apple leaves: Evidence for different water-use strategies between two cultivars. Ann. Bot. 2007, 100, 1347–1356. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Andersen, M.N.; Jacobsen, S.E.; Jensen, C.R. Stomatal control and water use efficiency of soybean (Glycine max L. Merr.) during progressive soil drying. Environ. Exp. Bot. 2005, 54, 33–40. [Google Scholar] [CrossRef]
- Asaf, S.; Khan, A.L.; Khan, M.A.; Imrana, Q.M.; Yuna, B.W.; Lee, I.J. Osmoprotective functions conferred to soybean plants via inoculation with Sphingomonas sp. LK11 and exogenous trehalose. Microbiol. Res. 2017, 205, 135–145. [Google Scholar] [CrossRef]
- Fenta, B.A.; Beebe, S.E.; Kunert, K.J.; Burridge, J.D.; Barlow, K.M.; Lynch, J.P.; Foyer, C.H. Field Phenotyping of Soybean Roots for Drought Stress Tolerance. Agronomy 2014, 4, 418–435. [Google Scholar] [CrossRef]
- Dong, S.; Jiang, Y.; Dong, Y.; Wang, L.; Wang, W.; Ma, Z.; Yan, C.; Ma, C.; Liu, L. A study on soybean responses to drought stress and rehydration. Saudi J. Biol. Sci. 2019, 26, 2006–2017. [Google Scholar] [CrossRef]
- Wijewardana, C.; Reddy, K.R.; Bellaloui, N. Soybean seed physiology, quality, and chemical composition under soil moisture stress. Food Chem. 2019, 278, 92–100. [Google Scholar] [CrossRef]
- Engineer, C.B.; Hashimoto-Sugimoto, M.; Negi, J.; Israelsson-Nordström, M.; Azoulay-Shemer, T.; Rappel, W.J.; Iba, K.; Schroeder, J. CO2 sensing and CO2 regulation of stomatal conductance: Advances and open questions. Trends Plant Sci. 2016, 21, 16–30. [Google Scholar] [CrossRef]
- Silva, P.C.; Ribeiro Junior, W.Q.; Ramos, M.L.G.; Celestino, S.M.C.; Silva, A.D.N.; Casari, R.A.D.C.N.; Santana, C.C.; Lima, C.A.; Williams, T.C.R.; Vinson, C.C. Quinoa for the Brazilian Cerrado: Agronomic Characteristics of Elite Genotypes under Different Water Regimes. Plants 2021, 10, 1591–1608. [Google Scholar] [CrossRef] [PubMed]
- Jayme-Oliveira, A.; Ribeiro Junior, W.Q.; Ramos, M.L.G.; Ziviani, A.C.; Jakelaitis, A. Amaranth, C. quinoa and millet growth and development under different water regimes in the Brazilian Cerrado. Pesqui. Agropecu. Bras. 2017, 52, 561571. [Google Scholar] [CrossRef]
- Soares, G.F.; Ribeiro, W.Q., Jr.; Pereira, L.F.; Lima, C.A.; Soares, D.D.S.; Muller, O.; Rascher, U.; Ramos, M.L.G. Characterization of wheat genotypes for drought tolerance and water use efficiency. Sci. Agric. 2021, 78, e20190304. [Google Scholar] [CrossRef]
- Pereira, L.F.; Ribeiro, W.Q., Jr.; Ramos, M.L.G.; dos Santos, N.Z.; Soares, G.F.; das Chagas Noquelli Casari, R.; Muller, O.; Tavares, C.J.; de Souza Martins, É.; Rascher, U.; et al. Physiological changes in soybean cultivated with soil remineralizer in the Cerrado under variable water regimes. Pesqui. Agropecu. Bras. 2021, 56, e01455. [Google Scholar] [CrossRef]
- Pejić, B.; Maksimović, L.; Cimpeanu, S.; Bucur, D.; Milić, S.; Ćupina, B. Response of soybean to water stress at specific growth stages. J. Food Agric. Environ. 2011, 9, 280–284. [Google Scholar]
- Ball, R.A.; Purcell, L.C.; Vories, E.D. Short-season soybean yield compensation in response to population and water regime. Crop Sci. 2000, 40, 1070–1078. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought Stress in Plants: A Review on Morphological Characteristics and Pigments Composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Junior, C.P.; Kawakami, J.; Bridi, M.; Müller, M.M.L.; Conte, M.V.D.; Michalovicz, L. Phenological and quantitative plant development changes in soybean cultivars caused by sowing date and their relation to yield. Afr. J. Agric. Res. 2015, 10, 515–523. [Google Scholar]
- Jumrani, K.; Bhatia, V.S. Impact of combined stress of high temperature and water deficit on growth and seed yield of soybean. Physiol. Mol. Biol. Plants 2018, 24, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Menezes-Silva, P.E.; Sanglard, L.M.V.P.; Ávila, R.T.; Morais, L.E.; Martins, S.L.C.V.; Nobres, P.; Patreze, C.M.; Ferreira, M.; Araújo, W.L.; Fernie, A.R.; et al. Photosynthetic and metabolic acclimation to repeated drought events play key roles in drought tolerance in coffee. J. Exp. Bot. 2017, 68, 4309–4322. [Google Scholar] [CrossRef]
- Zadražnik, T.; Moen, A.; Šuštar-Vozlič, J. Chloroplast proteins involved in drought stress response in selected cultivars of common bean (Phaseolus vulgaris L.). 3 Biotech 2019, 9, 331. [Google Scholar] [CrossRef] [PubMed]
- Abedi-Koupai, J.; Sohrab, F.; Swarbrick, G. Evaluation of hydrogel application on soil water retention characteristics. J. Plant Nutr. 2008, 31, 318–331. [Google Scholar] [CrossRef]
- Nunes-Nesi, A.; Araújo, W.L.; Obata, T.; Fernie, A. Regulation of the mitochondrial tricarboxylic acid cycle. Curr. Opin. Plant Biol. 2013, 16, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Jiang, S.; Jin, J.; Ning, S.; Feng, P. Quantitative assessment of soybean drought loss sensitivity at different growth stages based on S-shaped damage curve. Agric. Water Manag. 2019, 213, 821–832. [Google Scholar] [CrossRef]

| Depth | pH | Ca | Mg | P | K | Al | OM |
|---|---|---|---|---|---|---|---|
| (cm) | (H2O) | cmolc dm−3 | cmolc dm−3 | mg L−1 | cmolc dm−3 | cmolc dm−3 | (%) |
| 0–20 | 5.74 | 2.317 | 1.146 | 9.868 | 118 | 0.069 | 2.246 |
| 20–40 | 5.62 | 1.51 | 0.931 | 2.725 | 53 | 0.089 | 1.823 |
| V | S | BRS 5980IPRO | NA 5909RG | BRS 7280RR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WR1 | WR2 | WR3 | WR4 | WR1 | WR2 | WR3 | WR4 | WR1 | WR2 | WR3 | WR4 | ||
| A | C | 2.09 Ad | 4.99 Ac | 15.10 Ab | 18.52 Aa | 2.89 Ac | 6.90 Ac | 16.83 Ab | 19.10 Aa | 3.57 Ac | 6.68 Ac | 17.87 Ab | 21.26 Aa |
| P | 3.2 Ad | 5.64 Ac | 9.09 Bb | 15.65 Aa | 3.87 Ac | 4.52 Ac | 10.65 Bb | 17.52 Aa | 3.05 Ac | 4.53 Bc | 8.58 Bb | 17.96 Ba | |
| gs | C | 0.114 Ab | 0.119 Ab | 0.243 Ab | 0.366 Aa | 0.059 Ac | 0.119 Ac | 0.251 Ab | 0.340 Aa | 0.069 Ad | 0.085 Ac | 0.201 Ab | 0.341 Aa |
| P | 0.043 Bb | 0.069 Bb | 0.082 Bb | 0.196 Ba | 0.037 Bc | 0.052 Bc | 0.123 Bb | 0.239 Ba | 0.050 Bd | 0.054 Bc | 0.085 Bb | 0.238 Ba | |
| E | C | 2.72 Ac | 2.84 Abc | 5.16 Ab | 6.82 Aa | 1.82 Ac | 2.93 Ac | 5.54 Ab | 6.61 Aa | 2.04 Ac | 2.4 Ac | 4.86 Ab | 6.86 Aa |
| P | 1.23 Bc | 1.88 Bbc | 2.13 Bb | 4.21 Ba | 1.16 Bc | 1.58 Bc | 3.07 Bb | 5.05 Ba | 1.55 Bc | 1.65 Bc | 2.34 Bb | 5.24 Ba | |
| Fv/Fm | C | 0.71 Bb | 0.73 Bab | 0.81 Aa | 0.79 Aa | 0.73 Ab | 0.77 Aa | 0.80 Aa | 0.79 Aa | 0.75 Ab | 0.80 Aab | 0.82 Aa | 0.80 Aa |
| P | 0.76 Ab | 0.80 Aab | 0.81 Aa | 0.79 Aa | 0.74 Ab | 0.80 Aa | 0.81 Aa | 0.81 Aa | 0.76 Ab | 0.79 Aab | 0.82 Aa | 0.82 Aa | |
| Fv′/Fm′ | C | 0.44 Ab | 0.45 Ab | 0.53 Ba | 0.52 Aa | 0.42 Ab | 0.45 Aab | 0.44 Bab | 0.54 Aa | 0.29 Ab | 0.45 Aab | 0.47 Ba | 0.52 Aa |
| P | 0.46 Ab | 0.49 Ab | 0.57 Aa | 0.57 Aa | 0.44 Ab | 0.47 Aab | 0.53 Aab | 0.55 Ba | 0.41 Ab | 0.44 Aab | 0.53 Aa | 0.53 Aa | |
| Cultivar | Polymer | WR4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | gs | E | ||||||||
| R3 | R5.1 | R5.5 | R3 | R5.1 | R5.5 | R3 | R5.1 | R5.5 | ||
| BRS 5980IPRO | Control | 18.68 Aa | 17.97 Aa | 15.64 Bb | 0.34 Aa | 0.27 Aa | 0.26 Ab | 6.97 Ab | 6.12 Ab | 8.58 Ba |
| Polymer | 18.73 Aa | 19.58 Aa | 18.63 Aa | 0.30 Aa | 0.25 Aa | 0.30 Aa | 6.99 Ab | 6.18 Ab | 9.58 Aa | |
| NA 5909RG | Control | 20.71 Aa | 18.56 Aa | 12.65 Bb | 0.30 Aa | 0.28 Aa | 0.19 Ab | 6.89 Ab | 6.77 Ab | 7.46 Ba |
| Polymer | 16.88 Aa | 18.69 Aa | 18.50 Aa | 0.23 Aa | 0.23 Aa | 0.29 Aa | 5.91 Ab | 6.09 Ab | 10.13 Aa | |
| BRS 7280RR | Control | 18.80 Aa | 18.18 Aa | 13.84 Bb | 0.28 Aa | 0.27 Aa | 0.22 Ab | 6.65 Ab | 6.69 Ab | 8.44 Ba |
| Polymer | 18.20 Aa | 20.70 Aa | 17.99 Aa | 0.27 Aa | 0.24 Aa | 0.27 Aa | 6.88 Ab | 6.46 Ab | 9.85 Aa | |
| WR3 | ||||||||||
| A | gs | E | ||||||||
| R3 | R5.1 | R5.5 | R3 | R5.1 | R5.5 | R3 | R5.1 | R5.5 | ||
| BRS 5980IPRO | Control | 19.82 Aa | 18.35 Aa | 15.20 Bb | 0.35 Aa | 0.35 Aa | 0.27 Ab | 7.15 Aa | 7.26 Aa | 8.35 Ba |
| Polymer | 19.71 Aa | 19.22 Aa | 19.26 Aa | 0.26 Ba | 0.22 Ba | 0.31 Aa | 6.36 Ab | 5.65 Ab | 9.93 Aa | |
| NA 5909RG | Control | 19.92 Aa | 17.20 Aa | 11.30 Bb | 0.29 Aa | 0.25 Aa | 0.18 Ab | 6.63 Aa | 6.34 Aa | 7.21 Ba |
| Polymer | 18.73 Aa | 18.26 Aa | 16.35 Aa | 0.25 Ba | 0.22 Ba | 0.23 Aa | 6.46 Ab | 6.10 Ab | 8.94 Aa | |
| BRS 7280RR | Control | 20.64 Aa | 18.84 Aa | 14.19 Bb | 0.31 Aa | 0.27 Aa | 0.20 Ab | 7.37 Aa | 6.78 Aa | 8.04 Ba |
| Polymer | 18.36 Aa | 18.75 Aa | 17.02 Aa | 0.20 Ba | 0.19 Ba | 0.25 Aa | 5.55 Ab | 5.72 Ab | 9.44 Aa | |
| WR2 | ||||||||||
| A | gs | E | ||||||||
| R3 | R5.1 | R5.5 | R3 | R5.1 | R5.5 | R3 | R5.1 | R5.5 | ||
| BRS 5980IPRO | Control | 19.94 Aa | 16.14 Ab | 11.29 Ac | 0.30 Aa | 0.24 Ab | 0.18 Ab | 6.55 Ab | 5.70 Ac | 6.94 Aa |
| Polymer | 15.17 Ba | 11.50 Bb | 8.90 Bc | 0.18 Ba | 0.12 Bb | 0.15 Bb | 5.01 Bb | 3.55 Bc | 6.12 Ba | |
| NA 5909RG | Control | 15.64 Aa | 12.39 Ab | 8.62 Ac | 0.20 Aa | 0.16 Ab | 0.16 Ab | 5.17 Ab | 4.76 Ac | 6.80 Aa |
| Polymer | 15.36 Aa | 8.82 Bc | 9.93 Ab | 0.17 Ba | 0.08 Bb | 0.14 Bb | 5.05 Bb | 2.89 Bc | 5.93 Ba | |
| BRS 7280RR | Control | 18.07 Aa | 14.32 Ab | 14.53 Ac | 0.22 Aa | 0.17 Ab | 0.22 Ab | 5.87 Ab | 4.83 Ac | 8.70 Aa |
| Polymer | 16.19 Ba | 13.94 Bb | 6.85 Bc | 0.18 Ba | 0.14 Bb | 0.10 Bb | 5.10 Bb | 4.55 Bc | 4.36 Ba | |
| WR1 | ||||||||||
| A | gs | E | ||||||||
| R3 | R5.1 | R5.5 | R3 | R5.1 | R5.5 | R3 | R5.1 | R5.5 | ||
| BRS 5980IPRO | Control | 16.0 Aa | 9.12 Ab | 8.27 Ab | 0.21 Aa | 0.11 Ab | 0.21 Aa | 4.98 Ab | 3.42 Ac | 7.52 Aa |
| Polymer | 12.21 Aa | 9.65 Ab | 3.68 Bb | 0.12 Aa | 0.10 Aab | 0.07 Bb | 3.63 Aa | 3.33 Aa | 3.10 Ba | |
| NA 5909RG | Control | 13.18 Aa | 6.70 Ab | 6.78 Ab | 0.14 Aa | 0.08 Ab | 0.15 Aa | 3.99 Ab | 2.62 Ac | 6.15 Aa |
| Polymer | 11.81 Aa | 9.09 Ab | 4.44 Bb | 0.12 Aa | 0.08 Aab | 0.07 Bb | 3.84 Aa | 2.68 Aa | 3.39 Ba | |
| BRS 7280RR | Control | 12.98 Aa | 6.90 Ab | 10.37 Ab | 0.14 Aa | 0.08 Ab | 0.16 Aa | 4.14 Ab | 2.75 Ac | 6.90 Aa |
| Polymer | 12.28 Aa | 12.02 Ab | 3.63 Bb | 0.13 Aa | 0.11 Aab | 0.07 Bb | 3.82 Aa | 4.08 Aa | 3.26 Ba | |
| Cultivar | Polymer | WR4 | |||||
|---|---|---|---|---|---|---|---|
| ϕPSII | ETR | ||||||
| R3 | R5.1 | R5.5 | R3 | R5.1 | R5.5 | ||
| BRS 5980IPRO | Control | 0.27 Ba | 0.27 Ba | 0.25 Aa | 144.63 Ba | 142.56 Aa | 133.21 Ba |
| Polymer | 0.29 Aa | 0.29 Aa | 0.26 Aa | 152.47 Aa | 152.47 Aa | 138.43 Aa | |
| NA 5909RG | Control | 0.29 Aa | 0.29 Aa | 0.25 Aa | 146.82 Ba | 146.82 Aa | 134.32 Ba |
| Polymer | 0.29 Aa | 0.29 Aa | 0.30 Aa | 154.25 Aa | 154.25 Aa | 157.72 Aa | |
| BRS 7280RR | Control | 0.27 Ba | 0.27 Ba | 0.25 Ba | 139.50 Ba | 139.50 Ba | 129.38 Ba |
| Polymer | 0.31 Aa | 0.31 Aa | 0.30 Aa | 165.35 Aa | 165.35 Aa | 159.65 Aa | |
| WR3 | |||||||
| ϕPSII | ETR | ||||||
| R3 | R5.1 | R5.5 | R3 | R5.1 | R5.5 | ||
| BRS 5980IPRO | Control | 0.27 Aa | 0.27 Aa | 0.22 Ab | 141.57 Aa | 141.57 Aa | 116.96 Aa |
| Polymer | 0.28 Aa | 0.28 Aa | 0.24 Ab | 145.69 Aa | 145.69 Aa | 126.89 Aa | |
| NA 5909RG | Control | 0.28 Aa | 0.28 Aa | 0.26 Aa | 148.07 Aa | 148.07 Aa | 133.82 Ab |
| Polymer | 0.30 Aa | 0.30 Aa | 0.26 Aa | 155.25 Aa | 155.25 Aa | 137.21 Ab | |
| BRS 7280RR | Control | 0.28 Aa | 0.28 Aa | 0.31 Aa | 148.86 Aa | 148.86 Aa | 160.48 Aa |
| Polymer | 0.30 Aa | 0.30 Aa | 0.26 Aa | 156.70 Aa | 156.70 Aa | 137.66 Aa | |
| WR2 | |||||||
| ϕPSII | ETR | ||||||
| R3 | R5.1 | R5.5 | R3 | R5.1 | R5.5 | ||
| BRS 5980IPRO | Control | 0.21 Aa | 0.21 Aa | 0.17 Aa | 109.24 Aa | 109.24 Aa | 90.55 Aa |
| Polymer | 0.19 Aa | 0.19 Aa | 0.17 Aa | 97.47 Ba | 97.47 Ba | 87.30 Aa | |
| NA 5909RG | Control | 0.22 Aa | 0.22 Aa | 0.23 Aa | 117.79 Aa | 117.79 Aa | 118.61 Aa |
| Polymer | 0.20 Ba | 0.20 Ba | 0.18 Ba | 106.26 Ba | 106.26 Ba | 92.80 Ba | |
| BRS 7280RR | Control | 0.25 Aa | 0.25 Aa | 0.26 Aa | 129.75 Aa | 132.58 Aa | 139.12 Aa |
| Polymer | 0.24 Ba | 0.24 Ba | 0.16 Ba | 126.58 Ba | 126.58 Ba | 85.62 Ba | |
| WR1 | |||||||
| ϕPSII | ETR | ||||||
| R3 | R5.1 | R5.5 | R3 | R5.1 | R5.5 | ||
| BRS 5980IPRO | Control | 0.12 Ba | 0.12 Ba | 0.12 Aa | 62.18 Ba | 62.18 Ba | 61.63 Aa |
| Polymer | 0.16 Aa | 0.16 Aa | 0.10 Aa | 86.11 Aa | 86.11 Aa | 51.74 Ba | |
| NA 5909RG | Control | 0.14 Ba | 0.14 Ba | 0.15 Aa | 75.00 Ba | 75.00 Ba | 76.96 Aa |
| Polymer | 0.16 Aa | 0.16 Aa | 0.10 Aa | 84.95 Aa | 84.95 Aa | 53.16 Ba | |
| BRS 7280RR | Control | 0.14 Ba | 0.14 Ba | 0.18 Aa | 71.27 Ba | 71.27 Ba | 92.46 Aa |
| Polymer | 0.21 Aa | 0.21 Aa | 0.11 Aa | 109.46 Aa | 109.46 Aa | 55.51 Ba | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, L.F.; Ribeiro Júnior, W.Q.; Ramos, M.L.G.; Soares, G.F.; de Lima Guimarães, C.A.; da Silva Neto, S.P.; Muller, O.; Vinson, C.C.; Pereira, A.F.; Williams, T.C.R. The Impact of Polymer on the Productivity and Photosynthesis of Soybean under Different Water Levels. Agronomy 2022, 12, 2657. https://doi.org/10.3390/agronomy12112657
Pereira LF, Ribeiro Júnior WQ, Ramos MLG, Soares GF, de Lima Guimarães CA, da Silva Neto SP, Muller O, Vinson CC, Pereira AF, Williams TCR. The Impact of Polymer on the Productivity and Photosynthesis of Soybean under Different Water Levels. Agronomy. 2022; 12(11):2657. https://doi.org/10.3390/agronomy12112657
Chicago/Turabian StylePereira, Lucas Felisberto, Walter Quadros Ribeiro Júnior, Maria Lucrecia Gerosa Ramos, Guilherme Filgueiras Soares, Cristiane Andréa de Lima Guimarães, Sebastião Pedro da Silva Neto, Onno Muller, Christina Cleo Vinson, André Ferreira Pereira, and Thomas Christopher Rhys Williams. 2022. "The Impact of Polymer on the Productivity and Photosynthesis of Soybean under Different Water Levels" Agronomy 12, no. 11: 2657. https://doi.org/10.3390/agronomy12112657
APA StylePereira, L. F., Ribeiro Júnior, W. Q., Ramos, M. L. G., Soares, G. F., de Lima Guimarães, C. A., da Silva Neto, S. P., Muller, O., Vinson, C. C., Pereira, A. F., & Williams, T. C. R. (2022). The Impact of Polymer on the Productivity and Photosynthesis of Soybean under Different Water Levels. Agronomy, 12(11), 2657. https://doi.org/10.3390/agronomy12112657







