Cloning and Functional Analysis of the Soybean GmRIQ2 Promoter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Promoter Cloning and Sequence Analysis
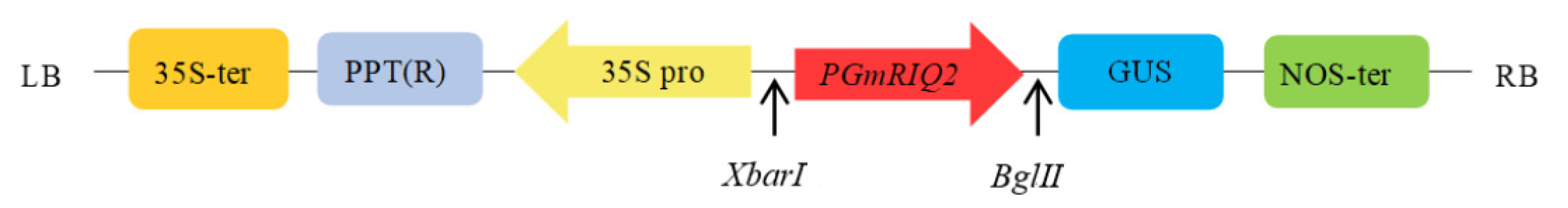
2.3. Construction and Transformation of a Promoter Expression Vector
2.4. Construction and Transformation of the Expression Vector for Promoter Deletion Fragments
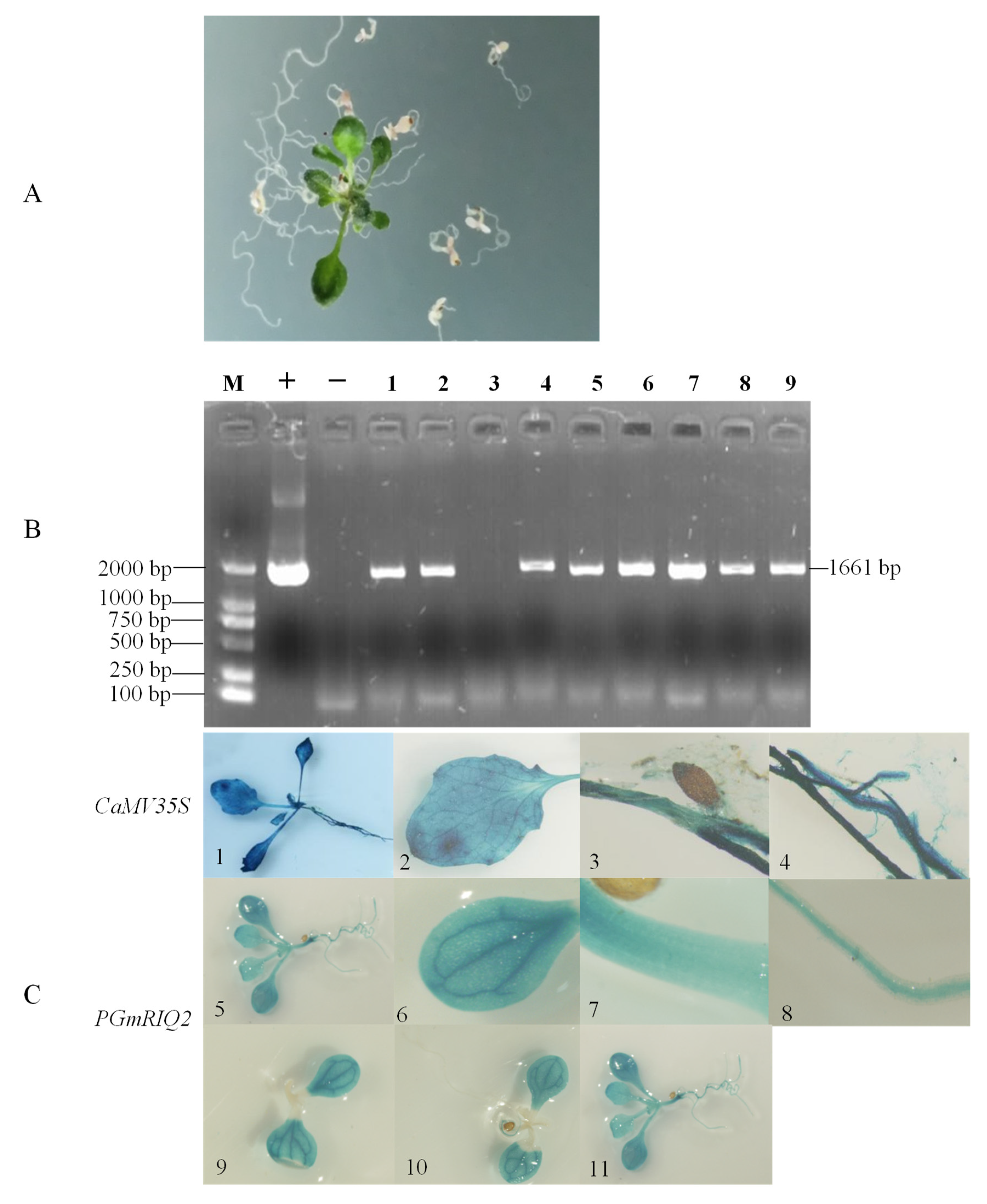
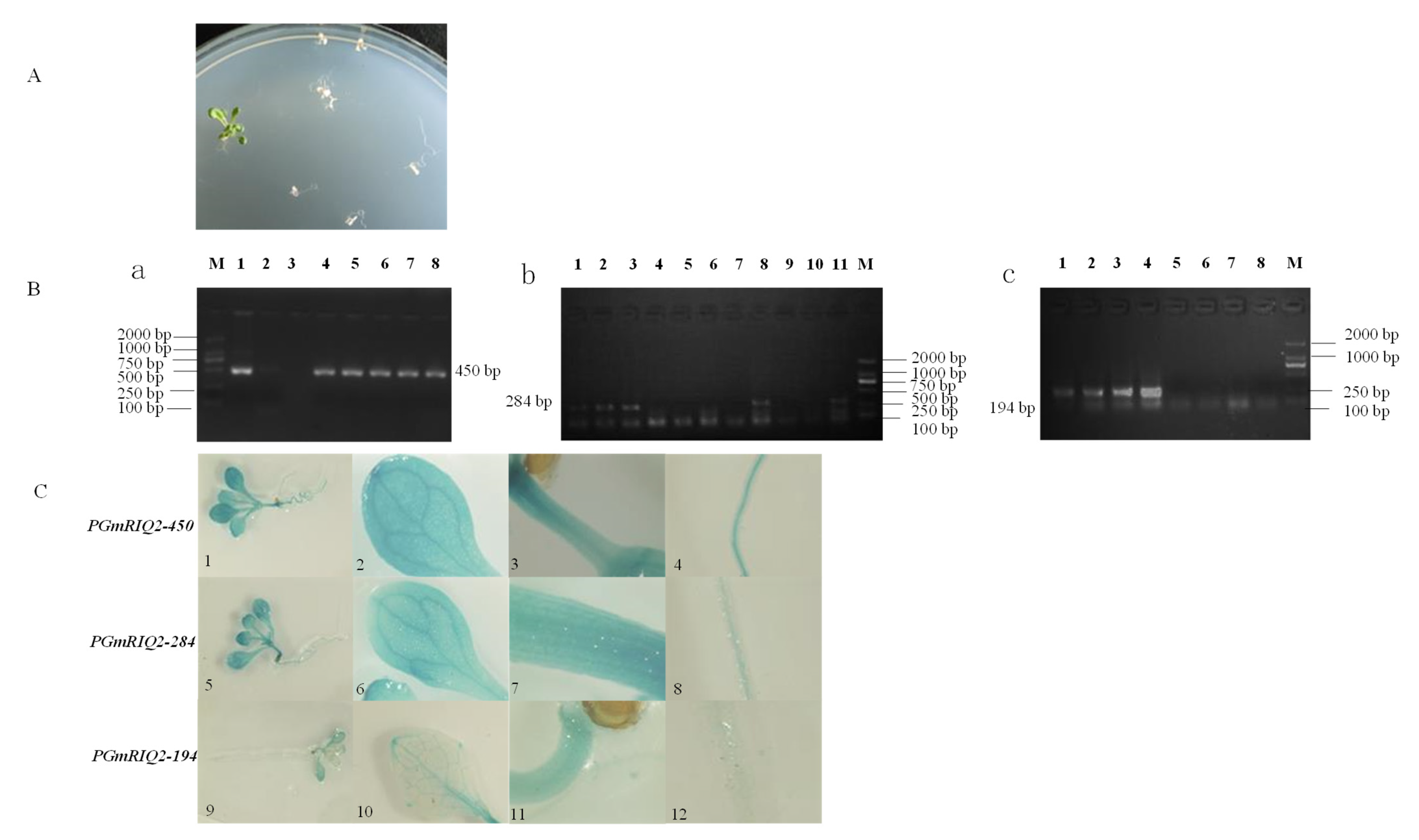
2.5. Cultivation and Identification of Transgenic Plants
2.6. Histochemical Staining
2.7. Hormone and Stress Management of Transgenic Arabidopsis Thaliana
2.8. GUS Fluorometric Quantitative Analysis
2.9. Hormone Treatment of Soybean Plants
2.10. Total RNA Extraction and Quantitative Real-Time (qRT)-PCR Analysis
3. Results
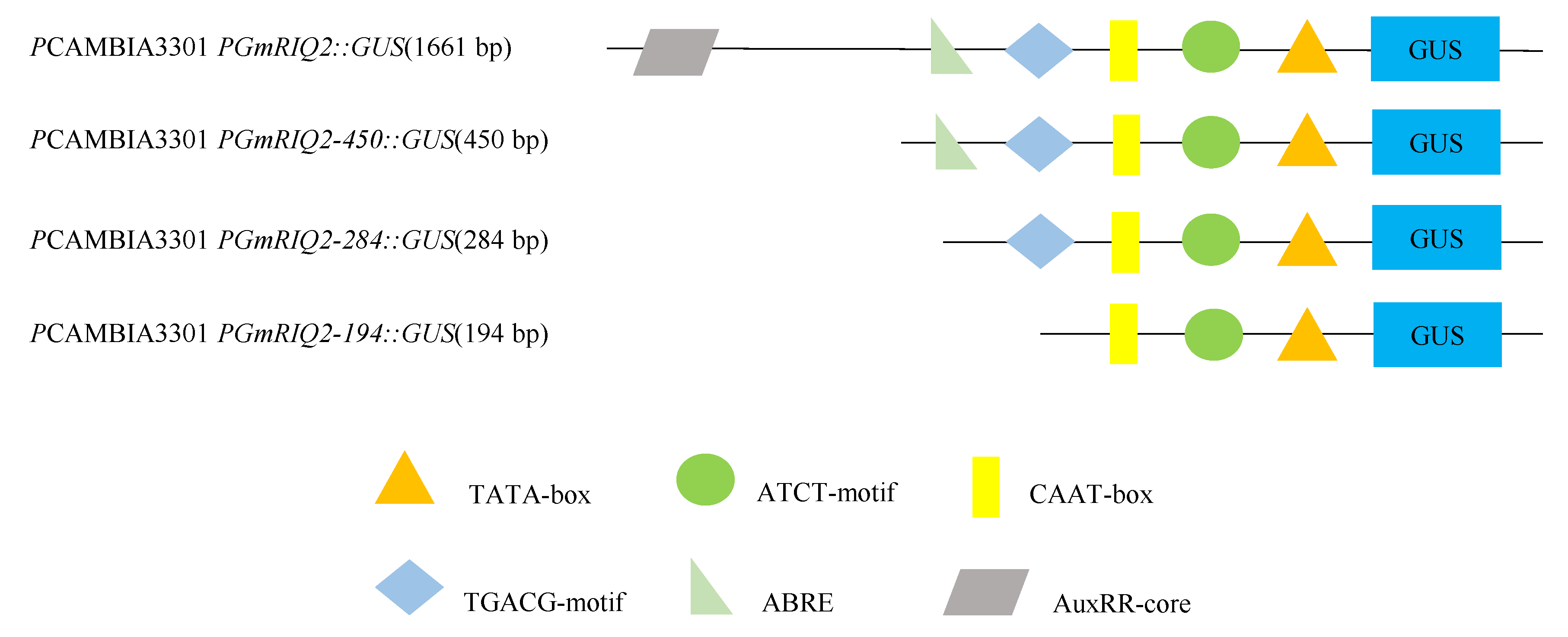
3.1. Bioinformatics Analysis of the PGmRIQ2 Sequence
3.2. GUS Gene Expression from PGmRIQ2 in Different Crops
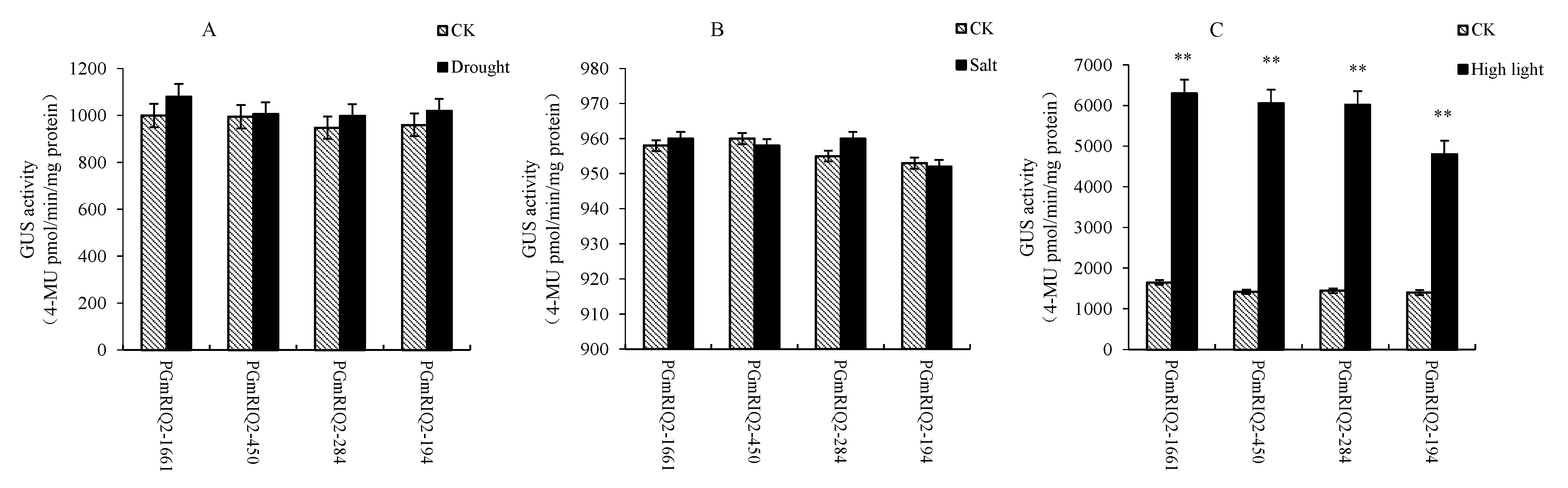
3.3. GUS Gene Expression Driven by PGmRIQ2 Deletion Mutants in Arabidopsis Thaliana
3.4. Functional Analysis of the GmRIQ2 Full-Length and Deletion Fragment Promoters
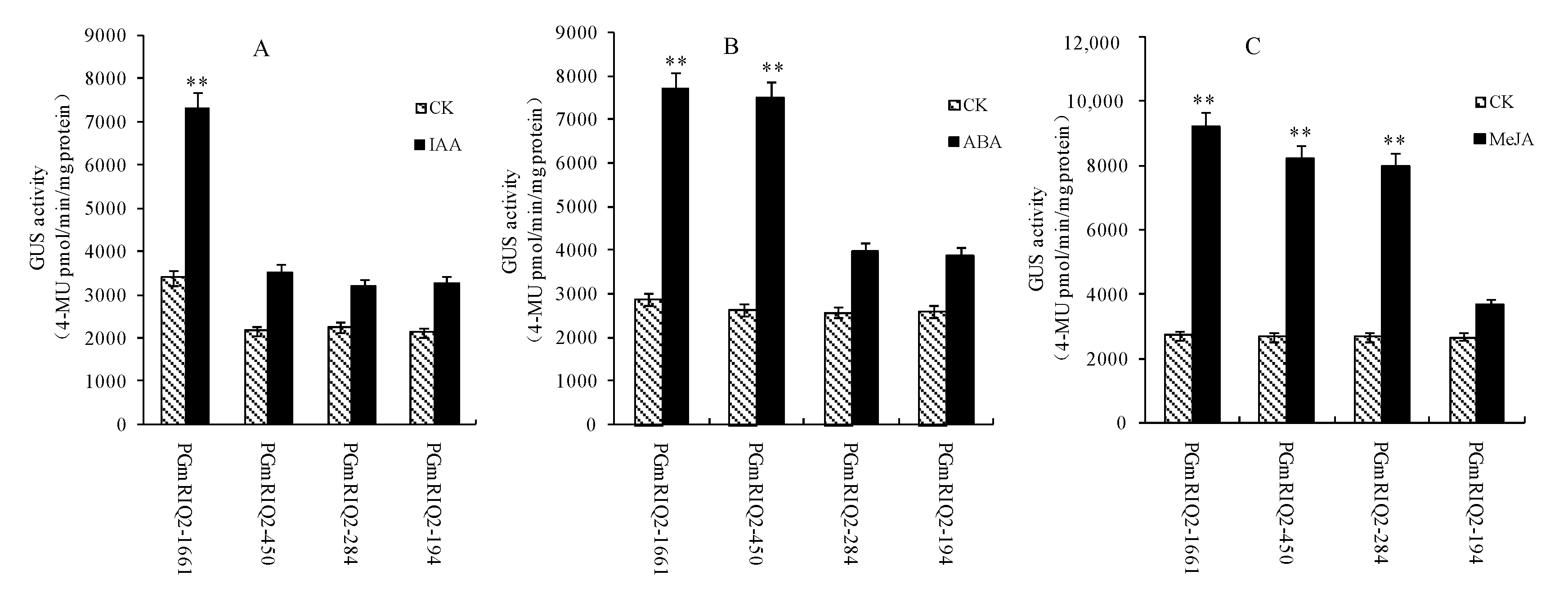
3.5. Gene Expression of GmRIQ2 in Hormone-Treated Soybean
4. Discussion
4.1. Application of the GUS Gene in Promoter Activity Analysis
4.2. Construction of Promoter Deletion Fragments by the 5′ End Deletion Method
4.3. Sequence Analysis of PGmRIQ2
4.4. GmRIQ2 Gene Promoter Expression of Vascular Tissue Specificity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.X.; Wang, W.Q.; Jiang, X.N.; Chen, X.M. Review on plant gene promoters. Acta Genetica. Sinica. 2004, 31, 1455–1464. [Google Scholar]
- Cabre, L.; Peyrard, S.; Sirven, C.; Gilles, L.; Pelissier, B.; Ducerf, S.; Poussereau, N. Identification and characterization of a new soybean promoter induced by Phakopsora pachyrhizi, the causal agent of Asian soybean rust. BMC Biotechnol. 2021, 21, 27. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, F.C.; Wang, W.Q.; Huang, L.X. Advance in the Study of Higher Plant Promoter. Biotechnol. Lett. 2006, 17, 658–661. [Google Scholar]
- Yokoyama, R.; Yamamoto, H.; Kondo, M.; Takeda, S.; Ifuku, K.; Fukao, Y.; Kamei, Y.; Nishimura, M.; Shikanai, T. Grana-localized proteins RIQ1, and RIQ2, affect the organization of light-harvesting complex II and grana stacking in Arabidopsis. Plant Cell. 2016, 28, 2261–2275. [Google Scholar] [CrossRef] [Green Version]
- Kadonaga, J.T. Perspectives on the RNA polymerase II core promoter. Wires Dev. Biol. 2012, 1, 40–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, R.J.; Bai, J.R.; Li, R.; Chang, L.F. Research progress of the inducible promoters in plant genetic engineering. J. Shanxi Agric. Sci. 2018, 46, 292–298. [Google Scholar]
- Li, T.; Sun, J.K.; Liu, J.T. Research progress of plant promoter. Biotechnol. Bull. 2015, 31, 18–25. [Google Scholar]
- Dutt, M.; Ananthakrishnan, G.; Jaromin, M.K.; Brlansky, R.H. Evaluation of four phloem-specific promoters in vegetative tissues of transgenic citrus plants. Tree Physiol. 2012, 32, 83–93. [Google Scholar] [CrossRef]
- Song, J.; Wang, D.; Zhang, F. Comparison of the Monte Carlo and guide to uncertainty in measurement methods in estimating measurement uncertainty: Indirect measurement of the CaMV35S promoter in mixed samples of genetically modified soybean. Food Control 2018, 90, 131–139. [Google Scholar] [CrossRef]
- Gittins, J.R.; Pellny, T.K.; Hiles, E.R.; Rosa, C.; James, D. Transgene expression driven by heterologous ribulose-1, 5-bisphosphate carboxylase/oxygenase small-subunit gene promoters in the vegetative tissues of apple (Malus pumila Mill.). Planta 2000, 210, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.J. Environmental risk assessment of releases of transgenic plants containing virus-derived inserts. Transgenic Res. 1996, 5, 359–362. [Google Scholar] [CrossRef]
- Kumpatla, S.P.; Chandrasekharan, M.B.; Iyer, L.M.; Li, G.; Hall, T.C. Genome intruder scanning and modulation systems and transgene silencing. Trends Plant Sci. 2012, 3, 97–104. [Google Scholar] [CrossRef]
- Teng, H.Y. Research progress of drought inducible promoters in rice. Mol. Plant Breed. 2020, 18, 450–458. [Google Scholar]
- Giuliano, G.; Pichersky, E.; Malik, M.P.; Timko, P.A.; Scolnik, A.R. An evolutionarily conserved protein binding sequence upstream of a plant light-regulated gene. Proc. Natl. Acad. Sci. USA 1988, 85, 7089–7093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.J.; Li, J.; Zhang, S.; Du, K.J. Research progress of plant promoter. North. Hortic. 2015, 22, 186–189. [Google Scholar]
- Qu, Y.; Zhang, N.; Chang, J.; Jin, X.; Wen, Y.K.; Si, H.J.; Wang, D. Cloning and Functional Analysis of Light-inducible, and Stem and Leaf-specific Expression Promoter ST-LS1 in Potato (Solanum tuberosumL.). J. Agric. Biotechnol. 2013, 21, 828–837. [Google Scholar]
- Kidokoro, S.; Watanabe, K.; Ohori, T.; Moriwaki, T.; Maruyama, K.; Mizoi, J.; Htwe, N.M.P.S.; Fujita, Y.; Sekita, S.; Shinozaki, K.; et al. Soybean DREB1/CBF-type transcription factors function in heat and drought as well as cold stress-responsive gene expression. Plant J. 2015, 81, 505–518. [Google Scholar] [CrossRef]
- Mahajan, S.; Tuteja, N.C. salinity and drought stresses: An overview. Arch. Biochem. Biophys. 2005, 444, 139–158. [Google Scholar] [CrossRef]
- Chen, Y.E.; Liu, W.J.; Su, Y.Q.; Cui, J.M.; Zhang, Z.W.; Yuan, M.; Zhang, H.Y.; Yuan, S. Different response of photosystem II to short and long term drought stress in Arabidopsis thaliana. Physiol. Plantarum. 2016, 158, 225–235. [Google Scholar] [CrossRef]
- Mengarelli, D.A.; Zanor, M.I. Genome-wide characterization and analysis of the CCT motif family genes in soybean (Glycine max). Planta 2021, 253, 1–17. [Google Scholar] [CrossRef]
- Mullineaux, C.W. Function and evolution of grana. Trends Plant Sci. 2005, 10, 521–525. [Google Scholar] [CrossRef]
- Shahmir, F.; Pauls, K.P. Identification gene structure and expression of bnmicemup: A gene upregulated in embryogenic brassica napus microspores. Front. Plant Sci. 2021, 11, 1899. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, D.; Yin, H.; Ma, L.; Zhang, J.; Zhang, B. Overexpression of GmRIQ2-like (Glyma.04G174400) enhances the tolerance of strong light stress and reduces photoinhibition in soybean (Glycine max (L.) Merr.). Agriculture 2020, 10, 157. [Google Scholar] [CrossRef]
- Spradling, A.C.; Stern, D.; Beaton, A.; Rhem, E.J.; Laverty, T.; Mozden, N.; Misra, S.; Rubin, G.M. The Berkeley Drosophila Genome Project gene disruption project: Single P-element insertions mutating 25% of vital Drosophila genes. Genetics 1999, 153, 135–177. [Google Scholar] [CrossRef] [PubMed]
- Petya, S.; Marieta, T.; Tzveta, G.; Velitchka, G.; Angel, A. A Modified CTAB Method for DNA Extraction from Soybean and Meat Products. Biotechnol. Biotechnol. Equip. 2013, 27, 3803–3810. [Google Scholar]
- Nakamura, S.I.; Suzui, N.; Ito-Tanabata, S.; Ishii, S.; Kawachi, N.; Rai, H.; Hattori, H.; Fujimaki, S. Application of glutathione and dithiothreitol to oil seed rape (Brassica napus L.) roots affects cadmium distribution in roots and inhibits Cd translocation to shoots. Soil Sci. Plant Nutr. 2016, 62, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Zhou, X.; Lu, J.; Wang, J.; Wang, X. Hairy roots induced by Agrobacterium rhizogenes and production of regenerative plants in hairy root cultures in maize. Sci. China (Ser. C: Life Sci.) 2006, 4, 305–310. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1999, 16, 735–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jefferson, R.A.; Burgess, S.M.; Hirsh, D. β-Glucuronidase from Escherichia coli as a gene-fusion marker. Proc. Natl. Acad. Sci. USA 1986, 83, 8447–8451. [Google Scholar] [CrossRef] [Green Version]
- Smale, S.T.; Kadonaga, J.T. The RNA polymerase II core promoter. Annu. Rev. Biochem. 2003, 72, 449–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, G.; Wei, D.Y.; Xu, B.B. Advances in Cis-element Analysis and Application of Plant Inducible Promoter Prd29A. Anhui Agri. Sci. Bull. 2020, 26, 32–36. [Google Scholar]
- Alam, J.; Cook, J.L. Reporter genes: Application to the study of mammalian gene transcription. Anal. Biochem. 1990, 188, 245–254. [Google Scholar] [CrossRef]
- Yang, Y.; Li, J.J.; Wang, S.; Qiu, G.Z. Progresses on reporter gene and its application. Life Sci. Res. 2011, 15, 277–282. [Google Scholar]
- Wang, Y.; Yang, Z.W.; Kong, Y.B.; Li, X.H.; Li, W.L.; Du, H.; Zhang, C.Y. GmPAP12 is required for nodule development and nitrogen fixation under phosphorus starvation in soybean. Front. Plant Sci. 2020, 11, 450. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.B.; Tang, G.Y.; Shan, L. Common methods and progress of plant type II promoter function research. Life Sci. 2013, 6, 40–47. [Google Scholar]
- Zeng, X.L.; Zhao, C.L.; Wen, G.S.; Ding, C.; Zhang, H.L. Research advances in prediction and validation methods for structures and functions of promoters. Mol. Plant Breed. 2018, 16, 3915–3925. [Google Scholar]
- Qin, L.L.; Zhang, X.; Jiang, C.; Li, L.I. Cloning and functional analysis of BpZFP4 Promoter from Birch (Betula platyphylla). Bull. Bot. Res. 2019, 39, 917–926. [Google Scholar]
- Li, H.Y. Cloning and Promoter Function Analysis of Somatic Embryogenesis Related Gene MIR171 in Lily. Doctoral Dissertation, Ornamental Horticulture of Shenyang Agricultural University, Shenyang, China, 2018. [Google Scholar]
- Bai, L.J.; Liu, W.; Wang, Z.L.; Wang, W.T.; Wu, C.X.; Feng, Y.J. Cloning and transient expression analysis of soybean GmbZIP33 gene promoter. Soybean Sci. 2019, 38, 511–516. [Google Scholar]
- Morffy, N.; Strader, L.C. Plant promoter-proximal pausing? Nat. Plants 2021, 7, 862–863. [Google Scholar] [CrossRef]
- Miyano, M.; Sayaman, R.W.; Shalabi, S.F.; Senapati, P.; Lopez, J.C.; Angarola, B.L.; Hinz, S.; Zirbes, O.; Yee, L.D.; Sedrak, M.S.; et al. Breast specific molecular clocks comprised of ELF5 expression and promoter methylation identify individuals susceptible to cancer initiation. Cancer Prev. Res. 2021, 14, 779–794. [Google Scholar] [CrossRef]
- Dell’Aquila, M.; Fiorentino, V.; Martini, M.; Capodimonti, S.; Cenci, T.; Lombardi, C.P.; Raffaelli, M.; Pontecorvi, A.; Fadda, G.; Pantanowitz, L.; et al. How limited molecular testing can also offer diagnostic and prognostic evaluation of thyroid nodules processed with liquid-based cytology: Role of TERT promoter and BRAF V600E mutation analysis. Cancer Cytopathol. 2021, 129, 819–829. [Google Scholar] [CrossRef] [PubMed]





| Primer Name | Primer Sequence (From 5′ to 3′) | Tm (°C) |
|---|---|---|
| PGmRIQ2-1661-F | GCTCTAGA(XbarI)CAGCACCAACCAAGCAGTTCTCTC | 55 |
| PGmRIQ2-1661-R | GAAGATCT(BglII)TACCATGAGGTTGCGCCTCAGAACCAGAGT | 55 |
| PGmRIQ2-450-F | GCTCTAGAGC(XbarI)CAGCACCAACCAAGCAGTTCTCTCT | 56 |
| PGmRIQ2-450-R | GAAGATCTTC(BglII)TACCATCCTGTTTGGGATTCTTTTA | 56 |
| PGmRIQ2-284-F | GCTCTAGAGC(XbarI)CAGCACCAACCAAGCAGT | 55 |
| PGmRIQ2-284-R | GAAGATCTTC(BglII)TACCATATTGGCGATTTGACCATG | 55 |
| PGmRIQ2-194-F | GCTCTAGAGC(XbarI)CAGCACCAACCAAGCAGT | 56 |
| PGmRIQ2-194-R | GAAGATCTTC(BglII)TACCATGCTAGGTGCAGGGAAACG | 56 |
| Bar-F | GCGGTACCGGCAGGCTGAAG | 55 |
| Bar-R | CCGCAGGAACCGCAGGAGTG | 55 |
| Medium | PH | Composition |
|---|---|---|
| GM | 5.8 | B5: 3.21 g; sucrose: 20 g; agar: 7.5 g |
| SCCM | 5.4 | Murashige and Skoog (MS): 0.449 g; sucrose: 30 g; MES: 3.9 g; agar: 7.5 g; acetosyringone (As): 100 mg/L; DTT: 154 mg/L |
| RM | 5.6 | MS: 0.449 g; sucrose: 30 g; MES: 0.59 g; agar: 7.5 g; cephalosporins: 250 mg/L |
| Primer Name | Primer Sequence (From 5′ to 3′) |
|---|---|
| Actin4-S | GTGTCAGCCATACTGTCCCCATT |
| Actin4-A | GTTTCAAGCTCTTGCTCGTAATCA |
| GmRIQ2-S | GTCACATAAAAACACACCACGA |
| GmRIQ2-A | TGCTTGATATCTCAACTCAGCT |
| Element | Core Sequence | Position | Number Present | Function |
|---|---|---|---|---|
| ABRE | ACGTG | 785 (+), 2012 (−) | 2 | Cis-acting element involved in ABA responsiveness |
| ARE | AAACCA | 311 (+), 1661 (+) | 2 | Cis-acting regulatory element essential for anaerobic induction |
| ATCT-motif | AATCTAATCC | 210 (+) | 1 | Part of a conserved DNA module involved in light responsiveness |
| AuxRR-core | GGTCCAT | 998 (−) | 1 | Cis-acting regulatory element involved in IAA responsiveness |
| Box II | CCACGTGGC | 2011 (+) | 1 | Part of a light responsive element |
| CGTCA-motif | CGTCA | 636 (−), 1913 (+) | 2 | Cis-acting regulatory element involved in MeJA responsiveness |
| G-box | CACGTG | 787 (+), 2010 (−) | 2 | Cis-acting regulatory element involved in light responsiveness |
| GATA-motif | AAGGATAAGG | 1862 (+) | 1 | Part of a light responsive element |
| RY-element | CATGCATG | 744 (+) | 1 | Cis-acting regulatory element involved in seed-specific regulation |
| TCT-motif | TCTTAC | 297 (+) | 1 | Part of a light responsive element |
| TGACG-motif | TGACG | 636 (+), 1913(−) | 2 | Cis-acting regulatory element involved in MeJA responsiveness |
| Agrobacterium rhizogenes Type | Number of Explants | Number of Hairy Roots | Positive Number | Conversion Rate | Positive Induction Rate |
|---|---|---|---|---|---|
| pCAMBIA3301PGmRIQ2::GUS | 90 | 38 | 27 | 42.22% | 71.05% |
| pCAMBIA3301::GUS | 90 | 36 | 28 | 40% | 77.78% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Yin, H.; Sun, Z.; Song, X.; Deng, J.; Zhang, Q.; Li, D. Cloning and Functional Analysis of the Soybean GmRIQ2 Promoter. Agronomy 2022, 12, 227. https://doi.org/10.3390/agronomy12010227
Zhang B, Yin H, Sun Z, Song X, Deng J, Zhang Q, Li D. Cloning and Functional Analysis of the Soybean GmRIQ2 Promoter. Agronomy. 2022; 12(1):227. https://doi.org/10.3390/agronomy12010227
Chicago/Turabian StyleZhang, Binbin, Huayi Yin, Zhihui Sun, Xiaohui Song, Jing Deng, Qian Zhang, and Dongmei Li. 2022. "Cloning and Functional Analysis of the Soybean GmRIQ2 Promoter" Agronomy 12, no. 1: 227. https://doi.org/10.3390/agronomy12010227
APA StyleZhang, B., Yin, H., Sun, Z., Song, X., Deng, J., Zhang, Q., & Li, D. (2022). Cloning and Functional Analysis of the Soybean GmRIQ2 Promoter. Agronomy, 12(1), 227. https://doi.org/10.3390/agronomy12010227






