Genotypic and Phenotypic Characterization of Lettuce Bacterial Pathogen Xanthomonas hortorum pv. vitians Populations Collected in Quebec, Canada
Abstract
1. Introduction
2. Materials and Methods
2.1. Purification of Bacterial Isolates
2.2. DNA Extraction and MultiLocus Sequence Analyses
2.3. Pathogenicity Assay
2.4. Purification of Xanthan
2.5. Purification of Xanthomonadins
2.6. Bacterial Genome Sequencing & De Novo Assembly
2.7. Genome-Wide Association Study (GWAS)
2.8. Diagnostic Tools
3. Results
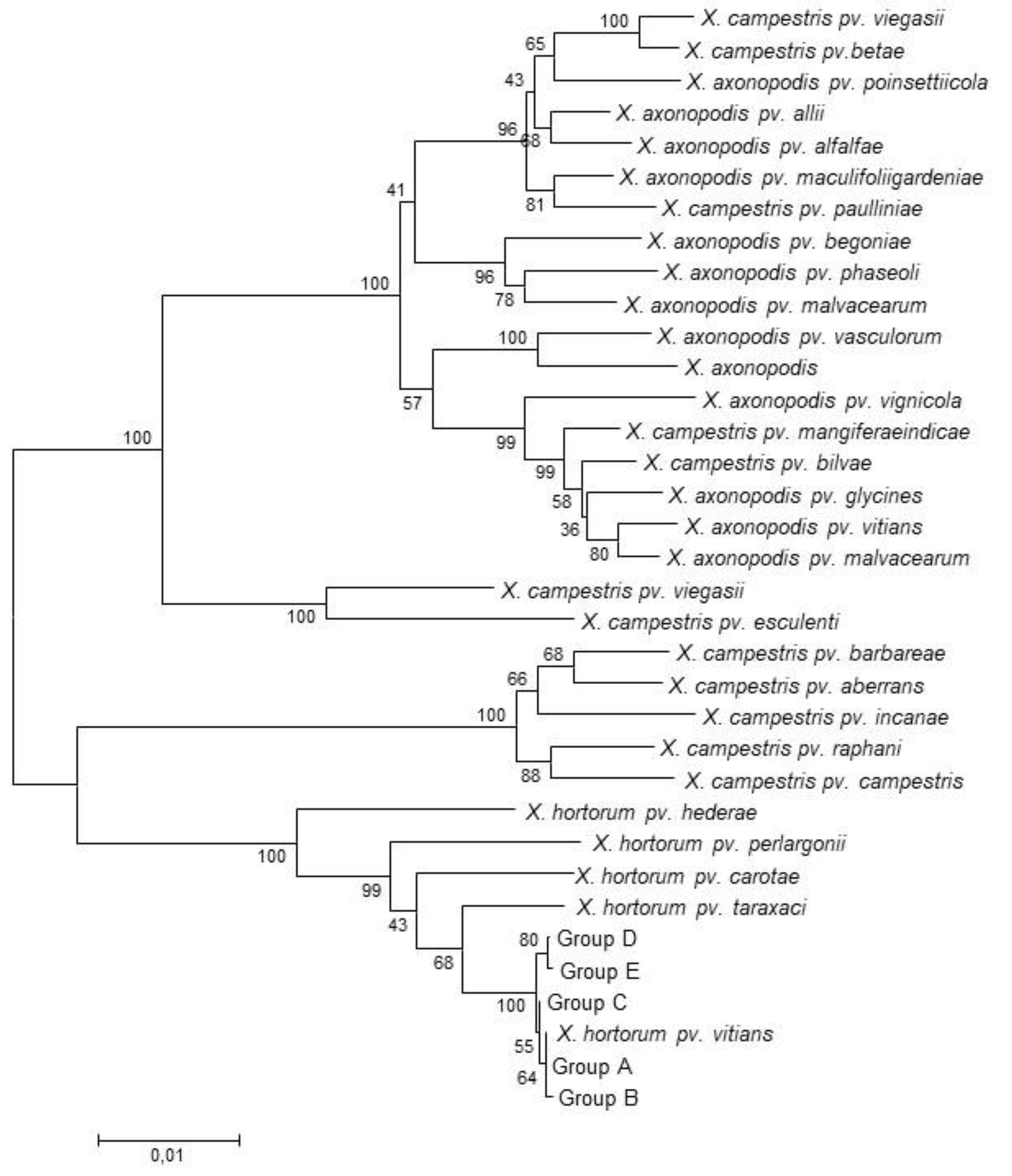
3.1. Multi Locus Sequence Analyses
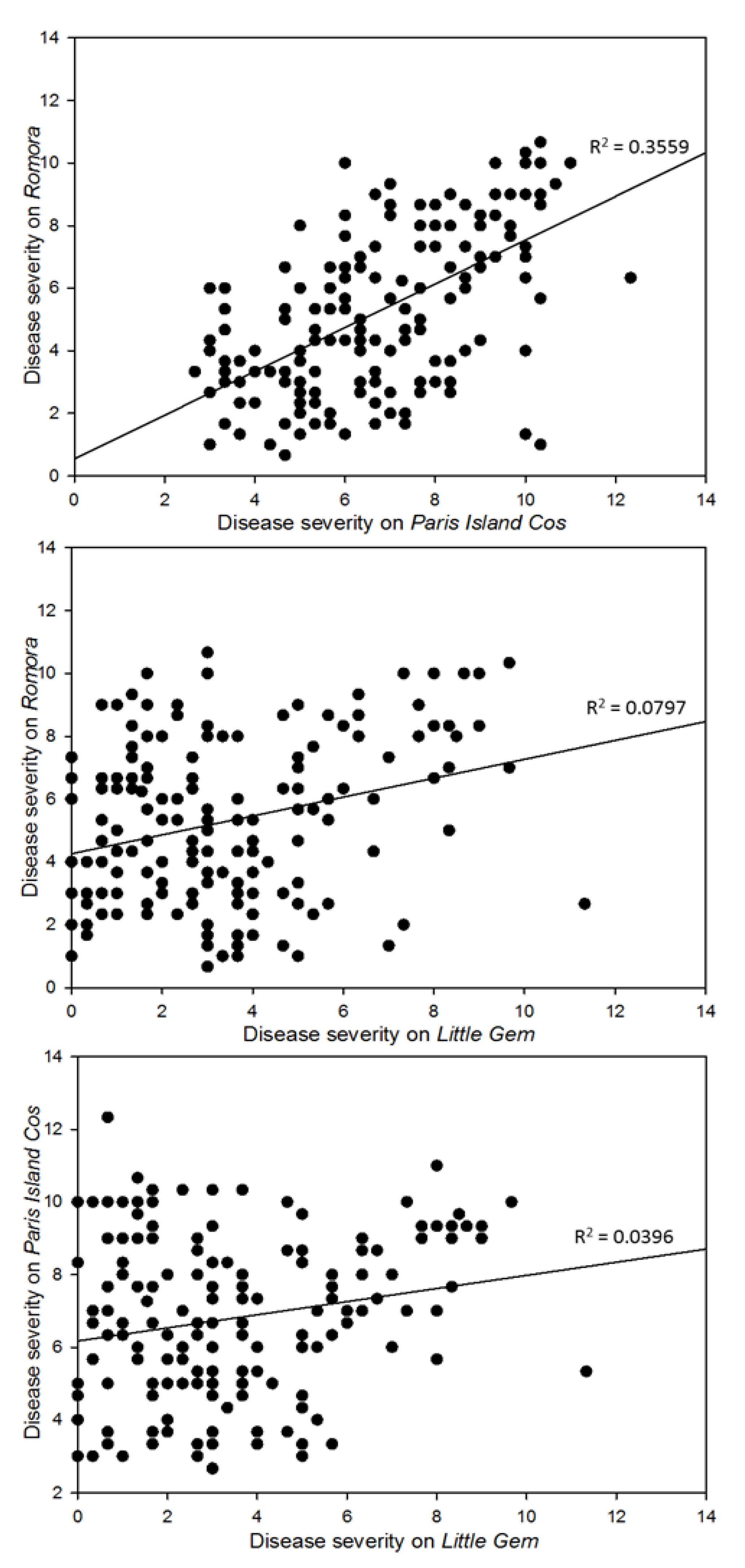
3.2. Pathogenicity Assay
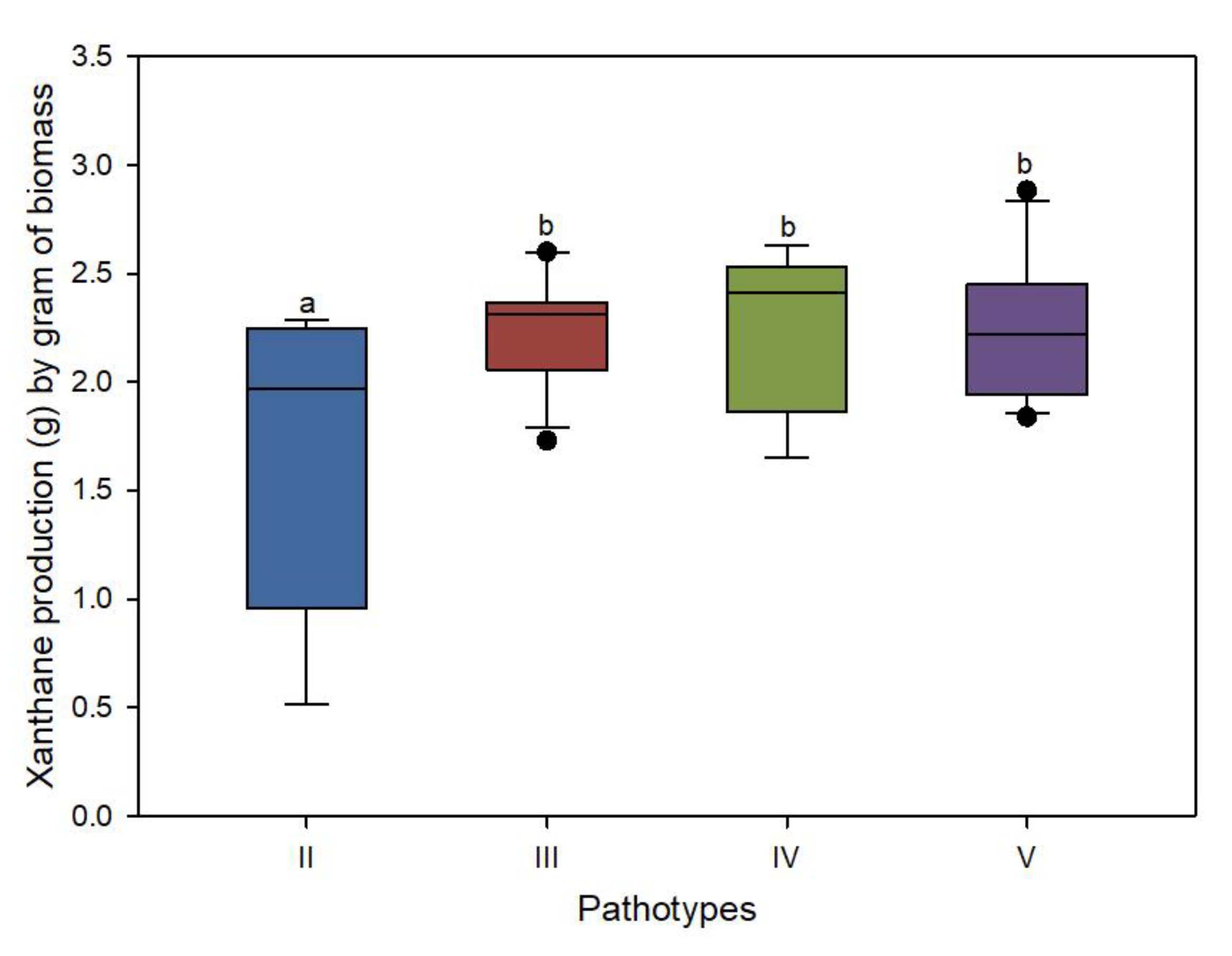
3.3. Xanthan Content Variation among Pathotypes
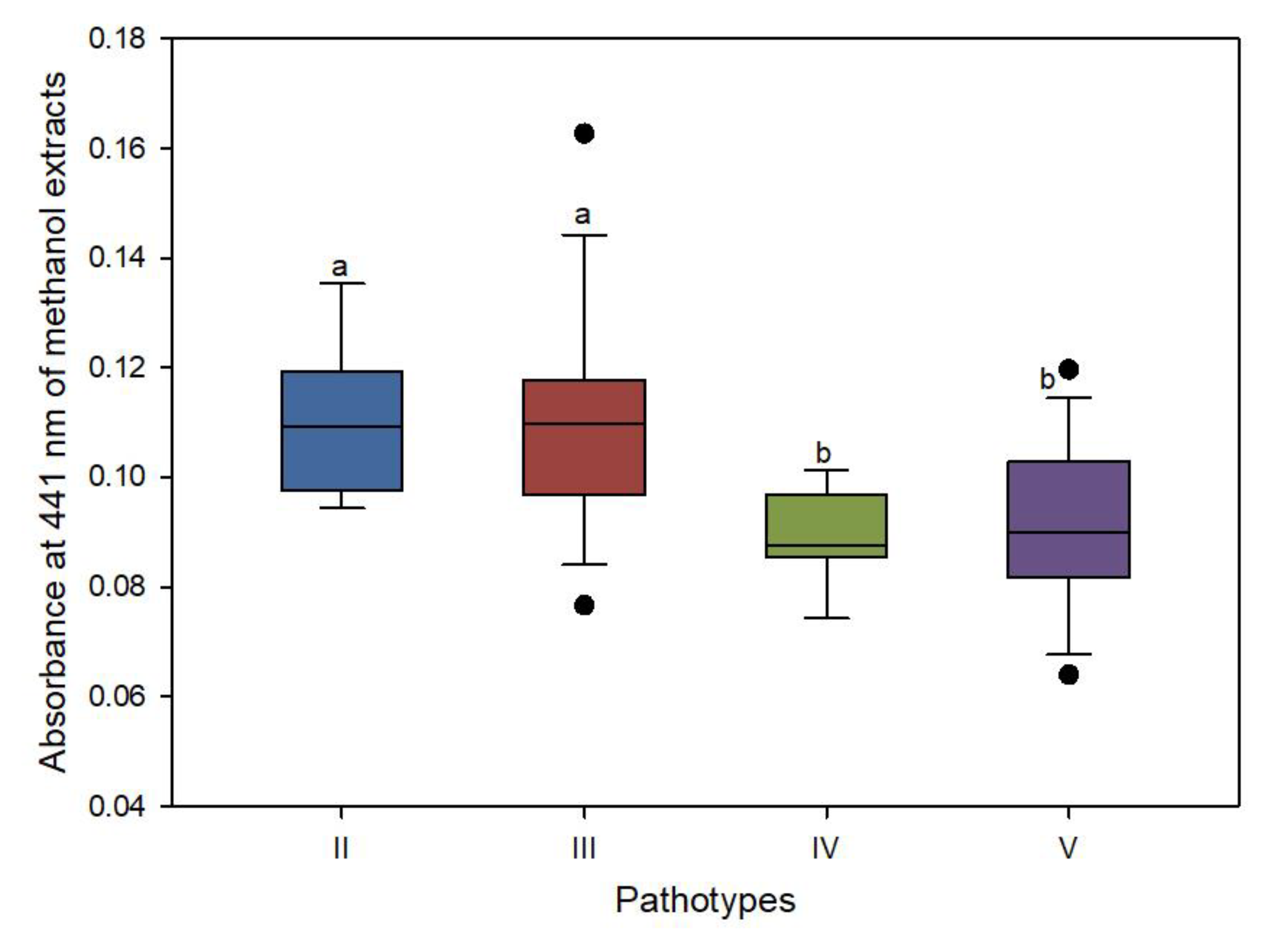
3.4. Xanthomonadin Content Variation among Pathotypes
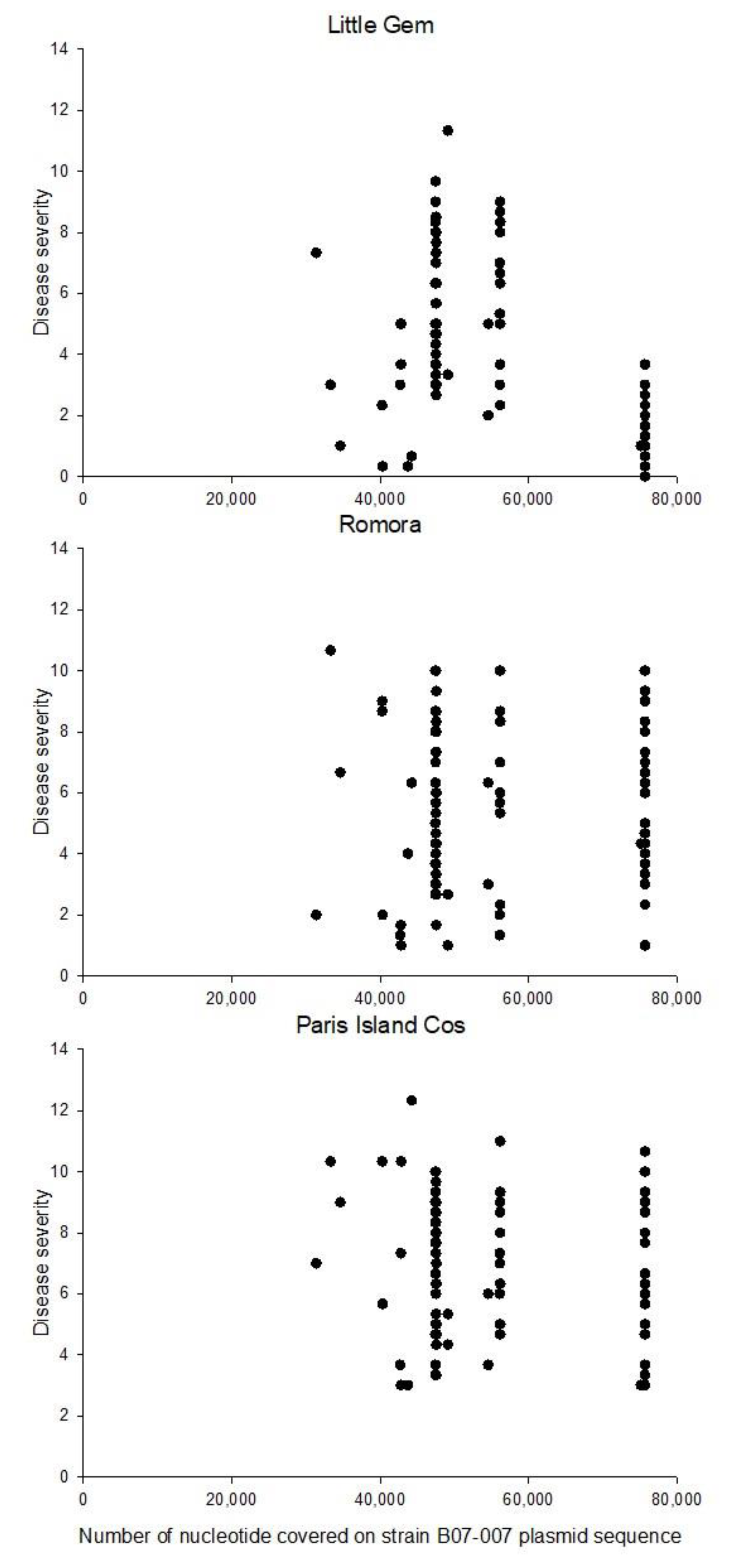
3.5. Genome Mapping, Contig De Novo Assembly & Genome Coverage Analysis
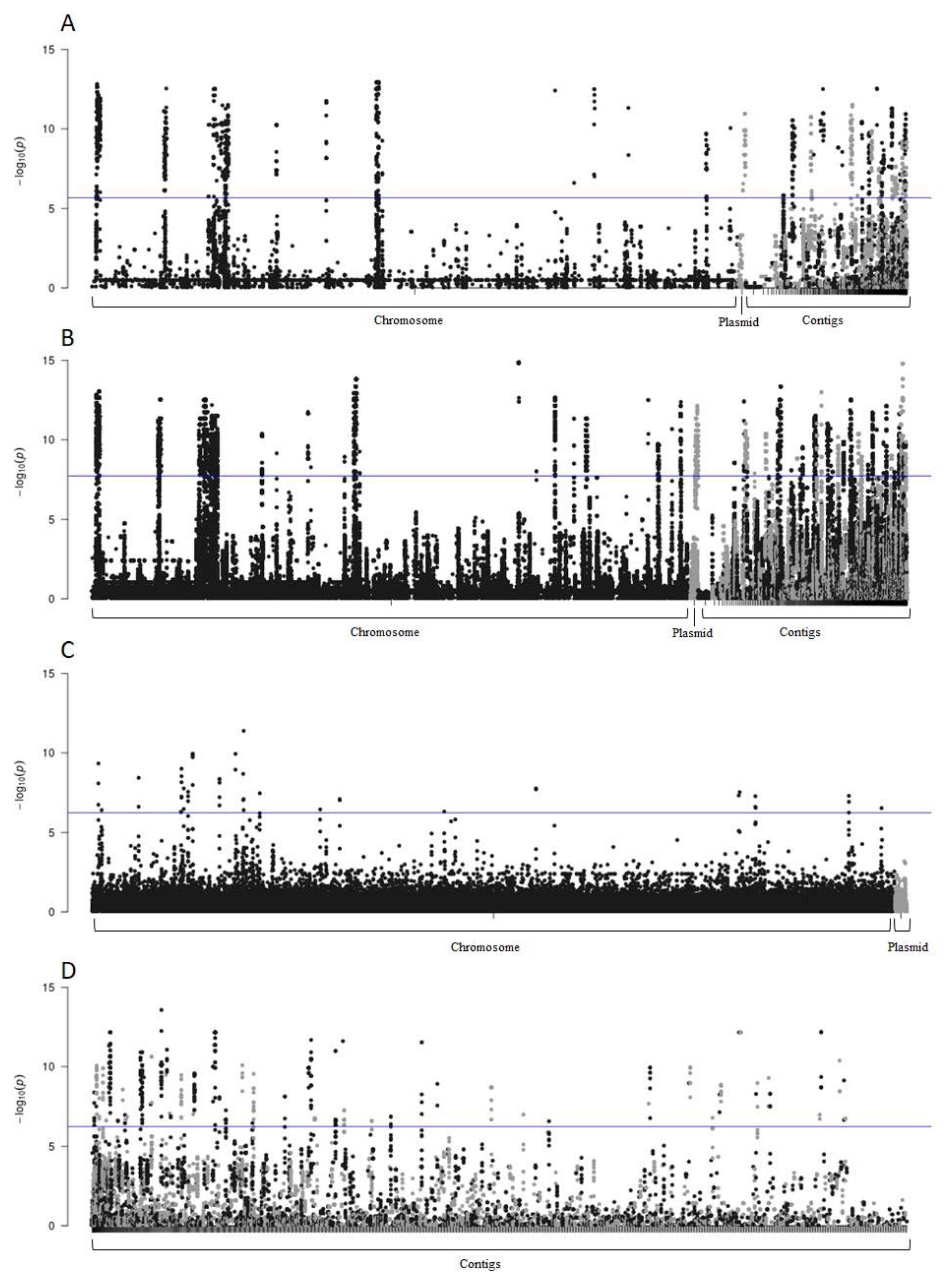
3.6. Genome-Wide Association Study (GWAS)
3.7. rhAmp® Genotyping for Detection of Pathogenic Isolates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Widmer, T.L.; Graham, J.H.; Mitchell, D.J. Histological comparison of fibrous root infection of disease-tolerant and susceptible citrus hosts by Phytophthora nicotianae and P. palmivora. Phytopathology 1998, 88, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Mandadi, K.K.; Scholthof, K.B. Plant immune responses against viruses: How does a virus cause disease? Plant Cell 2013, 25, 1489–1505. [Google Scholar] [CrossRef] [PubMed]
- Von Bodman, S.B.; Bauer, W.D.; Coplin, D.L. Quorum sensing in plant-pathogenic bacteria. Annu. Rev. Phytopathol. 2003, 41, 455–482. [Google Scholar] [CrossRef] [PubMed]
- Buttner, D.; He, S.Y. Type iii protein secretion in plant pathogenic bacteria. Plant Physiol. 2009, 150, 1656–1664. [Google Scholar] [CrossRef]
- Giraldo, M.C.; Valent, B. Filamentous plant pathogen effectors in action. Nat. Rev. Microbiol. 2013, 11, 800–814. [Google Scholar] [CrossRef]
- Buttner, D.; Bonas, U. Regulation and secretion of Xanthomonas virulence factors. FEMS Microbiol. Rev. 2010, 34, 107–133. [Google Scholar] [CrossRef]
- He, Y.-W.; Wu, J.; Zhou, L.; Yang, F.; He, Y.Q.; Jiang, B.-L.; Bai, L.; Xu, Y.; Deng, Z.; Tang, J.-L.; et al. Xanthomonas campestris diffusible factor is 3-hydroxybenzoic acid and is associated with xanthomonadin biosynthesis, cell viability, antioxidant activity, and systemic invasion. Mol. Plant-Microbe Interact. 2011, 24, 948–957. [Google Scholar] [CrossRef]
- Denny, T.P. Involvment of bacterial polysaccharides in plant pathogenesis. Annu. Rev. Phytopathol. 1995, 33, 173–197. [Google Scholar] [CrossRef]
- Brown, N.A. Some bacterial diseases of lettuce. J. Agric. Res. 1918, 13, 367–388. [Google Scholar]
- Davis, R.M.; Subbarao, K.V.; Kurtz, E.A.; Raid, R.N. Compendium of Lettuce Diseases; APS Press: Madison, WI, USA, 1997. [Google Scholar]
- Bradbury, J.F. Guide to Plant Pathogenic Bacteria; CAB International Mycological Institute: Slough, UK, 1986. [Google Scholar]
- Allex, D.; Rat, B. Les bactérioses des salades: Un problème omniprésent. PHM Rev. Hortic. 1990, 310, 45–50. [Google Scholar]
- Wallis, F.M.; Joubert, J.J. Bacterial spot of lettuce in natal. Landbouwet. S. Afr. Agric. Sci. S. Afr. Phytophylactica 1972, 4, 137–138. [Google Scholar]
- Zoina, A.; Volpe, E. Epidemiological Aspects of Lettuce Bacterial Spot Induced by Xanthomonas campestris pv. vitians, Plant Pathogenic Bacteria (Les Colloques INRA); INRA: Versailles, France, 1992; p. 797. [Google Scholar]
- Sahin, F. First report of bacterial spot of lettuce caused by Xanthomonas campestris pv. vitians in Turkey. Plant Dis. 2000, 84, 490. [Google Scholar] [CrossRef] [PubMed]
- Pernezny, K.; Raid, R.N.; Stall, R.E.; Hodge, N.; Collins, J. An outbreak of bacterial spot of lettuce in Florida caused by Xanthomonas campestris pv. vitians. Plant Dis. 1995, 79, 359–360. [Google Scholar] [CrossRef]
- Daboin, C.; Tortolero, O.J.F.V. Bacterial leaf spot of lettuce in some fields of the Venezuelan Andes. Fitopatol. Venezol. 1993, 6, 8–10. [Google Scholar]
- Toussaint, V.; Morris, C.E.; Carisse, O. Identification, resistance to antibiotics and utilization of carbon sources by Xanthomonas campestris pv. vitians strains isolated from different lettuce-growing areas. In Proceedings of the 7th International Congress of Plant Pathology, Edinburgh, UK, 1–31 July 1998. [Google Scholar]
- Robinson, P.; Jones, J.; Pernezny, K. Bacterial leaf spot of lettuce: Relationship of temperature to infection and potential host range of Xanthomonas campestris pv. vitians. Plant Dis. 2006, 90, 465–470. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barak, J.D.; Koike, S.T.; Gilbertson, R.L. Role of crop debris and weeds in the epidemiology of bacterial leaf spot of lettuce in california. Plant Dis. 2001, 85, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Sahin, F.; Miller, S.A. Identification of the bacterial leaf spot pathogen of lettuce, Xanthomonas campestris pv. vitians, in Ohio, and assessment of cultivar resistance and seed treatment. Plant Dis. 1997, 81, 1443–1446. [Google Scholar]
- FAO. Faostat Database; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Statistics Canada. Area, Production and Farm Gate Value of Marketed Vegetables; Statistics Canada: Ottawa, ON, Canada, 2020. [Google Scholar]
- USDA. Vegetables 2019 Summary; National Agricultural Statistics Service: Washington, DC, USA, 2020.
- Ritchie, D.F.; Dittapongpitch, V. Copper-and streptomycin-resistant strains and host differentiated races of Xanthomonas campestris pv. vesicatoria in North Carolina. Plant Dis. 1991, 75, 733–736. [Google Scholar]
- Cooksey, D.A. Genetics of bactericide resistance in plant pathogenic bacteria. Annu. Rev. Phytopathol. 1990, 28, 201–219. [Google Scholar] [CrossRef]
- Lu, H.; Hu, J.; Kwon, S.J. Association analysis of bacterial leaf spot resistance and SNP markers derived from expressed sequence tags (ESTs) in lettuce (Lactuca sativa L.). Mol. Breed. 2014, 34, 997–1006. [Google Scholar] [CrossRef]
- Wilson, E.E.; Zeitoun, F.M.; Fredrickson, D.L. Bacterial phloem canker, a new disease of Persian walnut trees. Phytopathology 1967, 57, 618–621. [Google Scholar]
- Young, J.M.; Park, D.C.; Shearman, H.M.; Fargier, E. A multilocus sequence analysis of the genus Xanthomonas. Syst. Appl. Microbiol. 2008, 31, 366–377. [Google Scholar] [CrossRef]
- Vauterin, L.; Hoste, B.; Kersters, K.; Swings, J. Reclassification of Xanthomonas. Int. J. Syst. Evol. Micr. 1995, 45, 472–489. [Google Scholar] [CrossRef]
- Carisse, O.; Ouimet, A.; Toussaint, V.; Philion, V. Evaluation of the effect of seed treatments, bactericides, and cultivars on bacterial leaf spot of lettuce caused by Xanthomonas campestris pv. vitians. Plant Dis. 2000, 84, 295–299. [Google Scholar] [CrossRef]
- Bull, C.T.; Goldman, P.H.; Hayes, R.; Madden, L.V.; Koike, S.T.; Ryder, E. Genetic diversity of lettuce for resistance to bacterial leaf spot caused by Xanthomonas campestris pv. vitians. Plant Health Prog. 2007, 8, 11. [Google Scholar] [CrossRef]
- Steffens, T.; Vorholter, F.J.; Giampa, M.; Hublik, G.; Puhler, A.; Niehaus, K. The influence of a modified lipopolysaccharide o-antigen on the biosynthesis of xanthan in Xanthomonas campestris pv. campestris b100. BMC Microbiol. 2016, 16, 93. [Google Scholar] [CrossRef]
- Starr, M.; Stephens, W.L. Pigmentation and taxonomy of the genus Xanthomonas. J. Bacteriol. 1964, 87, 293–302. [Google Scholar] [CrossRef]
- Poplawsky, A.R.; Chun, W. Pigb determines a diffusible factor needed for extracellular polysaccharide slime and xanthomonadin production in Xanthomonas campestris pv. campestris. J. Bacteriol. 1997, 179, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Andrews, S. FastQC. A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 28 March 2018).
- Ewels, P.; Magnusson, M.; Lundin, S.; Kaller, M. Multiqc: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with burrows-wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. Spades: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; Genome Project Data Processing, S. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- Li, H. A statistical framework for SNP calling, mutation discovery, association mapping and population genetical parameter estimation from sequencing data. Bioinformatics 2011, 27, 2987–2993. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. Plink: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Xie, C.; Tammi, M.T. CNV-seq, a new method to detect copy number variation using high-throughput sequencing. BMC Bioinform. 2009, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Schaad, N.W.; Jones, J.B.; Chun, W. Laboratory Guide for the Identification of Plant Pathogenic Bacteria, 3rd ed.; American Phytopathological Society: Saint Paul, MN, USA, 2001; p. 373. [Google Scholar]
- Hayes, R.J.; Trent, M.A.; Truco, M.J.; Antonise, R.; Michelmore, R.W.; Bull, C.T. The inheritance of resistance to bacterial leaf spot of lettuce caused by Xanthomonas campestris pv. vitians in three lettuce cultivars. Hortic. Res. 2014, 1, 14066. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, H.; Raid, R.N.; Nuessly, G.D.; Faroutine, G. Diverse responses of lettuce cultivars and germplasm lines to infections of three isolates of Xanthomonas campestris pv. vitians. Hortscience 2015, 50, 650. [Google Scholar] [CrossRef]
- Yun, M.H.; Torres, P.S.; El Oirdi, M.; Rigano, L.A.; Gonzalez-Lamothe, R.; Marano, M.R.; Castagnaro, A.P.; Dankert, M.A.; Bouarab, K.; Vojnov, A.A. Xanthan induces plant susceptibility by suppressing callose deposition. Plant. Physiol. 2006, 141, 178–187. [Google Scholar] [CrossRef]
- Souza, D.P.; Oka, G.U.; Alvarez-Martinez, C.E.; Bisson-Filho, A.W.; Dunger, G.; Hobeika, L.; Cavalcante, N.S.; Alegria, M.C.; Barbosa, L.R.; Salinas, R.K.; et al. Bacterial killing via a type iv secretion system. Nat. Commun. 2015, 6, 6453. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Canteros, B.I.; Minsavage, G.V.; Jones, J.B.; Stall, R.E. Diversity of plasmids in Xanthomonas campestris pv. vesicatoria. Phytopathology 1995, 85, 1482–1486. [Google Scholar] [CrossRef]
- Power, R.A.; Parkhill, J.; de Oliveira, T. Microbial genome-wide association studies: Lessons from human GWAS. Nat. Rev. Genet. 2017, 18, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Earle, S.G.; Wu, C.H.; Charlesworth, J.; Stoesser, N.; Gordon, N.C.; Walker, T.M.; Spencer, C.C.A.; Iqbal, Z.; Clifton, D.A.; Hopkins, K.L.; et al. Identifying lineage effects when controlling for population structure improves power in bacterial association studies. Nat. Microbiol. 2016, 1, 16041. [Google Scholar] [CrossRef] [PubMed]
- Brynildsrud, O.; Snipen, L.G.; Bohlin, J. CNOGpro: Detection and quantification of CNVs in prokaryotic whole-genome sequencing data. Bioinformatics 2015, 31, 1708–1715. [Google Scholar] [CrossRef]
- Zhang, F.; Hu, Z.; Wu, Z.; Lu, J.; Shi, Y.; Xu, J.; Wang, X.; Wang, J.; Zhang, F.; Wang, M.; et al. Reciprocal adaptation of rice and Xanthomonas oryzae pv. oryzae: Cross-species 2D GWAS reveals the underlying genetics. Plant Cell 2021, 33, 2538–2561. [Google Scholar] [PubMed]


| Diameter of Lesions | Score | Occurrence of Lesions | Score |
|---|---|---|---|
| <2 mm | 1 | On one inoculated leaf | 0.1 |
| 2 mm ≤ lesions ≤ 5 mm | 2 | On less than half of inoculated leaves but on more than one | 0.2 |
| 5 mm ≤ lesions ≤ 9 mm | 3 | On more than half of inoculated leaves | 0.3 |
| 9 mm ≤ lesions ≤ 20 mm | 4 | ||
| 20 mm ≤ lesions ≤ 41 mm | 5 |
| Pathotypes | Number of Isolates | Symptoms Observed | ||
|---|---|---|---|---|
| Little Gem | Paris Island Cos | Romora | ||
| I | 4 | − | − | + |
| II | 17 | − | + | − |
| III | 67 | − | + | + |
| IV | 21 | + | + | − |
| V | 60 | + | + | + |
| Isolates | Contig 245 | Genome 590,650 | Average Disease Severity | Isolates | Contig 245 | Genome 590,650 | Average Disease Severity |
|---|---|---|---|---|---|---|---|
| Ct | Ct | Little Gem | Ct | Ct | Little Gem | ||
| 190716A | 21.04 | 29.92 | 6.0 | 240914AN | 31.48 | 26.84 | 0.7 |
| 270716U | 21.23 | 40 | 3.0 | 170914I | 31.8 | 28.42 | 3.3 |
| 100816Q | 21.33 | 40 | 5.0 | 140916A | 31.85 | 37.36 | 0.7 |
| 190716C | 21.4 | 30.53 | 5.3 | 060814K | 32.1 | 29.65 | 0.7 |
| 100816U | 21.45 | 40 | 9.7 | 030914AG | 32.12 | 32.18 | 2.7 |
| 030914AC | 21.6 | 40 | 5.0 | 170914Y | 32.46 | 29.39 | 0.0 |
| 190716I | 21.69 | 40 | 4.7 | 240914F | 32.47 | 27.96 | 1.3 |
| 170914S | 21.77 | 40 | 4.0 | 170914AJ | 32.58 | 31.22 | 2.0 |
| 070815F | 21.83 | 40 | 3.7 | 060814AA | 32.8 | 30.78 | 2.7 |
| 100816C | 21.9 | 40 | 4.0 | 240914P | 32.89 | 28.03 | 2.3 |
| 140916O | 21.96 | 40 | 6.0 | 100914A | 33.09 | 29.5 | 2.0 |
| 030914A | 22.04 | 40 | 4.0 | 060814Z | 33.16 | 30.49 | 1.3 |
| 240914A | 22.05 | 40 | 8.0 | 030914K | 33.37 | 29.8 | 0.0 |
| 060814V | 22.05 | 40 | 3.3 | 030914T | 33.47 | 30.76 | 0.7 |
| 260814AC | 22.07 | 40 | 3.0 | 030914O | 33.49 | 29.83 | 1.3 |
| 170914AK | 22.12 | 40 | 3.0 | 040816B | 33.69 | 31.6 | 1.7 |
| 030914W | 22.13 | 39.97 | 5.3 | 100914L | 33.72 | 31.68 | 1.7 |
| 260814AF | 22.23 | 40 | 5.7 | 100914F | 33.94 | 29.03 | 1.7 |
| 100914X | 22.23 | 40 | 0.7 | 170914A | 34.13 | 29.96 | 2.7 |
| 030914E | 22.26 | 40 | 4.0 | 170914T | 34.36 | 29.46 | 1.7 |
| 030914AD | 22.27 | 40 | 5.0 | 200814I | 34.38 | 28.49 | 2.3 |
| 030914J | 22.28 | 40 | 3.7 | 170914Q | 34.67 | 30.34 | 0.7 |
| 240914AK | 22.31 | 40 | 3.0 | 030915I | 36.02 | 32.49 | 1.7 |
| 240914BC | 22.41 | 40 | 3.0 | 260814E | 36.79 | 29.24 | 3.0 |
| 200814N | 22.42 | 40 | 4.0 | 190716K | 36.9 | 28.24 | 1.0 |
| 110815K | 22.46 | 40 | 7.7 | 030915A | 37 | 39.09 | 1.7 |
| 160615A | 22.46 | 40 | 1.7 | 260814V | 37.02 | 29.35 | 0.0 |
| 100914Q | 22.47 | 31.21 | 2.7 | 260814AR | 37.14 | 28.55 | 0.3 |
| 110815S | 22.49 | 40 | 3.7 | 260814P | 38.01 | 28.54 | 2.7 |
| 240914Q | 22.54 | 40 | 4.0 | 070815H | 38.15 | 29.61 | 2.7 |
| 100914AG | 22.54 | 40 | 0.3 | 070815D | 38.33 | 31.73 | 2.7 |
| 100914V | 22.55 | 40 | 3.0 | 110815AH | 38.89 | 29.74 | 0.7 |
| 100816F | 22.59 | 40 | 3.0 | 110815AF | 39.04 | 30.97 | 1.3 |
| 070815B | 22.63 | 31.18 | 2.0 | 110815Z | 40 | 32.19 | 0.0 |
| 240914AT | 22.66 | 40 | 4.0 | 160615C | 40 | 27.7 | 1.0 |
| 110815D | 22.87 | 40 | 8.3 | 290715K | 40 | 38.74 | 2.7 |
| 110815AB | 23.67 | 40 | 6.7 | ||||
| 270716A | 24.58 | 29.41 | 0.7 | ||||
| 030914Y | 24.84 | 40 | 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hébert, P.-O.; Laforest, M.; Xu, D.; Ciotola, M.; Cadieux, M.; Beaulieu, C.; Toussaint, V. Genotypic and Phenotypic Characterization of Lettuce Bacterial Pathogen Xanthomonas hortorum pv. vitians Populations Collected in Quebec, Canada. Agronomy 2021, 11, 2386. https://doi.org/10.3390/agronomy11122386
Hébert P-O, Laforest M, Xu D, Ciotola M, Cadieux M, Beaulieu C, Toussaint V. Genotypic and Phenotypic Characterization of Lettuce Bacterial Pathogen Xanthomonas hortorum pv. vitians Populations Collected in Quebec, Canada. Agronomy. 2021; 11(12):2386. https://doi.org/10.3390/agronomy11122386
Chicago/Turabian StyleHébert, Pierre-Olivier, Martin Laforest, Dong Xu, Marie Ciotola, Mélanie Cadieux, Carole Beaulieu, and Vicky Toussaint. 2021. "Genotypic and Phenotypic Characterization of Lettuce Bacterial Pathogen Xanthomonas hortorum pv. vitians Populations Collected in Quebec, Canada" Agronomy 11, no. 12: 2386. https://doi.org/10.3390/agronomy11122386
APA StyleHébert, P.-O., Laforest, M., Xu, D., Ciotola, M., Cadieux, M., Beaulieu, C., & Toussaint, V. (2021). Genotypic and Phenotypic Characterization of Lettuce Bacterial Pathogen Xanthomonas hortorum pv. vitians Populations Collected in Quebec, Canada. Agronomy, 11(12), 2386. https://doi.org/10.3390/agronomy11122386






