Abstract
Biofortification has been widely used to increase mineral nutrients in staple foods, such as wheat (Triticum aestivum). In this study, a new approach has been used by analyzing the effect of inoculation with a plant growth-promoting rhizobacterium (PGPR), namely, Bacillus aryabhattai RSO25 and the addition of 1% (v/v) of organometallic Fe-containing polymeric nanoparticles (FeNPs) alone and in combination. Previously, the minimal inhibitory concentration of FeNPs for the bacterium was determined in order not to inhibit bacterial growth. All treatments had minor effects on seed germination and plant survival. Considering the physiology of plants, several photosynthetic parameters were significantly improved in individual treatments with FeNPs or the bacterium, particularly the efficiency of the photosystem II and the electron transport rate, which is indicative of a better photosynthetic performance. However, at the end of the experiment, a significant effect on final plant growth was not observed in shoots or in roots. When using FeNPs alone, earlier spike outgrow was observed and the final number of spikes increased by 20%. Concerning biofortification, FeNPs increased the concentration of Fe in spikes by 35%. In fact, the total amount of Fe per plant base rose to 215% with regard to the control. Besides, several side effects, such as increased Ca and decreased Na and Zn in spikes, were observed. Furthermore, the treatment with only bacteria decreased Na and Fe accumulation in grains, indicating its inconvenience. On its side, the combined treatment led to intermediate Fe accumulation in spikes, since an antagonist effect between RSO25 and FeNPs was observed. For this reason, the combined treatment was discouraged. In conclusion, of the three treatments tested, FeNPs alone is recommended for achieving efficient Fe biofortification in wheat.
1. Introduction
With the constant rise in human population, food security and quality are paramount issues for this century. It is estimated that one billion people are undernourished and more than a third of the total human population are malnourished [1]. In this context, “hidden hunger” is a widespread global problem affecting billions of people globally. Hidden hunger is not “hunger as we know”, but is related to strong deficiency in micronutrients and vitamins [2,3]. Iron deficiency is one of the most common micronutrient deficiencies and causes anemia, fatigue and hampers the cognitive development of children [2,3]. Causes are diverse and include poor and deficient diets, little diversified diets, elevated requirements at particular stages of life, such as infancy or pregnancy, or other problems, such as malabsorption, etc. [4].
Several factors, including soil degradation, desertification, salinization, acidification, and soil pollution are affecting the yield and quality of food globally; these conditions unfortunately will be even more adverse under future climate change scenarios. Consequently, one of the biggest challenges for sustaining global food security is to search for implemented methods or techniques to mitigate its effects on agricultural production, in terms of quantity and quality [5].
In this regard, biofortification is emerging as a solution to cope with the loss of food quality as it can increase the content of micronutrients (such as Fe, Zn, and Se) in plants for food security and new functional foods. Plant biofortification can be achieved by traditional crop breeding or transgenesis, among others [6]. More recently, the use of hybrid organic-metallic nanoparticles (NPs) has been developed as a very convenient strategy to improve the nutrient content of several microelements in plants [7]. This type of NPs, synthesized via different chemical methodologies [8], presents multiple and extensive applications in various fields, such as medicine, treatment of tumors, drug delivery, antimicrobial agents, and biomarkers, etc. [9]. At high concentrations, NPs induce the production of reactive oxygen species (ROS), inhibit germination, affect carbon fixation, photosynthesis, and water use efficiency [10,11]. However, at low concentrations, NPs sometimes improve seed germination, plant growth, and/or photosynthesis and induce the defense system towards pathogens or abiotic stress [12,13,14]. Biofortification of rice and wheat plants with Fe and Zn has been reported using metallic NPs [15,16]. However, the use of organometallic FeNPs containing tannic acid has not been tested for biofortification, in spite of the fact that these FeNPs have multiple applications in medicine [17,18]. However, despite the recognized potential of this methodology, there are gaps that must be filled to reliably determine its potential for improving production quality and determining its conditions for use. In this sense, it would be interesting to investigate if NPs would enhance the plant’s ability to accumulate more iron when supplied in the form of FeNPs prepared from tannic acid and stabilized with the biocompatible polymer, polyvinyl alcohol. It is worth mentioning that tannic acid is a harmless and environmentally friendly plant-derived polyphenolic compound with good reducing and stabilizing properties [19], able to form complexes with iron [20] and increasing the possibility to deliver iron to the plant.
Concerning the mechanism of the action of FeNPs, they can enter the roots either via root tips and rhizodermis or through wounds and lateral root junctions [21]. Once FeNPs are taken up by the plant, besides being accumulated in root cells, they can also be translocated to upper plant tissues via symplastic or apoplastic pathways. In the symplastic pathway, NPs move between the cytoplasm/vacuoles of adjacent cells, whereas in an apoplastic pathway NPs absorbed by the root are translocated through the cell walls and intercellular spaces and reach the Casparian strip and xylem [22,23]. In another scenario, it could be possible that NPs do not enter the plant. Grasses are known to secrete several phytosiderophores that act as Fe chelators (mugineic, hidroxi-mugenic, and deoxy-mugenic acids among others), that could extract Fe from the PVA-TA-FeNPs and transport it to the interior of the plant [24]. In this scenario, the constant for complex formation with these substances will determine the fate of Fe atoms in the rhizosphere [25]. This would mean that the nanoparticle would act only as an intermediate for releasing Fe in the rhizosphere. In previous studies, contrasting results are reported. Whereas in ryegrass (Lolium perenne L.) and pumpkin (Cucurbita mixta) no translocation of FeNPs into the plants was observed [26], other authors reported the uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants [25]. Indeed, it could be possible that plant genetics, nanoparticle composition and delivery methods can affect the uptake and translocation of FeNPs, as well as the biofortification possibilities of different treatments [27].
Furthermore, the employment of plant associated microorganisms is a powerful tool for facing the decrease of plant productivity related to multiple kinds of stress situations. In fact, the study of phytomicrobiota associated with plant roots is one of the most relevant areas of research, not only within plant sciences and cropping, but also in the environmental field [28]. In this line, some of these bacteria, known as rhizobacteria, live in the rhizosphere of plants where they can establish a relationship with plant roots and promote plant growth and, consequently, its production [29,30]. The mechanisms by which plant growth-promoting rhizobacteria (PGPR) help plants grow are divided into direct mechanisms (including nutrient acquisition, such as phosphate and potassium solubilization, nitrogen fixation, iron acquisition, and growth stimulation through the secretion of pf phytohormones, etc.), and indirect mechanisms (such as diminution of plant stress based on 1-aminocyclopropane-1-carboxylate (ACC) deaminase activity, biocontrol activity and induction of the plant resistance mechanisms) [30,31,32]. In addition, some PGPR can display biocontrol activity through the secretion of siderophores, production of substances with bactericidal action or inhibition of communication signals in the rhizosphere [29]. In the case of Fe, PGPR can mobilize Fe and increase the availability of this element, which in turn can be better absorbed by plant roots [31]. This is due to the secretion of organic acids, such as maleic, fumaric, citric, and succinic, etc. which acidify the rhizosphere and increase the availability of Fe. In addition, some PGPR secrete siderophores, which are molecules with a high affinity for Fe that form complexes with this element, making it available for the bacterium but also for the plant [33].
Therefore, this study was designed and conducted to contribute to filling some of the abovementioned gaps of knowledge. In particular, the following questions were assessed. (i) Will the application of FeNPs prepared from polyvinyl alcohol combined with tannic acid and/or bacterial inoculation improve the growth, physiology, and iron uptake in wheat plants? (ii) Do these treatments share the same physiological targets and levels of improvement? (iii) Is there a synergy effect using bacteria and FeNPs to reach a better balance between plant growth and iron uptake, particularly in grains? To answer these questions, the variations of plant growth and physiology, bacterial root colonization, and iron accumulation pattern in plant tissues were followed and studied in detail.
2. Materials and Methods
2.1. Synthesis and Characterization of Tannic Acid-Polyvinyl Alcohol Nanoparticles and Iron Nanoparticles (FeNPs)
Previous to the synthesis of FeNPs, tannic acid-PVA NPs were synthesized by scaling up the method previously described [8]. Two grams of polyvinyl alcohol (PVA) were dissolved in 200 mL of distilled water at 1000 rpm for 30 min. To this solution, 680 mg of tannic acid (TA) dissolved in 200 mL of distilled water were added dropwise for 20 min with vigorously stirring (1400 rpm). This mixture was additionally stirred for 30 min at 1400 rpm to obtain a white and stable suspension. To the previously described PVA-TANPs suspension, 2 g of anhydrous iron chloride (FeCl3) were added as a solid in small portions with vigorous stirring at 1400 rpm for 5 min. Once the addition finished, the mixture turned rapidly from white to dark blue-green and was stirred for an additional 15 min. The obtained suspension was dialyzed for 48 h at room temperature against distilled water using a cellulose membrane.
After dialysis, Fe analysis of the NPs was carried out following the colorimetric procedure based on the formation of a colored complex after Fe (II) reaction with o-phenanthroline [34]. First, Fe3+ was reduced to Fe2+ with ascorbic acid, which reacts with o-phenantroline in acidic medium to obtain a reddish-orange complex. The absorbance of this complex was measured at 512 nm to reveal 2200 ppm total Fe in NPs.
The characterization of NPs, both PVA-TANPs and FeNPs, was carried out by measuring the hydrodynamic size, Z potential and polydispersity index (PDI) of each NP using Dynamic Light Scattering (DLS) in a Zetasizer Nano ZS (Malvern Panalitical, Malvern, UK). DLS measurements gave a hydrodynamic diameter of 165.5 nm, a Z potential of −20.7 mV, and a PDI of 0.168 in PVA-TANPs. Likewise, FeNPs showed a size of 187 nm, a Z potential of 2.1 mV, and a PDI of 0.130. As the results demonstrated, the FeNPs size was greater than their Fe-free predecessors (Supplementary Information, Figure S1). The Z potential was considerably higher for the FeNPs due to the positive charges provided by the Fe2+ ions that can bind to the multiple negative charges present in tannic acid by ionic binding. The change in Z potential indicated that Fe2+ is located not only inside the NPs but is also attached to the FeNPs’ surface.
2.2. Bacterial Growth Conditions
The bacterium used in this study was Bacillus aryabhattai RSO25, which was previously isolated, identified, and characterized [35]. This bacterium has shown plant growth promoting properties and enhanced the growth of the host plant, Spartina densiflora [36], but was never used to inoculate crop plants. Moreover, this bacterium was able to enhance the accumulation of several heavy metals, such as Cu, Pb and Zn, in plants of S. densiflora [36].
Bacteria were cultured on TSA (tryptone soya agar) plates and incubated at 28 °C for 48 h. Plates could be kept at 4 °C for up to two weeks before subculturing them on fresh medium. For the preparation of liquid cultures, one individual colony was transferred to a TSB (tryptone soya broth) medium and cultivated overnight at 28 °C and 200 rpm. The absorbance at 600 nm of the cultures was determined and adjusted to 1.0 with the addition of a sterile TSB medium. Finally, 5 mL of the bacterial inoculum (diluted in 1 L of watering solution) were added per tray containing four pots × three plants per pot once a month during the experimental run-up.
2.3. Determination of Optimal FeNPs Doses
Tannic acid at high concentrations has been shown to inhibit bacterial growth on pathogenic microorganisms, such as Staphylococcus aureus [37]. However, in plant-associated bacteria, positive effects of tannic acid were reported [38]. For this reason, first, we tested the possible inhibitory effect of TA-PVA NPs (before and after adding Fe) on the bacterium by determining the minimal inhibitory concentration CMI to fix the maximum applicable concentration of NPs in the subsequent plant experiments. The CMI was tested in 96-well plates (see scheme in Supplementary Information Figure S2).
TA-PVA NPs or FeNPs were added to Müeller-Hinton medium [39] at 20% (v/v). The concentration of TA-PVA NPs or FeNPs in the wells followed a 2-base logarithm series, ranging from 20% to 0.3125% (v/v). Two hundred µL of suspensions were deposited in the wells of microtiter plates in triplicate. Wells were inoculated with 5 µL of overnight cultures of the bacterial strain Bacillus aryabhattai RSO25 (absorbance at 600 nm adjusted to 1.0). An additional row was kept without inoculation as control. Since initial turbidity was observed due to NPs, the initial absorbance at 600 nm was determined by using a microtiter plate reader (Synergy Neo2, BioTek, Agilent, Santa Clara, CA, USA). After incubating the plate for 24 h at 28 °C, the A600nm was determined and the initial value in each well was detracted from the final value.
To confirm the lack of inhibition, the effect of FeNPs on RSO25 growth and morphology was analyzed by using a low vacuum scanning electron microscope (SEM) (Pro, Phenom, Thermo Fisher Scientific, Waltham, MA, USA). For this purpose, RSO25 was incubated overnight at 28 °C in a TSB medium containing 1% FeNPs. After 24 h incubation, 50 µL of the culture were transferred to the metal plate of the microscope and frozen at −20 °C. The frozen metal plate was introduced into the low vacuum chamber of the microscope for observation.
2.4. Effect of Treatment on Seed Germination, Seedlings Survival, and Root Colonization by Bacillus Aryabhattai RSO25
Seeds of Triticum aestivum cv. Mulhacen (a commercial variety in Spain) were surface disinfected with ethanol (70%) for 2 min, followed by 5% sodium hypochlorite for 5 min. Finally, the seeds were washed four times with sterile distilled water.
To evaluate the effect of treatment on the germination rate, seeds were placed in square plates (five plates × ten seeds per plate) and exposed to four treatments: (i) Control: Hoagland solution-agar [40]; (ii) FeNPs: Hoagland solution-agar containing 1% FeNPs; (iii) RSO25: Hoagland solution agar and every seed was inoculated with 100 µL of an overnight culture of RSO25; (iv) TT combined: Hoagland solution-agar containing 1% FeNPs and every seed was inoculated with 100 µL of an overnight culture of RSO25. Plates were sealed and placed vertically, the bottom part was covered with black paper, and they were incubated in a light regime of 14 h light: 10 h dark at 22:18 °C in a plant growth cabinet. The germination rate and survival were recorded for the first five days. Moreover, seedling survival was evaluated after seven days.
Root colonization by RSO25 in the absence and presence of FeNPs was evaluated seven days after inoculation. Plant seedlings were taken out of the plates, washed with distilled water, and allowed to dry for 5 min at room temperature on sterile empty petri dishes. Small pieces of roots (approx. 2–3 mm) were cut, placed on the metal plate of a low vacuum scanning electron microscope (SEM) (Pro, Phenom, Thermo Fisher Scientific, Waltham, MA, USA), and observed as indicated in Section 2.3.
2.5. Effect of the Treatments on Plant Growth, Physiology, and Iron Accumulation
Surface disinfected seeds were pre-germinated for two days on water-agar plates in the dark at 18 °C and transferred to 13 cm high × 15 cm diameter plastic pots (three seeds per pot) using a mix of perlite, vermiculite, and sand (1:1:1) previously sterilized as a potting substrate. The experiment was conducted from September to November 2020 and it was done in the green house in Seville (Southwest Spain, latitude 37.358874°-longitude 5.986893°) with controlled growth conditions: day/night cycle of 16 h light: 8 h dark, and temperatures adjusted to 24 ± 2 °C during the day and 18 ± 2 °C during the night. Relative humidity was maintained at 45–55%. Concerning illumination, the green house has artificial illumination of fluorescent/incandescent lamps in order to supplement natural irradiation to reach a constant value of 1000–1200 micromoles photons m−2 s−1.
After a stabilization period of seven days, pots were placed on trays and randomly assigned to four different treatments (four pots per tray and three plants per pot, in total, n = 12 plants per treatment). Plants were placed in trays which were randomly arranged in different positions every week to avoid side effects. Every tray was watered once a week with the same treatments as described before: (i) Control: 1 L of Hoagland’s solution [40]; (ii) FeNPs: 1 L of Hoagland solution containing 1% (v:v) FeNPs; (iii) RSO25: 1 L of Hoagland solution and 5 mL of the bacterial culture (inoculation was performed once a month, the rest of the weeks plants were watered only with 1 L of the watering solution), and (iv) Combined TT: 1 L of Hoagland solution containing 1% (v/v) FeNPs and 5 mL of the bacterial culture (inoculation was performed only once a month). The experiment lasted for 45 days. It must be considered that, after the results of CMI, it has been established that 1% (v/v) FeNPs was below the CMI for the bacterium. Table 1 shows the design of the experiment and the parameters determined.

Table 1.
Scheme of the experimental design and the parameters determined.
At day 30 (before seed setting), photosynthetic activity was determined. Thus, leaf gas exchange and chlorophyll fluorescence parameters were measured in fully expanded leaves (n = 7) using an infrared gas analyzer (LI-6400-XT, Li-COR Inc., Lincoln, NE, USA) and a modulated fluorimeter (FMS-2; Hansatech Instruments Ltd., Norfolk, UK), respectively. Thus, the net photosynthetic rate (AN), stomatal conductance (gs), intercellular CO2 concentration (Ci), apparent carboxylation efficiency (Ce: ratio between AN and Ci) and instantaneous water use efficiency (iWUE: ratio between AN and gs) were obtained with the following settings: flux light density 1000 µmol photons m−2 s−1 (with 15% blue light to maximize stomatal aperture), ambient CO2 concentration (Ca) 400 µmol mol−1 air, leaf temperature of 25 ± 2 °C, 50 ± 5% relative humidity and vapor pressure deficit of 2.0–3.0 kPa. On the other hand, the saturation pulse method was used to determine the maximum quantum efficiency of PSII photochemistry (Fv/Fm) and the quantum efficiency of PSII (ΦPSII) [41]. As described by [42], a 0.8 s saturated actinic light pulse of 10,000 µmol m−2 s−1 was given at midday (1400 µmol photons m−2 s−1) in previously dark- and light-adapted leaves for 30 min using a modulate fluorimeter (FMS-2; Hansatech Instruments Ltd., Norfolk, UK). Using this information, the electron transport rate (ETR) was calculated [43].
At the end of the experiment, i.e., at day 45, plants were harvested and aboveground and belowground fractions were separated, dried at 80 °C for 48 h, and weighed for dry mass determination.
Finally, the concentrations of mineral nutrients in plant tissues were determined in spikes, shoots, and roots. Samples of 500 mg tissues (n = 2 per treatment) were digested with nitric acid, hydrochloric acid, and hydrogen peroxide in microwave at 240 °C in a closed glass (Milestone, Ultrawave, Sorisole, Italy) and Fe, Zn, Ca, Na, K, Mg, S and P concentrations were measured by a radial inductively coupled plasma (ICP) spectrometer (Horiba Jobin Yvon, Ultima 2, Tulln, Austria).
2.6. Statistical Analysis
Data of growth and physiological parameters are means ± medium standard error of 12 determinations, whereas the determination of Fe accumulation was done in duplicate, with each sample being a mix of six plants (two samples of six plants each). Statistical tests were performed in the software package Statistica v. 10.0 (Statsoft Inc., Hamburg, Germany). Two-way statistical models were used to analyze the interactive effects of bacterial inoculation and FeNPs application (as categorical factors) on the growth, physiological parameters and ion accumulation (as dependent variables) of Triticum aestivum plants. Multiple comparisons were analyzed by a LSD (post hoc) test. Before statistical analysis, Kolmogorov-Smirnov and Brown-Forsythe tests were used to verify the assumptions of normality and homogeneity of variances, respectively.
3. Results and Discussion
3.1. Effect of FeNPs on the Growth of the Bacterial Strain
First of all, the tolerance of the bacterium to FeNPs was assessed, since metallic NPs at high concentrations are known to be toxic to all living beings [44]. The effect of both TA-PVA NPs (before loading them with Fe) and FeNPs on bacterial growth was studied to determine the minimal inhibitory concentration (MIC) for RSO25. Data indicated that the presence of FeNPs affected bacterial growth. In fact, the growth was inhibited in a dose-dependent manner, being MIC 2.5% (v/v). Since FeNPs concentration in the plant experiment was 1% (v/v), this concentration was not inhibitory to bacterial growth. On its side, and since tannic acid had been reported as inhibitory for some bacteria [37], the CMI for TA-PVA NPs (without Fe) was also tested. In this case, the CMI was 2.5%, below the concentration used in the experiments, which was 1% (v/v).
Furthermore, microscopy observation was done to confirm the survival of bacteria after incubating RSO25 overnight in the presence of 1% FeNPs. Microscopy observation under a low vacuum microscope showed the presence of dense colonies, indicating bacterial multiplication. Moreover, the morphology of the cells (long and thick rods in pairs or short chains of 4–6 bacteria) seemed not to be affected (Figure 1). The appearance of round structures could indicate the formation of spores since this species is a spore-forming rod [45].
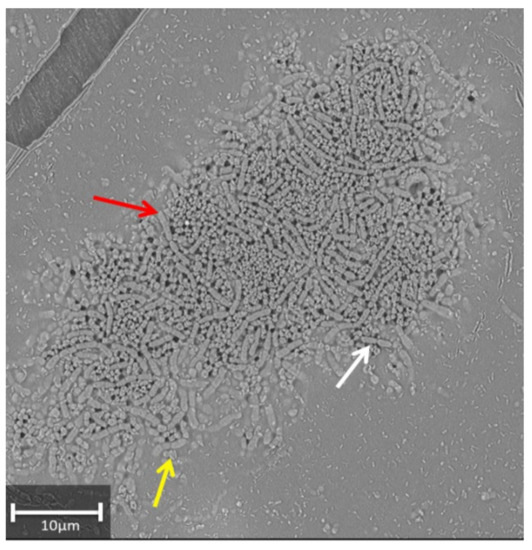
Figure 1.
Observation of Bacillus aryabhattai cultivated in the presence of 1% (v/v) Fe-NPs by low-vacuum SEM microscopy. Red and white arrows point the rods in chains and pairs, respectively. The presence of round structures could correspond to spores (yellow arrow).
In addition, the capacity of root colonization by RSO25 in the presence of FeNPs was evaluated. After growing the pre-germinated seeds in the presence of RSO25 and FeNPs at 1% (v/v) for seven days, and colonization was observed under low-vacuum SEM (Figure 2). In the control sample (non-inoculated) and in the samples treated with FeNPs alone, only a few bacteria could be observed (Figure 2A,B), probably reflecting endophytes (since the seeds were surface disinfected). By contrast, in the root of the plant previously inoculated with RSO25, the abundant presence of dense colonies of rods forming a biofilm onto the root surface and covering it was clearly identified (Figure 2C). When 1% FeNPs were included in the plant growth medium, the biofilm of bacteria colonizing the root was still observed, although it was not so dense as in the absence of FeNPs (Figure 2D). These results could suggest that FeNPs could affect biofilm formation by RSO25 at 1%, even though no growth inhibition was achieved at this concentration. Antibiofilm activity of metallic nanoparticles is reported [46].
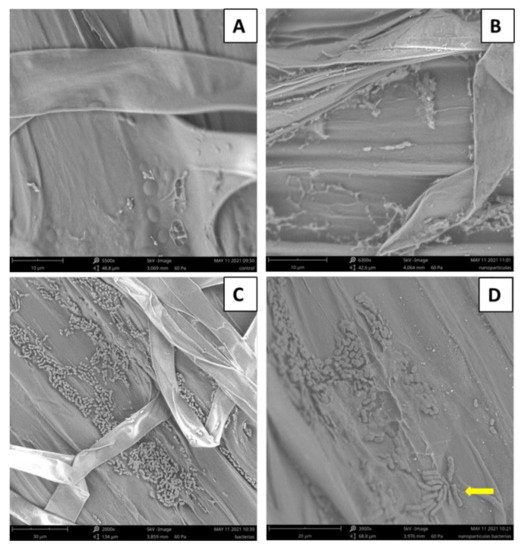
Figure 2.
Observation by low-vacuum SEM microscopy of wheat roots subjected to different inoculation treatments. (A): Non-inoculated control plants. (B): Non-inoculated plants in the presence of FeNPs (few bacteria are sporadically observed in A, B, probably reflecting the presence of endophytes or residual contamination). (C): Plants inoculated with Bacillus aryabhattai RSO25 in the absence of FeNPs. (D): Plants inoculated with Bacillus aryabhattai RSO25 in the presence of 1% FeNPs. Yellow arrow points a cell in division, indicating that the concentration of FeNPs was not inhibitory to bacterial growth.
3.2. Effect of Treatment on the Germination Rate, Initial Plant Survival, and Root Colonization
Treatment with FeNPs, both with or without bacteria, led to increased rates of germination (18% and 27%, respectively). However, only the combined treatment was significantly different (two-way Anova, p < 0.05; Figure 3). According to our results, previous studies revealed that nanoparticles of Fe-PVP syntethised from polyvinyl pyrrolidone had a positive effect on wheat seed germination at low concentrations ranging from 1–2 ppm but had toxic effects at higher doses [47]. On its hand, RSO25 had demonstrated a positive effect on seed germination of Spartina densiflora plants [48]. However, in our case, when considering final survival, no significant amelioration was recorded (two-way Anova, p < 0.05). This was related to a high dispersion of the data, particularly in control plates, since some fungal contamination arising from the seeds was observed. In this particular case, plates inoculated with bacteria showed less fungal contamination (not shown), indicating the possible role of this bacterium in biocontrol. Many Bacillus species are known biocontrol agents against fungal contamination [49].
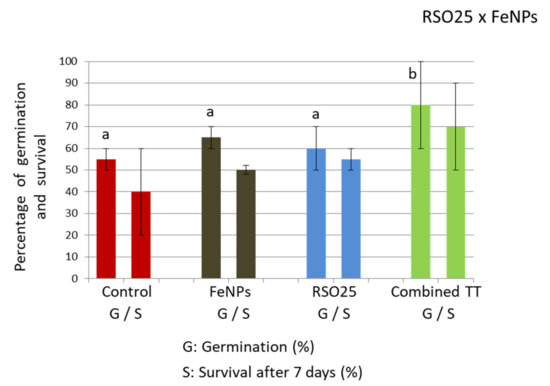
Figure 3.
Germination rate (final germination after five days) and survival after seven days of seedlings treated with different biofortification treatments: control; FeNPs treated with Fe-containing nanoparticles; RSO25, inoculated with Bacillus aryabhattai RSO25; TT combined, treatment including FeNPs and Bacillus aryabhattai RSO25. Data are means of 50 seeds ± standard deviations. RSO25, FeNPs or their interactions in the corner of the panel indicate main or interaction significant effects. Different letters indicate means that are significantly different from each other. (LSD test, p < 0.05).
3.3. Effect of Treatment on the Growth and Physiology of Wheat Plants
As displayed in Figure 4, none of the treatments caused remarkable effects on plant biomass or length, neither in shoots nor in roots (two-way Anova, p > 0.05). Thus, the aboveground dry mass and length did not vary between experimental treatments, showing in all cases mean values between 0.4–0.5 g and 35–45 cm, respectively. Concerning root biomass, all data ranged between 0.4–0.5 g.
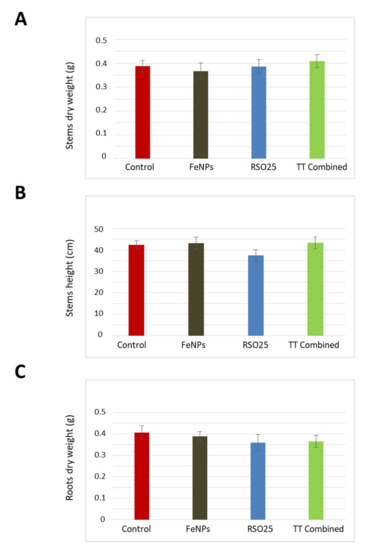
Figure 4.
Dry mass of stem (A), roots (B), and height of the stems (C) of Triticum aestivum plants treated with metallic Fe-containing polymeric nanoparticles (FeNPs), inoculated with Bacillus aryabhattai (RSO25) and exposed to the combination of both treatments (TT Combined) for 45 days. Values represent means ± SD, n = 12.
In spite of this, no differences in growth between experimental treatments were observed, although some variations in the performance of the photosynthetic apparatus of plants were registered. Whereas the net photosynthetic rate (AN) showed no significant differences between treatments (Two-way Anova, p > 0.05; Table 2), the stomatal conductance (gs) and intercellular CO2 concentration (Ci) markedly decreased with FeNPs application, bacterial inoculation and its combination (Two-way Anova: RSO25 x FeNPs, p < 0.05; Table 2). These data indicate a higher ability of plants to maintain a better carbon and water balance, as indicated by greater values of apparent carboxilation efficiency (Ce) and better water use efficiency (iWUE) (two-way Anova: RSO25 x FeNPs, p < 0.05; Table 2). These results suggest a better physiological state of plants treated with either of the treatments. The mechanism by which the different treatments could ameliorate plant physiology was further investigated, revealing that, although the maximum quantum efficiency of PSII photochemistry (Fv/Fm) did not vary between treatments, it was linked to higher light harvesting and transport efficiency, as deduced from the quantum efficiency of the photosystem II (ΦPSII) and from the electron transport rate (ETR) respectively. This effect was particularly important in plants treated with FeNPs and in plants inoculated with RSO25, whereas the effect seemed to be abolished in the combined treatment (two-way Anova: RSO25, FeNPs; p < 0.05; Table 2).

Table 2.
Results of different physiological parameters. Net photosynthetic rate (AN); stomatal conductance (gs); intercellular CO2 concentration (Ci); apparent carboxylation efficiency (Ce); intrinsic water use efficiency (iWUE); maximum quantum efficiency of PSII photochemistry (Fv/Fm); quantum efficiency of PSII (ΦPSII) and electron transport rate (ETR) in Triticum aestivum plants watered with metallic Fe-containing polymeric nanoparticles (FeNPs), inoculated with Bacillus aryabhattai (RSO25) and exposed to the combination of both treatments (TT Combined) for 45 days. Values represent means ± SD, n = 7, respectively. Different letters indicate means that are significantly different from each other (LSD test, p < 0.05). Cells in gray indicate significant differences regarding the control.
Some previous works reported the positive effect of diverse FeNPs on Capsicum annus, for which an increase of chloroplast number and grana stacking were reported [23]. However, in our case, the effect on physiology was not finally correlated with measurable growth. Regarding bacterial inoculation, previous studies with wheat reported that the application of a bacterial consortium ameliorated plant growth and yield under nutrient deficiency conditions [50]. Moreover, Bacillus aryabhattai has been widely used as a plant growth promoting rhizobacterium [36,51,52]. Finally, the possibility of sinergy using FeNPs and RSO25 is discouraged considering the results, since it does not ameliorate plant growth nor plant physiology.
3.4. Effect of the Experimental Treatments on the Concentration of Mineral Nutrients in Plant Tissues
The nutritional state of the plants was analyzed, in particular Na, K, P, S, Mg, Ca, and Zn in three types of tissues of the plants, namely, stems, roots, and spikes (Table 3). The results significantly different from control plants are highlighted in gray. In global terms, after visual inspection of Table 3, it can be seen that the most affected tissue is the root, precisely the tissue where the treatments were applied (from 21 nutrient concentrations: 7 nutrients × 3 treatments; 13 out of 21 nutrient concentrations were significantly affected). Moreover, the treatment with RSO25 led to decreased levels of almost all nutrients in roots (two-way Anova: RSO25; p < 0.05). On its side, treatment with FeNPs diminished the concentrations of several nutrients in roots, such as P, S, and Na (two-way Anova: FeNPs, p < 0.05). In the second place, the concentration of nutrients in the stems showed also alterations, but at a lower extent (9 out 21 nutrient concentrations were significantly affected). Finally, only 3 out of 21 nutrient concentrations were significanly affected in spikes (Table 3).

Table 3.
Content of nutrients in plant tissues in mg.kg−1. Data are means ± SD of two independent determinations corresponding to the mix of six plants each. Different letters indicate means that are significantly different from each other in each specific tissue (LSD test, p < 0.05). Cells in gray indicate significant differences regarding the control.
The most affected element was Na, whose concentration diminished after almost every treatment in shoots and roots (from 50% less in the stem to 20–30% less in the spike and root). In spikes, only the treatment with RSO25 had an effect on the concentration of this element (two-way Anova: RSO25, p < 0.05). The other elements that majoritarily diminished in shoots and roots were P and S (between 20–40%), although, again, there was no effect in the spikes. On its side, the concentration of Zn was much increased in roots after the three treatments (Two-way Anova: RSO25 × FeNPs, p < 0.05). It has to be remembered that RSO25 is a Zn resistant bacterium, as previously reported [35]. In plants of Spartina densiflora, incoulation with this bacterium led to an increased concentration of several metals, including Zn [36], particularly in the roots.
Regarding the spike, which is the most relevant tissue concerning biofortification, Table 3 shows an increment in Ca concentration in the spike (between 15–30%) by treatment containing FeNPs. It is known that cation channels are involved, not only in the nutritional aspects of the plant, but in the management of salt stress development and signaling [53]. In this regard, it could be interesting to analyze the possible modulation of the expression of Ca channels that could affect the distribution of this element among different plant tissues, particularly those involved in mobilizing Ca to the spike, as has been done for Zn transporters in wheat grains [54]. Besides, a diminution in Na levels was recorded. The collateral effects of other FeNPs on the assimilation of different elements in several plants are noteworthy. In this context, FeS2 NPs (600–700 nm) applied to spinach (Spinacia oleracea) led to a higher content of calcium, manganese, and zinc in the leaves [55]. Similarly, nano-chelated iron fertilizer led to increased concentrations of, not only iron, but nitrogen, phosphorus, potassium, and zinc in rice of between 25% and 50%, depending on the element [18]. Analogously, a set of nutrient deficiencies in plants watered with FeNPs stand out from this experiment as possible detrimental effects, most of which are outside the spike, where the only serious deficiency is related to Na. Interestingly, this deficit of stem and root nutrients does not seem to affect the physiology or physical characteristics of the plant but may be a consequence of an ionic reorganization due to the imbalance provoked by FeNPs. In fact, in other plants, such as sunflower, an imbalance of nutrients was recorded upon application of iron oxide NPs [56].
The kinetics of spike development varied between different treatments (Figure 5A). In the control treatment, the development of spikes followed a linear trend and finally 2.7 spikes per plant on average were produced. The treatment with RSO25 led to the lowest spike production, with, finally, 1.7 spikes per plant (two-way Anova, RSO25, p < 0.05). On the other hand, both treatments with NPs, either inoculated or non-inoculated, led to the highest production, at up to a maximum of 3.3 spikes per plant. Moreover, the treatment with FeNPs was the one that favored the fastest spikes outgrow (Figure 5A).

Figure 5.
Kinetics of spikes outgrow (A), biomass of spikes (B), and Fe content in spikes (C) of Triticum aestivum plants treated with metallic Fe-containing polymeric nanoparticles (FeNPs), inoculated with Bacillus aryabhattai (RSO25) and exposed to the combination of both treatments (TT Combined) for 45 days. Values represent means ± SD, n = 12. RSO25, FeNPs or their interactions in the corner of the panel indicate main or interaction significant effects. Different letters indicate means that are significantly different from each other (LSD test, p < 0.05). In the case of Figure 5A, the letters correspond to data of day 10 after spike outgrow.
The average biomass of spikes in the different treatments was also determined and the results are shown in Figure 5B. The treatment with FeNPs led to the highest biomass, with a net increase of 20% regarding the control. Still, this difference was not significant (two-way Anova, p > 0.05). In addition, Fe was determined in the dry mass of the spikes. The results (Figure 5C) pointed to significant differences between the treatments, including FeNPS or not, regardless of the presence of the bacteria. In fact, both treatments including FeNPs led to increases of around 33–35% in the concentration of Fe (two-way Anova: RSO25 x FeNPs, p < 0.05), thus suggesting the effectiveness of biofortification. These data are similar to other data provided by different authors using different FeNPs [57]. For instance, biofortification of 8% and 20% were reported after using FeEDTA and FeSO4 NPs, respectively [58]. Moreover, biofortification could rise to 56% when NPs were applied in the form of foliar spray and wheat plants were supplemented with additional nitrogen (such as urea) in the soil. In the same work, biofortification with FeEDTA, FeSO4, FeEDDHA (N, N′-ethylenediamine-bisacetic acid), and Fe contained in a citrate salt led to a less than 20% increase in Fe content after application of urea as a foliar spray [59]. On their side, [16] used Fe2O3-NPs applied as irrigation and obtained enhancements in the amount of Fe by 26% and 45% in two wheat varieties, thus probing the contribution of the plant to the biofortication process, besides the type of FeNPs and the application method. Finally, the findings of other authors on the biofortification of another cereal, Oryza sativa (rice), with foliar application of Fe nanochelants showed an increase of 25% in the amount of Fe in the grain [15].
Finally, the total amount of Fe in the spikes per plant was calculated by multiplying the three parameters in Figure 5, i.e., the product of [spikes production] × [biomass of spikes] × [Fe accumulation in spikes] (two-way Anova: RSO25, FeNPs, RSO25 × FeNPs, p < 0.05; Figure 6). In the treatment with only FeNPs, an efficient increase of 214% in the total amount of Fe per plant was found. By contrast, the treatment with only bacteria led to a 40% decrease in Fe accumulation in spikes. Finally, the combination of both treatments seemed to cause an antagonist effect and is not recommended, since the final Fe accumulation regarding the treatment with only FeNPs was significantly reduced by 19%.
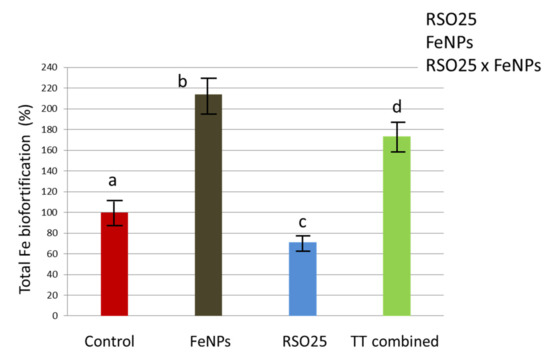
Figure 6.
Net Fe biofortification per plant in Triticum aestivum treated with metallic Fe-containing polymeric nanoparticles (FeNPs), inoculated with Bacillus aryabhattai RSO25 (RSO25) and exposed to the combination of both treatments (TT Combined) for 45 days. Data are obtained after multiplication of the three parameters in Figure 5, i.e., the product of [spikes production] × [biomass of spikes] × [Fe accumulation in spikes]. RSO25, FeNPs or their interactions in the corner of the panel indicate main or interaction significant effects. Different letters indicate means that are significantly different from each other (LSD test, p < 0.05).
Particularly, it is worth mentioning that, in our experiment, both the number of spikes and the per spike biomass were improved after treatment with FeNPs, which led to a total increase of 214% in the Fe accumulated in grains per plant. A similar effect was observed in previous publications using iron oxide NPs [59] or nano Fe-fertilizer [60]. Besides, in soybean, an increased number of pods and higher size of pods were recorded after treatment with metallic FeNPs [61]. Furthermore, FeNPs based on poly-vinyl-alcohol and tannic acid show high stability and low toxicity, since the compounds used in their synthesis are biocompatible [62,63]. In addition to this, several parameters, such as using FeNPs in foliar spray or adding nitrogen, can be adjusted to improve Fe absorption [58].
In contrast to the expected results, application of Bacillus aryabhattai did not led to improved growth in spite of the fact that this species is well known PGPR [51]. In particular, the strain RSO25 has proved good PGP properties in Spartina densiflora [35], being able to increase the concentration of several metals in this plant [36]. This strain was isolated from halophyte Spartina maritima in saltmarshes, being adapted to thrive in conditions of moderate salinity and heavy metals [35]. It is known that both the habitat and the plant through the secretion of specific root exudates can modulate rhizosphere populations [64,65]. In fact, root exudates produced by Brachypodium distachyon, a plant which is considered as a model for wheat, are being deciphered [66]. Thus, different plants can secrete different exudates and modulate the effect of rhizosphere bacteria, which in turn can affect the expression of genes in the plant [52]. Besides, genotypic characteristics determine the higher or lower ability of plants for ions translocation from root to shoots [67]. These reasons may explain why this bacterium, which was reported to increase metal concentration in S. densiflora [36], has not been useful to increase Fe concentration in wheat.
Finally, although the possibility of combinatorically using FeNPs and RSO25 was explored, considering the results, it was discouraged, since the amelioration of plant growth, physiology or accumulation either of Fe or other essential nutrients in grains were not detected. One possible explanation could be related to the antimicrobial and antibiofilm effects of NPs in general, and tannic acid in particular, that may affect bacterial communities in the rhizosphere [68,69]. Thus, even though the concentration of FeNPs used for the experiments (1%) was below the CMI, a cumulative effect could have been produced after repeated watering. Different types of NPs, such as amine-modified polystyrene nanospheres and titanium dioxide nanoparticles, caused significant decreases in rhizosphere bacterial counts and decreased root and stem growth of lettuce plants [70]. In other microbe-plant associations, such as mycorrhizal and rhizobial symbioses, a negative effect of some metal NPs was reported, depending mainly on the concentration, but also on the type of NPs, size, soil physicochemical properties and sensitivity of the plant species [71]. In a metatranscriptomic study on maize, drastic changes in the composition and functionality of the root microbiome upon long treatment with silver NPs was observed, finally leading to plant stress, fungal attack and impaired nitrogen cycling [72]. All of these data point to the negative effects of different kinds of NPs on root microbiome health and functionality, finally leading to negative effects on plant growth. In our case, even though colonization of the root in the presence of NPs was observed, the amount of bacteria was lower than in the control, suggesting a certain toxicity of FeNPs to the bacteria and/or to their capacity to colonize the roots [70]. In this regard, the treatment with only FeNPs seems to be the most adequate not only to maintain plant growth, but also to improve plant physiology, and enhance Fe biofortification at the same time.
4. Conclusions
In spite that both strategies, the use of PGPR and the use of NPs, are widely utilized for plant biofortification, in our hands this is one of the few studies concerning the combinatory use of both treatments for this purpose. Our results led us to conclude that both treatments and their combination had minor effects on germination, plant survival and final growth, in spite of the fact that several photosynthetic parameters were ameliorated. Considering final biofortification, it is possible to conclude that the biofortification resulting from the use of FeNPs based on PVA-tannic acid is superior or similar to that obtained by other Fe nanoparticles previously described in the literature. Besides, the lateral effects of some other macro- and micronutrients in spikes, namely an increase in Ca and deficit of Na, were recorded upon treatment with FeNPs. On the other hand, the bacterium Bacillus aryabhattai RSO25 has not demonstrated good plant growth promoting properties in wheat plants. Finally, the combined use of FeNPs and RSO25 is discouraged, since the amelioration of plant growth and physiology was not achieved, but the amount of total Fe accumulated in grains was diminished by 19%. The application of only FeNPs is indeed the best option of the three treatments investigated. The possibility of other application methods, such as foliar spray may be investigated, since nutrient concentrations of several elements followed a gradient roots > stems > spikes.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agronomy12010228/s1. Supplementary Information Figure S1: Size distribution of A) PVA-TANPs y B) FeNps. It can be observed that PVA-TANPs intensity peaks at a lower size than FeNps. Supplementary Information Figure S2: Scheme of the microtiter plate for determination of the CMI of the bacterium B. aryabhattai RSO25 towards the FeNPs nanoparticles. A: TSB; B: TSB + 20% FeNPs; C: TSB + 10% FeNPs; D: TSB + 5% FeNPs; E: TSB + 2.5% NPs of FeNPs; F: TSB + 1.25% FeNPs; G: TSB + 0.625% FeNPs; H: TSB + 0.3125% FeNPs.
Author Contributions
M.M.: Investigation and original draft preparation; A.A. and Y.T., project administration, supervision and draft revision and submission; B.B., nanoparticle characterization and draft review; G.M., nanoparticle synthesis; M.J.M.-V., analytical determination of Fe and nutrients; S.N.-T. and J.A.P.-R., determination of plant physiology parameters; E.M.-N. and S.R.-G.: analysis of results of physiology data; I.D.R.-L., draft review; F.M., funding for publication, analysis of data and draft review and E.P., project administration, microscopy studies, supervision and final draft review. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Innovation of Spain under the grant PID2019-109371GB-I00 and of the Junta de Andalucía (Spain) through the Project FQM-135 together with project PPIT-2020/00001095 (University of Seville, Spain).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The General Services of CITIUS (University of Seville), particularly Herbarium, Green House and Microanalysis are acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lowe, N. The global challenge of hidden hunger: Perspectives from the field. Proc. Nut. Soc. 2021, 80, 283–289. [Google Scholar] [CrossRef]
- Biesalki, H.K. Hidden hunger in the Developed World. In RTGN; Chapter 3; p. 40–50. Available online: https://www.nutri-facts.org/content/dam/nutrifacts/media/media-books/RTGN_chapter_03.pdf (accessed on 25 October 2021).
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3999603 (accessed on 25 October 2021).
- Gödecke, T.; Stein, A.J.; Qaim, M. The global burden of chronic and hidden hunger: Trends and determinants. Glob. Food Secur. 2018, 17, 21–29. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, Y.; Wu, Z.; Lv, T. A Bibliometric Analysis on Land Degradation: Current Status, Development, and Future Directions. Land 2020, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Sect. 2017, 12, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Elemike, E.E.; Uzoh, I.M.; Onwudiwe, D.C.; Babalola, O.O. The Role of Nanotechnology in the Fortification of Plant Nutrients and Improvement of Crop Production. Appl. Sci. 2019, 9, 499. [Google Scholar] [CrossRef] [Green Version]
- Aguilera, J.R.; Venegas, V.; Oliva, J.M.; Sayagués, M.J.; de Miguel, M.; Sánchez-Alcázar, J.A.; Arévalo-Rodríguez, M.; Zaderenko, A.P. Targeted multifunctional tannic acid nanoparticles. RSC Adv. 2016, 6, 7279–7287. [Google Scholar] [CrossRef]
- Bundschuh, M.; Filser, J.; Lüderwald, S.; McKee, M.S.; Metreveli, G.; Schaumann, G.E.; Schulz, R.; Wagner, S. Nanoparticles in the environment: Where do we come from, where do we go to? Environ. Sci. Eur. 2018, 30, 6. [Google Scholar] [CrossRef] [Green Version]
- Lurthy, T.; Pivato, B.; Lemanceau, P.; Mazurier, S. Importance of the Rhizosphere Microbiota in Iron Biofortification of Plants. Front. Plant. Sci. 2021, 12, 744445. [Google Scholar] [CrossRef]
- Rico, C.M.; Majumdar, S.; Duarte-Gardea, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Interaction of nanoparticles with edible plants and their possible implications in the food chain. J. Agric. Food Chem. 2011, 59, 3485–3498. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Hernández, H.; González-Morales, S.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Effects of Chitosan-PVA and Cu Nanoparticles on the Growth and Antioxidant Capacity of Tomato under Saline Stress. Molecules 2018, 23, 178. [Google Scholar] [CrossRef] [Green Version]
- Quiterio-Gutiérrez, T.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Hernández-Fuentes, A.D.; Sandoval-Rangel, A.; Benavides-Mendoza, A.; Cabrera-de la Fuente, M.; Juárez-Maldonado, A. The Application of Selenium and Copper Nanoparticles Modifies the Biochemical Responses of Tomato Plants under Stress by Alternaria solani. Int. J. Mol. Sci. 2019, 20, 1950. [Google Scholar] [CrossRef] [Green Version]
- Palmqvist, N.G.M.; Seisenbaeva, G.A.; Svedlindh, P.; Kessler, V.G. Maghemite Nanoparticles Acts as Nanozymes, Improving Growth and Abiotic Stress Tolerance in Brassica napus. Nanoscale Res. Lett. 2017, 12, 631. [Google Scholar] [CrossRef] [PubMed]
- Fakharzadeh, S.; Hafizi, M.; Baghaei, M.; Etesami, M.; Khayamzadeh, M.; Kalanaky, S.; Akbari, M.; Nazaran, M. Using Nanochelating Technology for Biofortification and Yield Increase in Rice. Sci. Rep. 2020, 10, 4351. [Google Scholar] [CrossRef]
- Sundaria, N.; Singh, M.; Upreti, P.; Chauhan, R.; Jaiswal, J.P.; Kumar, A. Seed Priming with Iron Oxide Nanoparticles Triggers Iron Acquisition and Biofortification in Wheat (Triticum aestivum L.) Grains. J. Plant. Growth Reg. 2019, 38, 122–131. [Google Scholar] [CrossRef]
- Krzyzowska, M.; Tomaszewska, E.; Ranoszek-Soliwoda, K.; Bien, K.; Orlowski, P.; Celichowski, G.; Grobelny, J. Tannic acid modification of metal nanoparticles: Possibility for new antiviral applications. In Micro and Nano Technologies, Nanostructures for Oral Medicine; Andronescu, E., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 12; pp. 335–363. [Google Scholar] [CrossRef]
- Saowalak, K.; Titipun, T.; Somchai, T.; Chalermchai, P. Iron(III)-Tannic Molecular Nanoparticles Enhance Autophagy effect and T1 MRI Contrast in Liver Cell Lines. Sci. Rep. 2018, 8, 6647. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, T. Reviewing the Tannic Acid Mediated Synthesis of Metal Nanoparticles. J. Nanotechnol. 2014, 954206. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Chen, R. Study of Complexes of Tannic Acid with Fe(III) and Fe(II). J. Anal. Methods Chem. 2019, 3894571. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Jaramillo, J.E.; Carrión, V.J.; de Hollander, M.; Raaijmakers, J.M. The wild side of plant microbiomes. Microbiome 2018, 6, 143. [Google Scholar] [CrossRef] [Green Version]
- Curie, C.; Briat, J.F. Iron Transport and Signaling in Plants. Ann. Rev. Plant. Biol. 2003, 54, 183–206. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Chen, Y.; Li, H.; Lu, J.; Zhao, H.; Liu, M.; Nechitaylo, G.S.; Glushchenko, N.N. New insights into the cellular responses to iron nanoparticles in Capsicum annuum. Sci. Rep. 2018, 8, 3228. [Google Scholar] [CrossRef]
- Hider, R.C.; Yoshimura, E.; Khodr, H.; von Wirén, N. Competition or complementation: The iron-chelating abilities of nicotianamine and phytosiderophores. New Phytol. 2004, 164, 201–204. [Google Scholar] [CrossRef]
- Zhu, H.; Han, J.; Xiao, J.Q.; Jin, Y. Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J. Environ. Monit. 2008, 10, 713–717. [Google Scholar] [CrossRef]
- Wang, H.; Kou, X.; Pei, Z.; Xiao, J.Q.; Shan, X.; Xing, B. Physiological effects of magnetite (Fe3O4) nanoparticles on perennial ryegrass (Lolium perenne L.) and pumpkin (Cucurbita mixta) plants. Nanotoxicology 2011, 5, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Yashveer, S.; Singh, V.; Kaswan, V.; Kaushik, A.; Tokas, J. Green biotechnology, nanotechnology and biofortification: Perspectives on novel environment-friendly crop improvement strategies. Biotechnol. Genet. Eng. Rev. 2014, 30, 113–126. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant. Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oleńska, E.; Małek, W.; Wójcik, M.; Swiecicka, I.; Thijs, S.; Vangronsveld, J. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: A methodical review. Sci. Total Environ. 2020, 743, 140682. [Google Scholar] [CrossRef]
- Glick, B.R. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol. Res. 2014, 169, 30–39. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 3, 197. [Google Scholar] [CrossRef] [Green Version]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Kloepper, J.; Leong, J.; Teintze, M.; Schroth, M.N. Enhanced plant growth by siderophores produced by plant growth-promoting rhizobacteria. Nature 1980, 286, 885–886. [Google Scholar] [CrossRef]
- Lee, T.S.; Kolthoff, I.M.; Leussing, D.L. Reaction of Ferrous and Ferric Ions with 1,10-Phenanthroline. II. Kinetics of Formation and Dissociation of Ferrous Phenanthroline. J. Am. Chem. Soc. 1948, 70, 3596–3600. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Páliz, K.I.; Caviedes, M.A.; Doukkali, B.; Mateos-Naranjo, E.; Rodríguez-Llorente, I.D.; Pajuelo, E. Screening beneficial rhizobacteria from Spartina maritima for phytoremediation of metal polluted salt marshes: Comparison of gram-positive and gram-negative strains. Environ. Sci. Pollut. Res. 2016, 23, 19825–19837. [Google Scholar] [CrossRef] [PubMed]
- Paredes-Páliz, K.I.; Mateos-Naranjo, E.; Doukkali, B.; Caviedes, M.A.; Redondo-Gómez, S.; Rodríguez-Llorente, I.D.; Pajuelo, E. Modulation of Spartina densiflora plant growth and metal accumulation upon selective inoculation treatments: A comparison of gram negative and gram positive rhizobacteria. Mar. Pollut. Bull. 2017, 125, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Liu, H.; Yu, X.; Zhang, X.; Lu, H.; Zhou, T.; Cao, J. Antimicrobial and anti-biofilm activity of tannic acid against Staphylococcus aureus. Nat. Prod. Res. 2018, 32, 2225–2228. [Google Scholar] [CrossRef]
- Wang, C.; Pian, R.; Chen, X.; Lv, H.; Zhou, W.; Zhang, Q. Beneficial Effects of Tannic Acid on the Quality of Bacterial Communities Present in High-Moisture Mulberry Leaf and Stylo Silage. Front. Microbiol. 2020, 11, 2754. Available online: https://www.frontiersin.org/article/10.3389/fmicb.2020.586412 (accessed on 25 October 2021). [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; CLSI Document M07; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Hoagland, D.R.; Arnon, I. The water-culture method for growing plants without soil. In California Agricultural Experiment Station Circular; University of California, College of Agriculture, Agricultural Experiment Station: Berkeley, CA, USA, 1950; Volume 347. [Google Scholar]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta (BBA) Gen. Subj. 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Schreiber, U.; Bilger, W.; Neubauer, C. Chlorophyll Fluorescence as a Nonintrusive Indicator for Rapid Assessment of In Vivo Photosynthesis. In Ecophysiology of Photosynthesis; Springer Study Edition; Schulze, E.D., Caldwell, M.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1995; Volume 100. [Google Scholar] [CrossRef]
- Krall, J.P.; Edwards, G.E. Environmental Effects on the Relationship Between the Quantum Yields of Carbon Assimilation and in vivo PsII Electron Transport in Maize. Func. Plant. Biol. 1991, 18, 267–278. [Google Scholar] [CrossRef]
- Martínez, G.; Merinero, M.; Pérez-Aranda, M.; Pérez-Soriano, E.M.; Ortiz, T.; Begines, B.; Alcudia, A. Environmental Impact of Nanoparticles’ Application as an Emerging Technology: A Review. Materials 2020, 14, 166. [Google Scholar] [CrossRef] [PubMed]
- Elarabi, N.I.; Abdelhadi, A.A.; Ahmed, R.H.; Saleh, I.; Arif, I.A.; Osman, G.; Ahmed, D.S. Bacillus aryabhattai FACU: A promising bacterial strain capable of manipulate the glyphosate herbicide residues. Saudi J. Biol. Sci. 2020, 27, 2207–2214. [Google Scholar] [CrossRef]
- Shkodenko, L.; Kassirov, I.; Koshel, E. Metal Oxide Nanoparticles against Bacterial Biofilms: Perspectives and Limitations. Microorganisms 2020, 8, 1545. [Google Scholar] [CrossRef]
- Alam, J.; Sultana, F.; Iqbal, T. Potential of Iron Nanoparticles to Increase Germination and Growth of Wheat Seedling. J. Nanosci. Adv. Tech. 2015, 1, 14–20. [Google Scholar] [CrossRef]
- Paredes-Páliz, K.I.; Pajuelo, E.; Doukkali, B.; Caviedes, M.A.; Rodríguez-Llorente, I.D.; Mateos-Naranjo, E. Bacterial inoculants for enhanced seed germination of Spartina densiflora: Implications for restoration of metal polluted areas. Mar. Pollut. Bull. 2016, 110, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Li, R.; Zhang, H.; Gehong, W.; Zhefei, L. Beneficial bacteria activate nutrients and promote wheat growth under conditions of reduced fertilizer application. BMC Microbiol. 2020, 20, 38. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, C.; Bakshi, U.; Mallick, I.; Mukherji, S.; Bera, B.; Ghosh, A. Genome-Guided Insights into the Plant Growth Promotion Capabilities of the Physiologically Versatile Bacillus aryabhattai Strain AB211. Front. Microbiol. 2017, 8, 411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paredes-Páliz, K.; Rodríguez-Vázquez, R.; Duarte, B.; Caviedes, M.A.; Mateos-Naranjo, E.; Redondo-Gómez, S.; Caçador, M.I.; Rodríguez-Llorente, I.D.; Pajuelo, E. Investigating the mechanisms underlying phytoprotection by plant growth-promoting rhizobacteria in Spartina densiflora under metal stress. Plant. Biol. 2018, 20, 497–506. [Google Scholar] [CrossRef]
- Demidchik, V.; Maathuis, F.J.M. Physiological roles of nonselective cation channels in plants: From salt stress to signalling and development. New Phytol. 2007, 175, 387–404. [Google Scholar] [CrossRef]
- Alomari, D.Z.; Eggert, K.; von Wirén, N.; Alqudah, A.M.; Polley, A.; Plieske, J.; Ganal, M.W.; Pillen, K.; Röder, M.S. Identifying Candidate Genes for Enhancing Grain Zn Concentration in Wheat. Front. Plant. Sci. 2018, 9, 1313. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, G.; Das, C.K.; Das, A.; Singh, S.K.; Roy, M.; Kim, H.; Sethy, N.; Kumar, A.; Sharma, R.K.; Singh, S.K.; et al. Seed treatment with iron pyrite (FeS2) nanoparticles increases the production of spinach. RSC Adv. 2014, 4, 58495–58504. [Google Scholar] [CrossRef]
- Martínez-Fernández, D.; Barroso, D.; Komarek, M. Root water transport of Helianthus annuus L. under iron oxide nanoparticle exposure. Environ. Sci. Pollut. Res. 2016, 23, 1732–1741. [Google Scholar] [CrossRef]
- Riaz, A.; Huda, N.; Abbas, A.; Raza, S. Biofortification of Wheat with Iron. Int. J. Adv. Sci. Res. 2017, 3, 69–76. Available online: https://ssjournals.com/index.php/ijasr/article/view/4275 (accessed on 25 October 2021). [CrossRef] [Green Version]
- Aciksoz, S.B.; Yarici, A.; Ozturk, L.; Cakmak, I. Biofortification of wheat with iron through soil and foliar application of nitrogen and iron fertilizers. Plant. Soil 2011, 349, 215–225. [Google Scholar] [CrossRef]
- Siva, G.V.; Benita, L.F.J. Iron Oxide Nanoparticles Promotes Agronomic Traits of Ginger (Zingiber officinale Rosc.). Int. J. Adv. Res. Biol. Sci. 2016, 3, 230–237. [Google Scholar]
- Gomaa, M.A.; Kandil, E.E.; Abdelsalam, N.R.; Al-Jaddadi, M.A.M. Growth, productivity of some rice cultivars in relation to nano-zinc and iron fertilizer. Middle East. J. Agric. Res. 2018, 7, 1352–1358. Available online: http://www.curresweb.com/mejar/mejar/2018/1352-1358.pdf (accessed on 25 October 2021).
- Sheykhbaglou, R.; Sedghi, M.; Shishevan, M.T.; Sharifi, R.S. Effects of nano-iron oxide particles on agronomic traits of soybean. Not. Sci. Biol. 2010, 2, 112–113. [Google Scholar] [CrossRef] [Green Version]
- Paradossi, G.; Cavalieri, F.; Chiessi, E.; Spagnoli, C.; Cowman, M.K. Poly(vinyl alcohol) as versatile biomaterial for potential biomedical applications. J. Mater. Sci. Mater. Med. 2003, 14, 687–691. [Google Scholar] [CrossRef]
- Sahiner, N.; Sagbas, S.; Sahiner, M.; Silan, C.; Aktas, N.; Turk, M. Biocompatible and biodegradable poly(Tannic Acid) hydrogel with antimicrobial and antioxidant properties. Int. J. Biol. Macromol. 2016, 82, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Haichar, F.; Marol, C.; Berge, O.; Rangel-Castro, J.I.; Prosser, J.I.; Balesdent, J.; Heulin, T.; Achouak, W. Plant host habitat and root exudates shape soil bacterial community structure. ISME J. 2008, 2, 1221–1230. [Google Scholar] [CrossRef]
- Dennis, P.G.; Miller, A.J.; Hirsch, P.R. Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol. Ecol. 2010, 72, 313–327. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, A.; Donn, S.; Ryan, P.R.; Mathesius, U.; Devilla, R.; Jones, A.; Watt, M. Microbiome and Exudates of the Root and Rhizosphere of Brachypodium distachyon, a Model for Wheat. PLoS ONE 2016, 11, e0164533. [Google Scholar] [CrossRef] [Green Version]
- Rengel, Z. Genotypic differences in micronutrient use efficiency in crops. Commun. Soil Sci. Plant. Anal. 2001, 32, 1163–1186. [Google Scholar] [CrossRef]
- Sagbas, S.; Aktas, N.; Sahiner, N. Modified biofunctional p-(tannic acid) microgels and their antimicrobial activity. Appl. Surf. Sci. Part B 2015, 354, 306–313. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Sushkova, S.; Tsitsuashvili, V.; Mandzhieva, S.; Gorovtsov, A.; Nevidomskyaya, D.; Gromakova, N. Effect of nanoparticles on crops and soil microbial communities. J. Soils Sediments 2018, 18, 2179–2187. [Google Scholar] [CrossRef]
- Kibbey, T.C.G.; Strevett, K.A. The effect of nanoparticles on soil and rhizosphere bacteria and plant growth in lettuce seedlings. Chemosphere 2019, 221, 703–707. [Google Scholar] [CrossRef]
- Tian, H.; Kah, M.; Kariman, K. Are Nanoparticles a Threat to Mycorrhizal and Rhizobial Symbioses? A Critical Review. Front. Microbiol. 2019, 10, 1660. Available online: https://www.frontiersin.org/article/10.3389/fmicb.2019.01660 (accessed on 25 October 2021). [CrossRef] [Green Version]
- Sillen, W.M.A.; Thijs, S.; Abbamondi, G.R.; De La Torre Roche, R.; Weyens, N.; White, J.C.; Vangronsveld, C. Nanoparticle treatment of maize analyzed through the metatranscriptome: Compromised nitrogen cycling, possible phytopathogen selection, and plant hormesis. Microbiome 2020, 8, 127. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).