1. Introduction
Objective and standardized analysis of phenotypic traits is one of the most crucial requirements of modern plant breeding and genetic mapping programs. Association studies like QTL analysis and GWAS are highly dependent on precise genotypic and phenotypic data. However, in breeding research it is still common to rely on visual assignment of categorical classes describing for example disease severity caused by certain pathogens. To reduce the possibility of human errors, it is advisable that these class assignments are performed by multiple people on multiple biological replicates of the genetic material under study and in multiple repetitions of the tests. Further, in the case of host/pathogen interactions it is sometimes important to screen material with different isolates or races of the pathogen at different stages of the infection to identify the durability or resistance spectrum of a novel resistance source. These considerations make obvious, that manual phenotyping is a tedious and time-consuming task when done properly. In addition, albeit a high degree of experience of personnel performing the scorings, subjectivity of individual persons always introduces a certain degree of bias.
This is where computer vision comes into play. The advancements in this field during the last decades have made it possible to automatically collect large numbers of high-quality images to record and store different stages e.g., during infection progression. Moreover, machine learning approaches have been developed over the last one and half decades that exceed former technology. Machine learning is now available in all sorts of technologies allowing e.g., speech recognition, face recognition or pattern recognition in written text, and more applications. Additionally, the accessibility of machine learning algorithms has been greatly facilitated when the free and open source library TensorFlow was made available by Google in 2015 [
1,
2]. For image classification, deep convolutional neural networks (DCNN) have been developed starting from Alexnet [
3] going to VGG [
4] over to Inception and GoogLeNet [
5], always increasing the complexity and the number of layers making them even “deeper”. These state of the art DCNNs have an excellent accuracy when trained properly. However, they require comprehensive computational resources. In recent years more and more articles were published using such networks in various combinations and adaptations to identify and quantify diseases on a wide variety of plants under various scenarios (Review articles on these topics [
6,
7]). The first attempt of implicating deep learning for image-based plant disease assessment carried out in 2016 successfully classified 14 different crops and 26 diseases with an average accuracy of 99% [
8]. A recent study combines several of these DCNNs for detecting and to distinguish different grapevine diseases on whole leaves [
9]. In a further study deep learning approaches using CNNs were employed to detect grapevine downy mildew and spider mites under field conditions on images of whole plants [
10].
In this study we decided to use so-called “shallow convolutional neural networks” (SCNNs) which use only a small number of image convolutions and subsequent fully-connected layers thereby reducing the requirement of high computational resources and training time [
11,
12]. As proof of concept, the SCNNs were trained to detect DM infections on RGB images of experimentally inoculated leaf discs.
DM caused by
P. viticola is one of the major diseases viticulture is facing annually in temperate climate areas [
13,
14]. In 2016 and 2021 very heavy epidemics occurred in German viticulture with a strong economic impact due to intensified plant protection regimes and yield losses. The obligate biotrophic oomycete invades green plant tissue via zoospores, which emerge from vegetatively generated sporangia or sexually produced oospores during wet and humid conditions. Using their flagella, zoospores swim to stomata, encyst at their rim and form a primary hypha, which enters the substomatal cavity, branches between spongy mesophyll cells and forms haustoria. After three to four days under optimal conditions, the oomycete forms so-called sporangiophores that emerge through stomata. Sporangia are formed at their tips and finally dispersed to restart the infection cycle under humid and wet conditions [
15]. DM can have a devastating impact on yield when young inflorescences up to pea-sized berries are infected. The cultivated grapevine
Vitis vinifera ssp.
vinifera which is grown world-wide has no natural resistance against this pathogen. Grapevine growers therefore rely on strict plant protection regimes that require high amounts of fungicides [
13,
14]. To reduce the amount of plant protection chemicals used in viticulture, breeding programs have been established that focus on the introgression of natural resistances against this and many other pathogens into new breeding lines, eventually creating new resistant cultivars for future sustainable viticulture [
16,
17,
18].
These breeding efforts first require screening of segregating crosses for the identification of novel resistance traits, which then, in the next step, can be genetically identified and introgressed into existing elite lines. For experimental inoculations of DM under controlled environmental conditions, leaf disc assays are preferably used and the degree of disease severity determined by visual assessment [
19,
20,
21,
22,
23]. The amount of leaf discs for these screenings easily reaches several thousand per year making the need for a high-throughput screening method obvious.
Following the approach used by Bierman et al. [
24] where they used a DCNN for quantification of powdery mildew infections on leaf discs, we trained SCNNs to automatically detect areas on leaf discs with DM infections using small number of images. To ensure accuracy of the trained SCNNs we created ground truth data and calculated the true positives. After observing a true positive ratio of 95% in average, we implemented an easy to use leaf-disc-scoring pipeline that can be run on any given Unix system. The pipeline is available as an open-source GitHub repository with instructions included on how to train SCNNs for other traits or pathogen symptoms
https://www.github.com/Daniel-Ze/Leaf-disc-scoring, accessed on 20 August 2021.
2. Materials and Methods
2.1. Plant Material
Infection experiments and image capturing was carried out on F1 individuals of two cross populations with different genetic backgrounds. The first population consisted of 497 F1 individuals of a cross between ‘Morio Muskat’ (‘Silvaner grün’ × ‘Muscat à petits grains blancs’) and a genotype COxGT2 (V. coignetiae × ‘Gewürztraminer’). The second population was derived by a cross between ‘Cabernet Dorsa’ (‘Blaufränkisch’ × ‘Dornfelder’) and Couderc 13 (Vitis aestivalis var. lincecumii × Couderc 162-5) and comprises 314 F1 individuals. The breeding line COxGT2 (Nursery E. Schrank, Dackenheim, Germany) and the cultivar Couderc 13 were described to exhibit a high degree of field resistance against downy mildew. Both crosses were performed in 2018 and seeds germinated in spring of 2019. All individuals were grown as potted plants in the greenhouse at the Institute for Grapevine Breeding Geilweilerhof in Siebeldingen (49°13′05.0″ N 8°02′45.0″ E) in the southwest of Germany with a single shoot of approx. 40 cm height and a pot diameter of 16 cm. The plantation was protected against powdery mildew until one week before an artificial leaf disc inoculation experiment.
2.2. Inoculation Experiments
Leaf disc inoculation assays were performed in two and three independent replicates, respectively, for both cross populations during summer of 2020 to determine the levels of leaf resistance to downy mildew in the F
1 progenies. Two leaves of each individual were sampled from the third and fourth apical node respectively and four leaf discs (one cm diameter) were punched using a cork borer. Leaf discs were arrayed upside-down on 1% water agar (Insula Agar Agar Pulver E406, Gustav Essig GmbH & Co. KG, Mannheim, Germany) in 245 mm Square BioAssay Dishes (Corning
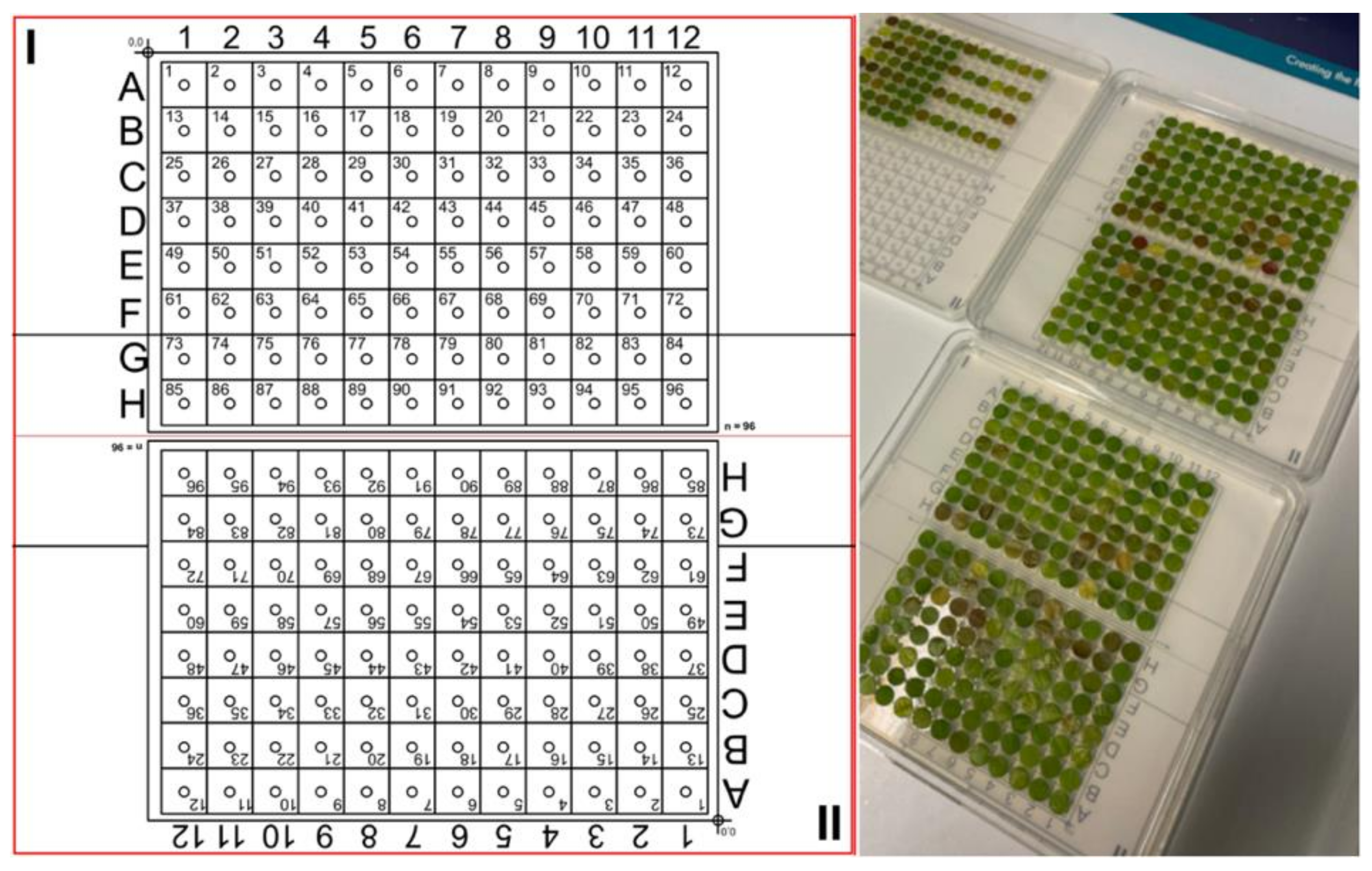
®, Corning, New York, NY, USA). They were laid out according to a template of two 96 sample grids as shown in
Figure 1. The template was designed to fit the dimensions of a Zeiss AxioZoom v16 motorized table.
Three of the four leaf discs were artificially inoculated with a centered drop (30 µL) of a P. viticola sporangial suspension (18,000 sporangia per mL), the fourth leaf disc served as a water inoculation control. Sporangia were collected from leaves of un-sprayed susceptible V. vinifera vines from the field at the Julius Kuehn Institute, Institute for Grapevine Breeding Geilweilerhof, Germany by vacuum suction using a water jet pump. Fresh sporulation was obtained by incubation of leaves that showed characteristic oil spots over-night under dark and wet conditions. After an overnight incubation at room temperature in the dark, spore suspension drops were removed using a water jet pump. Infected leaf discs were incubated for four days at 22 °C with a photoperiod of 16 h (full spectrum light) and high relative humidity within the dishes.
2.3. Automated Imaging of Leaf Discs
For automated imaging of leaf discs after inoculation with P. viticola a Zeiss Axio Zoom V16 (Jena, Germany) with motorized table and a 0.5× magnification lens was used (PlanApo Z 0.5×/0.125; Free Working Distance 114 mm). Images were recorded in 10.5-fold magnification. A movement template was generated with the ZenBlue version 3.4 (Zeiss, Jena, Germany) software which allowed to automatically access the 96 positions. A sample template which can be directly loaded into Zen Blue 3.0 is available in the associated GitHub repository. The spacing of the template was calculated to be sufficient for leaf discs with one cm diameter. A LED ring light, two gooseneck lights and a backlight were used for illumination of the leaf discs. The gooseneck lights were positioned in an 45° angle to properly illuminate sporangiophores standing out from the leaf discs. The three lights combined allowed an exposure time of only 18–20 ms, making the whole imaging process fast. Consistent lighting of leaf discs was crucial for the trained CNN to reliably classify images.
Together with the motorized table, the Zeiss Axio Zoom (Jena, Germany) V16 is equipped with a software-autofocus, which was used to find the proper focal height for each leaf disc prior to imaging. To gain the highest focal depth, the smallest aperture possible was chosen. For each leaf disc one image was taken. In total 96 leaf discs could be imaged in roughly 15 min. A detailed protocol for the whole imaging process is available in the associated GitHub repository together with the complete workflow, which can be directly imported into Zen Blue 3.0 (Zeiss, Jena, Germany). With the workflow imported, imaging of leaf discs can be performed right away (
https://github.com/Daniel-Ze/Leaf-disc-scoring/tree/main/microscope_instructions, accessed on 20 August 2021).
After imaging the first 96 samples, the plate was rotated 180° and the second half of the plate was recorded. Images were exported as JPEG files with a resolution of 2752 × 2208 pixels for further analysis. The exported images were renamed using a naming template and a Python script (available in the GitHub repository).
2.4. Manual Phenotyping
Disease symptoms on leaf discs were captured at 4 dpi (days post-inoculation) using the AxioZoom V16 stereo microscope and were visually rated for disease severity. A reversed five-class OIV 452-1 descriptor (OIV, 2nd edition 2001,
https://www.oiv.int/en/technical-standards-and-documents/description-of-grape-varieties/oiv-descriptor-list-for-grape-varieties-and-vitis-species-2nd-edition (last accessed 20 August 2021) scale was used for the visual assessment. Disease severity was determined by the intensity of sporangia developed on the leaf discs. Where, score 1: no sporangiophores (highly resistant), score 3: one to five sporangiophores (resistant), score 5: six to twenty sporangiophores (moderate), score 7: more than twenty (susceptible), and score 9: dense uniform cloud of sporangiophores (highly susceptible).
2.5. SCNN: Training, Evaluation and Pipeline Building
2.5.1. Training and Validation of the SCNNs
Leaf disc images representing the five possible inverse OIV452-1 classes were chosen from inoculation experiments with F
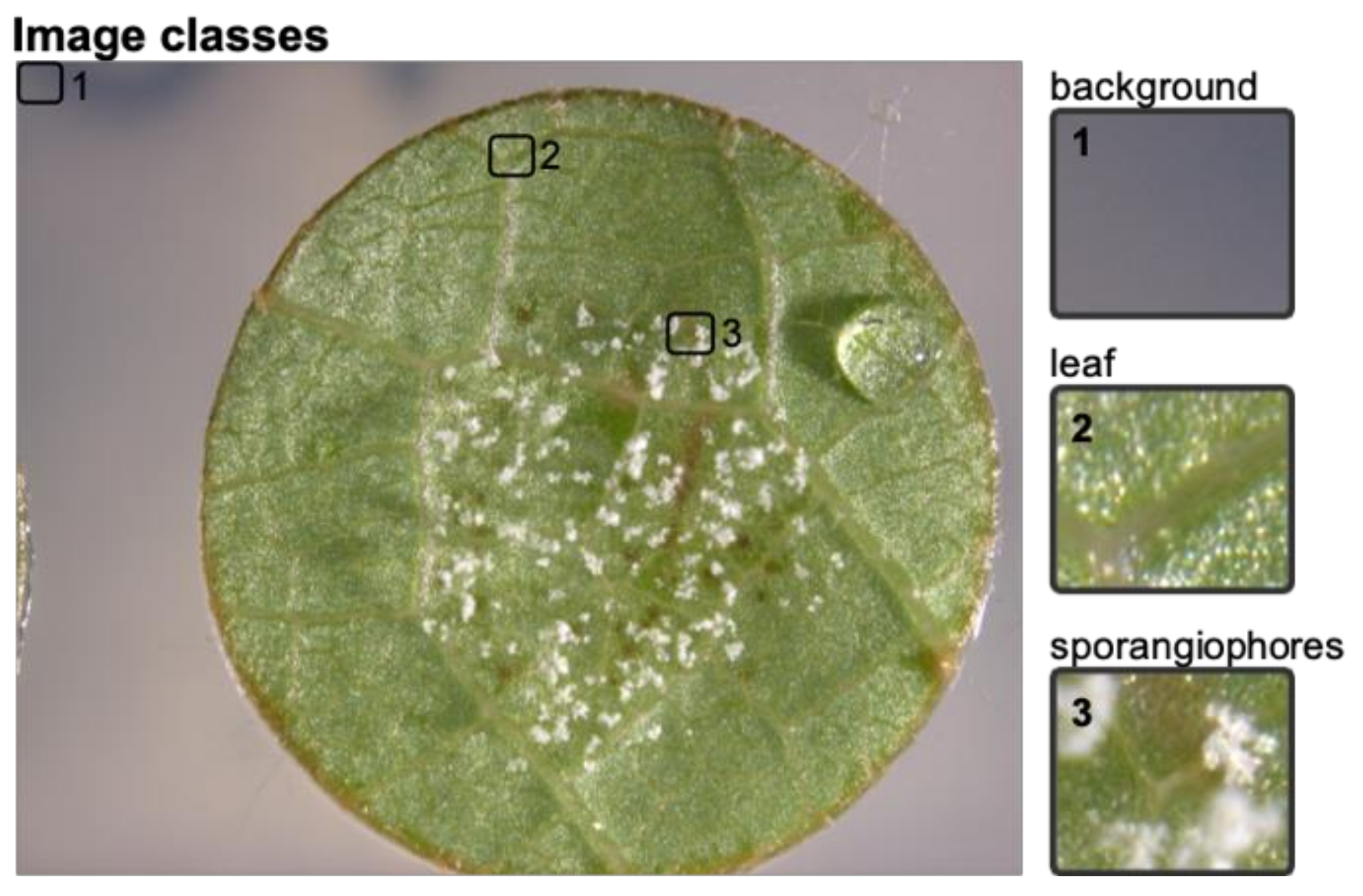
1 individuals of the cross ‘Morio Muskat’ × (COxGT2). The leaf discs were each sliced into 506 segments and manually classified into the classes background, leaf and leaf disc with sporangiophores (
Figure 2) using image-sorter2 [
25].
A script implemented in Python was used for random sampling of image slices from the respective classes for training and validation. The script is available in the associated GitHub repository.
The first binary SCNN has the classes “background” and “leaf disc” (CNN1). It was trained with 2919 images and validated with 947 images per class. The second binary SCNN was trained to classify images as “sporangiophores” and “no sporangiophores” (CNN2). The final dataset comprised 968 images per class for training and 437 images per class for validation. All datasets are available in the GitHub repository. All SCNN trainings were performed on a desktop PC running Ubuntu 20.04, a 24 thread CPU and 32 Gb of RAM. Due to the use of SCNNs all calculations were performed using the CPU.
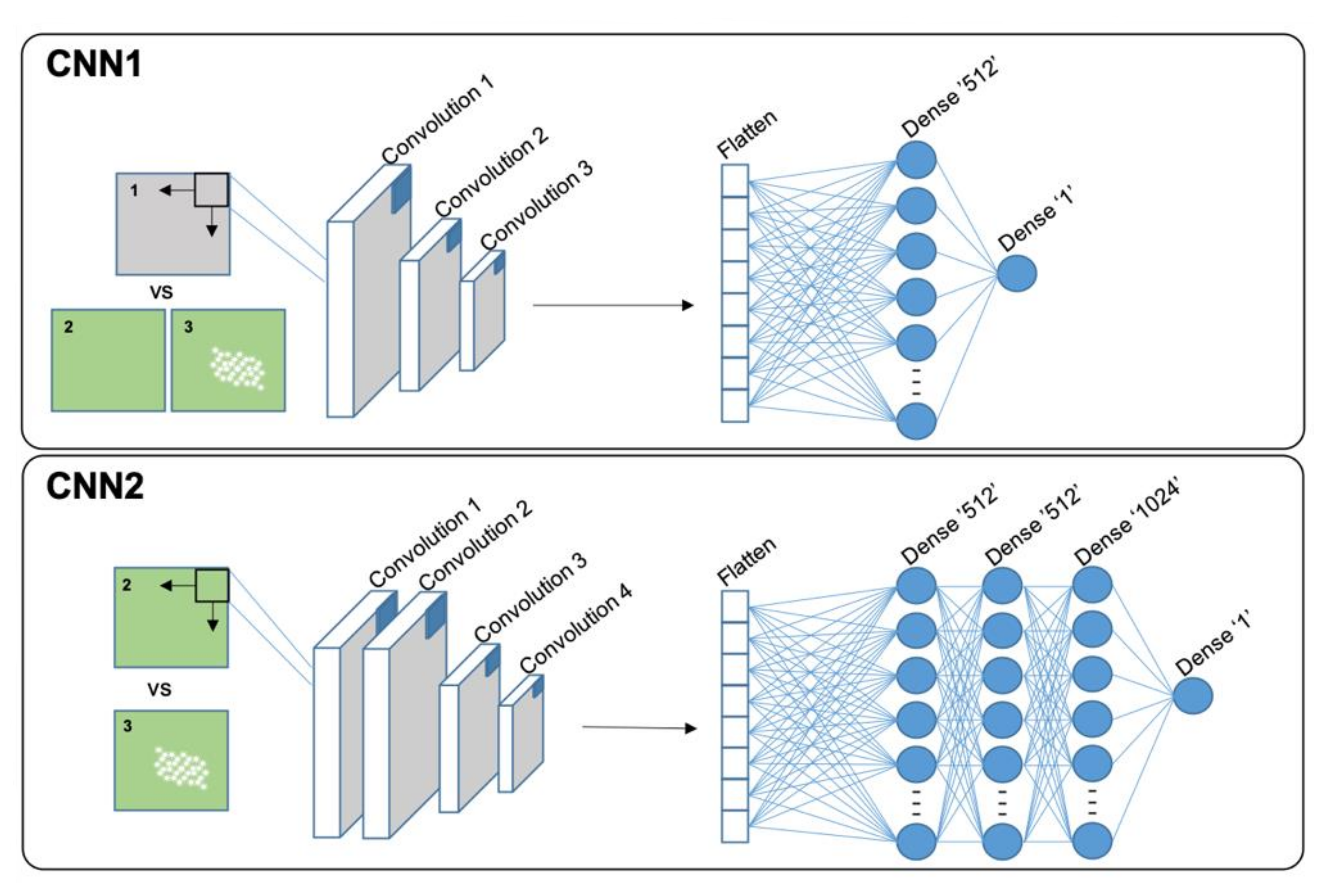
The detailed structure of the SCNN layers can be found in the GitHub repository. To retrain the SCNNs for special applications the scripts can be used with Tensorflow (v2.3.1) and Keras (v2.4.3) (GitHub repository) [
2,
26]. For the generated CNN1 model we used three image convolutions and one fully connected dense layer with 512 nodes (
Figure 3, CNN1). As optimizer, “SGD” with “Nesterov momentum” with a learning rate of 0.1 and a dropout of 0.4 was chosen (GitHub repository, CNN1.py). The structure of CNN2 was different. An additional convolution layer and two dense layers with 512 and 1024 nodes respectively were added (
Figure 3, CNN2). Best results for the CNN2 were achieved with the “Nadam” optimizer with a learning rate of 0.0001 and a dropout of 0.5 (GitHub repository, CCN2.py). For training of the two SCNNs 30 epochs each were enough to achieve sufficient accuracy. Final image datasets for training the two SCNNs are available for download together with a Jupyter notebook containing detailed instructions (
https://github.com/Daniel-Ze/Leaf-disc-scoring/tree/main/scripts, accessed on 20 August 2021).
2.5.2. Evaluating the Performance of the Trained SCNNs
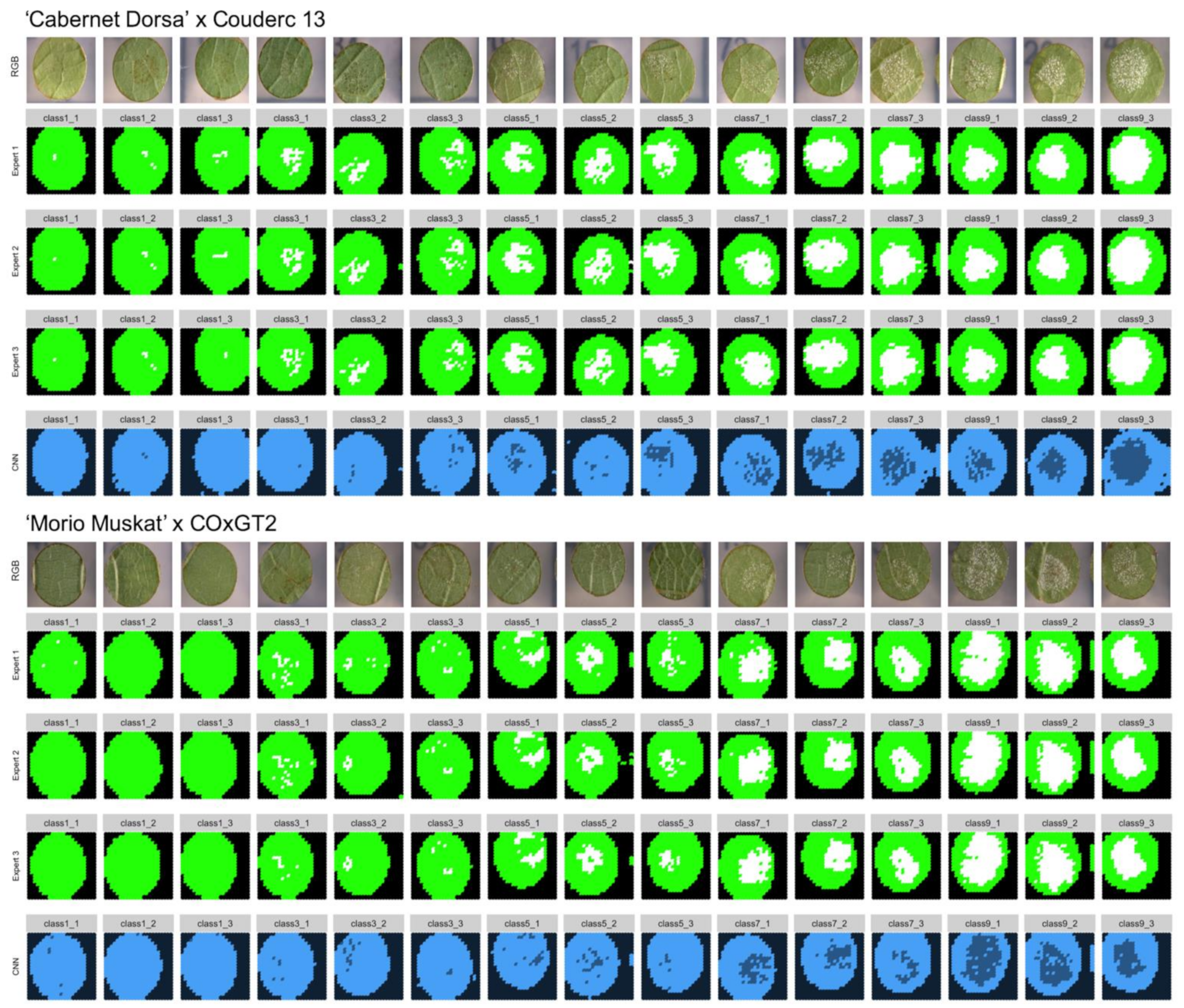
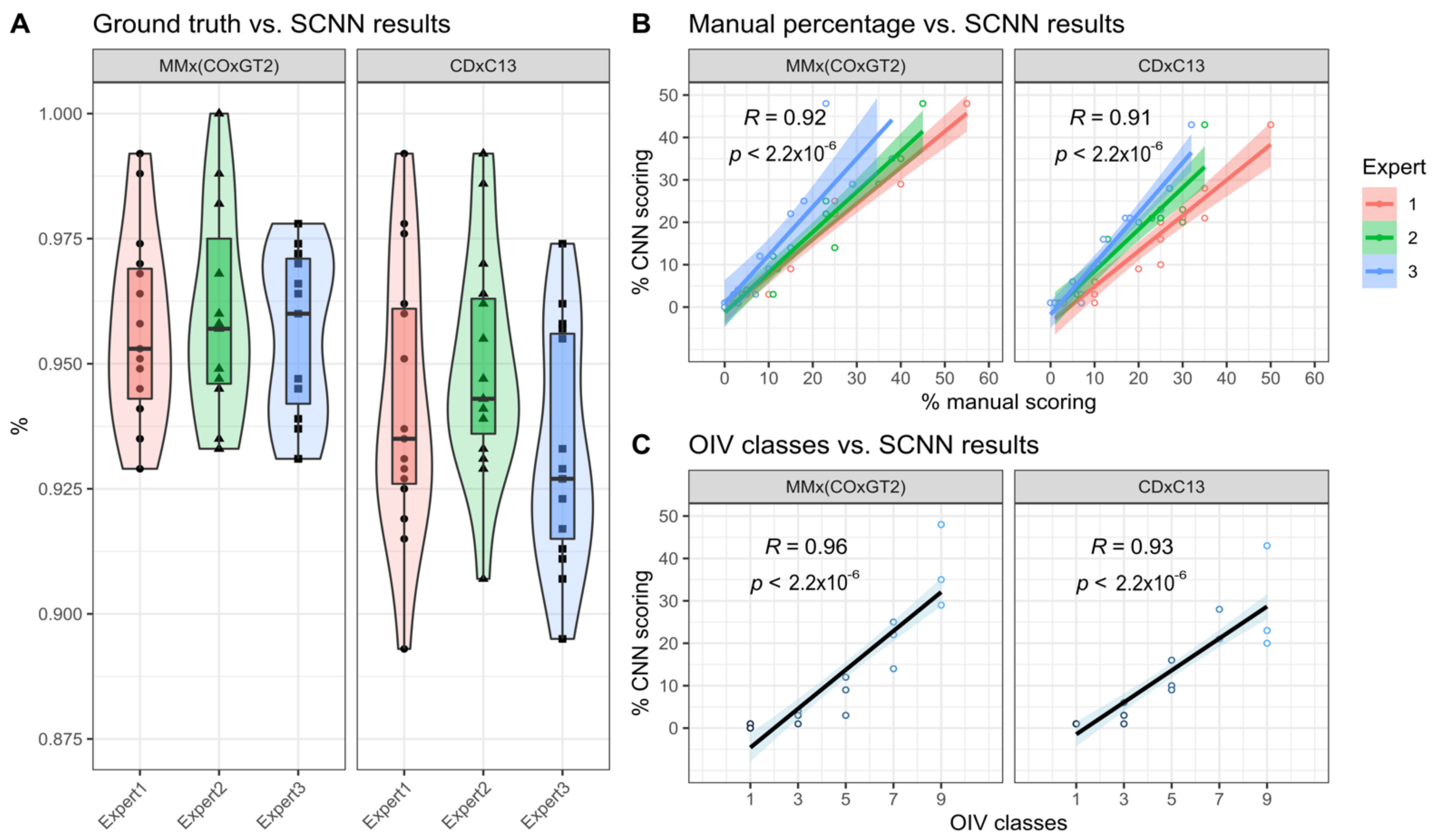
To confirm the accuracy of the trained neural network, ground truth data was generated. Three independent experts classified slices of 30 leaf discs manually. For this test two different genetic backgrounds were used. First, 15 leaf disc images were chosen from the ‘Morio Muskat’ × (COxGT2) population (three of each inverse OIV452-1 phenotypic class). The two SCNNs were originally trained with leaf disc images from this cross. To test the performance of the two SCNNs additional 15 images from a second independent cross (‘Cabernet Dorsa’ × Couderc 13) were analyzed (three of each inverse OIV class).
In total, 15,180 image slices were sorted into the three different classes (“background” = 1, “sporangiophores” = 2, “no sporangiophores” = 3). It was ensured that a set of leaf discs was chosen which has not been used in the earlier training of the CNNs. From this manually assigned set of scores, the true positive percentage was calculated by comparing the manually assigned classes of three independent experts with the classes of the two CNNs.
Further, the neural network calculated percentage leaf disc area with sporangiophores was correlated with either the recorded inverse OIV452-1 score or the manually scored percentage leaf disc area with sporangiophores. The data was correlated using the R cor() package with the method “spearman” (OIV) and “pearson”, respectively (percentage leaf disc area with sporangiophores) [
27].
2.5.3. The Classification Pipeline
To make the analysis of a large set of images easier a simple one-line command line pipeline was implemented (
Figure 4).
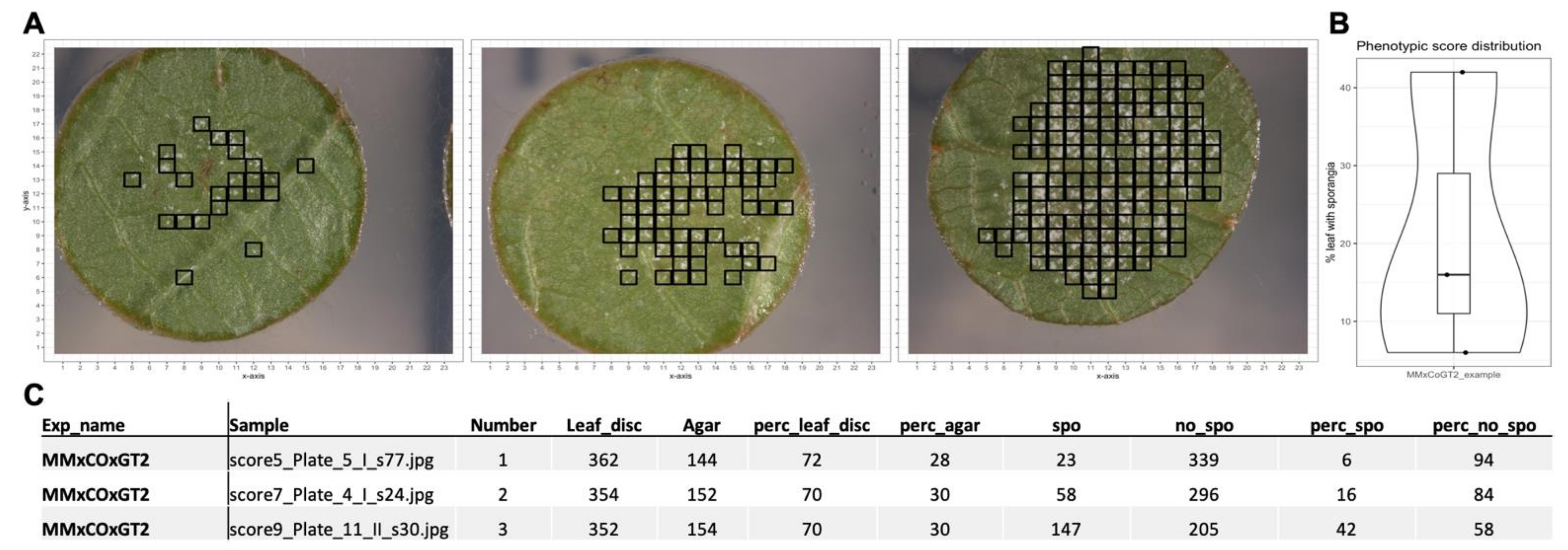
First, a leaf disc image was sliced into 506 pieces, which were then classified in water agar (background) or leaf disc. Leaf disc images are further analyzed with the second CNN2, which classifies them into leaf disc without and leaf disc with sporangiophores (
Figure 4). The pipeline loops over all images in a supplied folder and after classification of all images an R script plots the location of sporangiophores on the original RGB image, generates a plot with the percentage of leaf disc area with sporangiophores and a table of all generated values (
Figure 4). As control, all images of classified sporangiophores are stored in a folder for manual inspection. The pipeline together with detailed installation instructions is available under
www.github.com/Daniel-Ze/Leaf-disc-scoring, accessed on 20 August 2021. Additional information on how the CNNs were trained are included in the repository.
4. Discussion
We present here an automated leaf disc scoring pipeline using RGB images and trained shallow convolutional neural networks (SCNNs). We further supply detailed instructions on how to train these SCNNs for novel traits to adjust the pipeline accordingly. As proof of concept, we trained the neural networks to efficiently detect grapevine downy mildew emerging from inoculated leaf discs from two segregating populations generated from different genetic backgrounds. A similar approach was already successfully implemented for the detection of grapevine powdery mildew caused by
Erysiphe necator [
24]. The pipeline presented here allows the objective high-throughput screening of leaf discs with controlled infections, which is a crucial part of any plant breeding or quantitative trait locus (QTL) mapping workflow. In recent years, the use of deep learning for image classification in the area of plant pathogen detection has received much attention. For reviews on this topic see [
6,
7,
28,
29]. For visually easily detectable diseases located on the leaf surface like powdery mildew several deep learning image segmentation approaches have been published in recent years [
24,
30,
31].
As downy mildew is a major problem in temperate climates where relatively cold and humid spring times are common, a major effort is made to breed new grapevine cultivars resistant against this pathogen. As for all breeding programs, objective phenotyping of the offspring of a segregating cross is essential. This requires highly trained personnel who have many years of experience with the pathogen of choice. However, in the meantime, breeding programs are forced to increase their capacity and the need for high-throughput phenotyping methods emerges. Leaf disc assays have already been an important part of resistance scoring under controlled laboratory conditions for a long time. The evaluation of these tests however, was done manually in most of the cases [
32,
33,
34]. The advancements in computer vision and machine learning in recent years enabled a mainstream application of previously highly complex and very computationally intense algorithms. The well documented open source projects TensorFlow [
2] and Keras [
26] built on top of the latter have made it easy for people with a limited knowledge in Python programming to apply these principles to their own datasets.
The pipeline downstream of the image acquisition presented in this study was designed as open source repository for anybody to be used and can be adjusted to any individual needs. We assume that image acquisition will differ from laboratory to laboratory; therefore, we put together a workflow for training new SCNN models with image data available from other image acquisition pipelines. However, we also included detailed instructions and a template for automatic imaging with a Zeiss Axio Zoom v16 with ZenBlue3.0 software.
4.1. SCNN Versus Expert Evaluation
The goal of this study was to create an efficient method for scoring disease severity on leaf discs using a limited amount of image data and a limited amount of computational power. To achieve this, we used simple SCNNs and trained these with small numbers of images. Further, we decided to use only a simple binary classification of the images reducing the complexity and allowing to first distinguish background from leaf disc and then, in the second step, the detection of infected leaf disc areas. This approach will allow specific training of the second SCNN for additional traits or pathogens therefore increasing the modularity of the pipeline.
Training of the first SCNN shows that the detection of leaf disc image slices is a fairly easy task for the neural network as the leaf slices differ greatly from the background. Training of this SCNN showed a high specificity with a 98% validation accuracy and a validation loss below 10%. The training of the second SCNN was thought to be much more complicated in terms of distinguishing leaf hairs from sporangiophores. However, after adjustments to the model such as increasing the number of image convolutions together with two additional fully connected layers and a different model optimizer, training of this neural network also showed a very high validation accuracy of 95% with a validation loss around 15% (
Figure 4).
To evaluate the performance of these trained SCNNs, three independent experts sorted 15,180 image slices (30 leaf discs) into the three categories: background, leaf disc with and leaf disc without sporangiophores (
Figure 2). A feature of trained SCNNs is their transferability, meaning that in optimal conditions the SCNNs should have learned distinct features allowing a categorization of images previously not observed by the SCNN. To test this, we chose 15 new images from the same genetic background plus 15 images from another cross with an unrelated genetic background as testing set. The test set of images was analyzed with the two SCNNs and the ratio of true positives was calculated from that as means of performance evaluation. The median ratio of true positives was around 96% for the trained genetic background and 93% for the unrelated genetic background. This indicates that the transferability of the two SCNNs is given with only a slightly decreased accuracy.
Correlating the results of the two SCNNs with real world applications such as manual estimation of sporangiophores-covered area and OIV452-1 classes showed highly significant correlations again with a slight decrease for the unrelated genetic background. The subjectivity of the manually estimated area covered with sporangiophores is indicated by the limited overlap of the three linear regressions (
Figure 7B). This was not the case for the assigned inverse OIV452-1 classes due to the reduction of complexity by the limited number of assignable classes. For these two correlations again, a slight decrease was observed for the second unrelated genetic background as it was seen for the true positive ratio.
To counteract this observed difference, we propose to generate a diverse training set composed of multiple different genetic backgrounds to maximize the transferability and increase the already very good performance of the two SCNNs. The results of the true positive ratio and the performed correlations indicate that the results of the two SCNNs are a well-suited objective substitute for these two subjective scoring methods.
4.2. The Leaf Disc Scoring Pipeline
We demonstrated that the two SCNNs can efficiently detect grapevine DM infections on images of leaf discs. This allows their incorporation as a valuable tool for the evaluation of resistance traits in breeding processes and for the characterization of genetic resources in germplasm repositories. The new leaf disc scoring method is particularly applicable for specific research purposes like the phenotyping of large populations needed for the detection and QTL mapping of novel resistances to e.g., DM. Therefore, we wrote a simple open-source pipeline incorporating the two SCNNs. The pipeline is an easy to execute command-line application that was tested to work on Unix systems.
With the modular two-step detection process, it will be easy to train the second SCNN to detect and quantify other grapevine pathogens, where leaf infections are currently only visually assed like
Guignardia bidwellii causing black rot,
Colletotrichum gloeosporioides causing ripe rot,
Phakopsora euvitis causing leaf rust or
Elsinoë ampelina causing anthracnose [
35,
36,
37,
38]. Grapevine DM is by far one of the easiest pathogens to be detected with its very white sporangiophores. For other pathogens such as
E. necator the used SCNN model layers of CNN2 most probably have to be adjusted to a DCNN like in Biermann et al. [
24] to fit the different fungal structures formed during the infection. However, we are confident that this scoring pipeline will find a multitude of applications, not only for resistance assessment of grapevine diseases, but also for other plant-pathogen systems where leaf disc assays are already established or will be developed in the future. The whole process for training the SCNNs is documented in an easy to use Jupyter notebook (see GitHub repository).
4.3. Further Improvements
A step in improving the leaf disc-scoring pipeline would be to switch from binary to categorical classification. This would allow the detection of several different traits with the second CNN at the same time. An even better way of categorical classification would be semantic labeling of the traits analyzed on the leaf disc image slices. This would allow a much better accuracy in terms of detection. For example, the investigated leaf discs infected with DM showed different sporangiophore densities. Such differences are neglected by the CNNs presented here. To further improve the detection accuracy a switch to alternative deep learning techniques such as semantic labelling is inevitable. In a recent study this approach was demonstrated for black rot, black measles, leaf blight and mites on grapevine leaves [
39]. Further studies have shown promising detection of leaf localized diseases using semantic segmentation for coffee and rice [
40,
41]
However, the results obtained with the simple binary classification setup presented here clearly show a high correlation with standard scoring methods indicating its suitability for high-throughput phenotyping of leaf discs. Furthermore, it keeps the requirements in terms of computational resources and image training sets to a minimum allowing a fast adaptation to new plant-pathogen systems.