First Report of Amaranthus palmeri S. Wats. in Cotton, Maize and Sorghum in Greece and Problems with Its Management
Abstract
:1. Introduction
2. Materials and Methods
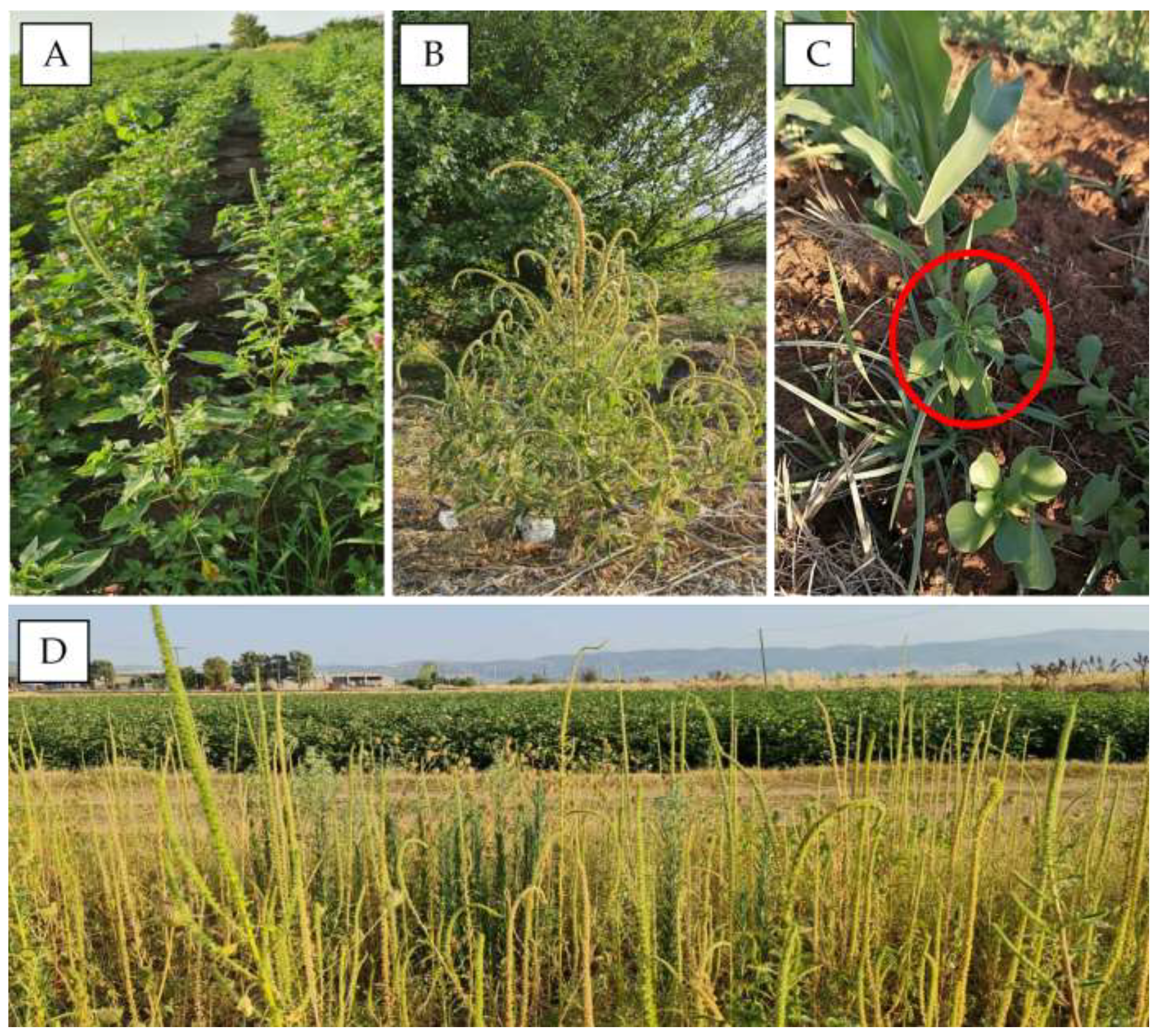
2.1. Weed Detection and First Surveys
2.2. Seed Collection and Plant Samples
2.3. Experimental Design
2.4. Measurements
2.5. Statistical Analysis
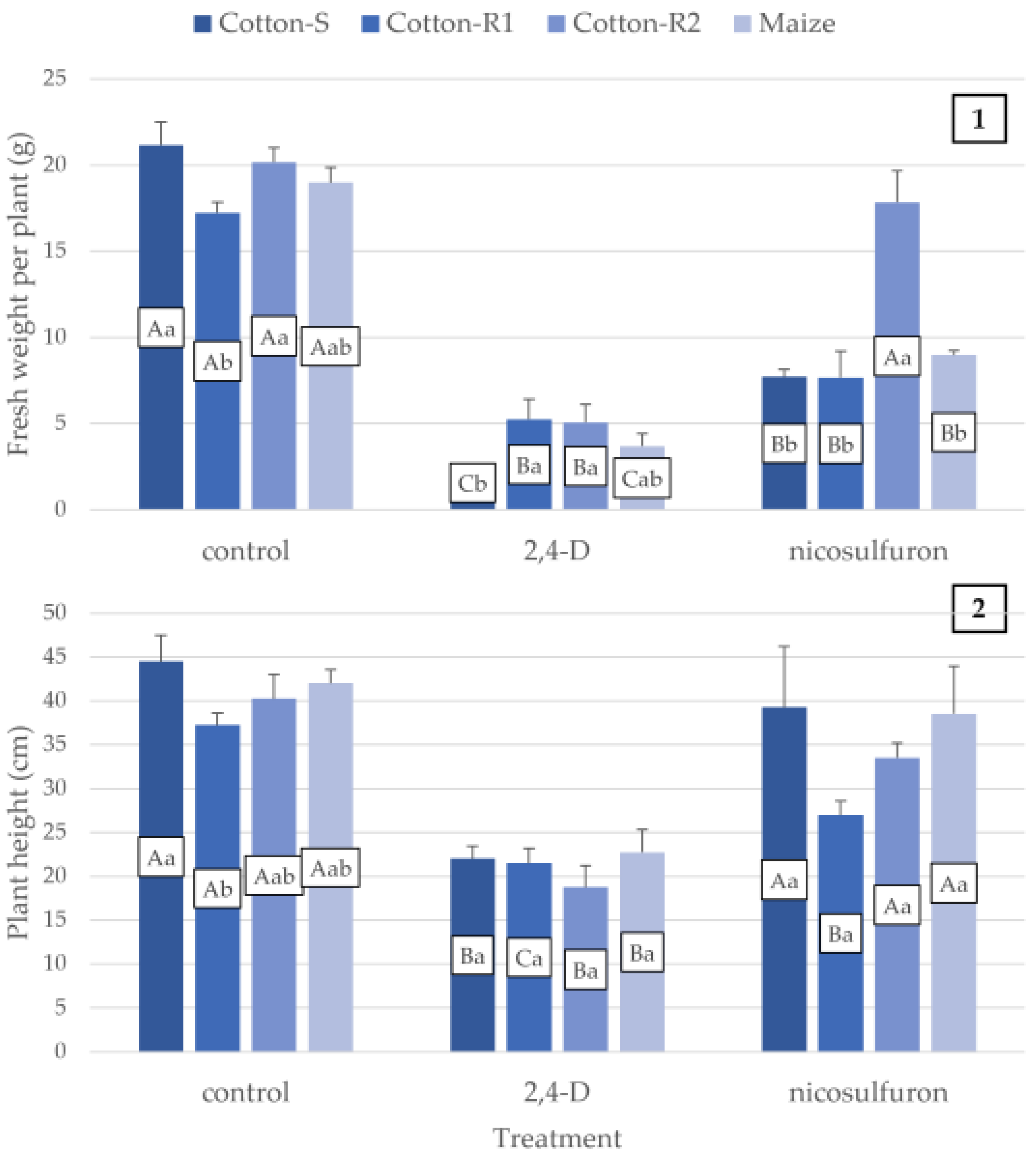
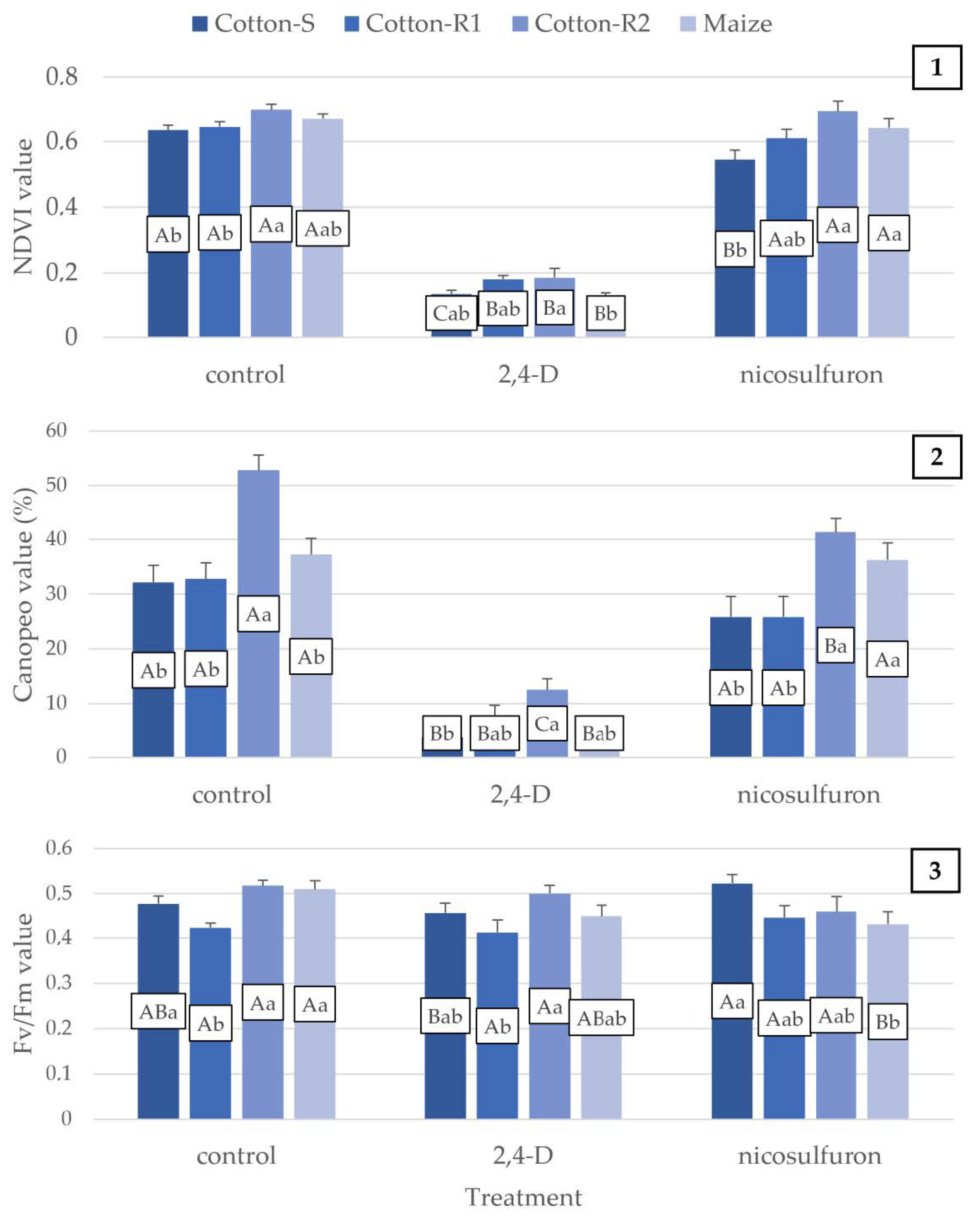
3. Results
3.1. Weed Detection
3.2. Management
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ward, S.M.; Webster, T.M.; Steckel, L.E. Palmer amaranth (Amaranthus palmeri): A review. Weed Technol. 2013, 27, 12–27. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Liu, R.; Boyer, G.; Stahlman, P.W. Confirmation of 2,4-D resistance and identification of multiple resistance in a Kansas Palmer amaranth (Amaranthus palmeri) population. Pest Manag. Sci. 2019, 75, 2925–2933. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. 2021. Available online: www.weedscience.org (accessed on 15 May 2021).
- Torra, J.; Royo-Esnal, A.; Romano, Y.; Osuna, M.D.; León, R.G.; Recasens, J. Amaranthus palmeri a new invasive weed in Spain with herbicide resistant biotypes. Agronomy 2020, 10, 993. [Google Scholar] [CrossRef]
- Milani, A.; Panozzo, S.; Farinati, S.; Iamonico, D.; Sattin, M.; Loddo, D.; Scarabel, L. Recent discovery of Amaranthus palmeri S. Watson in Italy: Characterization of ALS-resistant populations and sensitivity to alternative herbicides. Sustainability 2021, 13, 7003. [Google Scholar] [CrossRef]
- Schwartz-Lazaro, L.M.; Norsworthy, J.K.; Scott, R.C.; Barber, L.T. Resistance of two Arkansas Palmer amaranth populations to multiple herbicide sites of action. Crop Prot. 2017, 96, 158–163. [Google Scholar] [CrossRef]
- Webster, T.M.; Simmons, D.B.; Culpepper, A.S.; Grey, T.L.; Bridges, D.C.; Scully, B.T. Factors affecting potential for Palmer amaranth (Amaranthus palmeri) suppression by winter rye in Georgia, USA. Field Crops Res. 2016, 192, 103–109. [Google Scholar] [CrossRef]
- Travlos, I.; Tsekoura, A.; Antonopoulos, N.; Kanatas, P.; Gazoulis, I. Novel sensor-based method (quick test) for the in-season rapid evaluation of herbicide efficacy under real field conditions in durum wheat. Weed Sci. 2021, 69, 147–160. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; Smith, K.L.; Steckel, L.E.; Koger, C.H. Weed seed contamination of cotton gin trash. Weed Technol. 2009, 23, 574–580. [Google Scholar] [CrossRef]
- Yu, E.; Blair, S.; Hardel, M.; Chandler, M.; Thiede, D.; Cortilet, A.; Gunsolus, J.; Becker, R. Timeline of Palmer amaranth (Amaranthus palmeri) invasion and eradication in Minnesota. Weed Technol. 2021, 1–9. [Google Scholar] [CrossRef]
- Vieira, B.C.; Luck, J.D.; Amundsen, K.L.; Werle, R.; Gaines, T.A.; Kruger, G.R. Herbicide drift exposure leads to reduced herbicide sensitivity in Amaranthus spp. Sci. Rep. 2020, 10, 2146. [Google Scholar] [CrossRef] [Green Version]
- Shyam, C.; Chahal, P.; Jhala, A.; Jugulam, M. Management of glyphosate-resistant Palmer amaranth (Amaranthus palmeri) in 2,4-D–, glufosinate-, and glyphosate-resistant soybean. Weed Technol. 2021, 35, 136–143. [Google Scholar] [CrossRef]
- Lawrence, B.H.; Bond, J.A.; Eubank, T.W.; Golden, B.R.; Cook, D.R.; Mangialardi, J.P. Evaluation of 2, 4-D–based herbicide mixtures for control of glyphosate-resistant Palmer amaranth (Amaranthus palmeri). Weed Technol. 2019, 33, 263–271. [Google Scholar] [CrossRef]
- Kumar, V.; Liu, R.; Stahlman, P.W. Differential sensitivity of Kansas Palmer amaranth populations to multiple herbicides. Agron. J. 2020, 112, 2152–2163. [Google Scholar] [CrossRef]
- Hay, M.M.; Shoup, D.E.; Peterson, D.E. Herbicide options for control of Palmer amaranth (Amaranthus palmeri) and common waterhemp (Amaranthus rudis) in double-crop soybean. Weed Technol. 2019, 33, 106–114. [Google Scholar] [CrossRef]
- Arianoutsou, M.; Bazos, I.; Delipetrou, P.; Kokkoris, Y. The alien flora of Greece: Taxonomy, life traits and habitat preferences. Biol. Invasions 2010, 12, 3525–3549. [Google Scholar] [CrossRef]
- Smith, S.C.; Jennings, K.M.; Monks, D.W.; Chaudhari, S.; Schultheis, J.R.; Reberg-Horton, C. Critical timing of Palmer amaranth (Amaranthus palmeri) removal in sweetpotato. Weed Technol. 2020, 34, 547–551. [Google Scholar] [CrossRef]
- Korres, N.E.; Norsworthy, J.K.; Mauromoustakos, A.; Williams, M.M. Soybean density and Palmer amaranth (Amaranthus palmeri) establishment time: Effects on weed biology, crop yield, and economic returns. Weed Sci. 2020, 68, 467–475. [Google Scholar] [CrossRef]
- Kanatas, P.; Travlos, I.S.; Gazoulis, I.; Tataridas, A.; Tsekoura, A.; Antonopoulos, N. Benefits and limitations of decision support systems (DSS) with a special emphasis on weeds. Agronomy 2020, 10, 548. [Google Scholar] [CrossRef] [Green Version]
- Reinhardt Piskackova, T.A.; Reberg-Horton, S.C.; Richardson, R.J.; Jennings, K.M.; Franca, L.; Young, B.G.; Leon, R.G. Windows of action for controlling palmer amaranth (Amaranthus palmeri) using emergence and phenology models. Weed Res. 2021, 61, 188–198. [Google Scholar] [CrossRef]
- Houston, M.M.; Norsworthy, J.K.; Barber, T.; Brabham, C. Field evaluation of preemergence and postemergence herbicides for control of protoporphyrinogen oxidase-resistant Palmer amaranth (Amaranthus palmeri S. Watson). Weed Technol. 2019, 33, 610–615. [Google Scholar] [CrossRef] [Green Version]
- Palma-Bautista, C.; Tataridas, A.; Kanatas, P.; Travlos, I.S.; Bastida, F.; Domínguez-Valenzuela, J.A.; De Prado, R. Can control of glyphosate susceptible and resistant Conyza sumatrensis populations be dependent on the herbicide formulation or adjuvants? Agronomy 2020, 10, 1599. [Google Scholar] [CrossRef]
- Palma-Bautista, C.; Vazquez-Garcia, J.G.; Travlos, I.; Tataridas, A.; Kanatas, P.; Domínguez-Valenzuela, J.A.; De Prado, R. Effect of adjuvant on glyphosate effectiveness, retention, absorption and translocation in Lolium rigidum and Conyza canadensis. Plants 2020, 9, 297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webster, T.M.; Scully, B.T.; Grey, T.L.; Culpepper, A.S. Winter cover crops influence Amaranthus palmeri establishment. Crop Prot. 2013, 52, 130–135. [Google Scholar] [CrossRef]
- Leon, R.G.; Wright, D.L. Recurrent changes of weed seed bank density and diversity in crop—Livestock systems. Agron. J. 2018, 110, 1068–1078. [Google Scholar] [CrossRef]
- Travlos, I.; Rapti, E.; Gazoulis, I.; Kanatas, P.; Tataridas, A.; Kakabouki, I.; Papastylianou, P. The herbicidal potential of different pelargonic acid products and essential oils against several important weed species. Agronomy 2020, 10, 1687. [Google Scholar] [CrossRef]
- Kanatas, P.J.; Travlos, I.S.; Gazoulis, J.; Antonopoulos, N.; Tsekoura, A.; Tataridas, A.; Zannopoulos, S. The combined effects of false seedbed technique, post-emergence chemical control and cultivar on weed management and yield of barley in Greece. Phytoparasitica 2020, 48, 131–143. [Google Scholar] [CrossRef]

| Active Ingredient | Site of Action | Classification HRAC Group |
|---|---|---|
| Foramsulfuron, iodosulfuron-methyl, mesosulfuron-methyl, rimsulfuron, trifloxysulfuron, nicosulfuron, halosulfuron-methyl, pyrithiobac-sodium | Inhibition of acetolactate synthase (ALS) | B |
| Pendimethalin | Microtubule assembly inhibition | K1 |
| S-metolachlor | Inhibition of cell division | K3 |
| Mesotrione | Inhibition of 4-hydroxyphenyl-pyruvate-dioxygenase (4-HPPD) | F2 |
| Glyphosate | Inhibition of EPSPS synthase | G |
| 2,4-D, dicamba | Synthetic auxins | O |
| Biotype | Region | Crop | Location Coordinates |
|---|---|---|---|
| Cotton-S | Central Greece | cotton | 39°21′42″ N, 22°28′79″ E |
| Cotton-R1 | Central Greece | cotton | 39°22′83″ N, 22°28′22″ E |
| Cotton-R2 | Central Greece | cotton | 39°22′46″ N, 22°28′04″ E |
| Maize | Western Greece | maize | 38°52′12″ N, 20°51′51″ E |
| Treatment | Biotype | NDVI | Canopeo | Plant Height | Fresh Weight | Fv/Fm |
|---|---|---|---|---|---|---|
| 2,4-D | Cotton-S | −78.7% | −88.5% | −50.6% | −93.4% | −4.2% |
| Cotton-R1 | −72.1% | −80.2% | −42.3% | −69.5% | −2.9% | |
| Cotton-R2 | −73.5% | −76.3% | −53.4% | −74.9% | −3.4% | |
| Maize | −80.6% | −88.4% | −45.8% | −80.5% | −11.8% | |
| Nicosulfuron | Cotton-S | −14.2% | −19.8% | −11.8% | −63.5% | 9.4% |
| Cotton-R1 | −5.4% | −21.2% | −27.5% | −55.5% | 5.3% | |
| Cotton-R2 | −0.7% | −21.8% | −16.8% | −11.6% | −11.1% | |
| Maize | −4.1% | −2.6% | −8.3% | −52.6% | −15.2% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanatas, P.; Tataridas, A.; Dellaportas, V.; Travlos, I. First Report of Amaranthus palmeri S. Wats. in Cotton, Maize and Sorghum in Greece and Problems with Its Management. Agronomy 2021, 11, 1721. https://doi.org/10.3390/agronomy11091721
Kanatas P, Tataridas A, Dellaportas V, Travlos I. First Report of Amaranthus palmeri S. Wats. in Cotton, Maize and Sorghum in Greece and Problems with Its Management. Agronomy. 2021; 11(9):1721. https://doi.org/10.3390/agronomy11091721
Chicago/Turabian StyleKanatas, Panagiotis, Alexandros Tataridas, Vyronas Dellaportas, and Ilias Travlos. 2021. "First Report of Amaranthus palmeri S. Wats. in Cotton, Maize and Sorghum in Greece and Problems with Its Management" Agronomy 11, no. 9: 1721. https://doi.org/10.3390/agronomy11091721
APA StyleKanatas, P., Tataridas, A., Dellaportas, V., & Travlos, I. (2021). First Report of Amaranthus palmeri S. Wats. in Cotton, Maize and Sorghum in Greece and Problems with Its Management. Agronomy, 11(9), 1721. https://doi.org/10.3390/agronomy11091721








