UAV Multispectral Imaging Potential to Monitor and Predict Agronomic Characteristics of Different Forage Associations
Abstract
:1. Introduction
2. Materials and Methods
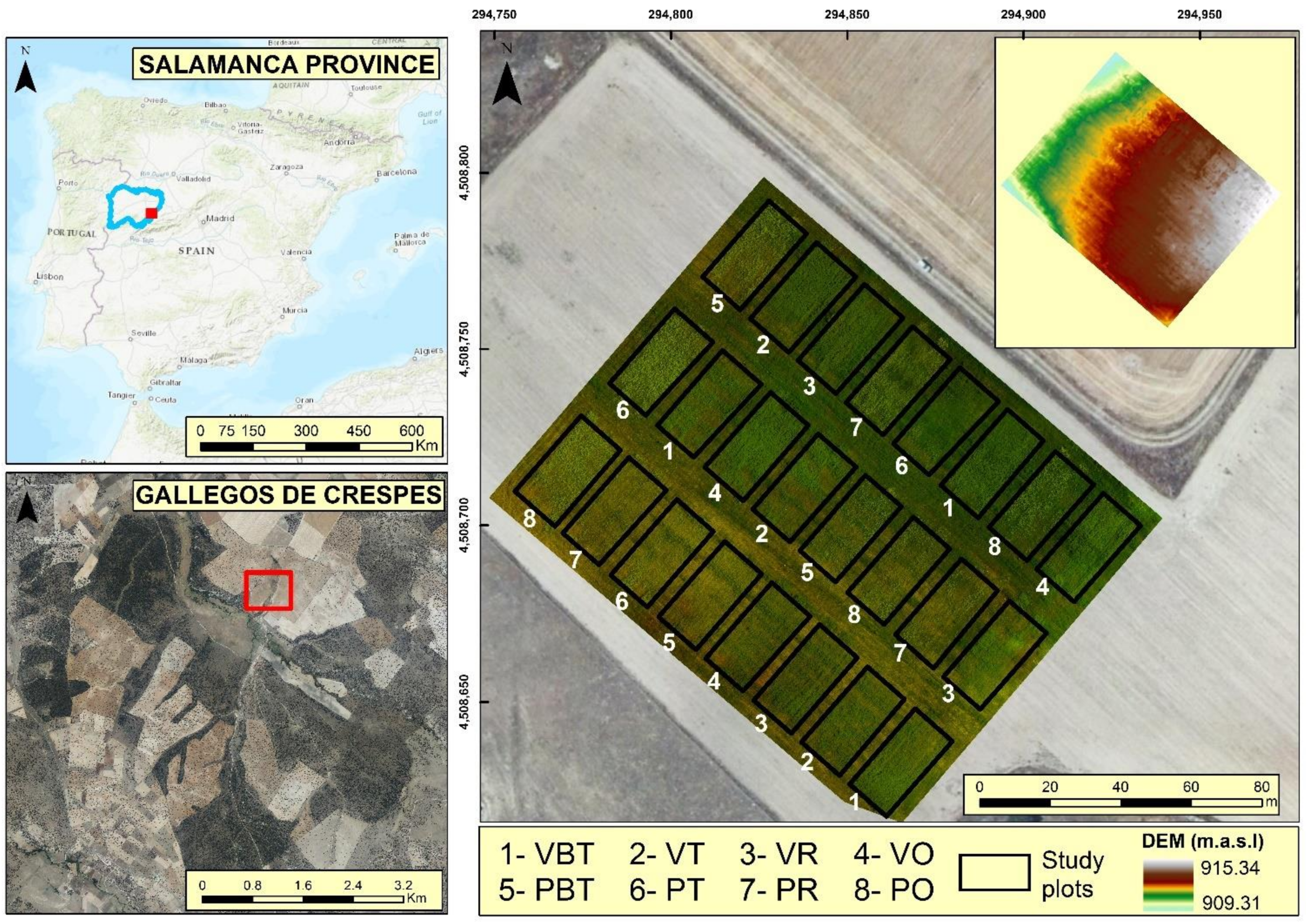
2.1. Experimental Design
2.2. Field and Laboratory Estimations
2.3. Multispectral Imaging Collection and Vegetation Indices
2.4. Statistical Analysis
2.4.1. Exploratory Analysis of Data Variability
2.4.2. Correlation Analysis
2.4.3. Prediction Model of In Situ Agronomic Parameters along the Growing Cycle Based on Partial Least Squares (PLS) Regression
3. Results and Discussion
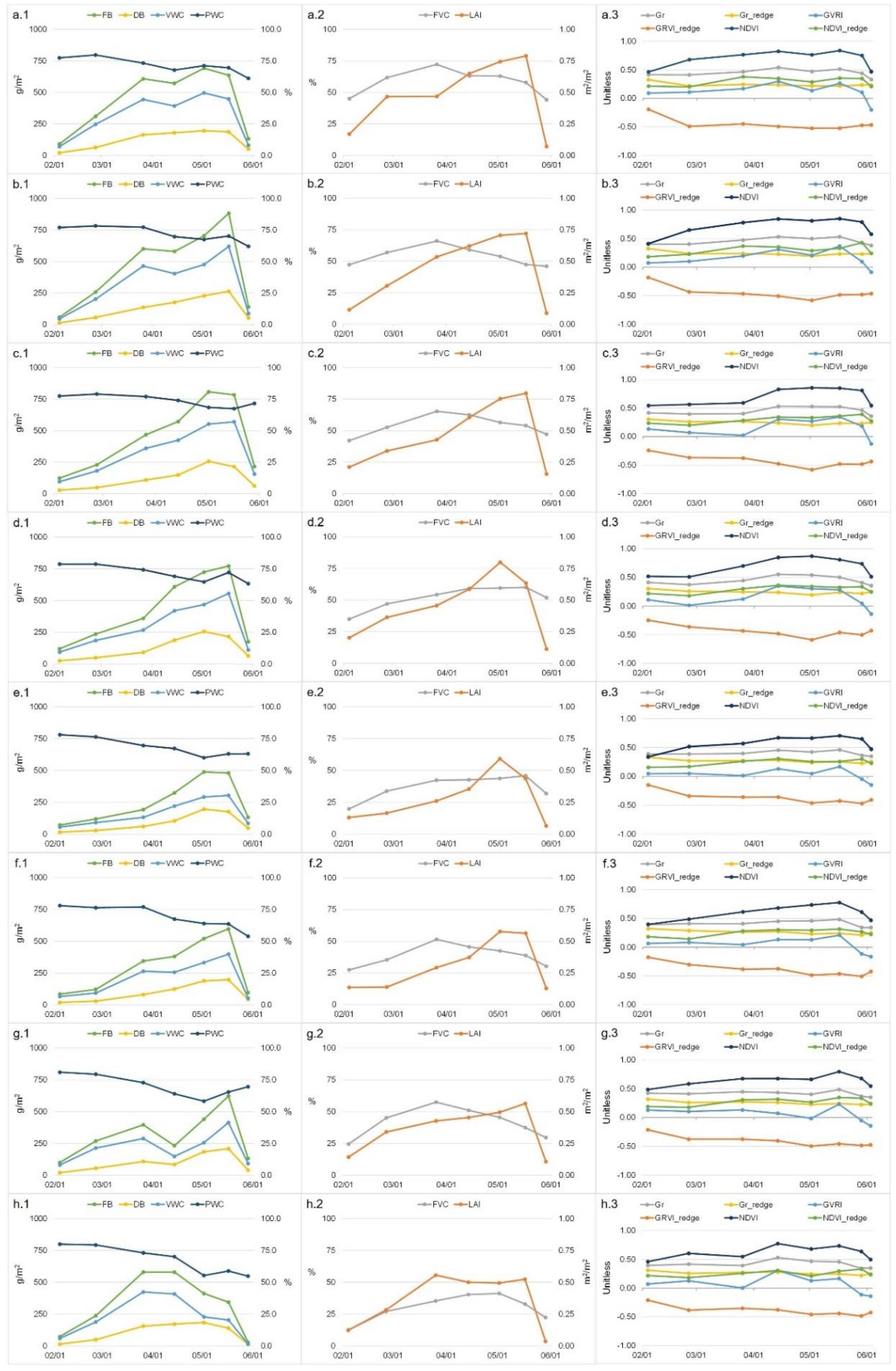
3.1. Spatio-Temporal Patterns
3.1.1. Field Measurements
3.1.2. Spectral Indices
3.2. Correlation Analysis
3.3. Modeling and Prediction of the Associations Behaviour PLS Regression
3.3.1. Global PLS Analysis for All Associations as a Whole
3.3.2. PLS Analysis for the Vetch-Based Associations (VBT, VT, VR and VO)
3.3.3. PLS Analysis for the Pea-Based Associations (PBT, PT, PR and PO)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B
| Meas. Date | Parameter | FB | DB | VWC | PWC | FCV | LAI | Meas. Date | Parameter | FB | DB | VWC | PWC | FCV | LAI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 February 2020 | Gr | 0.244 | 0.275 | 0.232 | −0.204 | 0.374 | 0.265 | 26 February 2020 | Gr | 0.173 | 0.190 | 0.168 | 0.036 | 0.039 | 0.010 |
| Gr_redge | −0.587 * | −0.533 * | −0.597 * | −0.111 | −0.027 | −0.594 * | Gr_redge | −0.618 * | −0.638 ** | −0.0610 * | −0.360 | −0.715 ** | −0.642 ** | ||
| GRVI | 0.383 | 0.402 | 0.373 | −0.172 | 0.316 | 0.385 | GRVI | 0.288 | 0.311 | 0.281 | 0.104 | 0.133 | 0.108 | ||
| GRVI_redge | −0.566 * | −0.530 * | −0.570 * | −0.026 | −0.174 | −0.593 * | GRVI_redge | −0.641 ** | −0.664 ** | −0.633 ** | −0.361 | −0.716 ** | −0.635 ** | ||
| NDVI | 0.512 * | 0.512 * | 0.506 * | −0.118 | 0.294 | 0.555 * | NDVI | 0.591 * | 0.619 * | 0.582 * | 0.330 | 0.594 * | 0.497 | ||
| NDVI_redge | 0.475 | 0.498 * | 0.463 | −0.209 | 0.303 | 0.577 * | NDVI_redge | 0.428 | 0.461 | 0.419 | 0.300 | 0.639 ** | 0.399 | ||
| 26 March 2020 | Gr | 0.399 | 0.330 | 0.415 | 0.231 | 0.587 * | 0.309 | 14 April 2020 | Gr | 0.821 ** | 0.765 ** | 0.817 ** | 0.629 ** | 0.518 * | 0.708 ** |
| Gr_redge | −0.400 | −0.292 | −0.430 | −0.396 | −0.597 * | −0.389 | Gr_redge | −0.446 | −0.426 | −0.440 | −0.296 | −0.836 ** | −0.811 ** | ||
| GRVI | 0.401 | 0.334 | 0.417 | 0.226 | 0.578 * | 0.308 | GRVI | 0.829 ** | 0.756 ** | 0.829 ** | 0.665 ** | 0.492 | 0.673 ** | ||
| GRVI_redge | −0.407 | −0.307 | −0.434 | −0.366 | −0.621 * | −0.377 | GRVI_redge | −0.548 * | −0.530 * | −0.538 * | −0.358 | −0.837 ** | −0.871 ** | ||
| NDVI | 0.459 | 0.390 | 0.475 | 0.253 | 0.655 ** | 0.340 | NDVI | 0.836 ** | 0.782 ** | 0.830 ** | 0.616 * | 0.675 ** | 0.848 ** | ||
| NDVI_redge | 0.627 ** | 0.573 * | 0.634 ** | 0.239 | 0.743 ** | 0.402 | NDVI_redge | 0.594 * | 0.560 * | 0.588 * | 0.376 | 0.809 ** | 0.749 ** | ||
| 2 May 2020 | Gr | 0.606 * | 0.566 * | 0.585 * | 0.434 | 0.573 * | 0.572 * | 17 May 2020 | Gr | 0.635 ** | 0.528 * | 0.662 ** | 0.610 * | 0.518 * | 0.651 ** |
| Gr_redge | −0.701 ** | −0.584 * | −0.699 ** | −0.631 ** | −0.691 ** | −0.638 ** | Gr_redge | −0.302 | −0.195 | −0.336 | −0.545 * | −0.483 | −0.460 | ||
| GRVI | 0.606 * | 0.576 * | 0.581 * | 0.429 | 0.553 * | 0.580 * | GRVI | 0.742 ** | 0.639 ** | 0.766 ** | 0.643 ** | 0.504 * | 0.698 ** | ||
| GRVI_redge | −0.710 ** | −0.591 * | −0.708 ** | −0.631 ** | −0.712 ** | −0.643 ** | GRVI_redge | −0.297 | −0.194 | −0.329 | −0.517 * | −0.463 | −0.452 | ||
| NDVI | 0.712 ** | 0.617 * | 0.702 ** | 0.598 * | 0.691 ** | 0.655 ** | NDVI | 0.597 * | 0.499 * | 0.622 * | 0.628 ** | 0.459 | 0.608 * | ||
| NDVI_redge | 0.661 ** | 0.563 * | 0.655 ** | 0.608 * | 0.645 ** | 0.608 * | NDVI_redge | 0.501 * | 0.457 | 0.508 * | 0.514 * | 0.396 | 0.513 * | ||
| 29 May 2020 | Gr | 0.699 ** | 0.524 * | 0.723 ** | 0.553 * | 0.790 ** | 0.314 | ||||||||
| Gr_redge | 0.382 | 0.143 | 0.454 | 0.625 ** | 0.303 | −0.075 | |||||||||
| GRVI | 0.713 ** | 0.523 * | 0.742 ** | 0.582 * | 0.797 ** | 0.332 | |||||||||
| GRVI_redge | 0.187 | −0.032 | 0.263 | 0.521 * | 0.021 | −0.180 | |||||||||
| NDVI | 0.582 * | 0.478 | 0.584 * | 0.400 | 0.812 ** | 0.342 | |||||||||
| NDVI_redge | 0.266 | 0.221 | 0.267 | 0.234 | 0.585 * | 0.172 |
| Assoc. | Parameter | FB | DB | VWC | PWC | FVC | LAI | Assoc. | Parameter | FB | DB | VWC | PWC | FVC | LAI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VBT | Gr | 0.661 ** | 0.693 ** | 0.637 * | −0.203 | 0.426 | 0.643 * | PBT | Gr | 0.708 ** | 0.656 * | 0.728 ** | −0.320 | 0.474 | 0.652 * |
| Gr_redge | −0.537 * | −0.530 | −0.530 | 0.245 | −0.481 | −0.553 * | Gr_redge | −0.395 | −0.448 | −0.352 | 0.764 ** | −0.491 | −0.256 | ||
| GRVI | 0.573 * | 0.555 * | 0.570 * | −0.003 | 0.456 | 0.587 * | GRVI | 0.548 * | 0.483 | 0.579 * | −0.075 | 0.297 | 0.481 | ||
| GRVI_redge | −0.590 * | −0.605 * | −0.573 * | 0.325 | −0.497 | −0.587 * | GRVI_redge | −0.523 | −0.562 * | −0.486 | 0.821 ** | −0.604 * | −0.387 | ||
| NDVI | 0.667 ** | 0.710 ** | 0.638 * | −0.423 | 0.560 * | 0.593 * | NDVI | 0.682 ** | 0.680 ** | 0.670 ** | −0.799 ** | 0.718 ** | 0.522 | ||
| NDVI_redge | 0.496 | 0.585 * | 0.452 | −0.585 * | 0.392 | 0.254 | NDVI_redge | 0.427 | 0.425 | 0.419 | −0.733 ** | 0.573 * | 0.278 | ||
| VT | Gr | 0.700 ** | 0.741 ** | 0.678 ** | −0.237 | 0.165 | 0.771 ** | PT | Gr | 0.773 ** | 0.744 ** | 0.772 ** | 0.027 | 0.520 | 0.713 ** |
| Gr_redge | −0.422 | −0.479 | −0.397 | 0.494 | −0.216 | −0.459 | Gr_redge | −0.366 | −0.460 | −0.303 | 0.835 ** | −0.191 | −0.409 | ||
| GRVI | 0.729 ** | 0.747 ** | 0.715 ** | −0.104 | 0.112 | 0.790 ** | GRVI | 0.675 ** | 0.637 * | 0.682 ** | 0.186 | 0.415 | 0.608 * | ||
| GRVI_redge | −0.481 | −0.535 * | −0.456 | 0.504 | −0.231 | −0.515 | GRVI_redge | −0.504 | −0.580 * | −0.448 | 0.814 ** | −0.323 | −0.526 | ||
| NDVI | 0.584 * | 0.621 * | 0.565 * | −0.507 | 0.185 | 0.595 * | NDVI | 0.846 ** | 0.872 ** | 0.812 ** | −0.596 * | 0.578 * | 0.809 ** | ||
| NDVI_redge | 0.213 | 0.234 | 0.203 | −0.634 * | 0.078 | 0.152 | NDVI_redge | 0.731 ** | 0.736 ** | 0.711 ** | −0.570 * | 0.569 * | 0.697 ** | ||
| VR | Gr | 0.660 * | 0.683 ** | 0.645 * | −0.792 ** | 0.209 | 0.676 ** | PR | Gr | 0.688 ** | 0.579 * | 0.729 ** | −0.064 | 0.331 | 0.614 * |
| Gr_redge | −0.550 * | −0.580 * | −0.532 | 0.817 ** | −0.328 | −0.551 * | Gr_redge | −0.368 | −0.452 | −0.299 | 0.663 ** | −0.195 | −0.279 | ||
| GRVI | 0.654 * | 0.683 ** | 0.636 * | −0.725 ** | 0.061 | 0.658 * | GRVI | 0.530 | 0.373 | 0.609 * | 0.247 | 0.186 | 0.398 | ||
| GRVI_redge | −0.584 * | −0.606 * | −0.570 * | 0.834 ** | −0.359 | −0.576 * | GRVI_redge | −0.452 | −0.536 * | −0.380 | 0.733 ** | −0.281 | −0.390 | ||
| NDVI | 0.631 * | 0.658 * | 0.615 * | −0.887 ** | 0.199 | 0.589 * | NDVI | 0.721 ** | 0.723 ** | 0.689 ** | −0.638 * | 0.421 | 0.580 * | ||
| NDVI_redge | 0.499 | 0.519 | 0.486 | −0.817 ** | 0.189 | 0.380 | NDVI_redge | 0.438 | 0.492 | 0.384 | −0.644 * | 0.275 | 0.323 | ||
| VO | Gr | 0.841 ** | 0.854 ** | 0.817 ** | −0.319 | 0.735 ** | 0.732 ** | PO | Gr | 0.653 * | 0.704 ** | 0.586 * | −0.028 | 0.613 * | 0.641 * |
| Gr_redge | −0.566 * | −0.679 ** | −0.498 | 0.802 ** | −0.771 ** | −0.496 | Gr_redge | 0.156 | −0.020 | 0.231 | 0.743 ** | −0.214 | 0.065 | ||
| GRVI | 0.823 ** | 0.807 ** | 0.814 ** | −0.174 | 0.678 ** | 0.704 ** | GRVI | 0.548 * | 0.537 * | 0.518 | 0.193 | 0.435 | 0.537 * | ||
| GRVI_redge | −0.653 * | −0.750 ** | −0.591 * | 0.793 ** | −0.838 ** | −0.557 * | GRVI_redge | −0.086 | −0.279 | 0.012 | 0.794 ** | −0.445 | −0.164 | ||
| NDVI | 0.797 ** | 0.845 ** | 0.756 ** | −0.631 * | 0.872 ** | 0.630 * | NDVI | 0.413 | 0.532 | 0.329 | −0.508 | 0.569 * | 0.429 | ||
| NDVI_redge | 0.603 * | 0.673 ** | 0.555 * | −0.727 ** | 0.769 ** | 0.400 | NDVI_redge | 0.062 | 0.064 | 0.058 | −0.454 | 0.077 | −0.034 |
References
- Ghanbari-Bonjar, A.; Lee, H.C. Intercropped wheat (Triticum aestivum L.) and bean (Vicia faba L.) as a whole-crop forage: Effect of harvest time on forage yield and quality. Grass Forage Sci. 2003, 58, 28–36. [Google Scholar] [CrossRef]
- Nadeem, M.; Ansar, M.; Anwar, A.; Hussain, A. Performance of Winter Cereal-Legumes Fodder Mixtures and Their Pure Stand at Different Growth Stages Under Rainfed Conditions of Pothowar. J. Agric. Res. 2010, 48, 181–192. [Google Scholar]
- Eskandari, H.; Ghanbari, A.; Javanmard, A. Intercropping of Cereals and Legumes for Forage Production. Not. Sci. Biol. 2009, 1, 07–13. [Google Scholar] [CrossRef] [Green Version]
- Willey, R.W. Intercropping: Its Importance and Research Needs. Part 1, Competition and Yield Advantages. Field Crop. Abstr. 1979, 32, 1–10. [Google Scholar]
- Staniak, M.; Ksiak, J.; Bojarszczuk, J. Mixtures of Legumes with Cereals as a Source of Feed for Animals. In Organic Agriculture Towards Sustainability; Pilipavicius, V., Ed.; InTech: Kaunas, Lithuania, 2014; pp. 123–145. ISBN 978-953-51-1340-9. [Google Scholar]
- Chandel, A.K.; Khot, L.R.; Yu, L.-X. Alfalfa (Medicago sativa L.) crop vigor and yield characterization using high-resolution aerial multispectral and thermal infrared imaging technique. Comput. Electron. Agric. 2021, 182, 105999. [Google Scholar] [CrossRef]
- Undersander, D.; Grau, C.; Cosgrove, D.; Doll, J.; Martin, N. Alfalfa Stand Assessment: Is This Stand Good Enough to Keep? University of Wisconsin-Extension: Madison, WI, USA, 2011; pp. 3–6. [Google Scholar]
- Puangbut, D.; Jogloy, S.; Vorasoot, N. Association of photosynthetic traits with water use efficiency and SPAD chlorophyll meter reading of Jerusalem artichoke under drought conditions. Agric. Water Manag. 2017, 188, 29–35. [Google Scholar] [CrossRef]
- Pereira, L.S.; Paredes, P.; Melton, F.; Johnson, L.; Wang, T.; López-Urrea, R.; Cancela, J.J.; Allen, R.G. Prediction of Crop Coefficients from Fraction of Ground Cover and Height. Background and Validation Using Ground and Remote Sensing Data; Elsevier B.V.: Amsterdam, The Netherlands, 2020; Volume 241. [Google Scholar]
- Guan, J.; Nutter, F.W. Relationships between defoliation, leaf area index, canopy reflectance, and forage yield in the alfalfa-leaf spot pathosystem. Comput. Electron. Agric. 2003, 37, 97–112. [Google Scholar] [CrossRef]
- Orloff, S.B. Methods to assess alfalfa forage quality in the field. In Proceedings of the 27th National Alfalfa Symposium, San Diego, CA, USA, 9–10 December 1996; pp. 183–193. [Google Scholar]
- Salama, H.S.A. Mixture cropping of berseem clover with cereals to improve forage yield and quality under irrigated conditions of the Mediterranean basin. Ann. Agric. Sci. 2020, 65, 159–167. [Google Scholar] [CrossRef]
- Mulla, D.J. Twenty five years of remote sensing in precision agriculture: Key advances and remaining knowledge gaps. Biosyst. Eng. 2013, 114, 358–371. [Google Scholar] [CrossRef]
- Chang, A.; Jung, J.; Maeda, M.M.; Landivar, J. Crop height monitoring with digital imagery from Unmanned Aerial System (UAS). Comput. Electron. Agric. 2017, 141, 232–237. [Google Scholar] [CrossRef]
- Pádua, L.; Vanko, J.; Hruška, J.; Adão, T.; Sousa, J.J.; Peres, E.; Morais, R. UAS, sensors, and data processing in agroforestry: A review towards practical applications. Int. J. Remote Sens. 2017, 38, 2349–2391. [Google Scholar] [CrossRef]
- Li, X.; Lee, W.S.; Li, M.; Ehsani, R.; Mishra, A.R.; Yang, C.; Mangan, R.L. Spectral difference analysis and airborne imaging classification for citrus greening infected trees. Comput. Electron. Agric. 2012, 83, 32–46. [Google Scholar] [CrossRef]
- Verger, A.; Vigneau, N.; Chéron, C.; Gilliot, J.M.; Comar, A.; Baret, F. Green area index from an unmanned aerial system over wheat and rapeseed crops. Remote Sens. Environ. 2014, 152, 654–664. [Google Scholar] [CrossRef]
- Zhou, X.; Zheng, H.B.; Xu, X.Q.; He, J.Y.; Ge, X.K.; Yao, X.; Cheng, T.; Zhu, Y.; Cao, W.X.; Tian, Y.C. Predicting grain yield in rice using multi-temporal vegetation indices from UAV-based multispectral and digital imagery. ISPRS J. Photogramm. Remote Sens. 2017, 130, 246–255. [Google Scholar] [CrossRef]
- Castro, W.; Junior, J.M.; Polidoro, C.; Osco, L.P.; Gonçalves, W.; Rodrigues, L.; Santos, M.; Jank, L.; Barrios, S.; Valle, C.; et al. Deep Learning Applied to Phenotyping of Biomass in Forages with Uav-Based RGB Imagery. Sensors 2020, 20, 4802. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.H.; Schell, J.A.; Deering, D.W.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation (Green Wave Effect) of Natural Vegetation. In Final Report, Type III; NASA/GSFC: Greenbelt, MD, USA, 1974; p. 371. [Google Scholar]
- Becker-Reshef, I.; Vermote, E.; Lindeman, M.; Justice, C. A generalized regression-based model for forecasting winter wheat yields in Kansas and Ukraine using MODIS data. Remote Sens. Environ. 2010, 114, 1312–1323. [Google Scholar] [CrossRef]
- Maresma, A.; Chamberlain, L.; Tagarakis, A.; Kharel, T.; Godwin, G.; Czymmek, K.J.; Shields, E.; Ketterings, Q.M. Accuracy of NDVI-derived corn yield predictions is impacted by time of sensing. Comput. Electron. Agric. 2020, 169, 105236. [Google Scholar] [CrossRef]
- Shafiee, S.; Lied, L.M.; Burud, I.; Dieseth, J.A.; Alsheikh, M.; Lillemo, M. Sequential forward selection and support vector regression in comparison to LASSO regression for spring wheat yield prediction based on UAV imagery. Comput. Electron. Agric. 2021, 183, 106036. [Google Scholar] [CrossRef]
- Drucker, H.; Burges, C.J.C.; Kaufman, L.; Smola, A.; Vapnik, V. Support Vector Regression Machines. In Advances in Neural Information Processing Systems 9; MIT Press: Cambridge, MA, USA, 1997; pp. 155–161. [Google Scholar]
- Wold, H. Soft modelling: The Basic Design and Some Extensions. In Systems Under Indirect Observation: Causality-Structure-Prediction. Part II; North-Holland Publishing Company: Amsterdam, The Netherlands, 1982; pp. 1–54. [Google Scholar]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Ma, D.; Maki, H.; Neeno, S.; Zhang, L.; Wang, L.; Jin, J. Application of non-linear partial least squares analysis on prediction of biomass of maize plants using hyperspectral images. Biosyst. Eng. 2020, 200, 40–54. [Google Scholar] [CrossRef]
- Montes, J.M.; Technow, F.; Dhillon, B.S.; Mauch, F.; Melchinger, A.E. High-throughput non-destructive biomass determination during early plant development in maize under field conditions. Field Crops Res. 2011, 121, 268–273. [Google Scholar] [CrossRef]
- Zhou, Z.; Morel, J.; Parsons, D.; Kucheryavskiy, S.V.; Gustavsson, A.-M. Estimation of yield and quality of legume and grass mixtures using partial least squares and support vector machine analysis of spectral data. Comput. Electron. Agric. 2019, 162, 246–253. [Google Scholar] [CrossRef]
- Osborne, S.L.; Schepers, J.S.; Francis, D.D.; Schlemmer, M.R. Use of Spectral Radiance to Estimate In-Season Biomass and Grain Yield in Nitrogen- and Water-Stressed Corn. Crop. Sci. 2002, 42, 165–171. [Google Scholar] [PubMed]
- Helfer, G.A.; Victória Barbosa, J.L.; dos Santos, R.; da Costa, A.B. A computational model for soil fertility prediction in ubiquitous agriculture. Comput. Electron. Agric. 2020, 175, 105602. [Google Scholar] [CrossRef]
- Abdi, H. Partial least squares regression and projection on latent structure regression (PLS Regression). WIREs Comput. Stat. 2010, 2, 97–106. [Google Scholar] [CrossRef]
- Krishnan, A.; Williams, L.J.; McIntosh, A.R.; Abdi, H. Partial Least Squares (PLS) methods for neuroimaging: A tutorial and review. NeuroImage 2011, 56, 455–475. [Google Scholar] [CrossRef]
- Barnsley, M.J.; Lewis, P.; O’Dwyer, S.; Disney, M.I.; Hobson, P.; Cutter, M.; Lobb, D. On the potential of CHRIS/PROBA for estimating vegetation canopy properties from space. Remote Sens. Rev. 2000, 19, 171–189. [Google Scholar] [CrossRef]
- Jackson, T.J.; Chen, D.; Cosh, M.; Li, F.; Anderson, M.; Walthall, C.; Doriaswamy, P.; Hunt, E.R. Vegetation water content mapping using Landsat data derived normalized difference water index for corn and soybeans. Remote Sens. Environ. 2004, 92, 475–482. [Google Scholar] [CrossRef]
- Jiang, Z.; Huete, A.R.; Chen, J.; Chen, Y.; Li, J.; Yan, G.; Zhang, X. Analysis of NDVI and scaled difference vegetation index retrievals of vegetation fraction. Remote Sens. Environ. 2006, 101, 366–378. [Google Scholar] [CrossRef]
- Valcarce-Diñeiro, R.; Lopez-Sanchez, J.M.; Sánchez, N.; Arias-Pérez, B.; Martínez-Fernández, J. Influence of Incidence Angle in the Correlation of C-band Polarimetric Parameters with Biophysical Variables of Rain-fed Crops. Can. J. Remote Sens. 2018, 44, 643–659. [Google Scholar] [CrossRef]
- Sánchez, N.; Martínez-Fernández, J.; González-Piqueras, J.; González-Dugo, M.P.; Baroncini-Turrichia, G.; Torres, E.; Calera, A.; Pérez-Gutiérrez, C. Water balance at plot scale for soil moisture estimation using vegetation parameters. Agric. For. Meteorol. 2012, 166–167, 1–9. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S.J. Image Processing with Image. J. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Martin, T.N.; Marchese, J.A.; Fernandes de Sousa, A.K.; Curti, G.L.; Fogolari, H.; Dos Santos, V. Using the imagej software to estimate leaf area in bean crop. Interciencia 2013, 38, 843–848. [Google Scholar]
- García-Estévez, I.; Quijada-Morín, N.; Rivas-Gonzalo, J.C.; Martínez-Fernández, J.; Sánchez, N.; Herrero-Jiménez, C.M.; Escribano-Bailón, M.T. Relationship between hyperspectral indices, agronomic parameters and phenolic composition of Vitis vinifera cv Tempranillo grapes: Hyperspectral indices, agronomic parameters and phenolic composition of V. vinifera. J. Sci. Food Agric. 2017, 97, 4066–4074. [Google Scholar] [CrossRef] [Green Version]
- Abdelkader, M.M.M.; Puchkov, M.; Loktionova, E. Applying a digital method for measuring leaf area index of tomato plants. In Proceedings of the International Scientific and Practical Conference “Digital agriculture—Development strategy” (ISPC 2019), Ekaterinburg, Russia, 21–22 March 2019; Atlantis Press: Ekaterinburg, Russia, 2019. [Google Scholar]
- Calera, A.; Martínez, C.; Melia, J. A procedure for obtaining green plant cover: Relation to NDVI in a case study for barley. Int. J. Remote Sens. 2001, 22, 3357–3362. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, N.; Alonso-Arroyo, A.; Martínez-Fernández, J.; Piles, M.; González-Zamora, Á.; Camps, A.; Vall-llosera, M. On the Synergy of Airborne GNSS-R and Landsat 8 for Soil Moisture Estimation. Remote Sens. 2015, 7, 9954–9974. [Google Scholar] [CrossRef] [Green Version]
- Hunt, E.R.; Doraiswamy, P.C.; McMurtrey, J.E.; Daughtry, C.S.T.; Perry, E.M.; Akhmedov, B. A visible band index for remote sensing leaf chlorophyll content at the Canopy scale. Int. J. Appl. Earth Obs. Geoinform. 2013, 21, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Sumesh, K.C.; Ninsawat, S.; Som-ard, J. Integration of RGB-based vegetation index, crop surface model and object-based image analysis approach for sugarcane yield estimation using unmanned aerial vehicle. Comput. Electron. Agric. 2021, 180, 105903. [Google Scholar] [CrossRef]
- Khan, Z.; Rahimi-Eichi, V.; Haefele, S.; Garnett, T.; Miklavcic, S.J. Estimation of vegetation indices for high-throughput phenotyping of wheat using aerial imaging. Plant. Methods 2018, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Jannoura, R.; Brinkmann, K.; Uteau, D.; Bruns, C.; Joergensen, R.G. Monitoring of crop biomass using true colour aerial photographs taken from a remote controlled hexacopter. Biosyst. Eng. 2015, 129, 341–351. [Google Scholar] [CrossRef]
- Lussem, U.; Bolten, A.; Gnyp, M.L.; Jasper, J.; Bareth, G. Evaluation of RGB-based vegetation indices from UAV imagery to estimate forage yield in grassland. ISPRS Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2018, XLII–3, 1215–1219. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, B.D.S.; Ferraz, G.a.S.; Gonçalves, L.M.; Marin, D.B.; Maciel, D.T.; Ferraz, P.F.P.; Rossi, G. RGB vegetation indices applied to grass monitoring: A qualitative analysis. Agron. Res. 2019, 17, 2. [Google Scholar] [CrossRef]
- Bendig, J.; Yu, K.; Aasen, H.; Bolten, A.; Bennertz, S.; Broscheit, J.; Gnyp, M.L.; Bareth, G. Combining UAV-based plant height from crop surface models, visible, and near infrared vegetation indices for biomass monitoring in barley. Int. J. Appl. Earth Obs. Geoinform. 2015, 39, 79–87. [Google Scholar] [CrossRef]
- Sripada, R.P.; Heiniger, R.W.; White, J.G.; Meijer, A.D. Aerial Color Infrared Photography for Determining Early In-Season Nitrogen Requirements in Corn. Agron. J. 2006, 98, 968–977. [Google Scholar] [CrossRef]
- Motohka, T.; Nasahara, K.N.; Oguma, H.; Tsuchida, S. Applicability of Green-Red Vegetation Index for Remote Sensing of Vegetation Phenology. Remote Sens. 2010, 2, 2369–2387. [Google Scholar] [CrossRef] [Green Version]
- Pearson, K. On the Theory of Contingency and Its Relation to Association and Normal Correlation; Dulau and Company: London, UK, 1904; Volume 1, p. 46. [Google Scholar]
- Hauggaard-Nielsen, H.; Jensen, E.S. Evaluating pea and barley cultivars for complementarity in intercropping at different levels of soil N availability. Field Crops Res. 2001, 72, 185–196. [Google Scholar] [CrossRef]
- Roberts, S.J. Effect of soil moisture on the transmission of pea bacterial blight (Pseudomonas syringae pv. pisi) from seed to seedling. Plant. Pathol. 1992, 41, 136–140. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Z.; Tang, W.; Huang, Y.; Coulter, J.A.; Nan, Z. Common vetch cultivars improve yield of oat row intercropping on the Qinghai-Tibetan plateau by optimizing photosynthetic performance. Eur. J. Agron. 2020, 117, 126088. [Google Scholar] [CrossRef]
- Calera, A.; González-Piqueras, J.; Melia, J. Monitoring barley and corn growth from remote sensing data at field scale. Int. J. Remote Sens. 2004, 25, 97–109. [Google Scholar] [CrossRef]
- Gutierrez, M.; Escalante Estrada, J.A.; And, E.-E.; Rodriguez-Gonzalez, M. Canopy Reflectance, Stomatal Conductance, and Yield of Phaseolus vulgaris L. and Phaseolus coccinues L. Under Saline Field Conditions. Int. J. Agric. Biol. 2005, 7, 491–494. [Google Scholar]
- Hunt, E.R.; Cavigelli, M.; Daughtry, C.S.T.; Mcmurtrey, J.E.; Walthall, C.L. Evaluation of Digital Photography from Model Aircraft for Remote Sensing of Crop Biomass and Nitrogen Status. Precis. Agric. 2005, 6, 359–378. [Google Scholar] [CrossRef]
- Fu, Z.; Jiang, J.; Gao, Y.; Krienke, B.; Wang, M.; Zhong, K.; Cao, Q.; Tian, Y.; Zhu, Y.; Cao, W.; et al. Wheat Growth Monitoring and Yield Estimation based on Multi-Rotor Unmanned Aerial Vehicle. Remote Sens. 2020, 12, 508. [Google Scholar] [CrossRef] [Green Version]
- Gamon, J.A.; Field, C.B.; Goulden, M.L.; Griffin, K.L.; Hartley, A.E.; Joel, G.; Peñuelas, J.; Valentini, R. Relationships Between NDVI, Canopy Structure, and Photosynthesis in Three Californian Vegetation Types. Ecol. Appl. 1995, 5, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Bater, C.W.; Coops, N.C.; Wulder, M.A.; Hilker, T.; Nielsen, S.E.; McDermid, G.; Stenhouse, G.B. Using digital time-lapse cameras to monitor species-specific understorey and overstorey phenology in support of wildlife habitat assessment. Environ. Monit. Assess. 2011, 180, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bacsa, C.M.; Martorillas, R.M.; Balicanta, L.P.; Tamondong, A.M. Correlation of UAV-based multispectral vegetation indices and leaf color chart observations for nitrogen concentration assesment on rice crops. In Proceedings of the International Archives of the Photogrammetry, Remote Sensing and Spatial Information Sciences, Copernicus GmbH, Manila, Philippines, 14–15 November 2019; Volume XLII-4-W19, pp. 31–38. [Google Scholar]
- Janoušek, J.; Jambor, V.; Marcoň, P.; Dohnal, P.; Synková, H.; Fiala, P. Using UAV-Based Photogrammetry to Obtain Correlation between the Vegetation Indices and Chemical Analysis of Agricultural Crops. Remote Sens. 2021, 13, 1878. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Kim, J.H.; Nguyen, A.T.; Nguyen, L.T.; Shin, J.C.; Lee, B.-W. Using canopy reflectance and partial least squares regression to calculate within-field statistical variation in crop growth and nitrogen status of rice. Precis. Agric. 2006, 7, 249–264. [Google Scholar] [CrossRef]
- Abdel-Rahman, E.M.; Mutanga, O.; Odindi, J.; Adam, E.; Odindo, A.; Ismail, R. A comparison of partial least squares (PLS) and sparse PLS regressions for predicting yield of Swiss chard grown under different irrigation water sources using hyperspectral data. Comput. Electron. Agric. 2014, 106, 11–19. [Google Scholar] [CrossRef]
- Kawamura, K.; Ikeura, H.; Phongchanmaixay, S.; Khanthavong, P. Canopy Hyperspectral Sensing of Paddy Fields at the Booting Stage and PLS Regression can Assess Grain Yield. Remote Sens. 2018, 10, 1249. [Google Scholar] [CrossRef] [Green Version]
- Erler, A.; Riebe, D.; Beitz, T.; Löhmannsröben, H.-G.; Gebbers, R. Soil Nutrient Detection for Precision Agriculture Using Handheld Laser-Induced Breakdown Spectroscopy (LIBS) and Multivariate Regression Methods (PLSR, Lasso and GPR). Sensors 2020, 20, 418. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Huang, Y.; Shang, G.; Zhou, C.; Cheng, T.; Tian, Y.; Cao, W.; Zhu, Y. Evaluation of Six Algorithms to Monitor Wheat Leaf Nitrogen Concentration. Remote Sens. 2015, 7, 14939–14966. [Google Scholar] [CrossRef] [Green Version]
- Du, L.; Shi, S.; Yang, J.; Sun, J.; Gong, W. Using Different Regression Methods to Estimate Leaf Nitrogen Content in Rice by Fusing Hyperspectral LiDAR Data and Laser-Induced Chlorophyll Fluorescence Data. Remote Sens. 2016, 8, 526. [Google Scholar] [CrossRef] [Green Version]





| Field/Laboratory Parameters | Units | |
|---|---|---|
| Fraction of Vegetation Cover | FVC | % |
| Leaf Area Index | LAI | m2/m2 |
| Fresh biomass | FB | gr/m2 |
| Dry biomass | DB | gr/m2 |
| Vegetation Water Content | VWC | gr/m2 |
| Percentage of Water Content | PWC | % |
| Vegetation Indices | Equation | |
|---|---|---|
| Greenness | Gr | |
| Greenness_Red_Edge | Gr_redge | |
| Normalized Difference Vegetation Index | NDVI | |
| Normalized Difference Vegetation Index_Red_Edge | NDVI_redge | |
| Green-Red Vegetation Index | GRVI | |
| Green-Red Vegetation Index_Red_Edge | GRVI_redge | |
| % Variance | % Accumulated | % Variance | % Accumulated | Mean Predicted | |
|---|---|---|---|---|---|
| 1 | 69.94 | 69.94 | 44.43 | 44.44 | 42.50 |
| 2 | 22.09 | 92.03 | 7.01 | 51.45 | 48.74 |
| 3 | 7.13 | 99.16 | 2.07 | 53.52 | 49.97 |
| 4 | 0.51 | 99.67 | 4.23 | 57.75 | 53.38 |
| 5 | 0.32 | 99.99 | 0.34 | 58.09 | 53.06 |
| 6 | 0.01 | 100.00 | 1.13 | 59.22 | 52.83 |
| Explanatory | Mean | Predicted | |
|---|---|---|---|
| Variable | PRESS | ||
| FB (g/m2) | 65.75 | 22,520.30 | 62.23 |
| DB (g/m2) | 70.40 | 2017.47 | 67.07 |
| VWC (g/m2) | 61.12 | 12,493.50 | 57.32 |
| PWC (%) | 52.47 | 31.23 | 47.34 |
| LAI (m2/m2) | 62.02 | 0.02 | 57.79 |
| FVC (%) | 34.77 | 133.34 | 28.54 |
| % Variance | % Accumulated | % Variance | % Accumulated | Mean Predicted | |
|---|---|---|---|---|---|
| 1 | 76.03 | 76.03 | 44.15 | 44.15 | 40.81 |
| 2 | 12.58 | 88.61 | 8.29 | 52.45 | 45.97 |
| 3 | 10.67 | 99.28 | 1.63 | 54.08 | 46.42 |
| 4 | 0.51 | 99.89 | 2.10 | 56.18 | 46.37 |
| 5 | 0.18 | 99.98 | 0.63 | 56.82 | 45.74 |
| 6 | 0.02 | 100.00 | 3.18 | 60.01 | 47.85 |
| Explanatory | Mean | Predicted | |
|---|---|---|---|
| Variable | PRESS | ||
| FB (g/m2) | 62.65 | 31,210.60 | 56.77 |
| DB (g/m2) | 67.90 | 2590.51 | 62.71 |
| VWC (g/m2) | 58.26 | 17,213.00 | 51.74 |
| PWC (%) | 49.11 | 22.17 | 38.90 |
| LAI (m2/m2) | 64.09 | 0.03 | 58.86 |
| FVC (%) | 22.49 | 81.60 | 9.50 |
| % Variance | % Accumulated | % Variance | % Accumulated | Mean Predicted | |
|---|---|---|---|---|---|
| 1 | 54.70 | 54.70 | 41.70 | 41.70 | 37.02 |
| 2 | 37.43 | 92.14 | 8.10 | 49.81 | 44.29 |
| 3 | 6.86 | 99.01 | 1.83 | 51.65 | 44.92 |
| 4 | 0.82 | 99.8 | 5.81 | 57.46 | 48.62 |
| 5 | 0.15 | 99.99 | 1.65 | 59.11 | 48.97 |
| 6 | 0.01 | 100.000 | 3.70 | 62.82 | 49.80 |
| Explanatory | Mean | Predicted | |
|---|---|---|---|
| Variable | PRESS | ||
| FB (g/m2) | 62.19 | 16,471.20 | 54.89 |
| DB (g/m2) | 69.70 | 1802.05 | 63.16 |
| VWC (g/m2) | 54.96 | 8598.87 | 46.65 |
| PWC (%) | 66.10 | 30.90 | 59.36 |
| LAI (m2/m2) | 58.21 | 0.02 | 48.68 |
| FVC (%) | 33.61 | 101.64 | 18.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plaza, J.; Criado, M.; Sánchez, N.; Pérez-Sánchez, R.; Palacios, C.; Charfolé, F. UAV Multispectral Imaging Potential to Monitor and Predict Agronomic Characteristics of Different Forage Associations. Agronomy 2021, 11, 1697. https://doi.org/10.3390/agronomy11091697
Plaza J, Criado M, Sánchez N, Pérez-Sánchez R, Palacios C, Charfolé F. UAV Multispectral Imaging Potential to Monitor and Predict Agronomic Characteristics of Different Forage Associations. Agronomy. 2021; 11(9):1697. https://doi.org/10.3390/agronomy11091697
Chicago/Turabian StylePlaza, Javier, Marco Criado, Nilda Sánchez, Rodrigo Pérez-Sánchez, Carlos Palacios, and Francisco Charfolé. 2021. "UAV Multispectral Imaging Potential to Monitor and Predict Agronomic Characteristics of Different Forage Associations" Agronomy 11, no. 9: 1697. https://doi.org/10.3390/agronomy11091697
APA StylePlaza, J., Criado, M., Sánchez, N., Pérez-Sánchez, R., Palacios, C., & Charfolé, F. (2021). UAV Multispectral Imaging Potential to Monitor and Predict Agronomic Characteristics of Different Forage Associations. Agronomy, 11(9), 1697. https://doi.org/10.3390/agronomy11091697








