Effect of Salinity Stress on Physiological Changes in Winter and Spring Wheat
Abstract
1. Introduction
2. Results
2.1. Response of Wheat Genotypes against Different Salinity Levels
2.2. Value of % Control
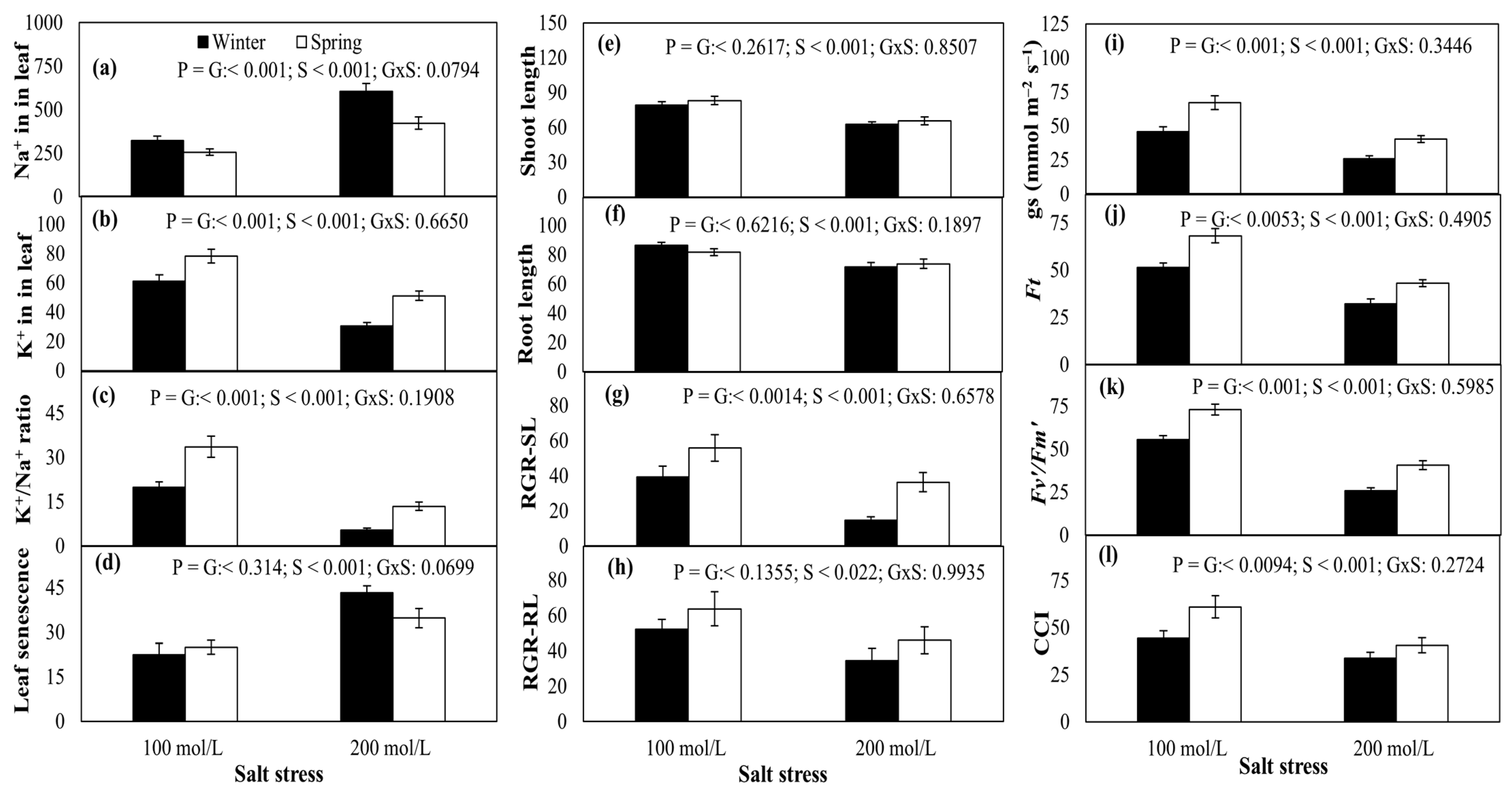
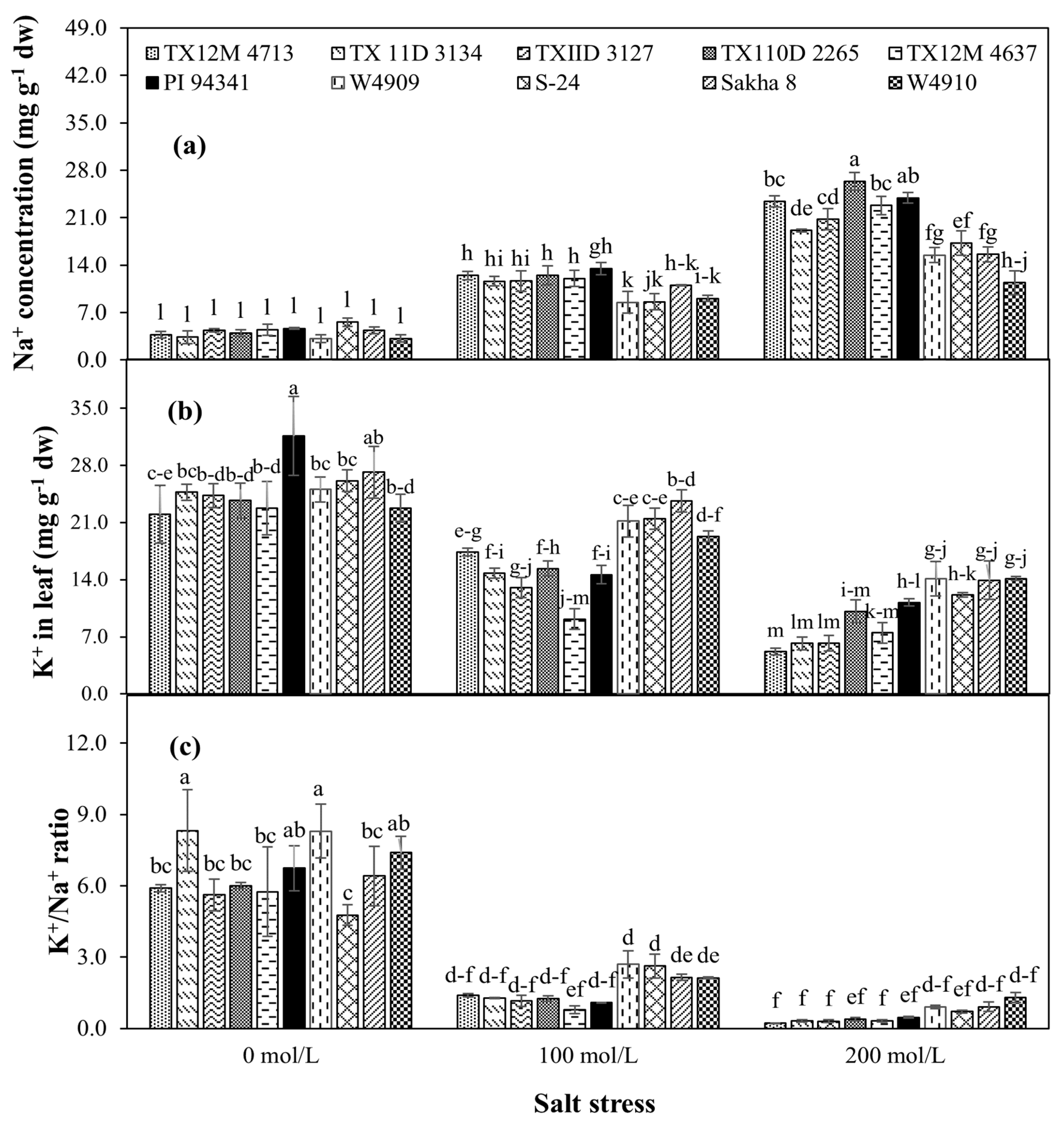
2.3. Leaf Na+ and K+ Contents
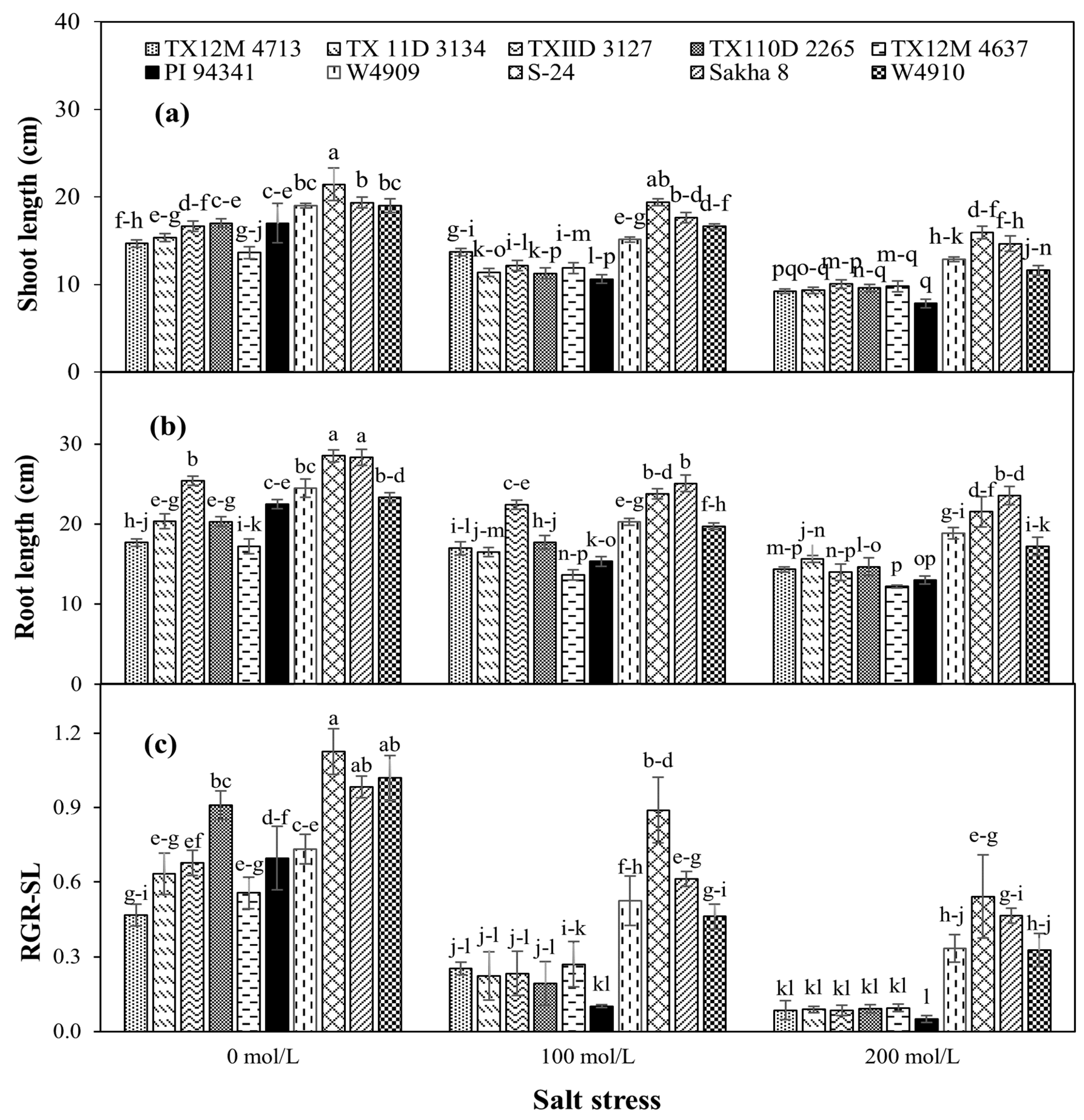
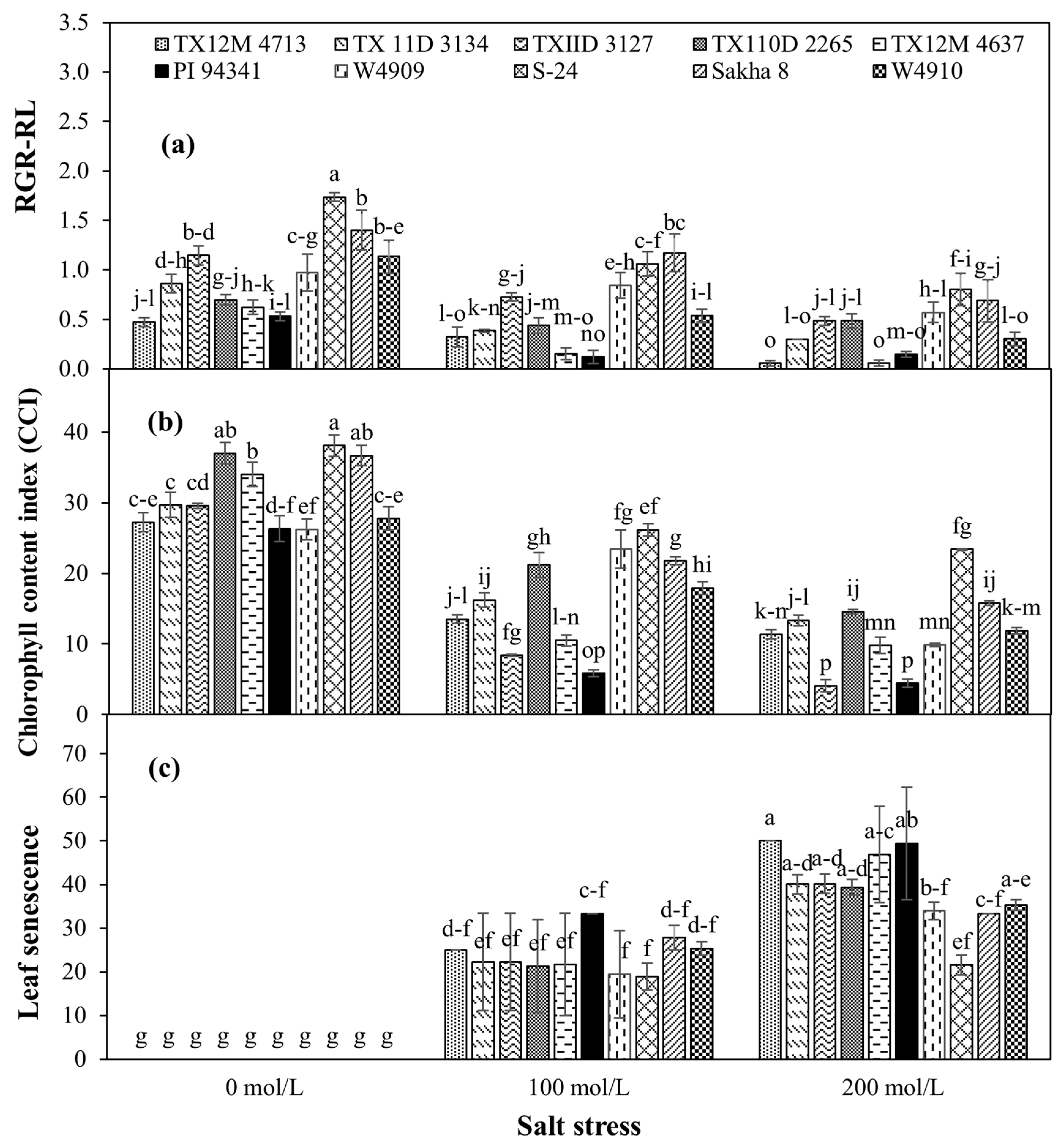
2.4. Shoot and Root Growth
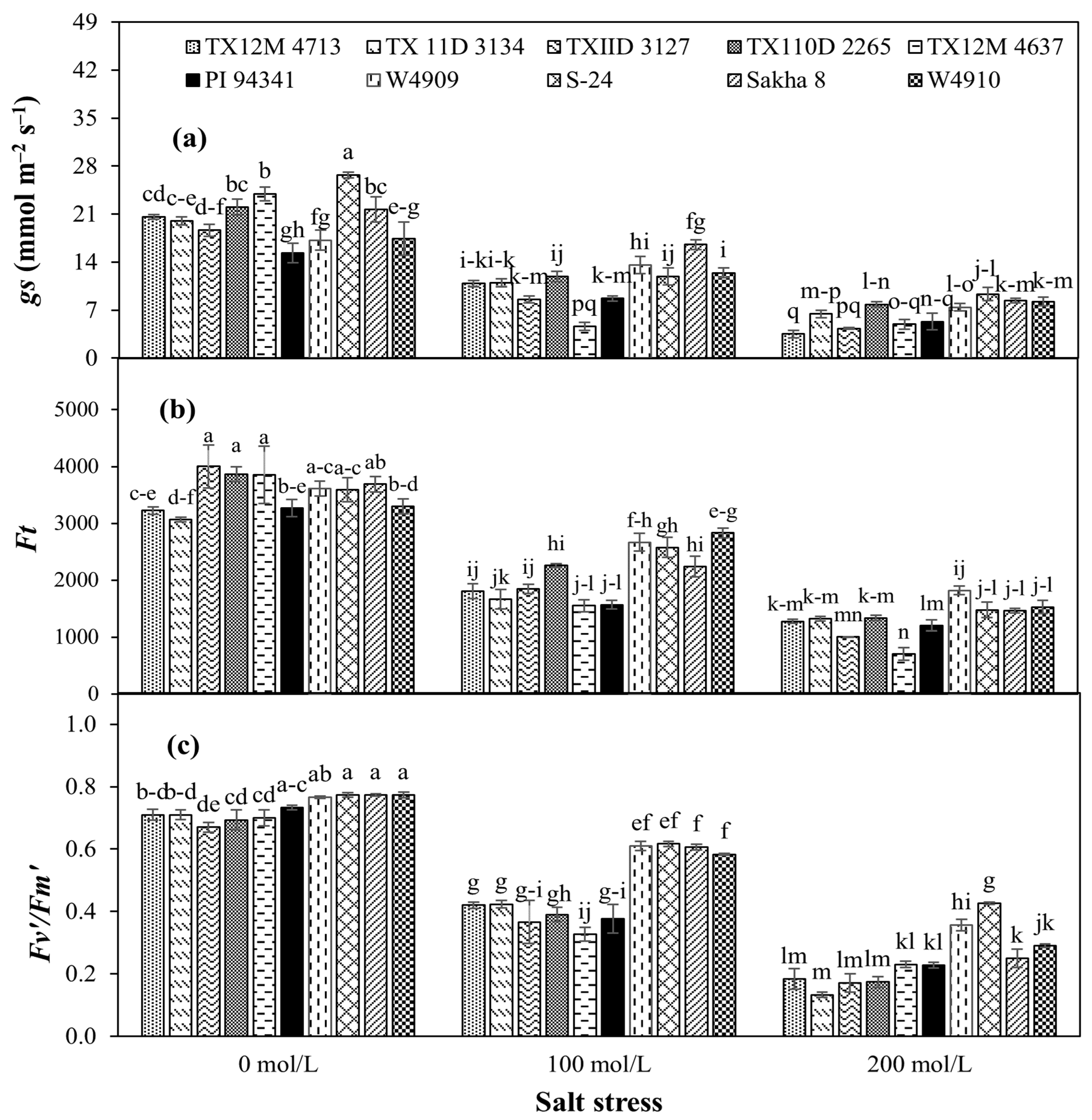
2.5. Physiological Traits
2.6. Trait Correlations
3. Discussion
4. Materials and Methods
4.1. Germplasm Collection
4.2. Hydroponic Culture
4.3. Determination of Leaf Na+ and K+ Concentrations
4.4. Morphological Traits
4.5. Chlorophyll Index and Stomatal Conductance
4.6. Chlorophyll Fluorescence
4.7. Leaf Senescence
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wasaya, A.; Manzoor, S.; Yasir, T.A.; Sarwar, N.; Mubeen, K.; Ismail, I.A.; Raza, A.; Rehman, A.; Hossain, A.; EL Sabagh, A. Evaluation of Fourteen Bread Wheat (Triticum aestivum L.) Genotypes by Observing Gas Exchange Parameters, Relative Water and Chlorophyll Content, and Yield Attributes under Drought Stress. Sustainability 2021, 13, 4799. [Google Scholar] [CrossRef]
- Bhusal, N.; Han, S.-G.; Yoon, T.-M. Impact of drought stress on photosynthetic response, leaf water potential, and stem sap flow in two cultivars of bi-leader apple trees (Malus× domestica Borkh.). Sci. Hortic. 2019, 246, 535–543. [Google Scholar] [CrossRef]
- Woodrow, P.; Pontecorvo, G.; Ciarmiello, L.F.; Annunziata, M.G.; Fuggi, A.; Carillo, P. Transcription factors and genes in abiotic stress. In Crop Stress and Its Management: Perspectives and Strategies; Springer: Dordrecht, The Netherlands, 2012; pp. 317–357. [Google Scholar]
- Alamri, S.; Hu, Y.; Mukherjee, S.; Aftab, T.; Fahad, S.; Raza, A.; Ahmad, M.; Siddiqui, M.H. Silicon-induced postponement of leaf senescence is accompanied by modulation of antioxidative defense and ion homeostasis in mustard (Brassica juncea) seedlings exposed to salinity and drought stress. Plant Physiol. Biochem. 2020, 157, 47–59. [Google Scholar] [CrossRef]
- Hafeez, M.B.; Raza, A.; Zahra, N.; Shaukat, K.; Akram, M.Z.; Iqbal, S.; Basra, S.M.A. Gene regulation in halophytes in conferring salt tolerance. In Handbook of Bioremediation; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2021; pp. 341–370. [Google Scholar]
- Raza, A. Eco-physiological and Biochemical Responses of Rapeseed (Brassica napus L.) to Abiotic Stresses: Consequences and Mitigation Strategies. J. Plant Growth Regul. 2020. [Google Scholar] [CrossRef]
- Mahmood, Q.; Bilal, M.; Jan, S. Herbicides, pesticides, and plant tolerance: An overview. Emerg. Technol. Manag. Crop. Stress Toler. 2014, 423–448. [Google Scholar]
- Viehweger, K. How plants cope with heavy metals. Bot. Stud. 2014, 55, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil salinity: Historical perspectives and a world overview of the problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Cham, Switzerland, 2018; pp. 43–53. [Google Scholar]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, J.; Zahid, M.; Parveen, S.; Naseem, A. Salt-tolerance of Wheat (Triticum aestivum L.) Genotypes: A Lysimeter Study. Pak. J. Biol. Sci. 2000, 3, 1627–1629. [Google Scholar] [CrossRef]
- Salim, N.; Raza, A. Nutrient use efficiency (NUE) for sustainable wheat production: A review. J. Plant Nutri. 2020, 43, 297–315. [Google Scholar] [CrossRef]
- Zahra, N.; Mahmood, S.; Raza, Z.A. Salinity stress on various physiological and biochemical attributes of two distinct maize (Zea mays L.) genotypes. J. Plant Nutr. 2018, 41, 1368–1380. [Google Scholar] [CrossRef]
- Javaid, T.; Farooq, M.A.; Akhtar, J.; Saqib, Z.A.; Anwar-ul-Haq, M. Silicon nutrition improves growth of salt-stressed wheat by modulating flows and partitioning of Na+, Cl− and mineral ions. Plant Physiol. Biochem. 2019, 141, 291–299. [Google Scholar] [CrossRef]
- Yadav, T.; Kumar, A.; Yadav, R.; Yadav, G.; Kumar, R.; Kushwaha, M. Salicylic acid and thiourea mitigate the salinity and drought stress on physiological traits governing yield in pearl millet-wheat. Saudi J. Biol. Sci. 2020, 27, 2010–2017. [Google Scholar] [CrossRef]
- Iqbal, S.; Basra, S.M.; Afzal, I.; Wahid, A.; Saddiq, M.S.; Hafeez, M.B.; Jacobsen, S.E. Yield potential and salt tolerance of quinoa on salt-degraded soils of Pakistan. J. Agron. Crop Sci. 2019, 205, 13–21. [Google Scholar] [CrossRef]
- Ami, K.; Planchais, S.; Cabassa, C.; Guivarc’h, A.; Véry, A.-A.; Khelifi, M.; Djebbar, R.; Abrous-Belbachir, O.; Carol, P. Different proline responses of two Algerian durum wheat cultivars to in vitro salt stress. Acta Physiol. Plant. 2020, 42, 1–16. [Google Scholar] [CrossRef]
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Sec. 2013, 5, 291–317. [Google Scholar] [CrossRef]
- Chaves, M.S.; Martinelli, J.A.; Wesp-Guterres, C.; Graichen, F.A.S.; Brammer, S.P.; Scagliusi, S.M.; da Silva, P.R.; Wiethölter, P.; Torres, G.A.M.; Lau, E.Y. The importance for food security of maintaining rust resistance in wheat. Food Sec. 2013, 5, 157–176. [Google Scholar] [CrossRef]
- Becker-Reshef, I.; Justice, C.; Barker, B.; Humber, M.; Rembold, F.; Bonifacio, R.; Zappacosta, M.; Budde, M.; Magadzire, T.; Shitote, C. Strengthening agricultural decisions in countries at risk of food insecurity: The GEOGLAM Crop Monitor for Early Warning. Remote Sens. Environ. 2020, 237, 111553. [Google Scholar] [CrossRef]
- Aslam, M.; Prathapar, S.A.; Aslam, M.; Prathapar, S. Strategies to Mitigate Secondary Salinization in the Indus Basin of Pakistan: A Selective Review; Research Report 97; International Water Management Institute (IWMI): Colombo, Sri Lanka, 2006; p. 33. ISBN 92-9090-616-2. [Google Scholar]
- Woodrow, P.; Ciarmiello, L.F.; Annunziata, M.G.; Pacifico, S.; Iannuzzi, F.; Mirto, A.; D'Amelia, L.; Dell’Aversana, E.; Piccolella, S.; Fuggi, A. Durum wheat seedling responses to simultaneous high light and salinity involve a fine reconfiguration of amino acids and carbohydrate metabolism. Physiol. Plant. 2017, 159, 290–312. [Google Scholar] [CrossRef]
- Oyiga, B.C.; Sharma, R.; Shen, J.; Baum, M.; Ogbonnaya, F.; Léon, J.; Ballvora, A. Identification and characterization of salt tolerance of wheat germplasm using a multivariable screening approach. J. Agron. Crop Sci. 2016, 202, 472–485. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; Sadaqat, N.; Jin, W. Arabidopsis thaliana: Model Plant for the Study of Abiotic Stress Responses. In The Plant Family Brassicaceae; Springer: Singapore, 2020; pp. 129–180. [Google Scholar]
- Li, M.; Yang, Y.; Raza, A.; Yin, S.; Wang, H.; Zhang, Y.; Dong, J.; Wang, G.; Zhong, C.; Zhang, H. Heterologous expression of Arabidopsis thaliana rty gene in strawberry (Fragaria× ananassa Duch.) improves drought tolerance. BMC Plant Biol. 2021, 21, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, H.; Ashraf, M.; Shahbaz, M. Assessment of salt tolerance of some newly developed and candidate wheat (Triticum aestivum L.) cultivars using gas exchange and chlorophyll fluorescence attributes. Pak. J. Bot. 2011, 43, 2693–2699. [Google Scholar]
- Allakhverdiev, S.I.; Sakamoto, A.; Nishiyama, Y.; Inaba, M.; Murata, N. Ionic and osmotic effects of NaCl-induced inactivation of photosystems I and II in Synechococcus sp. Plant Physiol. 2000, 123, 1047–1056. [Google Scholar] [CrossRef]
- Genc, Y.; Oldach, K.; Taylor, J.; Lyons, G.H. Uncoupling of sodium and chloride to assist breeding for salinity tolerance in crops. New Phytol. 2016, 210, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Genc, Y.; Mcdonald, G.K.; Tester, M. Reassessment of tissue Na+ concentration as a criterion for salinity tolerance in bread wheat. Plant Cell Environ. 2007, 30, 1486–1498. [Google Scholar] [CrossRef] [PubMed]
- Bhutta, W.M.; Hanif, M. Genetic variability of salinity tolerance in spring wheat (Triticum aestivum L.). Acta Agric. Scand. Section B Soil Plant Sci. 2010, 60, 256–261. [Google Scholar]
- Ashraf, M. Registration of ‘S-24’Spring Wheat with Improved Salt Tolerance. J. Plant Regis. 2010, 4, 34–37. [Google Scholar] [CrossRef]
- Saddiq, M.S.; Afzal, I.; Basra, S.M.; Ali, Z.; Ibrahim, A.M. Sodium exclusion is a reliable trait for the improvement of salinity tolerance in bread wheat. Arch. Agron. Soil Sci. 2018, 64, 272–284. [Google Scholar] [CrossRef]
- Majeed, A.; Haq, M.; Niaz, A.; Mahmood, A.; Ahmad, N.; Khan, H. Comparison of Soil and Hydroponic Based Screening Process to Select Salt Tolerant Wheat Varieties. Sarhad J. Agric. 2019, 35, 1107–1112. [Google Scholar] [CrossRef]
- Mahboob, W.; Khan, M.A.; Shirazi, M.U.; Mumtaz, S.; Shereen, A. Using growth and ionic contents of wheat seedlings as rapid screening tool for salt tolerance. J. Crop Sci. Biotechnol. 2018, 21, 173–181. [Google Scholar] [CrossRef]
- Elkelish, A.A.; Soliman, M.H.; Alhaithloul, H.A.; El-Esawi, M.A. Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol. Biochem. 2019, 137, 144–153. [Google Scholar] [CrossRef]
- James, R.A.; Davenport, R.J.; Munns, R. Physiological characterization of two genes for Na+ exclusion in durum wheat, Nax1 and Nax2. Plant Physiol. 2006, 142, 1537–1547. [Google Scholar] [CrossRef]
- James, R.A.; Blake, C.; Byrt, C.S.; Munns, R. Major genes for Na+ exclusion, Nax1 and Nax2 (wheat HKT1; 4 and HKT1; 5), decrease Na+ accumulation in bread wheat leaves under saline and waterlogged conditions. J. Exp. Bot. 2011, 62, 2939–2947. [Google Scholar] [CrossRef]
- Bulle, M.; Yarra, R.; Abbagani, S. Enhanced salinity stress tolerance in transgenic chilli pepper (Capsicum annuum L.) plants overexpressing the wheat antiporter (TaNHX2) gene. Mol. Breed. 2016, 36, 36. [Google Scholar] [CrossRef]
- Shabala, S.; Pottosin, I. Regulation of potassium transport in plants under hostile conditions: Implications for abiotic and biotic stress tolerance. Physiol. Plant. 2014, 151, 257–279. [Google Scholar] [CrossRef]
- Saddiq, M.S.; Iqbal, S.; Afzal, I.; Ibrahim, A.M.; Bakhtavar, M.A.; Hafeez, M.B.; Jahanzaib; Maqbool, M.M. Mitigation of salinity stress in wheat (Triticum aestivum L.) seedlings through physiological seed enhancements. J. Plant Nutri. 2019, 42, 1192–1204. [Google Scholar] [CrossRef]
- Janmohammadi, M.; Enayati, V.; Sabaghnia, N. Impact of cold acclimation, de-acclimation and re-acclimation on carbohydrate content and antioxidant enzyme activities in spring and winter wheat. Icel. Agric. Sci. 2012, 25, 3–11. [Google Scholar]
- Zheng, Y.; Jia, A.; Ning, T.; Xu, J.; Li, Z.; Jiang, G. Potassium nitrate application alleviates sodium chloride stress in winter wheat cultivars differing in salt tolerance. J. Plant Physiol. 2008, 165, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Qiong, Y.; Guo, Y.; Zhixia, X.; Ke, S.; Jin, X.; Ting, Y.; Xiaojing, L. Effects of salt stress on tillering nodes to the growth of winter wheat (Triticum aestivum L.). Pak. J. Bot. 2016, 48, 1775–1782. [Google Scholar]
- Brestic, M.; Zivcak, M.; Kalaji, H.M.; Carpentier, R.; Allakhverdiev, S.I. Photosystem II thermostability in situ: Environmentally induced acclimation and genotype-specific reactions in Triticum aestivum L. Plant Physiol. Biochem. 2012, 57, 93–105. [Google Scholar] [CrossRef]
- Živčák, M.; Brestič, M.; Olšovská, K.; Slamka, P. Performance index as a sensitive indicator of water stress in Triticum aestivum L. Plant Soil Environ. 2008, 54, 133–139. [Google Scholar] [CrossRef]
- Mehta, P.; Jajoo, A.; Mathur, S.; Bharti, S. Chlorophyll a fluorescence study revealing effects of high salt stress on Photosystem II in wheat leaves. Plant Physiol. Biochem. 2010, 48, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Husain, S.; Munns, R.; Condon, A.T. Effect of sodium exclusion trait on chlorophyll retention and growth of durum wheat in saline soil. Aust. J. Agric. Res. 2003, 54, 589–597. [Google Scholar] [CrossRef]
- Loutfy, N.; Sakuma, Y.; Gupta, D.K.; Inouhe, M. Modifications of water status, growth rate and antioxidant system in two wheat cultivars as affected by salinity stress and salicylic acid. J. Plant Res. 2020, 133, 549–570. [Google Scholar] [CrossRef] [PubMed]
- Arfan, M.; Athar, H.R.; Ashraf, M. Does exogenous application of salicylic acid through the rooting medium modulate growth and photosynthetic capacity in two differently adapted spring wheat cultivars under salt stress? J. Plant Physiol. 2007, 164, 685–694. [Google Scholar] [CrossRef]
- Baker, N.R.; Rosenqvist, E. Applications of chlorophyll fluorescence can improve crop production strategies: An examination of future possibilities. J. Exp. Bot. 2004, 55, 1607–1621. [Google Scholar] [CrossRef]
- Nawaz, K.; Hussain, K.; Majeed, A.; Khan, F.; Afghan, S.; Ali, K. Fatality of salt stress to plants: Morphological, physiological and biochemical aspects. Afr. J. Biotechnol. 2010, 9, 5475–5480. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Bhusal, N.; Lee, M.; Lee, H.; Adhikari, A.; Han, A.R.; Han, A.; Kim, H.S. Evaluation of morphological, physiological, and biochemical traits for assessing drought resistance in eleven tree species. Sci. Total Environ. 2021, 779, 146466. [Google Scholar] [CrossRef]
- Geissler, N.; Hussin, S.; El-Far, M.M.; Koyro, H.-W. Elevated atmospheric CO2 concentration leads to different salt resistance mechanisms in a C3 (Chenopodium quinoa) and a C4 (Atriplex nummularia) halophyte. Environ. Exp. Bot. 2015, 118, 67–77. [Google Scholar] [CrossRef]
- Zahra, N.; Raza, Z.A.; Mahmood, S. Effect of salinity stress on various growth and physiological attributes of two contrasting maize genotypes. Brazilian Arch. Biol. Technol. 2020, 63. [Google Scholar] [CrossRef]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.E.; Salami, S.A.; Babalar, M. Morphological, physiochemical and antioxidant responses of Maclura pomifera to drought stress. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Zeng, L.; Shannon, M.C.; Lesch, S.M. Timing of salinity stress affects rice growth and yield components. Agric. Water Manag. 2001, 48, 191–206. [Google Scholar] [CrossRef]
- Munns, R.; James, R.A. Screening methods for salinity tolerance: A case study with tetraploid wheat. Plant Soil 2003, 253, 201–218. [Google Scholar] [CrossRef]
- Mansour, E.; Moustafa, E.S.; Desoky, E.-S.M.; Ali, M.; Yasin, M.A.; Attia, A.; Alsuhaibani, N.; Tahir, M.U.; El-Hendawy, S. Multidimensional evaluation for detecting salt tolerance of bread wheat genotypes under actual saline field growing conditions. Plants 2020, 9, 1324. [Google Scholar] [CrossRef] [PubMed]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Shavrukov, Y.; Langridge, P.; Tester, M. Salinity tolerance and sodium exclusion in genus Triticum. Breed. Sci. 2009, 59, 671–678. [Google Scholar] [CrossRef]
- Gardner, F.; Pearce, R.; Mitchell, R. Physiology of Crop Plants, 2nd ed.; Iowa State University Press: Ames, IA, USA, 1985. [Google Scholar]
- Fanizza, G.; Gatta, C.D.; Bagnulo, C. A non-destructive determination of leaf chlorophyll in Vitis vinifera. Ann. Appl. Biol. 1991, 119, 203–205. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QA redox state and excitation energy fluxes. Photosy Res. 2004, 79, 209–218. [Google Scholar] [CrossRef]
| Among the Five Spring Wheat Genotypes | |||||||||||||
| Source | Df | RL | SL | RGR-RL | RGR-SL | Na+ | K+ | K+/ Na+ | Fv′/Fm′ | gs | Ft | Leaf Senescence | CCI |
| G | 4 | 46.6 *** | 2.1 * | 0.399 *** | 0.02 n.s. | 10.71 ** | 12.41 n.s | 1.49 n.s | 0.002 n.s | 15.38 *** | 353972 ** | 36.24 n.s. | 126.39 *** |
| SL | 1 | 136.1 ** | 130.2 *** | 0.943 *** | 1.26 *** | 1292.7 *** | 1023.0 *** | 158.14 *** | 0.621 *** | 989.45 *** | 2.441 × 101 *** | 7022.2 *** | 1886.48 ** |
| G*SL | 3 | 10.1 *** | 3.5 *** | 0.034 *** | 0.033 ** | 6.63 n.s. | 15.42 n.s. | 1.26 n.s. | 0.004 n.s. | 15.81 *** | 310460 *** | 20.7 n.s. | 23.00 *** |
| Among the Five Winter Wheat Genotypes | |||||||||||||
| G | 4 | 111.5 ** | 61.9 *** | 1.233 *** | 0.39 *** | 47.74 *** | 10.91 n.s. | 2.66 * | 0.039 *** | 56.78 *** | 616640 *** | 236.8 ** | 368.36 *** |
| SL | 1 | 172.2 ** | 162.3 *** | 1.624 *** | 1.27 *** | 589.1 *** | 678.2 *** | 142.7 *** | 0.749 *** | 538.58 *** | 1.500 × 101 *** | 4806.9 *** | 1252.4 *** |
| G*SL | 3 | 2.96 n.s. | 3.9 n.s. | 0.075 n.s. | 0.028 n.s. | 15.6 *** | 30.62 ** | 2.00 n.s. | 0.008 *** | 18.63 *** | 196887 *** | 83.61 n.s. | 37.55 *** |
| Among the Ten Wheat Genotypes | |||||||||||||
| G | 9 | 119.3 ** | 58.9 *** | 0.984 *** | 0.36 *** | 41.46 *** | 74.6 *** | 2.86 * | 0.053 *** | 36.98 *** | 614174 *** | 131.5 n.s. | 233.68 *** |
| SL | 1 | 301.6 ** | 291 *** | 2.511 *** | 2.53 *** | 1812.7 *** | 1678.4 *** | 300.26 *** | 1.77 *** | 1483.1 *** | 3.853 × 101 *** | 11578 *** | 3081.1 *** |
| G*SL | 3 | 6.6 *** | 3.5 ** | 0.054 *** | 0.027 n.s. | 17.58 *** | 23.0 *** | 1.52 n.s | 0.0085 *** | 20.29 *** | 322609 *** | 74.3 n.s. | 33.33 *** |
| Among the Five Spring Wheat Genotypes | |||||||
|---|---|---|---|---|---|---|---|
| Traits | Senescence of Leaf | Na+ in Leaf | K+ in Leaf | gs | Ft | Fv′/Fm′ | Root Length |
| Na+ in leaf | 0.81 | ||||||
| K+ in leaf | −0.75 | −0.76 ** | |||||
| gs | −0.76 | −0.73 ** | 0.75 ** | ||||
| Ft | −0.83 | −0.87 ** | 0.77 ** | 0.82 ** | |||
| Fv′/Fm′ | −0.85 | −0.88 ** | 0.79 ** | 0.84 ** | 0.93 ** | ||
| Root length | −0.75 | −0.74 ** | 0.66 ** | 0.76 ** | 0.75 ** | 0.84 ** | |
| Shoot length | −0.67 | −0.65 ** | 0.58 ** | 0.75 ** | 0.69 ** | 0.70 ** | 0.83 ** |
| Among the Five Winter Wheat Genotypes | |||||||
| Na+ in leaf | 0.88 ** | ||||||
| K+ in leaf | −0.78 ** | −0.85 ** | |||||
| gs | −0.82 ** | −0.85 ** | 0.91 ** | ||||
| Ft | −0.79 ** | −0.85 ** | 0.89 ** | 0.93 ** | |||
| Fv′/Fm′ | −0.86 ** | −0.92 ** | 0.87 ** | 0.88 ** | 0.83 ** | ||
| Root length | −0.84 ** | −0.86 ** | 0.84 ** | 0.82 ** | 0.84 ** | 0.86 ** | |
| Shoot length | −0.49 ** | −0.47 ** | 0.52 ** | 0.47 ** | 0.55 ** | 0.43 ** | 0.58 ** |
| Among the Ten Wheat Genotypes | |||||||
| Na+ in leaf | 0.85 ** | ||||||
| K+ in leaf | −0.74 ** | −0.81 ** | |||||
| gs | −0.79 ** | −0.81 ** | 0.82 ** | ||||
| Ft | −0.81 ** | −0.86 ** | 0.83 ** | 0.89 ** | |||
| Fv′/Fm′ | −0.79 ** | −0.86 ** | 0.85 ** | 0.82 ** | 0.84 ** | ||
| Root length | −0.70 ** | −0.74 ** | 0.76 ** | 0.72 ** | 0.73 ** | 0.87 ** | |
| Shoot length | −0.54 ** | −0.56 ** | 0.61 ** | 0.59 ** | 0.60 ** | 0.66 ** | 0.80 ** |
| Spring Wheat | Winter Wheat |
|---|---|
| PI 94341 | TX12M 4713 |
| W4909 | TX 11D 3134 |
| W4910 | TXIID 3127 |
| S-24 | TX110D 2265 |
| Sakha 8 | TX12M 4637 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saddiq, M.S.; Iqbal, S.; Hafeez, M.B.; Ibrahim, A.M.H.; Raza, A.; Fatima, E.M.; Baloch, H.; Jahanzaib; Woodrow, P.; Ciarmiello, L.F. Effect of Salinity Stress on Physiological Changes in Winter and Spring Wheat. Agronomy 2021, 11, 1193. https://doi.org/10.3390/agronomy11061193
Saddiq MS, Iqbal S, Hafeez MB, Ibrahim AMH, Raza A, Fatima EM, Baloch H, Jahanzaib, Woodrow P, Ciarmiello LF. Effect of Salinity Stress on Physiological Changes in Winter and Spring Wheat. Agronomy. 2021; 11(6):1193. https://doi.org/10.3390/agronomy11061193
Chicago/Turabian StyleSaddiq, Muhammad Sohail, Shahid Iqbal, Muhammad Bilal Hafeez, Amir M. H. Ibrahim, Ali Raza, Esha Mehik Fatima, Heer Baloch, Jahanzaib, Pasqualina Woodrow, and Loredana Filomena Ciarmiello. 2021. "Effect of Salinity Stress on Physiological Changes in Winter and Spring Wheat" Agronomy 11, no. 6: 1193. https://doi.org/10.3390/agronomy11061193
APA StyleSaddiq, M. S., Iqbal, S., Hafeez, M. B., Ibrahim, A. M. H., Raza, A., Fatima, E. M., Baloch, H., Jahanzaib, Woodrow, P., & Ciarmiello, L. F. (2021). Effect of Salinity Stress on Physiological Changes in Winter and Spring Wheat. Agronomy, 11(6), 1193. https://doi.org/10.3390/agronomy11061193










