Abstract
In this study, black soybean was firstly processed by solid-state fermentation (SSF) with a probiotic fungus Eurotium cristatum YL-1. The effect of SSF on the nutritional components (including proximate, amino acids, minerals, and fatty acids), total phenolics, isoflavones, antioxidant activity, and volatile organic compounds of black soybeans were revealed. Results of this work demonstrated that black soybean processed by SSF with E. cristatum greatly increased the contents of protein, essential amino acids, and some minerals (e.g., calcium, phosphorus, and magnesium). GC results revealed that more than 80% of the total lipids from both fermented and non-fermented black soybeans were unsaturated fatty acids, and SSF influenced the fatty acids composition. Higher contents of total phenolics and aglycone isoflavones (i.e., genistein, daidzein, and glycitein) of fermented black soybeans were achieved by SSF. Furthermore, SSF with E. cristatum considerably augmented the ferric reducing antioxidant power, scavenging effects against ABTS·+ and DPPH radical, reducing power, and chelating ability of black soybeans, which evaluated with various polarity solvent extracts. HS-GC-IMS analysis detected a total of 66 volatile compounds in FBS and BS, and 56 volatile organic compounds were successfully identified. The intensities of main volatile compounds (i.e., 10 esters, 11 alcohols, and 19 aldehydes) differed remarkably by fermentation with E. cristatum. The intensities of seven alcohols and nine aldehydes considerably decreased, whereas higher levels of esters were achieved by SFF. Thus, our results confirmed that black soybeans processed by SSF with E. cristatum is a promising approach to substantially improve its nutritional value, flavor characteristics, and biological effect, and might have great potential in the development of new functional foods or be used as a new nutritional ingredient applied in food design.
1. Introduction
Legumes (including soybeans, chickpeas, lentils, peas, green beans, etc.) are considered as one of the most crucial staple foods for the diet of humans worldwide, particularly those for Asian and Mediterranean countries [1,2]. They could provide an excellent source of nutritional components such as fatty acids, minerals, protein, vitamins, etc., and are considered to be highly valuable in human nutrition and gaining considerable interest [1]. It was well documented that legumes play a vital role in agriculture and are the second most important source of food next to cereal grains [3,4]. Black soybean (Glycine max (L.) Merrill), a kind of soybean with a black seed coat, has been used as nutritionally rich food and folk medicine in Asia (mainly in Korea, China, and Japan) for hundreds of years. Black soybean contains plenty of fatty acids, vitamins, protein, minerals, and vitamins and is a nutritious food with functional properties [5]. Additionally, many phytochemicals, including isoflavones, flavonoids, phenolics, phytosterols, alkaloids, and saponins, are presented in black soybeans, which are known to exert diverse physiological activities such as anti-obesity, anti-inflammatory, antioxidant, and anticancer abilities, and these black soybean-based products are popular around the world recently [2,6]. In Asian countries, black soybeans were usually used to prepare fermented food products such as natto, doenjang, black soy sauce, and douchi [6]. These traditionally solid-state fermented foods have received considerable attention not only for their unique flavors but also for their great nutritional value and health beneficial effects and play a crucial part in human diets in most Asian countries.
Previous investigations have confirmed that the organoleptic and nutritional qualities of soybean-based products could be effectively improved by fermentation with microorganisms [7]. For example, previous literature showed that fermented soybeans demonstrated much higher soluble proteins, amino acids, total phenolics, isoflavone aglycones compared with non-fermented samples [8]. Especially, solid-state fermentation (SSF) is a widely used food processing technology due to its simple, non-toxicity, environmentally friendly, and cost-efficiently features, which has also been proved that could improve the nutritional and biological compounds, flavor, texture, sensorial, and physicochemical characteristics of food products. SSF with microorganisms could produce many hydrolytic enzymes that acted as catalysts and help complex reactions during the process and resulted in the hydrolyzation or transformation of complex organic compounds such as protein, polysaccharide, lipid, and phytochemicals, and then contributed to the processed products with the improvement of aroma, flavor, nutrition, and bioactivities [9,10]. For instance, Dey et al. [11] have reported that microorganisms (especially fungi) can secrete many enzymes that hydrolyze ether, ester, or glycosidic bonds to effectively disintegrate the cell wall structure components; thus, the insoluble phenolic compounds that bound covalently to cell wall structure components are released, and the biological activities of fermented legume products are improved during SSF. Lee et al. [9] have demonstrated that Tricholoma matsutake fungal mycelia used in the SSF of soybean can be beneficial to its nutritional value, bioactive compound, and potential antioxidant effect. Accordingly, SSF could be applied as a promising food processing technology that has the great potential to augment the nutritional value, phytochemical compounds, and biological activities of legume products [12,13,14].
Eurotium cristatum, commonly known as the “golden flower” that is non-toxic and safe, is the predominant probiotic fungus that has a long history of use in the manufacturing of Fu-brick dark tea in China [15,16,17]. It was reported that E. cristatum plays a vital role in the formation or conversion of biological and flavor compounds in Fu-brick dark tea during its post-fermentation process and have turned out to display numerous health beneficial effects (e.g., anti-obesity, antioxidant, anticancer, regulation of intestinal flora) after consumption of this tea [18,19]. In the past few years, the unique pharmacological health benefits of Fu-brick dark tea were widely attracted by many researchers, especially for the role of E. cristatum on the quality characteristics of tea [19,20,21]. As the dominant fungus, E. cristatum can secret large amounts of hydrolytic enzymes (e.g., α-amylase, β-glucosidase, cellulase, protease, pectinase, tanninase, etc.) during the post-fermentation stage, biotransformation of phytochemicals and thus directly improved the quality and bioactivity of Fu-brick dark tea. In fact, E. cristatum was also widely applied in the SSF bio-processing of other tea matrices such as Pu’er tea and loose leaf tea in recent years [22,23]. More interestingly, this fungus is characterized by its low free water content requirement of the substrate during SSF and is easily grown on various plant-derived food matrices or medicinal herbs. It has been reported that E. cristatum has great potential to improve the nutritional value and biological activity of substrates during SSF. For example, E. cristatum was recently used in our group in the processing of fermented buckwheat to enhance the antioxidant and α-glucosidase inhibitory activities for the accumulation of phenolic compounds during fermentation [24]. Furthermore, Gu et al. [21] reported that SSF of Hippophae rhamnoides leaves using the inoculum starter Eurotium sp. could convert rutin to three metabolites, namely, isorhamnetin, kaempferol, and quercetin, and augmented the antioxidant capacity. It has been found by Zou et al. [25] that E. cristatum secreted several enzymes including protease, cellulase, amylase, and hemicellulase during SSF of Ginkgo biloba leaves, thus decreased the ginkgolic acids, lignin, and cellulose, and increased flavonoids content, reducing power, scavenging activities of ABTS·+ and DPPH radical. Hence, we speculate that E. cristatum might be a suitable microbial candidate and have great potential for producing fermented foods with added value. Currently, as far as we know, no research is available on the SSF of black soybean using E. cristatum mycelium and its effect on the nutritional value, isoflavones composition, and antioxidant capacity.
In this work, SSF of black soybean using E. cristatum was performed for the first time. The main aim of the present research was to achieve fermented black soybean food with high nutritional characteristics as well as potent biological properties. The changes of nutritional components (proximate composition, fatty acid, amino acid, minerals, and isoflavones) and antioxidant property of solid-state fermented black soybeans by E. cristatum were firstly investigated in our work. Furthermore, advanced headspace-gas chromatography-ion mobility spectrometry (HS-GC-IMS) methodology was carried out to estimate the alterations of volatile organic compounds (VOCs) in fermented black soybean using E. cristatum. GC-IMS technology is an emerging approach for the analysis and characterization of VOCs that developed in these years and attracted great attention from food scientists. This analysis technique is high sensitivity, easy operation, high resolution, and high efficiency that combine the merits of ion mobility spectroscopy and gas chromatography, which can provide suitable analysis result in the separation and characterization of flavor components. Our findings for the current study will provide an innovative food processing approach for improving the nutritional potentials, bioactive compounds, and enhancing antioxidant capacity of black soybean through SSF using E. cristatum, as well as broaden the information of microbial metabolism and biotransformation with legume materials for the development of fermented food product.
2. Materials and Methods
2.1. Materials
Folin-Ciocalteuf’s reagent, 2,2-azinobis (3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt (ABTS), isoflavone standards including glucoside and aglycone types, 2,4,6-tris (2-pyridyl)-S-triazine (TPTZ), 17 standard amino acids, 2 2,2-diphenyl-1-picrylhydrazyl (DPPH), and gallic acid were bought from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). The fatty acids standards and mineral standards for quantitative analyses were bought from Merck (Darmstadt, Germany). Acetonitrile and methanol (Tedia, Fairfield, OH, USA) were of HPLC grade solvent. All of the other used reagents or chemicals in this study were of analytical grade. The E. cristatum YL-1 fungus that was previously isolated from Fu-brick dark tea in our laboratory [12,24] was employed as an inoculum starter for processing fermented black soybean. Black soybean seeds were obtained from a local supermarket in Changsha (Changsha, China).
2.2. Preparation of the Inoculum
Inoculum starter used for SSF was prepared based on the procedure as follows. In brief, the stored E. cristatum YL-1 was initially reactivated with successive inoculations with M40Y agar medium, incubated for 7 days at 28 °C, then transferred into another M40Y medium and incubated at the same temperature for 7 more days for sporulation of the E. cristatum on the surface of the culture medium. Sterile deionized water was then added into the plate medium and scraped the produced golden spores and mycelia with a sterile glass rod. The spores and mycelia were collected and filtered through sterilized absorbent cotton and shaken with a set of glass beads in an Erlenmeyer flask. Then, sterile deionized water was added into the filtrate to adjust the spore concentration of 106–107 spores/mL using a Neubauer cell-counting chamber (this spore suspension was used for inoculation). All of the above microbiological experiments were aseptically operated.
2.3. Solid-State Fermentation of Black Soybeans
Before being used for fermentation, the black soybean seeds were completely cleaned by washing with deionized water and soaked in deionized water at 25 °C for 1 h. After decanting the water, the seeds were sterilized in an autoclave at 121 °C for 25 min and cooled to room temperature prior to inoculation. Then, E. cristatum spores suspension inoculated into the sterilized black soybean substrates (5 mL/100 g wet seed) and placed at 28 °C for 8 days in a microbial incubator under static conditions. The non-fermented black soybean (BS) without inoculation of E. cristatum spore suspension was also prepared that used as a control. Both of the obtained fermented black soybeans (FBS) and BS samples were freeze-dried immediately and crushed by a grinding machine. The milled fours were then sieved with a 60 mesh sieve to obtain fine power and maintained at −20 °C in a refrigerator until analysis.
2.4. Proximate Analysis
The proximate analysis of BS and FBS, namely, ash, crude fat, and protein, were evaluated using the method of AOAC [26]. Fat content was determined by extraction with petroleum ether in a Soxhlet system. Proteins were assessed by the Kjeldahl method, and the nitrogen conversion factor into protein was 6.25. Finally, ash content was measured by incineration of known weights of the samples at 550 °C in a muffle furnace, and the total carbohydrates amount was determined by difference. The obtained data were expressed in g per 100 g of dry weight basis (g/100 g DW).
2.5. Analysis of Amino Acid Composition
The previously reported procedure of Xiao et al. [27] was performed to analyze the amino acid composition. Briefly, two hundred micrograms of each FBS or BS sample was hydrolyzed with 10 mL of 6 M HCl for 24 h in a sealed glass tube at 110 °C in an oven. After cooling to 25 °C, the collected supernatant was evaporated to dryness under vacuum, and the dried hydrolyzed sample was re-dissolved in 5 mL HCl (0.02 M). The solution was filtered using a 0.22 µm PVDF membrane syringe filter then analyzed by an automated amino acid analyzer (HITACHI L-8900) manufactured by Hitachi High-Technologies in Japan. The amino acid contents were calculated in milligram per g of dry weight basis (mg/g DW).
2.6. Fatty Acid Composition by Gas Chromatography
Fatty acid composition of BS and FBS were analyzed according to the method of Liu et al. [28] and Qi et al. [29]. The lipids of BS and FBS were extracted using a SOX 406 automatic lipid analyzer (Hanon Instruments, Rizhao, Shandong, China). FBS or BS powder was extracted with petroleum ether (chromatography grade) at 60 °C for 6 h. After extraction, the solvent was evaporated by a rotary evaporator RE-52 under reduced pressure at 40 °C to obtain the lipids of BS and FBS. The lipids were firstly converted into methyl esters by transesterification process and then separated using gas chromatography (GC-2010, Shimadzu, Tokyo, Japan) that equipped with an SP 2560 capillary column (100 × 0.25 mm, 0.20 μm film, Supelco, Bellefonte, PA, USA) and a flame ionization detector (FID). A total of 1 µL of sample volume was injected into the GC with a split ratio of 100:1, and the carrier gas was high-purity nitrogen with a flow rate of 1.0 mL/min. The column temperature of the gradient program was performed as follows: 60 °C held for 2 min, gradually increased to 170 °C at 10 °C/min, and maintaining for 10 min. Thereafter, it was linearly increased at 3 °C/min up to 230 °C and held for 50 min. The temperatures of injector and detector temperatures were both operated at 240 °C. Individual fatty acids were identified based on a comparison of the retention times with those of authentic standard mixture of methyl esters (Sigma, St. Louis, MO, USA). Quantification of fatty acids was calculated according to the GC peak areas of the internal standard (heptadecanoic acid, C17:0) and was presented as % of total lipids content.
2.7. Mineral Content Analysis
Mineral composition analysis of BS and FBS was conducted with atomic absorption spectroscopy (AAS) (AA 800, Perkin-Elmer, AnalytikjenaAG, Jena, Germany). Briefly, about two grams of sample flour was taken in a constantly weighed crucible and ignited in a muffle furnace for 10 h at 550–600 °C. The resulting ash residue was used for the determination of mineral contents, including potassium (K), magnesium (Mg), calcium (Ca), phosphorus (P), etc. The above-obtained ash of BS and FBS was then dissolved in 0.2 N of hydrochloric acid and filtered with a 0.22 µm syringe filter. Minerals in the filtrate were estimated by AAS analysis with respective hollow-cathode lamps (HCl). Standard calibration curves of each mineral were established by using a variety of standard solutions that measured with AAS.
2.8. Estimation of Total Phenolics Content (TPC), Isoflavones Compositions and Antioxidant Activity of Black Soybeans Processed by SSF with E. cristatum
2.8.1. Preparation of Solvent Extracts
The bioactive compounds were extracted according to the method as follows. Briefly, two grams of FBS or BS powder were extracted with 80 mL of three commonly used extraction solvents with variant polarities, namely, 80% methanol, 80% acetone, and 80% ethanol in an ultrasonic water bath (40 °C, 40 kHz) for 40 min, respectively. The supernatant was obtained after the extraction mixture centrifuged (3-18R, Hunan Hengnuo Instrument Equipment Co., Ltd., Changsha, China) for 15 min at 10,000× g (4 °C), and then the resulting residues were re-extracted twice at the assistance of ultrasonic oscillation under the same conditions. Then, a rotary evaporator RE-52 under reduced pressure (Biochemical Instrument Factory of Shanghai Yarong, Shanghai, China) at 40 °C was performed to concentrate the pooled extract. The extract volume was then adjusted to 50 mL with the respective solvent systems before they were stored at −20 °C in a freezer for further analysis.
2.8.2. Assay of TPC
The TPC of FBS and BS extracts were assayed using the method of Folin–Ciocalteu as previously reported by Xiao et al. [30]. Simply, 0.5 mL of Folin–Ciocalteu reagent was added by 0.2 mL of sample extract and 2.3 mL of deionized water in a reaction tube. After maintained for 1 min, 2 mL of Na2CO3 solution (75 g/L) was added into the reaction mixture and incubated at 25 °C for 1 h, and absorbance was detected at 760 nm. TPC was calculated based on a calibration curve using different gallic acid solutions, and data were expressed as µg gallic acid equivalent (GAE)/g DW.
2.8.3. HPLC Analysis of Isoflavone Compositions
Previous studies reported that black soybean products are rich in isoflavones that contributed to their bioactivity, such as antioxidant activity. Thus, the six main isoflavones (i.e., genistin, genistein, glycitin, glycitein, daidzin, and daidzein) presented in BS and FBS were analyzed by using high-performance liquid chromatography (Shimadzu LC-20AT, Kyoto, Japan). The collected extracts (which obtained in Part 2.8.1) were filtered through a 0.22 µm syringe filter, and then 20 µL of the sample was injected into an Agilent C18 reverse-phase column (ZORBAX SB-C18, 4.6 × 250 mm, 5 μm particle size) and maintained at 30 °C. Gradient elution was performed using a mixture of acetonitrile (eluent B) and deionized water (eluent A) at 0.8 mL/min following the gradient elution program as follows: beginning with 10% B at 0 min, then linearly ramped to 20% B over 3 min, 20–25% B in 5 min. After that, eluent B increased to 60% by linear gradient within 22 min, eluent B linearly ramped to 100% over 5 min and maintained for 2 min, and finally, eluent B linearly return to 10% in 1 min. The wavelength was monitored at 254 nm with a Shimadzu SPD-20A Prominence UV-Visible detector (Shimadzu, Kyoto, Japan). The UV spectra and retention times of those authentic standards were applied to the identification and quantification of the isoflavones [10,13,31]. The isoflavones content of BS and FBS were presented as µg/g DW.
2.8.4. Assay of Antioxidant Activity
The antioxidant effects of BS or FBS were tested by scavenging activities against ABTS·+ and DPPH radical, reducing power (RP), ferrous ion chelating ability (CHA), ferric reducing antioxidant power (FRAP). The ABTS·+ and DPPH radical scavenging effects were evaluated according to the methodology described by Chen et al. [12], and ascorbic acid (vitamin C) was used as the standard control. Radical scavenging effects were expressed as µg vitamin C equivalent (µg VCE) per g of dry weight black soybeans (µg VCE/g DW). The detailed procedure reported by Xiao et al. [30] was carried out to evaluate the FRAP, and different concentrations of FeSO4 solution (100–1600 µM) were applied to establish the calibration curve. The FRAP values were reported as µmol Fe (II) equivalents/g DW sample. The RP of BS and FBS was conducted by adapting the procedure of Liu et al. [32], and the results were expressed as µg VCE/g DW. The antioxidant capacity was also evaluated by chelating ability (CHA) on ferrous ions that were estimated following the procedure previously described by Xiao et al. [33]. Disodium ethylenediaminetetraacetate (EDTA-2Na) solution with a variety of concentrations was applied to plot the calibration curve, and the results were presented as µg EDTA-2Na equivalents per g dry weight basis.
2.9. Analysis of Volatile Organic Compounds (VOCs) by HS-GC-IMS Technology
Analyses of VOCs were carried out on an advanced FlavourSpec instrument of GC-IMS (G.A.S., Dortmund, Germany) that using an Agilent 490 gas chromatograph (Agilent Technologies, Wilmington, DE, USA). Tritium (3H) was used as the ionization source of the IMS. An auto-sampler (CTC Analytics AG, Zwingen, Switzerland) with a heated gas-tight injector and a headspace sampling unit was equipped on this device that was used in the following experiment. For the analysis, two grams of lyophilized FBS or BS powder was weighed and transferred into a headspace bottle (20 mL) and kept for 15 min at 60 °C with an incubation speed of 500 rpm. Subsequently, the headspace (300 μL) was sampled and automatically injected into the injector by a heated syringe (65 °C) under splitless injection mode. The VOCs were separated in an MXT-5 capillary column (15 m × 0.53 mm ID, 1 µm) with a column temperature of 60 °C and the drift tube temperature maintained at 45 °C. High-purity nitrogen was applied as sample carrier gas and flow programmed as follows: starting at 2 mL/min and maintained for 2 min, then gradually increased to 100 mL/min during the remaining 18 min. The 3H ionization source in an ionization chamber was applied to ionize the compounds prior to detection and ionization depending on the concentration and chemical nature of the VOCs that produce protonated monomers or proton-bound dimmers ions. Subsequently, the resulting ions were driven into a 9.8 cm in length drift tube and performed at a stable temperature of 45 °C and voltage of 5 kV. The flow of drift gas (N2) was maintained at 150 mL/min. An average of 16 scans was collected in each spectrum with a 30 ms repetition rate. All analyses for the VOCs were carried out in triplicate. The N-ketones C4-C9 were applied as external references (Beijing Sinopharm Chemical Reagent Co., Ltd., Beijing, China) to calculate the retention index (RI) of the VOCs. Identification of the detected VOCs was based on a comparison of drift time (RIP relative) and retention index (RI) with that of the standard in the library database of GC-IMS supplied by G.A.S. (Dortmund, Germany). Quantitative analysis (intensities) of VOCs was conducted according to previously reported by Lv et al. [34], who used the Gallery Plot analysis (v.1.0.7, G.A.S.) to select the signal peak and calculated the peak height.
2.10. Statistical Analysis
All analyses were performed independently for three replicates, and the results were reported as mean ± SD (standard deviation). The data were processed for one-way analysis of variance and Duncan’s multiple range tests or independent sample t-test using the SPSS 17.0 software (SPSS, IBM Corp, Chicago IL, USA) to determine the significant difference. The data of GC-IMS were viewed and processed by the following instrumental analysis software, including Gallery Plot analysis, Laboratory Analytical Viewer (LAV), Reporter plug-ins, Dynamic Principal Components Analysis, and GC×IMS Library Search (G.A.S., Dortmund, Germany).
3. Results and Discussion
3.1. Proximate Composition
The changes in the proximate composition of black soybean by SSF with E. cristatum are shown in Table 1. It can be observed that carbohydrate content, crude protein, crude fat, and ash changed significantly due to fermentation. Protein, fat and ash contents appeared to be significantly (p < 0.05) increased by 19.0%, 11.7% and 11.2%, respectively, whereas the carbohydrate content significantly (p < 0.05) decreased by 22.8% during SSF. These observed results are similar to those reported by Xiao et al. [27], Li et al. [13], and Mohapatra et al. [35], as they also noted that the protein content of legume or cereal increased due to fermentation. Furthermore, Dai et al. [8] reported that the crude protein content in soybean meal was increased by 17.18% after SSF with Bacillus subtilis. Comparable changes occurred in the present study. One explanation of the observed result was likely due to the microorganisms synthesizes some protein in the substrates, which have been reported in previous studies [35]. Another explanation of this observation might be caused by the fungi growth during fermentation, which reflected an increase in fungi biomass [13,27]. The carbohydrate constituents could be effectively used as an energy source for fungal growth, thus leading to a reduction during SSF. Furthermore, the increase in fat during fermentation is in accordance with the reports of Xiao et al. [27] whereas differed from the findings of Li et al. [13] and Mohapatra et al. [35], who noted that SSF resulted in a reduction in fat. This is mainly ascribed to the diverse metabolic effects of variant used microorganisms. Likewise, Abu et al. [36] observed that sweet potato fermented with Aspergillus oryzae and A. niger upgraded the total lipid content during fermentation, while the lipids decreased from 1.9% to 0.5% by the action of another fungus, Pleurotus ostreatus.

Table 1.
Proximate composition of E. cristatum-fermented and non-fermented black soybeans.
3.2. Changes of Amino Acids Composition during Fermentation
The amino acid profile of BS and FBS are depicted in Table 2. It was observed that five non-essential amino acids (i.e., Ala, Asp, Pro, Gly, Glu) and seven essential amino acids (i.e., Leu, Phe, Met, Val, Thr, Lys, Ile) significantly increased (p < 0.05) after SSF process. Furthermore, Table 2 also revealed that there are no amino acids significantly (p > 0.05) decreased after fermentation. The content of total essential amino acids and total amino acids of the black soybeans greatly improved by 11.4% and 10.8%, respectively, due to SSF with E. cristatum. A similar observation was recently reported by Li et al. [13], who found that the contents of Thr, Lys, Leu, and Phe + Tyr of soybean flour displayed a considerable increase through SSF with Lactobacillus casei. They further noted that the content of total essential amino acids increased 10.25% after 72 h fermentation, which is coincided with the result of our current study. Several other previous investigations have also proved that microbial fermentation showed great potential to increase levels of amino acid [35]. It was corroborated in earlier documents that the conversion of synthesis effects of amino acids might have occurred under the action of microbial enzymes such as transaminases during SSF, which might be in part responsible for the increase in amino acid contents [27]. Hence, greater levels of essential and total amino acids accumulated in FBS during SSF are likely attributed to the occurrence of transamination or synthesis effects. As far as we know, this study is the first time that investigated the amino acid composition of fermented black soybeans using the filamentous fungus E. cristatum. In general, SSF was a useful and effective approach to boost the nutritional value of FBS. Furthermore, it also noted that glutamic acid is the most predominant amino acid in BS and FBS, which agrees with those from previous findings of Xiao et al. [27] and Dai et al. [8], who had also observed the highest content of amino acids in fermented and non-fermented legumes was glutamic acid.

Table 2.
Amino acid compositions of E. cristatum-fermented and non-fermented black soybeans.
3.3. Effect of SSF on the Level of Minerals
Many previous studies elucidated that minerals performed a number of key functions for our body, such as making hormones, transmitting nerve impulses, building strong bones, etc. [37]. Five macro minerals (K, Mg, Na, Ca, P) and five micro minerals (Se, Mn, Fe, Zn, Cu) in FBS and BS were analyzed by AAS technology, and the data are revealed in Table 3. The result indicated that SSF with E. cristatum significantly (p < 0.05) augmented most of the mineral levels of black soybean in comparison with the non-fermented sample. For example, calcium, phosphorus, and magnesium of FBS were increased by 12.8%, 38.8%, and 6.1%, respectively, after SSF with E. cristatum. Our findings were in line with the earlier observations of Dhull et al. [38] and Chawla et al. [3], who demonstrated that lentil fermented with A. awamori or black-eyed pea seed fermented with A. oryzae led to the accumulation of some minerals, respectively. The increment of minerals in FBS might be due to the effect of the fungal growth in the black soybean. However, Table 3 revealed that fermentation greatly decreased the potassium content of black soybean by 21.1%.

Table 3.
Mineral elements content of E. cristatum-fermented and non-fermented black soybeans.
3.4. Changes of Fatty Acids Composition during Fermentation
The fatty acid is one of the most important nutritional components of soybean products in human health benefits [39]. Although many investigators have studied the fatty acids composition of this crop in previous researches, changes occurring in their composition during fermentation have not been extensively evaluated. Fatty acid compositions of BS and FBS are summarized in Table 4.

Table 4.
Fatty acids composition (%) of E. cristatum-fermented and non-fermented black soybeans.
It revealed that polyunsaturated fatty acids were at the highest level in both BS and FBS, followed by the monounsaturated fatty acids and then saturated fatty acids. Especially, it is clearly noted in Table 4 that the main fatty acids levels in both BS and FBS are in the descending order: linoleic acid (C18:2n6) > oleic acid (C18:1n9) > palmitic acid (C16:0) > linolenic acid (C18:3n3) > stearic acid (C18:0), which together accounted for over 90% of the total fatty acids. It is also observed that the predominant fatty acids (i.e., linolenic, oleic, palmitic, linoleic, and stearic acids) in FBS remained the same as before fermentation. The proportions of these fatty acids observed in BS and FBS indicated the suitable characteristic of black soybean oil for the great percent of unsaturated fatty acids, which was in agreement with previous findings by Feng et al. [40] and Shibata et al. [41] for soybean products. As shown in Table 4, the polyunsaturated fatty acid, namely, linoleic acid (C18:2n6), accounted for the largest proportion (more than 50% of the total lipid) among the fatty acids, while it showed a slight increase in its percentage in the FBS, from 52.63% to 53.22% of the total fatty acids during black soybean fermentation. Furthermore, Table 4 shows that the oleic acid (C18:1n9) was the predominant monounsaturated fatty acid in both BS and FBS, and it interestingly found that the relative content of oleic acid significantly increased (p < 0.05) via fermentation with E. cristatum. The obtained result is consistent with Lee et al. [9] that SSF of soybean with Tricholoma matsutake improved the oleic acid content. This observed phenomenon might be explained by the synthesis of oleic acid (C18:1n9) from de novo lipogenesis, or partly formed from the saturation/oxidation of linolenic acid (C18:3n3) because a reduction in the proportion of linolenic acid (C18:3n3) was accompanied by an increase in oleic acid [36]. Moreover, previous research reported that the existence of lipoprotein complexes in the soybean substrate could be hydrolyzed to produce fatty acid by fermented with fungi, which might also contribute to the observed phenomenon [40,42]. The main saturated fatty acid in both BS and FBS was palmitic acid (16:0), and it should be interestingly pointed out that the relative proportion of this fatty acid significantly decreased by fermentation, a similar observation was recently detected by Li et al. [13], which might be due to the reason that palmitic acid was used by fungus, possibly involved in the synthesis of phospholipids components in the fungal tissue cell membrane during SSF [43]. Furthermore, it was also noted that pentadecanoic acid (c15:0) and eicosatrienoic acid (C20:3n3) were formed while eicosadienoic acid (C20:2) was transformed during SSF. The result indicated that the fatty acid composition of black soybean lipid is influenced by fermentation with E. cristatum. Furthermore, this study found that an increase in linoleic content and a reduction in linolenic acid in comparison with unfermented sample (Table 4), which is coincided with the finding of Da Silveira et al. [44], who used wheat bran and rice as the substrate processed by SSF with Rhizopus oryzae fungus. Furthermore, fermentation with E. cristatum caused a reduction in saturated fatty acids and an increase in unsaturated fatty acids (Table 4), which is corroborated with previous studies for SSF of rice bran [43]. According to the above evidence, more than 80% of the total lipids from both BS and FBS were composed of unsaturated fatty acids that considerably greater compared with saturated fatty acids, similarly to several earlier reports for the soybean samples [45,46]. Furthermore, FBS exhibited greater unsaturated fatty acids than BS, implying that the SSF process affects the fatty acids composition of black soybeans. In the present study, the high level of oleic acid is of high importance because it is an essential nutrient due to its association with a reduction in the risk of blood pressure and coronary heart disease and regulation of coagulation and glucose homeostasis during the postprandial state.
3.5. Effect of SSF with E. cristatum on TPC and Isoflavone Profiles of Black Soybeans
It is known that the phenolics from legumes are a natural source of antioxidants and receiving much attention from scientists in the last decades. Numerous earlier investigations demonstrated that different polarities solvents depicted a great impact on the extraction ability of phenolic compounds [33,47]. Thus, BS and FBS were extracted with the three most-used solvents for phenolic compounds in this study for comparison. The result is shown in Figure 1.
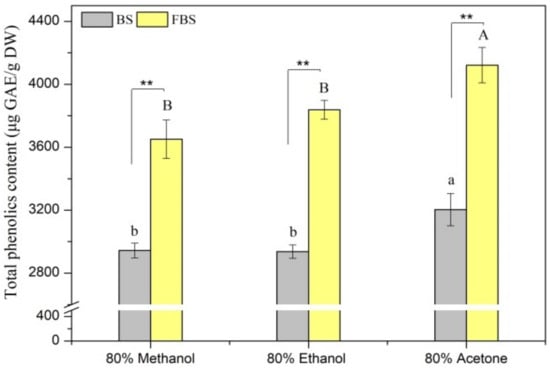
Figure 1.
Total phenolics content (TPC) of the various extracts from non-fermented black soybeans (BS) and E. cristatum-fermented black soybeans (FBS). Values followed by different capital letters or small letters are remarkably different (p < 0.05) among the different solvent extracts of FBS or BS, respectively. The symbols (**) denoted a significant difference at a level of 0.01 between FBS and BS regards the same extracted solvent.
It was noted that TPC of the extracts of BS and FBS ranged from 2936.51 to 3203.17 µg GAE/g DW and from 3650.79 to 4121.43 µg GAE/g DW, respectively, depending on the extraction solvents. These results confirmed that variant polarities solvents exhibited a great influence on phenolics extraction, which depends on their characteristics of chemical structure, polarity, and solubility. Furthermore, it was further found that 80% acetone extracts of BS or FBS showed the greatest TPC among the three solvent extracts, implying that 80% acetone was the best efficient solvent for extraction of phenolic compounds. This coincides with the studies reported by Zhao et al. [47] and Liu et al. [32] that 80% acetone displayed the best extraction capacity in extracting phenolic compounds from barley and pepper seeds, respectively. Moreover, it was interestingly noted that the TPC of FBS was significantly (p < 0.05) greater when compared with BS. Figure 1 displays that TPC of the acetone, ethanol, methanol extracts of FBS were almost 1.29-, 1.31-, and 1.24-fold greater, respectively, in comparison with the respective extract of BS. Results from this study are in agreement with those found by Santos et al. [10] and Chen et al. [12], where an improvement of TPC in fermented soybean samples with Saccharomyces cerevisiae or E. cristatum was observed. This result can be ascribed to the liberation of phenolics from cell wall structure during microbial fermentation. Many earlier studies reported that hydrolytic enzymes could be produced by fungus during SSF, which catalyzed the release of insoluble-bound phenolics from the substrate. Previous investigations have validated that only a small amount of phenolic compounds existed in the form of simple soluble-free esters in cereals or legumes. Most of them were covalently bound to the cell wall structure constituents (e.g., pectin, lignin, structural proteins, hemicellulose, arabinoxylans, and cellulose) that presented as insoluble-bound forms [48,49]. During the SSF process, a high amount of hydrolytic enzymes (such as amylases, cellulase, β-glucosidase, and protease) are secreted by the microbial that disintegrated the structure of the plant cell wall and hydrolyzed the covalent bonds such as ester or ether linkages between the cell wall macromolecules and insoluble-bound phenolics [11,49], leading to the release of phenolics and facilitating to extraction.
It is well evidenced that aglycone isoflavones are easily absorbed by the human body and have greater bioavailability and health effects (such as antioxidant ability) than their glucoside forms [50,51,52]. Several earlier studies proved that the isoflavone compositions could be distinctly affected by food processing technology such as cooking, frying, high-pressure treatment, germination, fermentation [9,31,53]. The present work investigated the changes of isoflavone composition during SSF to obtain valuable information on the quality of fermented black soybean, which was identified and quantified by HPLC analysis, and the result is shown in Table 5 and Figure 2.

Table 5.
HPLC analysis result of isoflavone compositions (μg/g DW) of E. cristatum-fermented and non-fermented black soybeans.
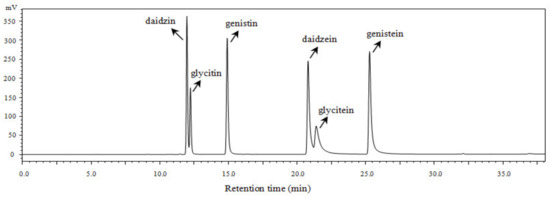
Figure 2.
High-performance liquid chromatographic (HPLC) chromatogram of the major six isoflavones presented in the non-fermented black soybeans (BS) and E. cristatum-fermented black soybeans (FBS).
Similar to TPC, it was revealed that the changes in solvent polarity differed greatly on the extraction capacities of isoflavones in the black soybean samples. Furthermore, it was interestingly found that the aglycone types significantly increased, whereas their glucoside types remarkably decreased during SSF with E. cristatum. For example, the contents of daidzein, glycitein, genistein, total aglycone isoflavones of the ethanol extract of FBS were 381.31, 60.34, 227.77, and 669.43 μg/g DW, respectively, which were about 4.40-, 6.13-, 4.07- and 4.39-fold greater, respectively, compared with those of unfermented samples. In contrast, the contents of glucosides types daidzin, glycitin, genistin, and total glucosides reduced by approximately 41.1%, 41.1%, 18.3%, and 30.0% after SSF with E. cristatum. This result elucidated that black soybean processed by SSF with E. cristatum promoted the conversion of glucosides isoflavone to aglycones forms. The biotransformation effect of glucoside isoflavones by SSF has been confirmed in several pieces of literature. Previous studies validated that the SSF process involved in the hydrolysis of the glycosidic bond of isoflavone glucosides into aglycone forms by the action of β-glucosidases [9,12]. Some microbes (such as T. matsutake, A. oryzae, and Saccharomyces cerevisiae) used in the processing of fermented soybean products have been reported that could secrete β-glucosidases, resulting in enhancement of aglycone isoflavones [9,10,53,54]. Furthermore, it has been demonstrated that E. cristatum could produce sufficient β-glucosidase during fermentation in some earlier studies [12,21]. Thus, the remarkable increase in aglycone isoflavones in the SSF of black soybeans by E. cristatum in the current study may be attributed to the activated β-glucosidases which involved in the conversion of isoflavone β-glucosides to aglycones. Aglycone types of isoflavone are proved to be easily absorbed by the human body and exert higher antioxidant, anticancer, and anti-atherosclerotic effects compared with their glucosides [9,52], greater aglycone isoflavones of fermented black soybean achieved by SSF with E. cristatum mycelia would enhance its biological activity. Herein, SSF of black soybean using E. cristatum was a useful strategy to increase isoflavone aglycones and thus enhance the bioactivity of black soybeans, which might be possibly used in the development of nutraceuticals and functional foods.
3.6. Effect of SSF on Antioxidant Activity
The antioxidant activities of BS and FBS were determined by the five most-used assays with discrepant reaction mechanisms, and the results are presented in Figure 3. All of these antioxidant assays are consistent with each other that FBS displayed stronger antioxidant activities than BS in spite of different solvents were used. For example, reducing power, radical scavenging activities against ABTS·+ and DPPH, and ferric reducing antioxidant power, the chelating ability of ethanol extract of unfermented black soybeans were estimated to be 976.97 µg VCE/g DW, 4497.85 µg VCE/g DW, 1770.25 µg VCE/g DW, 20.05 µmol Fe (II)/g DW, and 2.51 mg EDTA-2Na/g DW, respectively, which increased by approximately 76.1%, 15.9%, 33.4%, 70.4%, and 151.0%, respectively, after processing by SSF with E. cristatum. Up to now, several previous studies have confirmed that the promoting effects of antioxidant activity in the process of cereal or legume by SSF. Lee et al. [9] and Li et al. [13] proved that the antioxidant activity of legumes was distinctly augmented by SSF with T. matsutake or L. casei. The scavenging activities of DPPH radical and ABTS·+ of four cereal grains, namely, oat, brown rice, maize, and wheat, greatly improved by SSF with A. awamori, R. oryzae, or A. oryzae, as confirmed by Dey et al. [55]. It has been validated in earlier research that phenolic compounds displayed certain antioxidant properties of reducing power, chelating ability, and radicals scavenging [13,33]. Thus, the improved antioxidant capacity of FBS might be highly related to the accumulated phenolic contents by SSF with E. cristatum. Furthermore, to date, it is well elucidated in the literature that aglycone types of isoflavone (e.g., genistein, glytitein, daidzein) showed a greater antioxidant effect in comparison with their β-glucoside precursors (e.g., genistin, glycitin, daidzin). For example, it was noted that the improved antioxidant activity of fermented soybean is strongly connected with (r > 0.9) the considerably higher isoflavone aglycones content that accumulated during SSF, which was reported by Chen et al. [12]. Thus, the augmented antioxidant activity of FBS is likely ascribed to the great accumulation of isoflavone aglycones and TPC during SSF. Especially, Pearson’s correlation analysis was carried out to further clarify the relationships between antioxidant activities and TPC as well as isoflavone contents, and the results are listed in Table 6. As expected, it was revealed that highly (p < 0.05) positive correlation coefficients were observed between antioxidant activities and contents of isoflavone aglycones and TPC except for ABTS assay. For example, the correlation coefficients between TPC, isoflavone aglycones, and RP ranged from 0.933 to 0.993 (p < 0.01). Our findings are similar to those reported in several previously published data that the increased content of phenolics and isoflavone aglycones in fermented soybean products played a vital role in the improvement of antioxidant ability. Earlier literature has validated that the phenolics and isoflavone aglycones from soybean products are considered as potent antioxidant constituents, which antioxidant mechanism is capable of hydrogen donation and scavenging capacities of chain-breaking [51]. Hence, the augmentation of antioxidant activity of FBS is mainly attributed to the higher contents of phenolics and aglycones isoflavone (e.g., daidzein, genistein, and glycitein) that accumulated during fermentation with E. cristatum.
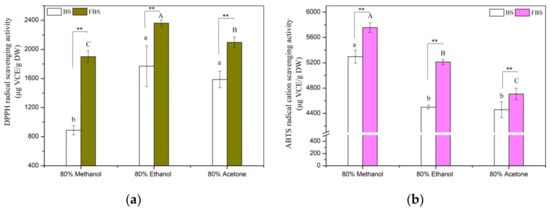
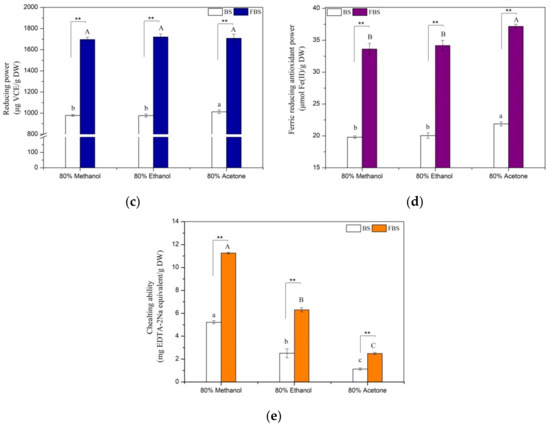
Figure 3.
Antioxidant activities of the various extracts from non-fermented black soybeans (BS) and E. cristatum-fermented black soybeans (FBS). (a) Scavenging effect of DPPH radical, (b) ABTS·+ scavenging effect, (c) ferric reducing antioxidant power (FRAP), (d) reducing power (RP), and (e) chelating ability. Values were represented as mean ± standard deviation of the triplicate assay. Values followed by different capital letters (A–C) or small letters (a–c) are remarkably different (p < 0.05) among the different solvent extracts of FBS or BS, respectively. The symbols (**) denoted a significant difference at a level of 0.01 between FBS and BS regards the same extracted solvent.

Table 6.
Pearson’s correlation coefficients among total phenolic content (TPC), isoflavones content, and antioxidant activity.
Figure 3 also displays that various polarities solvent extracts of BS or FBS differed significantly (p < 0.05) for their antioxidant activities. Earlier studies reported that antioxidant compounds extraction efficiency might be substantially influenced by the extraction solvent system. Changes in solvents polarity resulted in their different capacity to dissolve a selected class of antioxidant components and subsequently affected the antioxidant property. Albeit different solvent extracts exerted variant antioxidant activity, this effect was noted to be clearly associated with the contents of isoflavone aglycones and phenolics in the extracts. This statement was further supported by the correlation analysis and previous studies [12]. It is obvious noted from Table 6 that antioxidant activities were closely correlated with TPC and isoflavone aglycones contents. Discrepancies in antioxidant effect for the three varieties of solvent extracts from BS or FBS were likely ascribed to their different extraction capacity for phenolics and isoflavone aglycones. Thus, the variant levels of isoflavone aglycones and phenolics presented in the investigated extracts make a contribution to their discrepant antioxidant activity of the various extracts of BS or FBS. Additionally, it is worth mentioning that the results obtained from Figure 3 that methanol extract of BS or FBS showed the strongest CHA and ABTS·+ scavenging activity among the three test different solvent extracts, which did not coincide with their phenolics and isoflavone aglycones content. Previous research stated that the antioxidant potential of complex mixtures both depends on the antioxidant concentration and reactivity between reagents and the target compound [33]. Moreover, it has been reported that individual antioxidants can interact amongst themselves, which affected the antioxidant capacity of and creating synergistic, antagonistic, or even additive effects [32]. Furthermore, the presence of other bioactive compounds in the extract might also partly contribute to the observed results. Although different extraction solvents applied and the above-mentioned reasons might exist in this study, the result clearly revealed that all of the five antioxidant assays are in accordance with each other that FBS discovered greater antioxidant effects in comparison to their unfermented samples counterpart. In conclusion, we have confirmed that the antioxidant capacity of black soybeans was considerably enhanced through processing by SSF with E. cristatum.
Furthermore, a principal component analysis (PCA) model was created to obtain an insight of TPC and isoflavone variables that contributed to antioxidant activity, as well as to distinguish and classify fermented and non-fermented black soybeans extracted with diverse solvents, and the results are shown in Supplementary Figure S1. The first two validated variations of PC1 and PC2 were 76.06% and 18.64%, which explained 94.7% of the total variance. PCA loading plots (Figure S1A) revealed that DPPH, FRAP, RP, TPC, and aglycone isoflavones were similarly distributed and all have high loading on the first principal component (>0.8), suggesting a strong and positive correlation among DPPH, FRAP, RP, TPC, and aglycone isoflavones. CHA and ABTS distinctly separated with TPC and aglycone isoflavones; however, all of these parameters situated on the right side of the diagram, implying that TPC and aglycone isoflavones also contributed to the CHA and ABTS of black soybean samples, but these antioxidant effects mainly determined by other classes of compounds despite the fact that these compounds are generally known to be potent antioxidants. However, further studies are needed to verify this hypothesis. Some earlier studies also reported a moderate or negative correlation between phenolics and CHA [56]. Meanwhile, it was notably revealed that glucoside isoflavones (i.e., genistin, daidzin, glycitin) were inversely displayed with aglycone isoflavones (i.e., genistein, daidzein, glycitein) according to the direction of the variables, which suggested that this isoflavone-conjugated glucoside forms were hydrolyzed and subsequently leading to the releasing of aglycone types during fermentation. The PCA score plot of Supplementary Figure S1B illustrated that all the different polarities extracts of FBS distributed on the positive side of the first axis, which can be interpreted that these samples possessing great antioxidant activity, the content of phenolics and aglycone isoflavones. Furthermore, it also should be noted that discrepant polarities solvents of FBS or BS also clearly separated from each other in the diagram of the PCA score plot, which implied that different polarities solvents influenced the extraction capacity of bioactive compounds from FBS or BS and resulted in the variation of antioxidant activity. From the data obtained, PCA could be helpful to discriminate and classify fermented and non-fermented black soybean extracted with diverse solvents and provide the relationships between antioxidant capacity, TPC, and isoflavones.
3.7. Effect of SSF on Volatile Organic Compounds (VOCs)
The flavor is one of the most important characteristics of fermented products for consumers. Volatile compound changes of black soybeans during fermentation with E. cristatum were performed with HS-GC-IMS. Figure 4A shows the data of BS and FBS illustrated by the three-dimensional (3D) topographical visualization. It was noted that the VOCs of BS and FBS were similar, whereas some of the peak signal intensities were different between BS and FBS, which suggested that some of the certain VOCs changed during fermentation. Considering different kinds of VOCs were difficult to be detected by the 3D spectra; thus, a GC-IMS 2D spectrum obtained from the overhead view of the 3D-topographic plot was performed for further comparison, and the result is depicted in Supplementary Figure S2.
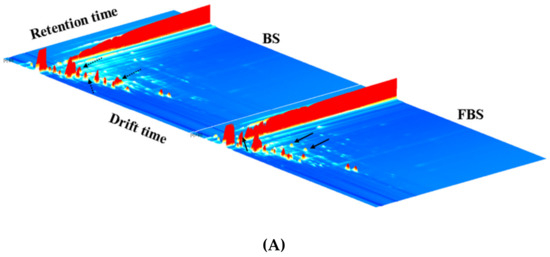
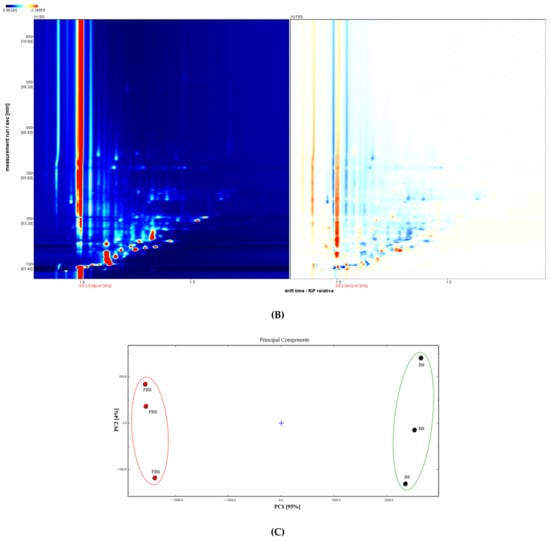
Figure 4.
(A) Representative three-dimensional topographical plot of volatile fingerprints in non-fermented black soybeans (BS) and E. cristatum-fermented black soybeans (FBS). (B) Changes volatile compounds in the two-dimensional (2D)-topographic of black soybeans during solid-state fermentation with E. cristatum. (C) Principal component analysis (PCA) of volatile organic compounds based on the signal intensity obtained with non-fermented black soybeans (BS) and E. cristatum-fermented black soybeans (FBS).
The result normalized the position of the ion migration time and the reactive ion peak (RIP), and all the headspace compounds of BS and FBS could be reflected in the whole 2D spectrum [57]. The signal intensity of the VOCs could be indicated by the color. The darker the color, the stronger the intensity [57]. White implies a lower intensity, whereas red indicates a higher intensity. Furthermore, differential comparison mode was also performed to illustrate the different VOCs between BS and FBS, as marked in Figure 4B. The spectrum of FBS was obtained by deducting the control spectral background (BS was used as a reference), in which the blue dot area suggested that the content was lower in comparison to that of the control, whereas the red dot area indicated the content was higher compared with that of the control. It was obviously observed that the content of VOCs of black soybeans notably changed by fermentation, which could also be supported by the PCA result (Figure 4C) that established by the VOCs signal intensity. It was observed that BS and FBS were well separated in the distribution plot, implying that the VOCs of BS and FBS exhibited substantially different. This methodology had been widely used in the food research field, such as identification and classification analysis of different fruit flavor compounds, fruit processing, fermentation of fruit and vegetable, as well as the other aspects [58,59]. The results revealed that some flavor compounds varied significantly throughout the fermentation processes, and others did not show distinct changes. SSF of black soybean with E. cristatum remarkably contributed to the alteration of VOCs, which might be indispensable for the unique flavor of the produced fermented products.
Furthermore, the compounds were characterized based on a comparison of drift time (RIP relative) and retention index (RI). A total of 66 volatile substances were observed in FBS and BS. Among them, 56 VOCs were successfully identified from the GC-IMS library database, as displayed in Table 7 and revealed in Supplementary Figure S2. It was noted that some of the certain compounds had produced various signals or spots (dimer or monomer) as a result of their different concentrations, similar to a previous report [57]. The identified VOCs belonged to several chemical families; specifically, there were 19 aldehydes, 11 alcohols, 10 esters, 3 hydrocarbons, 6 ketones, 2 acids, 3 heterocyclics, 1 phenol, and 1 terpenoid (Table 7). This result coincides with previous studies that reported that aldehydes, alcohols, and esters were the main volatile compounds for the bean products [7,60]. However, we found that the concentrations of these main VOCs were greatly influenced by fermentation with E. cristatum. Among them, it could be seen from Table 7 that the intensities of alcohols (3-methylsulfanylpropanol, ethanol-M), aldehydes (hexanal-M, hexanal-D, acetal, pentanal-M, pentanal-D, Methylpropanal), esters (butyl propanoate, propyl butanoate-M, butyl acetate-M, methyl 2-methylbutanoate-M, ethyl propanoate) significantly increased by fermentation. Compared with those detected in BS, the intensities of seven alcohols, nine aldehydes, four ketones remarkably decreased. The findings demonstrated that FBS displayed accepted flavor compounds, and E. cristatum used for fermentation of black soybeans achieved higher levels of esters. Microorganisms used for fermentation led to an increase in ester levels has also been observed by other researchers. Esters are generally considered as one of the most important volatile compounds that contributing to the flavor of food products [61]. Previous studies have verified that the esterification of alcohol is one of the sources of esters. Our present study revealed in Table 7 that most alcohols significantly decreased whereas esters greatly increased might possibility due to the esterification of alcohols during SSF. Some esters might be synthesized by microorganisms under the action of acetyltransferase that could use alcohol as the substrate [62]. GC-IMS fingerprint profiles of VOCs were analyzed to more directly clarify the different substances of BS and FBS, and the result is presented in Figure 5. All the signal peaks of each sample displayed in each row while each column indicated the same compounds in all the studied samples, and the color of each square suggested the content of VOCs. It was noted that the volatile compounds of BS and FBS are greatly different. With respect to other volatile constituents, it was found that only one phenol (maltol), two acids (pentanoic acid, heptanoic acid), three heterocyclics (2-pentylfuran, trimethylpyrazine, 2-ethylfuran) were detected from BS and FBS, and minor differences for these volatile constituents were recorded during fermentation. However, the content of limonene in FBS was at a low level, suggesting that fermentation facilitated its metabolism or transformation. These results confirmed that SSF with E. cristatum greatly influenced the volatile organic compounds of black soybeans. Thus, the GC-IMS data from the current study provided useful information and could be considered as an effective technique for the characterization of aroma profile and characteristic volatile organic compounds during legume fermentation. All of these obtained data would support and provide novel insights into the development of new fermented black soybean products in the future. Additionally, as can be seen from Figure 5, some VOCs in BS and FBS have not yet been identified but varied greatly, such as “1”, “2”, etc., which would be studied in our group in the future.

Table 7.
GC-IMS integration parameters of volatile compounds detected in E. cristatum-fermented and non-fermented black soybeans by GC-IMS.

Figure 5.
Gallery plot of volatile organic compounds (VOCs) fingerprints of non-fermented black soybeans (BS) and E. cristatum-fermented black soybeans (FBS).
4. Conclusions
The present work has studied the nutritional components (proximate composition, amino acids, minerals, and fatty acids), total phenolics, isoflavones, and antioxidant effects of fermented black soybeans using E. cristatum. Moreover, reliable information regards with the VOC changes during black soybean fermentation was firstly investigated in this work. The research showed that the amount of crude protein, total amino acids, most essential amino acids, total phenolics, and aglycone isoflavones of black soybean increased obviously after fermentation with E. cristatum. Furthermore, there is a clear indication that the antioxidant activities of black soybeans were considerably enhanced by SSF with E. cristatum, which were highly positive correlated with the augmentation of total phenolics and aglycone isoflavones. Furthermore, SSF significantly (p < 0.05) increased most of the minerals, including calcium, phosphorus, and magnesium, whereas decreased greatly decreased (by 21.1%) the potassium content of black soybean. SSF slightly influenced the fatty acids composition; however, the relative content of oleic acid significantly increased, and palmitic acid (16:0) significantly decreased by fermentation with E. cristatum, and FBS exhibited greater unsaturated fatty acids than BS. A total of 66 volatile components were observed in FBS and BS, of which 56 VOCs were successfully identified by GC-IMS analysis. Among them, aldehydes, alcohols, and esters were the main VOCs, but their concentrations were distinctly influenced by fermentation with E. cristatum. The findings revealed that E. cristatum used for fermentation of black soybeans achieved higher levels of esters (butyl propanoate, propyl butanoate-M, butyl acetate-M, methyl 2-methylbutanoate-M, ethyl propanoate). Collectively, data presented herein suggest that SSF with E. cristatum was an efficient and innovative processing strategy to considerably improve the nutritional value and bioactivity of black soybeans. From the overall obtained results, we believe that the black soybean processed by SSF using E. cristatum might have great potential to be applied in the production of nutraceuticals and functional foods. Fermented black soybean used as a dietary adjunct for the treatment and prevention of lifestyle-related oxidative diseases such as atherosclerosis, arthritis, and cancer might be recommended and thus was considered of great potential for the food and pharmaceutical industry. In addition, fermented black soybeans could be used as an ingredient in the design and development of various cereal- and legume-related food products (e.g., biscuits and bread). Furthermore, investigation of this fermented black soybean is indispensable to estimate its potential health beneficial effects by in vivo studies to develop functional foods.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agronomy11061029/s1. Figure S1. (A) Loading plot of principal component analysis (PCA) of total phenolic content (TPC), isoflavones content, and antioxidant activity. (B) Score plot of principal component analysis (PCA) of discrepant solvent extracts of non-fermented black soybean and E. cristatum-fermented black soybean. -M, -E, and -A indicate non-fermented black soybean (BS) and E. cristatum-fermented black soybean (FBS) were extracted with 80% methanol, 80% ethanol, and 80% acetone, respectively. Figure S2. Topographic plots of GC-IMS spectra with the 56 selected markers obtained from non-fermented black soybean (A) and E. cristatum-fermented black soybean (B).
Author Contributions
Project administration, data curation, validation, and formal analysis, Y.X.; methodology, Y.X., Y.H., and C.H.; writing—original draft preparation, Y.X. and Y.C.; writing—review and editing, Y.X. and Y.W.; investigation, Y.X., Y.H., R.C., Z.F., and Y.C.; supervision, Y.X. and Y.W.; Resources, Y.X., Z.L., and Y.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by Hunan Province Natural Science Foundation (No. 2020JJ5243), Excellent Youth Foundation of Education Department of Hunan Province (No. 19B269), and Natural Science Youth Foundation of Hunan Agricultural University (No. 19QN22).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the Hunan Agricultural University Research Startup Fund (20654-540741900399), the project of Key Research Technologies and Metabolomic studies of Traditional Fermented Food Manufacturing, Hunan Province Natural Science Foundation (No. 2020JJ5233), and Excellent Postdoctoral Innovative Talent Fund of Hunan Province (No. 2020RC2055).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bouchenak, M.; Lamri-Senhadji, M. Nutritional quality of legumes, and their role in cardiometabolic risk prevention: A review. J. Med. Food 2013, 16, 185–198. [Google Scholar] [CrossRef]
- Lee, K.J.; Baek, D.Y.; Lee, G.A.; Cho, G.T.; So, Y.S.; Lee, J.R.; Ma, K.H.; Chung, J.W.; Hyun, D.Y. Phytochemicals and antioxidant activity of Korean black soybean (Glycine max L.) landraces. Antioxidants 2020, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Chawla, P.; Bhandari, L.; Sadh, P.K.; Kaushik, R. Impact of solid-state fermentation (Aspergillus oryzae) on functional properties and mineral bioavailability of black-eyed pea (Vigna unguiculata) seed flour. Cereal Chem. 2017, 94, 437–442. [Google Scholar] [CrossRef]
- Marathe, S.A.; Rajalakshmi, V.; Jamdar, S.N.; Sharma, A. Comparative study on antioxidant activity of different varieties of commonly consumed legumes in India. Food Chem. Toxicol. 2011, 49, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cho, K.M. Changes occurring in compositional components of black soybeans maintained at room temperature for different storage periods. Food Chem. 2012, 131, 161–169. [Google Scholar] [CrossRef]
- Preedy, V.R.; Watson, R.R. (Eds.) Black soybean seed: Black soybean seed antioxidant capacity. In Nuts and Seeds in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2020; pp. 147–159. [Google Scholar]
- Jeong, D.W.; Heo, S.; Lee, B.; Lee, H.; Jeong, K.; Her, J.Y.; Lee, K.G.; Lee, J.H. Effects of the predominant bacteria from meju and doenjang on the production of volatile compounds during soybean fermentation. Int. J. Food Microbiol. 2017, 262, 8–13. [Google Scholar] [CrossRef]
- Dai, C.; Ma, H.; He, R.; Huang, L.; Zhu, S.; Ding, Q.; Luo, L. Improvement of nutritional value and bioactivity of soybean meal by solid-state fermentation with Bacillus subtilis. LWT 2017, 86, 1–7. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, C.E.; Son, K.S.; Cho, K.M. Comparisons of nutritional constituents in soybeans during solid state fermentation times and screening for their glucosidase enzymes and antioxidant properties. Food Chem. 2019, 272, 362–371. [Google Scholar] [CrossRef]
- Santos, V.A.Q.; Nascimento, C.G.; Schmidt, C.A.; Mantovani, D.; Dekker, R.F.; da Cunha, M.A.A. Solid-state fermentation of soybean okara: Isoflavones biotransformation, antioxidant activity and enhancement of nutritional quality. LWT 2018, 92, 509–515. [Google Scholar] [CrossRef]
- Dey, T.B.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: A review. Trends Food Sci. Tech. 2016, 53, 60–74. [Google Scholar]
- Chen, Y.; Wang, Y.; Chen, J.; Tang, H.; Wang, C.; Li, Z.; Xiao, Y. Bioprocessing of soybeans (Glycine max L.) by solid-state fermentation with Eurotium cristatum YL-1 improves total phenolic content, isoflavone aglycones, and antioxidant activity. RSC Adv. 2020, 10, 16928–16941. [Google Scholar] [CrossRef]
- Li, S.; Jin, Z.; Hu, D.; Yang, W.; Yan, Y.; Nie, X.; Lin, J.; Zhang, Q.; Gai, D.; Ji, Y.; et al. Effect of solid-state fermentation with Lactobacillus casei on the nutritional value, isoflavones, phenolic acids and antioxidant activity of whole soybean flour. LWT 2020, 153, 109264. [Google Scholar] [CrossRef]
- Vong, W.C.; Hua, X.Y.; Liu, S.Q. Solid-state fermentation with Rhizopus oligosporus and Yarrowia lipolytica improved nutritional and flavour properties of okara. LWT 2018, 90, 316–322. [Google Scholar] [CrossRef]
- Shi, J.; Liu, J.; Kang, D.; Huang, Y.; Kong, W.; Xiang, Y.; Zhu, X.; Duan, Y.; Huang, Y. Isolation and characterization of benzaldehyde derivatives with anti-inflammatory activities from Eurotium cristatum, the dominant fungi species in fuzhuan brick tea. ACS Omega 2019, 4, 6630–6636. [Google Scholar] [CrossRef]
- Kang, D.; Su, M.; Duan, Y.; Huang, Y. Eurotium cristatum, a potential probiotic fungus from Fuzhuan brick tea, alleviated obesity in mice by modulating gut microbiota. Food Funct. 2019, 10, 5032–5045. [Google Scholar] [CrossRef]
- Liu, G.; Duan, Z.; Wang, P.; Fan, D.; Zhu, C. Purification, characterization, and hypoglycemic properties of eurocristatine from Eurotium cristatum spores in Fuzhuan brick tea. RSC Adv. 2020, 10, 22234–22241. [Google Scholar] [CrossRef]
- Li, Q.; Huang, J.; Li, Y.; Zhang, Y.; Luo, Y.; Chen, Y.; Lin, H.; Wang, K.; Liu, Z. Fungal community succession and major components change during manufacturing process of Fu brick tea. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.Z.; Li, N.; Zhou, F.; Ouyang, J.; Lu, D.M.; Xu, W.; Li, J.; Lin, H.; Zhang, Z.; Xiao, J.B.; et al. Microbial bioconversion of the chemical components in dark tea. Food Chem. 2020, 312, 126043. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Wang, Q.; Yang, X. Fu Brick Tea alleviates chronic kidney disease of rats with high fat diet consumption through attenuating insulin resistance in skeletal muscle. J. Agric. Food Chem. 2019, 67, 2839–2847. [Google Scholar] [CrossRef]
- Gu, Q.; Duan, G.; Yu, X. Bioconversion of flavonoid glycosides from Hippophae rhamnoides leaves into flavonoid aglycones by Eurotium amstelodami. Microorganisms 2019, 7, 122. [Google Scholar] [CrossRef]
- Jiang, C.; Zeng, Z.; Huang, Y.; Zhang, X. Chemical compositions of Pu’er tea fermented by Eurotium Cristatum and their lipid-lowering activity. LWT 2018, 98, 204–211. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, M.; Huang, Y.; Li, C.; Pan, X.; Zhu, W.; Huang, Y. Appropriately raising fermentation temperature beneficial to the increase of antioxidant activity and gallic acid content in Eurotium cristatum-fermented loose tea. LWT Food Sci. Technol. 2017, 82, 248–254. [Google Scholar] [CrossRef]
- Xiao, Y.; Wu, X.; Yao, X.; Chen, Y.; Ho, C.T.; He, C.; Li, Z.; Wang, Y. Metabolite profiling, antioxidant and α-glucosidase inhibitory activities of buckwheat processed by solid-state fermentation with Eurotium cristatum YL-1. Food Res. Int. 2021, 143, 110262. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Guo, X.; Huang, Y.; Cao, F.; Su, E.; Wang, J. Improvement of the quality of Ginkgo biloba leaves fermented by Eurotium cristatum as high value-added feed. Processes 2019, 7, 627. [Google Scholar] [CrossRef]
- AOAC. Official Method of Analysis, 18th ed.; Association of Officiating Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- Xiao, Y.; Sun, M.; Zhang, Q.; Chen, Y.; Miao, J.; Rui, X.; Dong, M. Effects of Cordyceps militaris (L.) Fr. fermentation on the nutritional, physicochemical, functional properties and angiotensin I converting enzyme inhibitory activity of red bean (Phaseolus angularis [Willd.] WF Wight.) flour. J. Food Sci. Technol. 2018, 55, 1244–1255. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Xu, Y.F.; Gao, X.D.; Zhu, X.Y.; Du, M.Z.; Wang, Y.X.; Deng, R.X.; Gao, J.Y. Optimization of ultrasonic-assisted extraction of oil from the seed kernels and isolation of monoterpene glycosides from the oil residue of Paeonia lactiflora Pall. Ind. Crop. Prod. 2017, 107, 260–270. [Google Scholar] [CrossRef]
- Qi, Q.; Li, Y.; Xing, G.; Guo, J.; Guo, X. Fertility variation among Paeonia lactiflora genotypes and fatty acid composition of seed oil. Ind. Crop. Prod. 2020, 152, 112540. [Google Scholar] [CrossRef]
- Xiao, Y.; Xing, G.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enhancement of the antioxidant capacity of chickpeas by solid state fermentation with Cordyceps militaris SN-18. J. Funct. Foods 2014, 10, 210–222. [Google Scholar] [CrossRef]
- Chen, L.; Teng, H.; Xiao, J. A value-added cooking process to improve the quality of soybean: Protecting its isoflavones and antioxidant activity. Food Sci. Hum. Well. 2019, 8, 195–201. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Wang, Y.; Chen, J.; Huang, Y.; Yan, Y.; Li, L.; Li, Z.; Ren, Y.; Xiao, Y. Total phenolics, capsaicinoids, antioxidant activity, and α-glucosidase inhibitory activity of three varieties of pepper seeds. Int. J. Food Prop. 2020, 23, 1016–1035. [Google Scholar] [CrossRef]
- Xiao, Y.; Rui, X.; Xing, G.; Wu, H.; Li, W.; Chen, X.; Dong, M. Solid state fermentation with Cordyceps militaris SN-18 enhanced antioxidant capacity and DNA damage protective effect of oats (Avena sativa L.). J. Funct. Foods 2015, 16, 58–73. [Google Scholar] [CrossRef]
- Lv, W.; Lin, T.; Ren, Z.; Jiang, Y.; Zhang, J.; Bi, F.; Gu, L.; Hou, H.; He, J. Rapid discrimination of Citrus reticulata “Chachi” by headspace-gas chromatography-ion mobility spectrometry fingerprints combined with principal component analysis. Food Res. Int. 2020, 131, 108985. [Google Scholar] [CrossRef]
- Mohapatra, D.; Patel, A.S.; Kar, A.; Deshpande, S.S.; Tripathi, M.K. Effect of different processing conditions on proximate composition, anti-oxidants, anti-nutrients and amino acid profile of grain sorghum. Food Chem. 2019, 271, 129–135. [Google Scholar] [CrossRef]
- Abu, O.A.; Tewe, O.O.; Losel, D.M.; Onifade, A.A. Changes in lipid, fatty acids and protein composition of sweet potato (Ipomoea batatas) after solid-state fungal fermentation. Bioresour. Technol. 2000, 72, 189–192. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. The importance of minerals in human nutrition: Bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci. Tech. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Dhull, S.B.; Punia, S.; Kidwai, M.K.; Kaur, M.; Chawla, P.; Purewal, S.S.; Sangwan, M.; Palthania, S. Solid-state fermentation of lentil (Lens culinaris L.) with Aspergillus awamori: Effect on phenolic compounds, mineral content, and their bioavailability. Legume Sci. 2020, 2, e37. [Google Scholar] [CrossRef]
- De Almeida Chuffa, L.G.; Vieira, F.R.; da Silva, D.A.F.; Franco, D.M. Soybean Seed Oil: Nutritional Composition, Healthy Benefits and Commercial Applications. Seed Oil; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2014; pp. 1–24. [Google Scholar]
- Feng, Y.; Chen, Z.; Liu, N.; Zhao, H.; Cui, C.; Zhao, M. Changes in fatty acid composition and lipid profile during koji fermentation and their relationships with soy sauce flavour. Food Chem. 2014, 158, 438–444. [Google Scholar] [CrossRef]
- Shibata, M.; Takayama, K.; Ujiie, A.; Yamada, T.; Abe, J.; Kitamura, K. Genetic relationship between lipid content and linolenic acid concentration in soybean seeds. Breed. Sci. 2008, 58, 361–366. [Google Scholar] [CrossRef][Green Version]
- Wang, H.L.; Swain, E.W.; Wallen, L.L.; Hesseltine, C.W. Free fatty acids identified as antitryptic factor in soybeans fermented by Rhizopus oligosporus. J. Nutr. 1975, 105, 1351–1355. [Google Scholar] [CrossRef]
- Dos Santos Oliveira, M.; Feddern, V.; Kupski, L.; Cipolatti, E.P.; Badiale-Furlong, E.; de Souza-Soares, L.A. Changes in lipid, fatty acids and phospholipids composition of whole rice bran after solid-state fungal fermentation. Bioresour. Technol. 2011, 102, 8335–8338. [Google Scholar] [CrossRef]
- Da Silveira, C.M.; Oliveira, M.D.S.; Badiale-Furlong, E. Lipid content and fatty acid profile of defatted rice bran and wheat bran submitted to solid state fermentation by Aspergillus oryzae. Bol. Cent. Pesqui. Process. Aliment. 2010, 28, 133–140. [Google Scholar]
- Shi, H.; Nam, P.; Ma, A. Comprehensive profiling of isoflavones, phytosterols, tocopherols, minerals, crude protein, lipid, and sugar during soybean (Glycine max) germination. J. Agric. Food Chem. 2010, 58, 4970–4976. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, S.R.; Lee, Y.H.; Kim, K.; Cho, K.M.; Lee, Y.B. Changes occurring in compositions and antioxidant properties of healthy soybean seeds [Glycine max (L.) Merr.] and soybean seeds diseased by Phomopsis longicolla and Cercospora kikuchii fungal pathogens. Food Chem. 2015, 185, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Dong, J.; Lu, J.; Chen, J.; Li, Y.; Shan, L.; Lin, Y.; Fan, W.; Gu, G. Effects of extraction solvent mixtures on antioxidant activity evaluation and their extraction capacity and selectivity for free phenolic compounds in barley (Hordeum vulgare L.). J. Agric. Food Chem. 2006, 54, 7277–7286. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Bei, Q.; Chen, G.; Lu, F.; Wu, S.; Wu, Z. Enzymatic action mechanism of phenolic mobilization in oats (Avena sativa L.) during solid-state fermentation with Monascus anka. Food Chem. 2018, 245, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Izumi, T.; Piskula, M.K.; Osawa, S.; Obata, A.; Tobe, K.; Saito, M.; Kataoka, S.; Kubota, Y.; Kikuchi, M. Soy isoflavone aglycones are absorbed faster and in higher amounts than their glucosides in humans. J. Nutr. 2000, 130, 1695–1699. [Google Scholar] [CrossRef]
- Handa, C.L.; de Lima, F.S.; Guelfi, M.F.G.; da Silva Fernandes, M.; Georgetti, S.R.; Ida, E.I. Parameters of the fermentation of soybean flour by Monascus purpureus or Aspergillus oryzae on the production of bioactive compounds and antioxidant activity. Food Chem. 2019, 271, 274–283. [Google Scholar] [CrossRef]
- Hsiao, Y.H.; Ho, C.T.; Pan, M.H. Bioavailability and health benefits of major isoflavone aglycones and their metabolites. J. Funct. Foods 2020, 74, 104164. [Google Scholar] [CrossRef]
- Da Silva, L.H.; Celeghini, R.M.; Chang, Y.K. Effect of the fermentation of whole soybean flour on the conversion of isoflavones from glycosides to aglycones. Food Chem. 2011, 128, 640–644. [Google Scholar] [CrossRef]
- Handa, C.L.; Couto, U.R.; Vicensoti, A.H.; Georgetti, S.R.; Ida, E.I. Optimisation of soy flour fermentation parameters to produce β-glucosidase for bioconversion into aglycones. Food Chem. 2014, 152, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Dey, T.B.; Kuhad, R.C. Upgrading the antioxidant potential of cereals by their fungal fermentation under solid-state cultivation conditions. Let. App. Microbiol. 2014, 59, 493–499. [Google Scholar]
- Chakraborty, K.; Praveen, N.K.; Vijayan, K.K.; Rao, G.S. Evaluation of phenolic contents and antioxidant activities of brown seaweeds belonging to Turbinaria spp. (Phaeophyta, Sargassaceae) collected from Gulf of Mannar. Asian Pac. J. Trop. Bio. 2013, 3, 8–16. [Google Scholar] [CrossRef]
- Han, X.; Peng, Q.; Yang, H.; Hu, B.; Shen, C.; Tian, R. Influence of different carbohydrate sources on physicochemical properties and metabolites of fermented greengage (Prunus mume) wines. LWT 2020, 121, 108929. [Google Scholar] [CrossRef]
- Yang, K.M.; Chiang, P.Y. Effects of smoking process on the aroma characteristics and sensory qualities of dried longan. Food Chem. 2019, 287, 133–138. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, J.; Li, X.; Pan, J.; Xue, S.J.; Liu, D.; Ye, X. Differentiation of the volatile profiles of Chinese bayberry cultivars during storage by HS-SPME-GC/MS combined with principal component analysis. Postharvest Biol. Tec. 2015, 100, 59–72. [Google Scholar] [CrossRef]
- Bi, S.; Wang, A.; Lao, F.; Shen, Q.; Liao, X.; Zhang, P.; Wu, J. Effects of frying, roasting and boiling on aroma profiles of adzuki beans (Vigna angularis) and potential of adzuki bean and millet flours to improve flavor and sensory characteristics of biscuits. Food Chem. 2020, 339, 127878. [Google Scholar] [CrossRef]
- Fan, W.; Qian, M.C. Characterization of aroma compounds of Chinese “Wuliangye” and “Jiannanchun” liquors by aroma extract dilution analysis. J. Agric. Food Chem. 2006, 54, 2695–2704. [Google Scholar] [CrossRef]
- Mo, X.; Fan, W.; Xu, Y. Changes in volatile compounds of Chinese rice wine wheat Qu during fermentation and storage. J. Inst. Brew. 2009, 115, 300–307. (In Chinese) [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).