Nonadditive Effects on Decomposition of a Mixture of Rice Straw and Groundnut Stover Applied to a Sandy Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil and Plant Residues
2.2. Incubation Experiment
2.3. Analyses of Soil and Plant Residues
2.4. Calculations and Statistical Analysis
3. Results
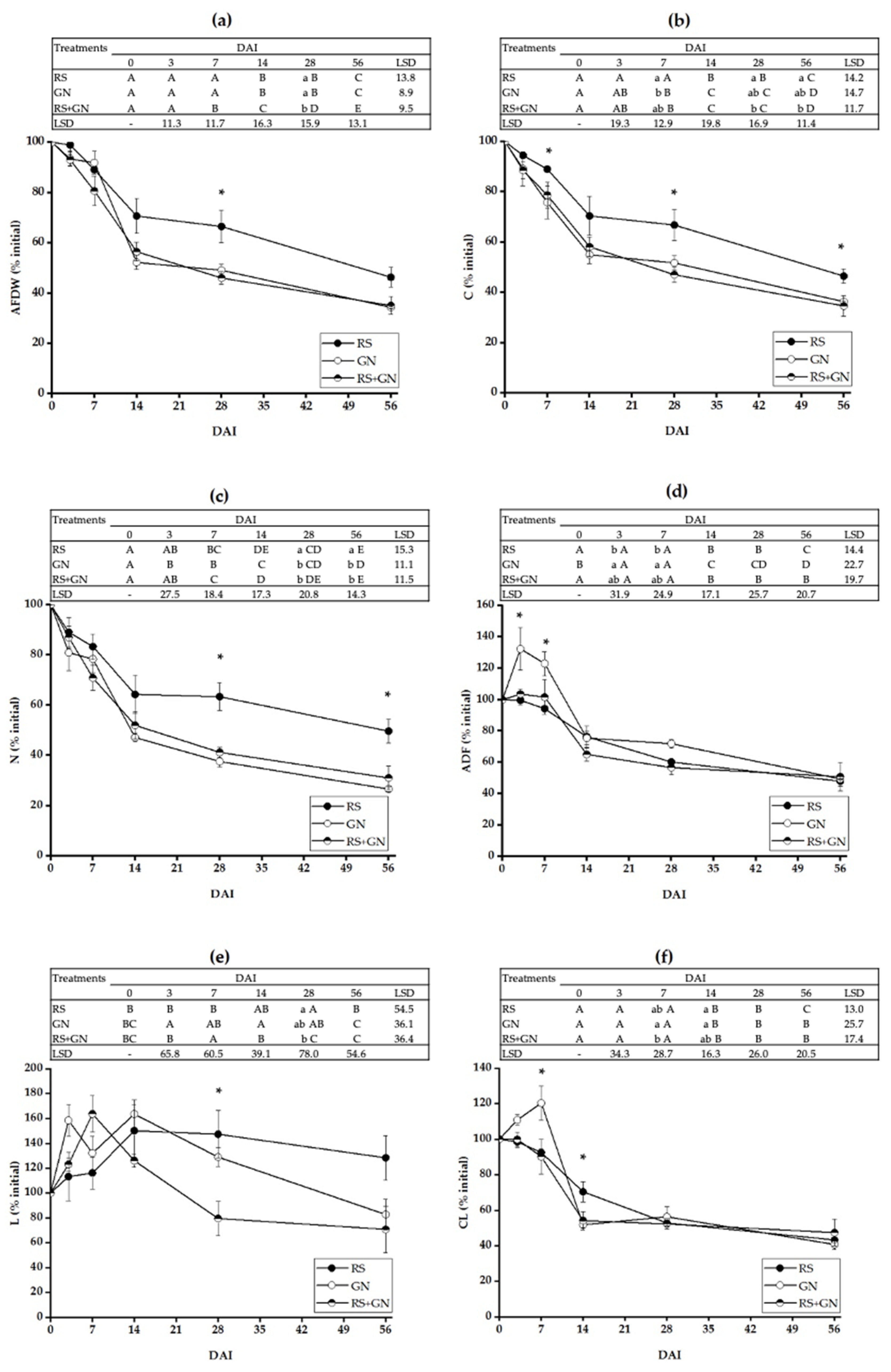
3.1. Decomposition Dynamics of Single versus Mixed Residues
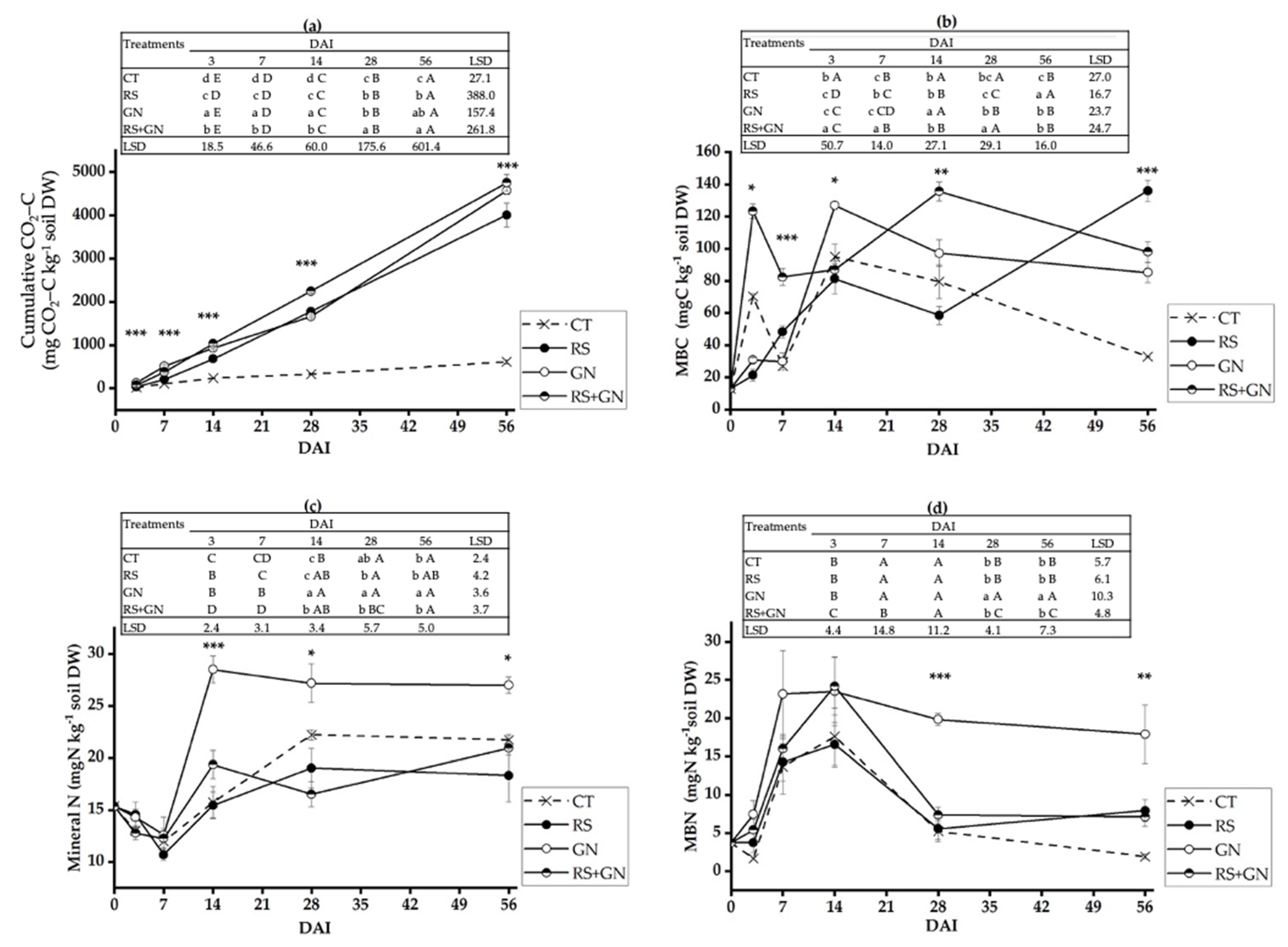
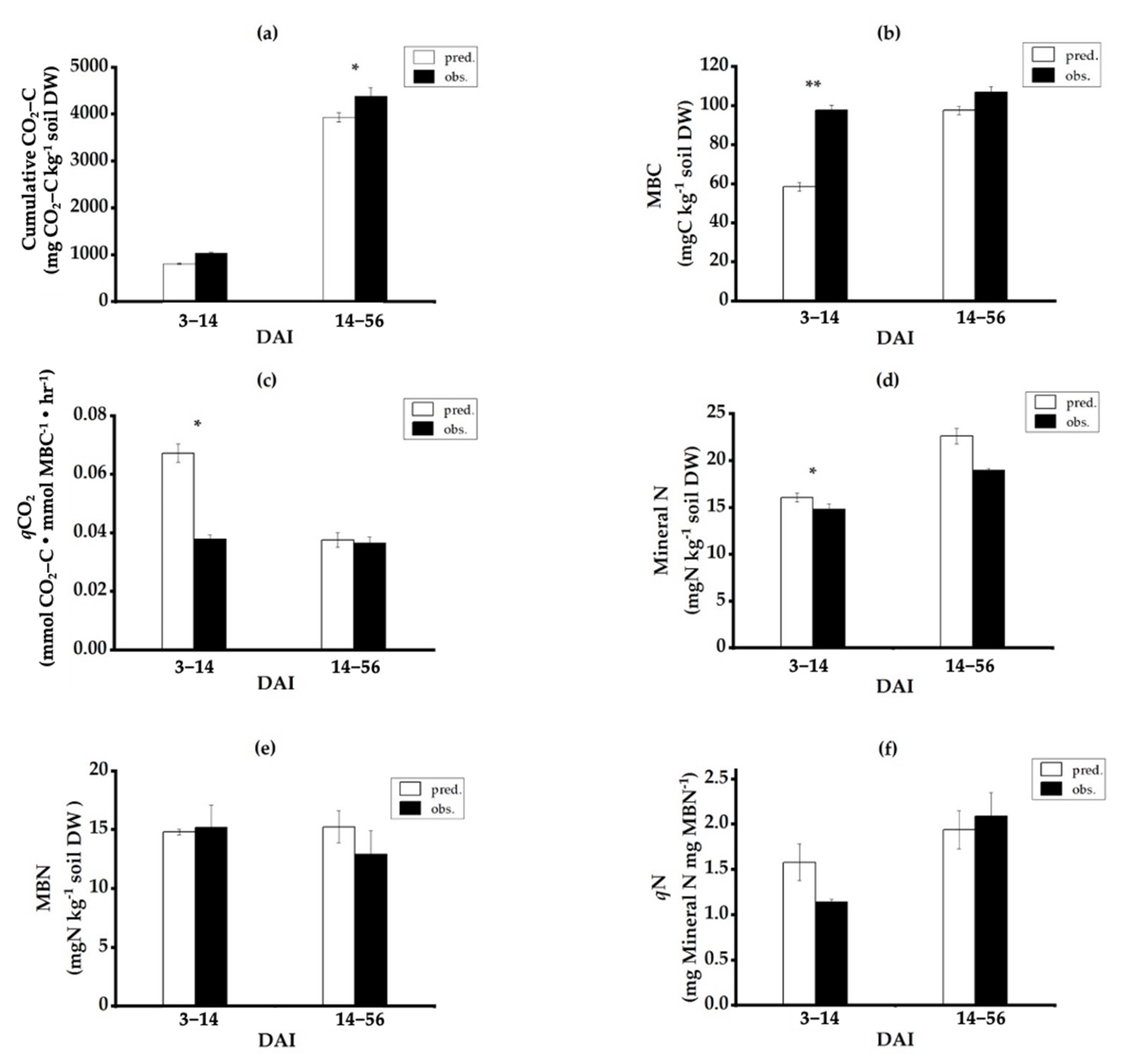
3.2. Dynamics of Soil Microbiological Processes with the Application of Single versus Mixed Residues
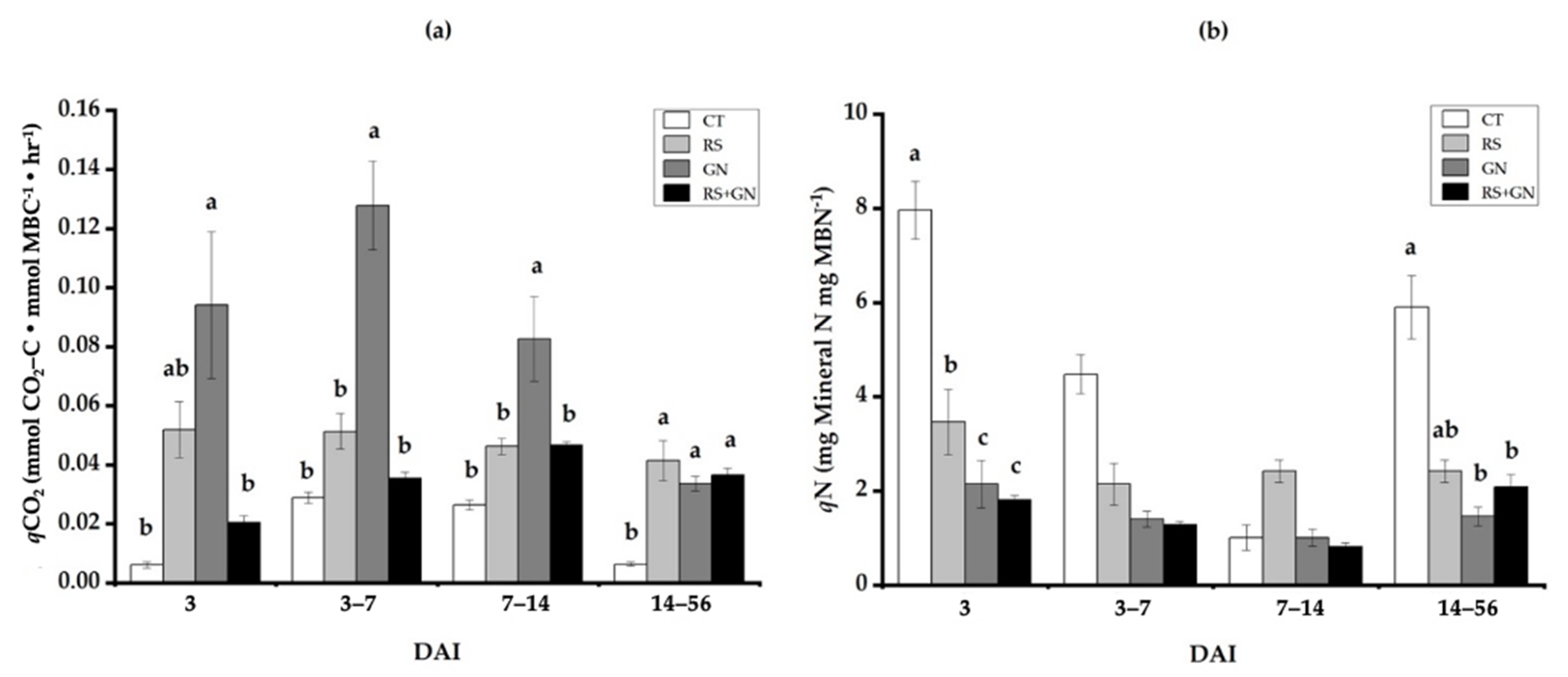
3.3. Dynamics of Microbial Metabolic and Microbial N Quotients under Single versus Mixed Residues
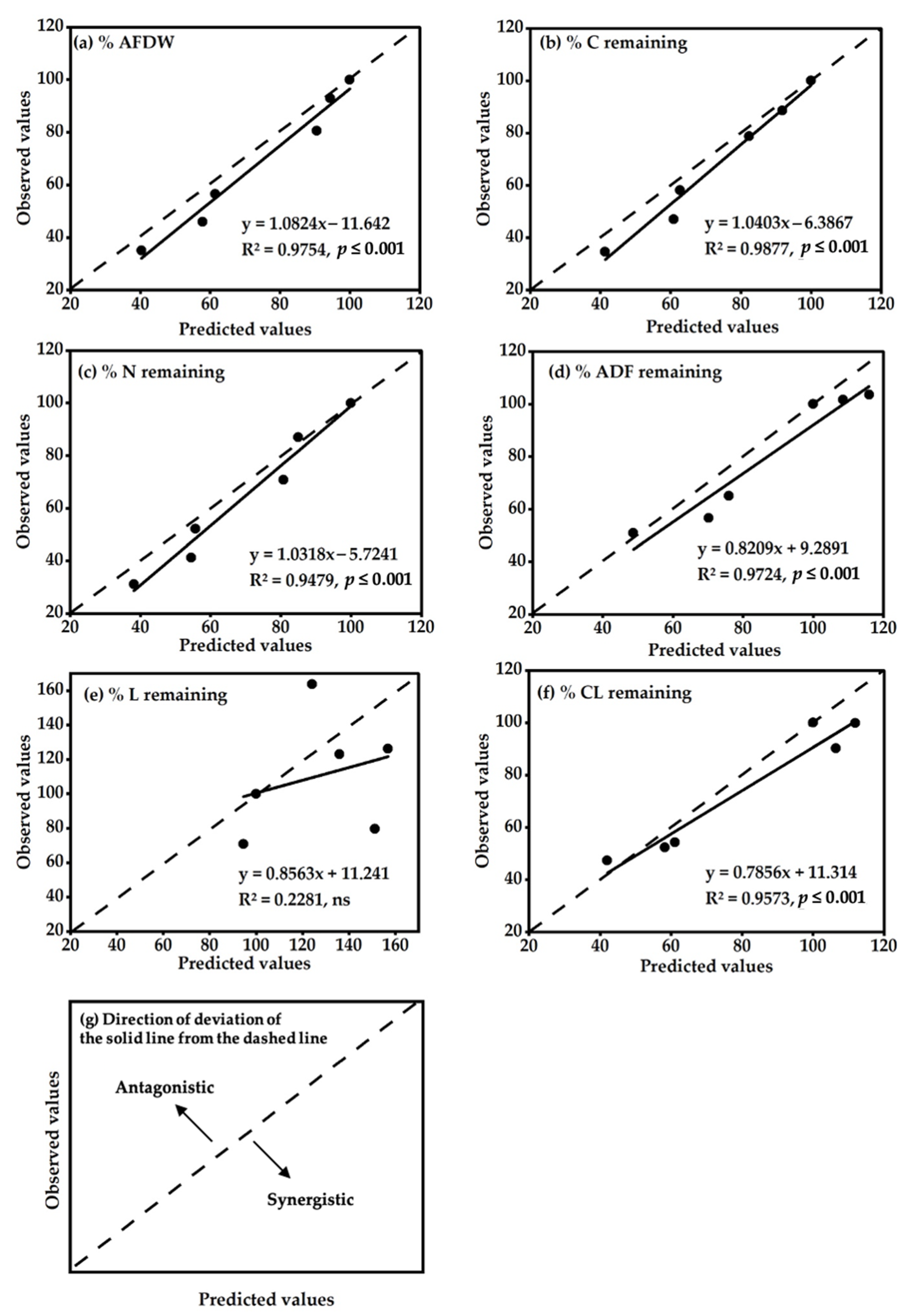
3.4. Nonadditive Effects of Mixed-Residue Decomposition Processes
4. Discussion
4.1. Decomposition of the Mixed Residues Is Altered as Compared with Single Residues due to Enhanced Availability of C and N in the Mixed Residues
4.2. Use of qCO2 and qN as Indicators of Microbial SUE, Which Is the Main Process That Alters Decomposition of Mixed Residues as Compared with Single Residues
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kunlanit, B.; Butnan, S.; Vityakon, P. Land-use changes influencing C sequestration and quality in topsoil and subsoil. Agronomy 2019, 9, 520. [Google Scholar] [CrossRef]
- Kumar, K.; Goh, K.M. Crop residues and management practices: Effects on soil quality, soil nitrogen dynamics and nitrogen recovery. Adv. Agron. 2000, 68, 197–319. [Google Scholar] [CrossRef]
- Puttaso, A.; Vityakon, P.; Seanjan, P.; Trelo-Ges, V.; Cadisch, G. Relationship between residue quality, decomposition patterns, and soil organic matter accumulation in a tropical sandy soil after 13 years. Nutr. Cycl. Agroecosyst. 2011, 89, 159–174. [Google Scholar] [CrossRef]
- Palm, C.A.; Gachengo, C.N.; Delve, R.J.; Cadisch, G.; Giller, K.E. Organic inputs for soil fertility management in tropical agroecosystems: Application of an organic resource database. Agric. Ecosyst. Environ. 2001, 83, 27–42. [Google Scholar] [CrossRef]
- Kunlanit, B.; Vityakon, P.; Puttaso, A.; Cadisch, G.; Rasche, F. Mechanisms controlling soil organic carbon composition pertaining to microbial decomposition of biochemically contrasting organic residues: Evidence from midDRIFTS peak area analysis. Soil Biol. Biochem. 2014, 76, 100–108. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Wallenstein, M.D.; Boot, C.M.; Denef, K.; Paul, E. The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: Do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 2013, 19, 988–995. [Google Scholar] [CrossRef]
- Cotrufo, M.F.; Soong, J.L.; Horton, A.J.; Campbell, E.E.; Haddix, M.L.; Wall, D.H.; Parton, W.J. Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 2015, 8, 776–779. [Google Scholar] [CrossRef]
- Office of Agricultural Economics, Ministry of Agriculture and Cooperatives. Available online: www.oae.go.th/download/journal/agri_eco56/agu-JAN56.pdf (accessed on 7 April 2020).
- Puttaso, A.; Vityakon, P.; Rasche, F.; Seanjan, P.; Trelo-Ges, V.; Cadisch, G. Does organic residue quality influence carbon retention in a tropical sandy soil. Soil Sci. Soc. Am. J. 2013, 77, 1001–1011. [Google Scholar] [CrossRef]
- Kunlanit, B.; Rasche, F.; Puttaso, A.; Cadisch, G.; Vityakon, P. Vertical dynamics of dissolved organic carbon in relation to organic input quality and microaggregate formation in a coarse-textured Ultisol. Eur. J. Soil Sci. 2020, 71, 459–471. [Google Scholar] [CrossRef]
- Keawpradit, W.; Toomsan, B.; Vityakon, P.; Limpinuntana, V.; Saenjan, P.; Jogloy, S.; Patanothai, A.; Cadisch, G. Regulating mineral N release and greenhouse gas emissions by mixing groundnut residues and rice straw under field conditions. Eur. J. Soil Sci. 2008, 59, 640–652. [Google Scholar] [CrossRef]
- Vityakon, P.; Meepech, S.; Cadisch, G.; Toomsan, B. Soil organic matter and nitrogen transformation mediated by plant residues of different qualities in sandy acid upland and paddy soils. Neth. J. Agric. Sci. 2000, 48, 75–90. [Google Scholar] [CrossRef]
- Jaman, M.S.; Muraina, T.O.; Dam, Q.; Zhang, X.; Jamil, M.; Bhattarai, S.; Islam, F. Effects of single and mixed plant types on soil carbon and nitrogen dynamics in homestead agroforestry systems in Northern Bangladesh. Agric. Ecosyst. Environ. 2021, 315, 107434. [Google Scholar] [CrossRef]
- Chen, X.; Chen, H.Y.H.; Chen, C.; Ma, Z.; Searle, E.B.; Yu, Z.; Huang, Z. Effects on plant diversity on soil carbon in diverse ecosystems: A global meta-analysis. Biol. Rev. 2020, 95, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, S.; Zhang, L.; Xin, X.; Liu, X. Litter mixing significantly affects decomposition in the Hulun Buir meadow steppe of Inner Mongolia. J. Plant Ecol. 2014, 7, 56–67. [Google Scholar] [CrossRef][Green Version]
- Redin, M.; Recous, S.; Aita, C.; Dietrich, G.; Skolaude, A.C.; Ludke, W.H.; Schmatz, R.; Giacomini, S.J. How the chemical composition and heterogeneity of crop residue mixtures decomposing at the soil surface affects C and N mineralization. Soil Biol. Biochem. 2014, 78, 65–75. [Google Scholar] [CrossRef]
- Almagro, M.; Ruiz-Navarro, A.; Díaz-Pereira, E.; Albaladejo, J.; Martínez-Mena, M. Plant residue chemical quality modulates the soil microbial response related to decomposition and soil organic carbon and nitrogen stabilization in a rainfed Mediterranean agroecosystem. Soil Biol. Biochem. 2021, 156, 108198. [Google Scholar] [CrossRef]
- Manzoni, S.; Capek, P.; Porada, P.; Thurner, M.; Winterdahl, M.; Beer, C.; Brüchert, V.; Frouz, J.; Herrmann, A.M.; Lindahl, B.D.; et al. Reviews and syntheses: Carbon use efficiency from organisms to ecosystems—Definitions, theories, and empirical evidence. Biogeosciences 2018, 15, 5929–5949. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Hämmerle, I.; Fuchslueger, L.; Hofhansl, F.; Knoltsch, A.; Schnecker, J.; Takriti, M.; Watzka, M.; Wild, B.; et al. Adjustment of microbial nitrogen use efficiency to carbon: Nitrogen imbalances regulates soil nitrogen cycling. Nat. Commun. 2014, 5, 3694. [Google Scholar] [CrossRef]
- Xu, X.; Schimel, J.P.; Janssens, I.A.; Song, X.; Song, C.; Yu, G.; Sinsabaugh, R.L.; Tang, D.; Zhang, X.; Thornton, P.E. Global pattern and controls of soil microbial metabolic quotient. Ecol. Monogr. 2017, 87, 429–441. [Google Scholar] [CrossRef]
- Anderson, T.-H.; Domsch, K.H. Maintenance carbon requirements of actively—Metabolizing microbial populations under in situ conditions. Soil Biol. Biochem. 1985, 17, 197–203. [Google Scholar] [CrossRef]
- Anderson, T.-H.; Domsch, K.H. Determination of ecophysiological maintenance carbon requirements of soil microorganisms in a dormant state. Biol. Fertil. Soils. 1985, 1, 81–89. [Google Scholar] [CrossRef]
- Anderson, T.-H.; Domsch, K.H. The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol. Biochem. 1993, 25, 393–395. [Google Scholar] [CrossRef]
- Wardle, D.A.; Ghani, A. A tale of two theories, a chronosequence and a bioindicator of soil quality. Soil Biol. Biochem. 2018, 121, A3–A7. [Google Scholar] [CrossRef]
- Zheng, Q.; Hu, Y.; Zhang, S.; Noll, L.; Böckle, T.; Richter, A.; Wanek, W. Growth explains microbial carbon use efficiency across soils differing in land use and geology. Soil Biol. Biochem. 2019, 128, 45–55. [Google Scholar] [CrossRef]
- Shimizu, K. Metabolic regulation of a bacterial cell system with emphasis on Escherichia coli metabolism. ISRN Biochem. 2013, 2013, 645983. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Yao, H.; Bowman, D. Soil microbial biomass, activity and nitrogen transformations in a turfgrass chronosequence. Soil Biol. Biochem. 2006, 38, 311–319. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, Z.; Tian, D.; Wang, J.; Fu, Z.; Wang, B.; Tang, Z.; Chen, W.; Chen, H.Y.H.; Wang, C.; et al. The stoichiometry of soil microbial biomass determines metabolic quotient of nitrogen mineralization. Environ. Res. Lett. 2020, 15, 034005. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Shah, J.J.F. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef]
- Sistla, S.A.; Schimel, J.P. Stoichiometric flexibility as a regulator of carbon and nutrient cycling in terrestrial ecosystems under change. New Phytol. 2012, 196, 68–78. [Google Scholar] [CrossRef]
- Gartner, T.B.; Cardon, Z.G. Decomposition dynamics in mixed-species leaf litter. Oikos 2004, 104, 230–246. [Google Scholar] [CrossRef]
- Bonanomi, G.; Capodilupo, M.; Incerti, G.; Mazzoleni, S. Nitrogen transfer in litter mixture enhances decomposition rate, temperature sensitivity, and C quality changes. Plant Soil 2014, 381, 307–321. [Google Scholar] [CrossRef]
- Mao, R.; Zeng, D.-H. Non-additive effects vary with the number of component residues and their mixing proportions during residue mixture decomposition: A microcosm study. Geoderma 2012, 170, 112–117. [Google Scholar] [CrossRef]
- Schimel, J.P.; Cates, R.G.; Ruess, R. The role of Balsam poplar secondary chemicals in controlling soil nutrient dynamics through succession in the Alaskan Taiga. Biogeochemistry 1998, 42, 221–234. [Google Scholar] [CrossRef]
- Hättenschwiler, S.; Vitousek, P.M. The role of polyphenols in terrestrial ecosystem nutrient cycling. Trends Ecol. Evol. 2000, 15, 238–243. [Google Scholar] [CrossRef]
- Soil Survey Staff. Keys to Soil Taxonomy; Natural Resources Conservation Service, United States Department of Agriculture: Washington, DC, USA, 2006.
- Butnan, S.; Deenik, J.L.; Toomsan, B.; Antal, M.J.; Vityakon, P. Biochar characteristics and application rates affecting corn growth and properties of soils contrasting in texture and mineralogy. Geoderma 2015, 237–238, 105–116. [Google Scholar] [CrossRef]
- Dewis, J.; Fretias, F. Physical and Chemical Methods of Soil and Water Analysis; FAO: Rome, Italy, 1970; p. 275. [Google Scholar]
- Pansu, M.; Gautheyrou, J. Handbook of Soil Analysis: Mineralogical, Organic and Inorganic Methods; Springer: Berlin/Heidelberg, Germany, 2006; p. 993. [Google Scholar]
- Anderson, J.P.E. Soil respiration. In Methods of Soil Analysis, 2nd ed.; Miller, R.H., Keeny, D.R., Eds.; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1982; pp. 831–871. [Google Scholar]
- Amato, M.; Ladd, J.N. Assay for microbial biomass based on ninhydrin-reactive nitrogen in extracts of fumigated soils. Soil Biol. Biochem. 1988, 20, 107–114. [Google Scholar] [CrossRef]
- Sparling, G.P.; West, A.W. A direct extraction method to estimate soil microbial C: Calibration in situ using microbial respiration and 14C labeled cells. Soil Biol. Biochem. 1988, 20, 337–343. [Google Scholar] [CrossRef]
- Wood, E.D.; Armstrong, F.A.J.; Richards, F.A. Determination of nitrate in sea water by cadmium-copper reduction to nitrite. J. Mar. Biol. Assoc. UK 1967, 47, 23–31. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass: Laboratory Analytical Prodedure (LAP); A National Laboratory of the U.S. Department of Energy Efficiency and Renewable Energy: Golden, CO, USA, 2008.
- Van Soest, P.J. Use of detergents in the analysis of fibrous feeds. II. A rapid method for the determination of fiber and lignin. J. Assoc. Off. Anal. Chem. 1963, 46, 829–835. [Google Scholar] [CrossRef]
- Talbot, J.M.; Treseder, K.K. Interactions among lignin, cellulose, and nitrogen drive litter chemistry-decay relationships. Ecology 2012, 93, 345–354. [Google Scholar] [CrossRef]
- Berglund, S.L.; Ågren, G.I.; Ekblad, A. Carbon and nitrogen transfer in leaf litter mixtures. Soil Biol. Biochem. 2013, 57, 341–348. [Google Scholar] [CrossRef]
- De Marco, A.; Meola, A.; Maisto, G.; Giordano, M.; Virzo De Santo, A. Non-additive effects of litter mixtures on decomposition of leaf litters in a Mediterranean maquis. Plant Soil 2011, 344, 305–317. [Google Scholar] [CrossRef]
- Hobara, S.; Osono, T.; Hirose, D.; Noro, K.; Hirota, M.; Benner, R. The roles of microorganisms in litter decomposition and soil formation. Biogeochemistry 2014, 118, 417–486. [Google Scholar] [CrossRef]
- Berg, B.; McClaugherty, C. Influences of chemical variation in the litter on decomposition. In Plant Litter: Decomposition, Humus Formation, Carbon Sequestration, 3rd ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 107–135. [Google Scholar]
- Manzoni, S.; Taylor, P.; Richter, A.; Porporato, A.; Ågren, G.I. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol. 2012, 196, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Agumas, B.; Blagodatsky, S.; Balume, I.; Musyoki, M.K.; Marhan, S.; Rasche, F. Microbial carbon use efficiency during plant residue decomposition: Integrating multi-enzyme stoichiometry and C balance approach. Appl. Soil Ecol. 2021, 159, 103820. [Google Scholar] [CrossRef]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Manzoni, S.; Trofymow, J.A.; Jackson, R.B.; Porporato, A. Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol. Monogr. 2010, 80, 89–106. [Google Scholar] [CrossRef]
- Haynes, R.J. The decomposition process: Mineralization, immobilization, humus formation and degradation. In Mineral Nitrogen in the Plant-Soil System, 1st ed.; Cameron, K.C., Goh, K.M., Sherlock, R.R., Eds.; Academic Press: Orlando, FL, USA, 1986; pp. 52–126. [Google Scholar]
| Residues 1 | Chemical Composition of Plant Residues 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | N | ADF g kg−1 | L | CL | C/N | L/N | CL/N | L/CL | |
| RS | 323.4 | 4.0 | 514.7 | 36.7 | 449.7 | 81.8 | 9.2 | 113.8 | 0.1 |
| GN | 347.5 | 17.5 | 546.7 | 108.7 | 408.0 | 19.9 | 6.2 | 23.3 | 0.3 |
| RS + GN | 341.6 | 14.3 | 532.3 | 76.7 | 427.0 | 24.0 | 5.4 | 29.9 | 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pingthaisong, W.; Vityakon, P. Nonadditive Effects on Decomposition of a Mixture of Rice Straw and Groundnut Stover Applied to a Sandy Soil. Agronomy 2021, 11, 1030. https://doi.org/10.3390/agronomy11061030
Pingthaisong W, Vityakon P. Nonadditive Effects on Decomposition of a Mixture of Rice Straw and Groundnut Stover Applied to a Sandy Soil. Agronomy. 2021; 11(6):1030. https://doi.org/10.3390/agronomy11061030
Chicago/Turabian StylePingthaisong, Wimonsiri, and Patma Vityakon. 2021. "Nonadditive Effects on Decomposition of a Mixture of Rice Straw and Groundnut Stover Applied to a Sandy Soil" Agronomy 11, no. 6: 1030. https://doi.org/10.3390/agronomy11061030
APA StylePingthaisong, W., & Vityakon, P. (2021). Nonadditive Effects on Decomposition of a Mixture of Rice Straw and Groundnut Stover Applied to a Sandy Soil. Agronomy, 11(6), 1030. https://doi.org/10.3390/agronomy11061030






