Traditional Sweet Chestnut and Hybrid Varieties: Chemical Composition, Morphometric and Qualitative Nut Characteristics
Abstract
1. Introduction
2. Materials and Methods
=100 − (w (moisture) +w (crude protein) + w (crude fat) + w (ash))
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, G.P. Revision of Castanea sect. Balanocastanon (Fagaceae). J. Arnold Arbor. 1988, 69, 25–49. [Google Scholar]
- Lang, P.; Dane, F.; Kubisiak, T.L. Phylogeny of Castanea (Fagaceae) based on chloroplast trnT-L-F sequence data. Tree Genet. Genomes 2006, 2, 132–139. [Google Scholar] [CrossRef]
- FAO. Faostat Database. 2018. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 20 January 2021).
- De Vasconcelos, M.C.B.M.; Bennett, R.N.; Rosa, E.A.; Ferreira-Cardoso, J.V. Composition of European chestnut (Castanea sativa Mill.) and association with health effects: Fresh and processed products. J. Sci. Food Agric. 2010, 90, 1578–1589. [Google Scholar] [CrossRef]
- Korel, F.; Balaban, M.Ö. Chemical composition and health aspects of chestnut (Castanea spp.). In Tree Nuts: Composition, Phytochemicals and Health Effects; Alasalvar, C., Shahidi, F., Eds.; CRC Press Taylor and Francis Group: Boca Raton, FL, USA, 2009. [Google Scholar]
- Barreira, J.C.M.; Casal, S.; Ferreira, I.C.F.R.; Peres, A.M.; Pereira, J.A.; Oliviera, M.B.P.P. Chemical characterization of chestnut cultivars from three consecutive years: Chemometrics and contribution for authentication. Food Chem. Toxicol. 2012, 50, 2311–2317. [Google Scholar] [CrossRef] [PubMed]
- De La Montaña Míguelez, J.; Miguez Bernàndez, M.; Garcia Quejeiro, J.M. Composition of varieties of chestnuts from Galicia (Spain). Food Chem. 2004, 84, 401–404. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; Ramos-Cabrer, A.M.; Díaz-Hernández, M.B.; Ciordia-Ara, M.; Ríos-Mesa, D. Chemical composition of chestnut cultivars from Spain. Sci. Hortic. 2006, 107, 306–314. [Google Scholar] [CrossRef]
- Peña-Méndez, E.M.; Hernández-Suárez, M.; Díaz-Romero, C.; Rodríguez-Rodríguez, E. Characterization of various chestnut cultivars by means of chemometrics approach. Food Chem. 2008, 107, 537–544. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Casal, S.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Nutritional, fatty acid and triacylglycerol profiles of Castanea sativa Mill. cultivars: A compositional and chemometric approach. J. Agric. Food Chem. 2009, 57, 2836–2842. [Google Scholar] [CrossRef]
- Dinis, L.T.; Ferreira-Cardoso, J.; Peixoto, F.; Costa, R.; Gomes Laranjo, J. Study of morphological and chemical diversity in chestnut trees (var. ‘Judia’) as a function of temperature sum. CyTA J. Food. 2011, 9, 192–199. [Google Scholar] [CrossRef]
- Mert, C.; Ertürk, Ü. Chemical compositions and sugar profiles of consumed chestnut cultivars in Marmara region, Turkey. Not. Bot. Horti Agrobot. Cluj Napoca 2017, 45, 203–207. [Google Scholar] [CrossRef]
- Bounous, G.; Botta, R.; Beccaro, G. The chestnut: The ultimate energy source nutritional value and alimentary benefits. Nucis 2000, 9, 44–50. [Google Scholar]
- Santos Rosa, E.A.; Seixas Martins Morais, I.V.; Oliveira, I.; Gonçalves, B.; Silva, A.P. Uses and health benefits of chestnuts. In Burleigh Dodds Series in Agricultural Science; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 69–108. ISBN 978-1-78676-224-5. [Google Scholar]
- Borges, O.; Gonçalves, B.; Carvalho, J.L.S.; Correia, P.; Silva, A.P. Nutritional quality of chestnut (Castanea sativa Mill.) cultivars from Portugal. Food Chem. 2008, 106, 976–984. [Google Scholar] [CrossRef]
- Piccioli, L. Monografia del Castagno. Suoi Caratteri Varieta, Coltivazione, Prodotti e Nemici; Tipografia di S. Landi: Firenze, Italy, 1902. [Google Scholar]
- Bellini, E.; Giordani, E.; Marinelli, C.; Perucca, B. Marrone del Mugello PGI chestnut nutritional and organoleptic quality. Acta Hortic. 2005, 693, 97–102. [Google Scholar] [CrossRef]
- Bounous, G. Italy. In Following Chestnut Footprints (Castanea spp.)–Cultivation and Culture, Folklore and History, Traditions and Uses; Avanzato, D., Ed.; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2009; Volume 9, pp. 72–84. [Google Scholar]
- Camus, A. Les chataigniers. Monographie des genres Castanea et Castanopsis. Encycl. Econ. Sylvic. 1929, 3, 1–604. [Google Scholar]
- Hennion, B. France. In Following Chestnut Footprints (Castanea spp.)–Cultivation and Culture, Folklore and History, Traditions and Uses; Avanzato, D., Ed.; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2009; Volume 9, pp. 44–47. [Google Scholar]
- Fernández, J.; Pereira, S. Inventario y Distribución de los Cultivares Tradicionales de Castaño (Castanea sativa Mill.) en Galicia; MAPA: Madrid, Spain, 1993. [Google Scholar]
- Pereira-Lorenzo, S.; Díaz-Hernández, B.; Ciordia-Ara, M.; Ascasibar-Errasti, J.; Ramos-Cabrer, A.; Sau, F. Spanish chestnut cultivars. Sci. Hortic. 2001, 36, 344–347. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; Díaz-Hernández, M.B.; Ramos-Cabrer, A.M. Spain. In Following Chestnut Footprints (Castanea spp.)–Cultivation and Culture, Folklore and History, Traditions and Uses; Avanzato, D., Ed.; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2009; Volume 9, pp. 134–141. [Google Scholar]
- Martin, M.A.; Moral, A.; Martin, L.M.; Alvarez, J.B. The genetic resources of European sweet chestnut (Castanea sativa Miller) in Andalusia, Spain. Genet. Resour. Crop Evol. 2007, 54, 379–387. [Google Scholar] [CrossRef]
- Martin, A.; Alvarez, J.B.; Martin, L.M.; Mattioni, C.; Cherubini, M.; Villani, F.; Ruiz, J.C. Traditional chestnut cultivars in Southern Spain: A case of endangered genetic resources. Acta Hortic. 2010, 866, 143–150. [Google Scholar] [CrossRef]
- Gomes-Laranjo, J.; Peixoto, F.; Costa, R.; Ferreira-Cardoso, J. Portugal. In Following Chestnut Footprints (Castanea spp.)–Cultivation and Culture, Folklore and History, Traditions and Uses; Avanzato, D., Ed.; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2009; Volume 9, pp. 106–111. [Google Scholar]
- Conedera, M.; Müller-Starck, G.; Fineschi, S. Genetic characterization of cultivated varieties of European chestnut (Castanea sativa Mill.) in Southern Switzerland. I. Inventory of chestnut varieties: History and perspectives. In Proceedings of the International Congress on Chestnut, Spoleto, Italy, 20–23 October 1994; pp. 299–302. [Google Scholar]
- Gobbin, D.; Hohl, L.; Conza, L.; Jermini, M.; Gessler, C.; Conedera, M. Microsatellite-based characterization of the Castanea sativa cultivar heritage of southern Switzerland. Genome 2007, 50, 1089–1103. [Google Scholar] [CrossRef]
- Conedera, M.; Krebs, P. Switzerland. In Following Chestnut Footprints (Castanea spp.)–Cultivation and Culture, Folklore and History, Traditions and Uses; Avanzato, D., Ed.; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2009; Volume 9, pp. 149–154. [Google Scholar]
- Bouffier, V.A.; Maurer, W.D. Germany. In Following Chestnut Footprints (Castanea spp.), Cultivation and Culture, Folklore and History, Traditions and Uses; Avanzato, D., Ed.; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2009; Volume 9, pp. 53–62. [Google Scholar]
- Bounous, G.; Barrel, A.; Beccaro, G.; Lovisolo, C.; Gomes Pereira, J.A. Inventory of Chestnut Research, Germplasm and References; FAO Ciheam Reu Technical Series; FAO: Rome, Italy, 2001; Volume 65, pp. 1–174. [Google Scholar]
- Martín, M.A.; Alvarez, J.B.; Mattioni, C.; Cherubini, M.; Villani, F.; Martín, L.M. Identification and characterization of traditional chestnut varieties of southern Spain using morphological and simple sequence repeats (SSRs) markers. Ann. Appl. Biol. 2009, 154, 389–398. [Google Scholar] [CrossRef]
- Torello Marinoni, D.; Akkak, A.; Beltramo, C.; Guaraldo, P.; Boccacci, P.; Bounous, G.; Ferrara, A.M.; Ebone, A.; Viotto, E.; Botta, R. Genetic and morphological characterization of chestnut (Castanea sativa Mill.) germplasm in Piedmont (north-western Italy). Tree Genet. Genomes 2013, 9, 1017–1030. [Google Scholar] [CrossRef]
- Neri, L.; Dimitri, G.; Sacchetti, G. Chemical composition and antioxidant activity of cured chestnuts from three sweet chestnut (Castanea sativa Mill.) ecotypes from Italy. J. Food Compost. Anal. 2010, 23, 23–29. [Google Scholar] [CrossRef]
- Poljak, I.; Vahčić, N.; Gačić, M.; Idžojtić, M. Morphology and chemical composition of fruits of the traditional Croatian chestnut variety ‘Lovran Marron’. Food Technol. Biotechnol. 2016, 54, 189–199. [Google Scholar] [CrossRef]
- Breisch, H. Harvesting, storage and processing of chestnuts in France and Italy. In Proceedings of the International Congress on Chestnut, Spoleto, Italy, 20–23 October 1994; pp. 429–436. [Google Scholar]
- Prospero, S.; Rigling, D. Chestnut blight. In Infectious Forest Diseases; Gonthier, P., Nicolotti, G., Eds.; CAB International: Wallingford, UK, 2013; pp. 318–338. [Google Scholar]
- Rigling, D.; Prospero, S. Cryphonectria parasitica, the causal agent of chestnut blight: Invasion history, population biology and disease control. Mol. Plant Pathol. 2017, 19, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Lorenzo, S.; Costa, R.L.; Ramos-Cabrer, A.; Marques Ribeiro, C.A.; Serra da Silva, M.F.; Manzano, G.; Barreneche, T. Variation in grafted European chestnut and hybrids by microsatellites reveals two main origins in the Iberian Peninsula. Tree Genet. Genomes 2010, 5, 701–715. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; Costa, R.; Anagnostakis, S.; Serdar, U.; Yamamoto, T.; Saito, T.; Ramos-Cabrer, A.M.; Ling, Q.; Barreneche, T.; Robin, C.; et al. Interspecific hybridization of chestnut. In Polyploidy and Hybridization for Crop Improvement; Mason, A.S., Ed.; Taylor & Francis Group, LLC: Abingdon, UK, 2016. [Google Scholar]
- Medak, J.; Idžojtić, M.; Novak-Agbaba, S.; Ćurković-Perica, M.; Mujić, I.; Poljak, I.; Juretić, D.; Prgomet, Ž. Croatia. In Following Chestnut Footprints (Castanea spp.)–Cultivation and Culture, Folklore and History, Traditions and Use; Avanzato, D., Ed.; International Society for Horticultural Science (ISHS): Leuven, Belgium, 2009; Volume 9, pp. 40–43. [Google Scholar]
- Idžojtić, M.; Zebec, M.; Poljak, I.; Šatović, Z.; Liber, Z. Analiza genetske raznolikosti “lovranskog maruna” (Castanea sativa Mill.) korištenjem mikrosatelitnih biljega. Sumar. List 2012, 136, 577–585. [Google Scholar]
- Ježić, M.; Krstin, L.; Poljak, I.; Liber, Z.; Idžojtić, M.; Jelić, M.; Meštrović, J.; Zebec, M.; Ćurković-Perica, M. Castanea sativa: Genotype-dependent recovery from chestnut blight. Tree Genet. Genomes 2014, 10, 101–110. [Google Scholar] [CrossRef]
- Oraguzie, N.; McNeil, D.; Paterson, A.M.; Chapman, H.M. Comparison of RAPD and morpho-nut markers for revealing genetic relationships between chestnut species (Castanea spp.) and New Zealand chestnut selections. New Zeal. J. Crop Hort. 1998, 26, 109–115. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; Fernández-López, J.; Moreno-Gonzalez, J. Variability and grouping of Northwestern Spanish chestnut cultivars. II. Isoenzyme traits. J. Am. Soc. Hortic. Sci. 1996, 121, 190–197. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; Díaz-Hernández, M.B.; Ramos-Cabrer, A. Use of highly discriminating morphological characters and isozymes in the study of Spanish chestnut cultivars. J. Am. Soc. Hortic. 2006, 131, 770–779. [Google Scholar] [CrossRef]
- Goulão, L.; Valdiviesso, T.; Santana, C.; Moniz Oliveira, C. Comparison between phenetic characterisation using RAPD and ISSR markers and phenotypic data of cultivated chestnut (Castanea sativa Mill.). Genet. Resour. Crop Evol. 2001, 48, 329–338. [Google Scholar] [CrossRef]
- Botta, R.; Akkak, A.; Guaraldo, P.; Bounous, G. Genetic characterization and nut quality of chestnut cultivars from Piemonte (Italy). Acta Hortic. 2005, 693, 395–401. [Google Scholar] [CrossRef]
- Ramos-Cabrer, A.M.; Pereira-Lorenzo, S. Genetic relationship between Castanea sativa Mill. trees from north-western to south Spain based on morphological traits and isoenzymes. Genet. Resour. Crop Evol. 2005, 52, 879–890. [Google Scholar] [CrossRef]
- Martín, M.A.; Mattioni, C.; Cherubini, M.; Taurchini, D.; Villani, F. Genetic characterization of traditional chestnut varieties in Italy using microsatellites (simple sequence repeats). Ann. Appl. Biol. 2010, 157, 37–44. [Google Scholar] [CrossRef]
- Martin, M.A.; Mattioni, C.; Cherubini, M.; Villani, F.; Martin, L.M. A comparative study of European chestnut varieties in relation to adaptive markers. Agrofor. Syst. 2017, 91, 97–109. [Google Scholar] [CrossRef]
- Beghè, D.; Ganino, T.; Dall’Asta, C.; Silvanini, A.; Cirlini, M.; Fabbri, A. Identification and characterization of ancient Italian chestnut using nuclear microsatellite markers. Sci. Hortic. 2013, 164, 50–57. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; Fernández-López, J.; Morengo-González, J. Variability and grouping of Northwestern Spanish chestnut cultivars. I. Morphological traits. J. Am. Soc. Hortic. 1996, 121, 183–189. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; Fernández-López, J. Description of 80 cultivars and 36 clonal selections of chestnut (Castanea sativa Mill.) from Northwestern Spain. Fruit Var. J. 1997, 51, 13–27. [Google Scholar]
- Queijeiro, J.M.; De la Montaña, J.; Míguez, M. Identification and morphological description of cultivars of chesnut (Castanea sativa Mill.) of the region of Verín-Monterrei (Ourense, Spain). J. Am. Pomol. Soc. 2006, 60, 37–45. [Google Scholar] [CrossRef]
- Álvarez-Álvarez, P.; Barrio-Anta, M.; Diéguez-Aranda, U. Differentiation of sweet chestnut (Castanea sativa Mill.) cultivars by leaf, nut and burr dimensions. Forestry 2006, 79, 149–158. [Google Scholar] [CrossRef]
- Furones-Pérez, P.; Fernández-López, J. Morphological and phenological description of 38 sweet chestnut cultivars (Castanea sativa Miller) in a contemporary collection. Span. J. Agric. Res. 2009, 7, 829–843. [Google Scholar] [CrossRef]
- Ertan, E. Variability in leaf and fruit morphology and in fruit composition of chestnuts (Castanea sativa Mill.) in the Nazilli region of Turkey. Genet. Resour. Crop Evol. 2007, 54, 691–699. [Google Scholar] [CrossRef]
- Saccheti, G.; Neri, L.; Dimitri, G.; Mastrocola, D. Chemical composition and functional properties of three sweet chestnut (Castanea sativa Mill.) ecotypes from Italy. Acta Hortic. 2009, 844, 41–46. [Google Scholar] [CrossRef]
- Piccolo, E.L.; Landi, M.; Ceccanti, C.; Mininni, A.N.; Marchetti, L.; Massai, R.; Guidi, L.; Remorini, D. Nutritional and nutraceutical properties of raw and traditionally obtained flour from chestnut fruit grown in Tuscany. Eur. Food Res. Technol. 2020, 246, 1867–1876. [Google Scholar] [CrossRef]
- Poljak, I.; Idžojtić, M.; Šatović, Z.; Ježić, M.; Ćurković-Perica, M.; Simovski, B.; Acevski, A.; Liber, Z. Genetic diversity of the sweet chestnut (Castanea sativa Mill.) in Central Europe and the western part of the Balkan Peninsula and evidence of marron genotype introgression into wild populations. Tree Genet. Genomes 2017, 13, 18. [Google Scholar] [CrossRef]
- Poljak, I.; Idžojtić, M.; Zebec, M.; Perković, N. The variability of European sweet chestnut (Castanea sativa Mill.) in the region of northwest Croatia according to morphology of fruits. Sumar. List 2012, 136, 479–489. [Google Scholar]
- UPOV. Guidelines for the Conduct of Tests for Distinctness, Homogenity and Stability. Chestnut (Castanea sativa Mill.); International Union for the Protection of New Varieties of Plants: Geneve, Switzerland, 1989. [Google Scholar]
- Martin, A.; Alvarez, J.B.; Mattioni, C.; Cherubini, M.; Villani, F.; Martin, L.M. On-farm conservation of sweet chestnut (Castanea sativa Mill.) in Andalusia. J. Agric. Sci. Technol. 2011, 5, 154–159. [Google Scholar]
- AOAC Official Method 925.40. Moisture in Nuts and Nut Products; AOAC International: Washington, DC, USA, 1995. [Google Scholar]
- AOAC Official Method 950.49. Ash of Nuts and Nut Products; AOAC International: Washington, DC, USA, 1995. [Google Scholar]
- AOAC Official Method 950.48. Protein (Crude) in Nuts and Nut Products; AOAC International: Washington, DC, USA, 1995. [Google Scholar]
- AOAC Official Method 948.22. Fat (Crude) in Nuts and Nut Products; AOAC International: Washington, DC, USA, 2000. [Google Scholar]
- Oliveira, I.; Sousa, A.; Morais, J.S.; Ferreira, I.C.; Bento, A.; Estevinho, L.; Pereira, J.A. Chemical composition, and antioxidant and antimicrobial activities of three hazelnut (Corylus avellana L.) cultivars. Food Chem. Toxicol. 2008, 46, 1801–1807. [Google Scholar] [CrossRef]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Ferreira, I.C.F.R.; Bento, A.; Estevinho, L.M. Bioactive properties and chemical composition of six walnut (Juglans regia L.) cultivars. Food Chem. Toxicol. 2008, 46, 2103–2111. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J. Biometry: The Principles and Practice of Statistics in Biological Research, 4th ed.; W.H. Freeman and Co.: New York, NY, USA, 2012; p. 937. [Google Scholar]
- Statistica (Data Analysis Software System), Version 13; TIBCO Software Inc.: Palo Alto, CA, USA, 2018.
- SAS/STAT 9.1 Users Guide; SAS Institute Inc.: Cary, NC, USA, 2004.
- R Core Team. R: A Language and Environment for Statistical Computing, v.3.2.2; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Koutecký, P. MorphoTools: A set of R functions for morphometric analysis. Plant Syst. Evol. 2015, 301, 1115–1121. [Google Scholar] [CrossRef]
- Botu, M.; Botu, I.; Neagoe, A.; Papachatzis, A. Evaluation of sweet chestnut cultivars and selections into the Vâlcea area. Acta Hortic. 2009, 844, 311–318. [Google Scholar] [CrossRef]
- Martín, M.A.; Mattioni, C.; Molina, J.R.; Alvarez, J.B.; Cherubini, M.; Herrera, M.A.; Villani, F.; Martín, L.M. Landscape genetic structure of chestnut (Castanea sativa Mill.) in Spain. Tree Genet. Genomes 2012, 8, 127–136. [Google Scholar] [CrossRef]
- Mattioni, C.; Martín, M.A.; Pollegioni, P.; Cherubini, M.; Villani, F. Microsatellite markers reveal a strong geographical structure in European populations of Castanea sativa (Fagaceae): Evidence for multiple glacial refugia. Am. J. Bot. 2013, 100, 951–961. [Google Scholar] [CrossRef]
- Mattioni, C.; Martin, M.A.; Chiocchini, F.; Cherubini, M.; Gaudet, M.; Pollegioni, P.; Velichkov, I.; Jarman, R.; Chambers, F.M.; Paule, L.; et al. Landscape genetics structure of European sweet chestnut (Castanea sativa Mill): Indications for conservation priorities. Tree Genet. Genomes 2017, 13, 39. [Google Scholar] [CrossRef]
- Lusini, I.; Velichkov, I.; Pollegioni, P.; Chiocchini, F.; Hinkov, G.; Zlatanov, T.; Cherubini, M.; Mattioni, C. Estimating the genetic diversity and spatial structure of Bulgarian Castanea sativa populations by SSRs: Implications for conservation. Conserv. Genet. 2014, 15, 283–293. [Google Scholar] [CrossRef]
- Bolvanský, M.; Užík, M. Morphometric variation and differentiation of European chestnut (Castanea sativa) in Slovakia. Biologia 2005, 60, 423–429. [Google Scholar]
- Solar, A.; Podjavoršek, A.; Štampar, F.S. Fenotypic and genotypic diversity of European chestnut (Castanea sativa Mill.) in Slovenia–opportunity for genetic improvement. Genet. Resour. Crop Evol. 2005, 52, 391–394. [Google Scholar] [CrossRef]
- Idžojtić, M.; Zebec, M.; Poljak, I.; Medak, J. Variation of sweet chestnut (Castanea sativa Mill.) populations in Croatia according to the morphology of fruits. Sauteria 2009, 18, 232–333. [Google Scholar]
- Bounous, G. Among the Chestnut Trees in Cuneo Province; Edizioni Metafore: Cuneo, Italy, 1999. [Google Scholar]
- Tonelli, N.; Gallouin, F. Des Fruits et des Graines Comestibles du Monde Entire; Lavoisier: Paris, France, 2013. [Google Scholar]
- Riondato, I.; Akyüz, B.; Beccaro, G.; Casey, J.; Conedera, M.; Coulié, J.; Diamandis, S.; Gomes-Laranjo, J.; Nishio, S.; Ramos-Cabrer, A.; et al. Cultivars list and breeding. In The Chestnut Handbook: Crop and Forest Management; Beccaro, G., Alma, A., Bounous, G., Gomes-Laranjo, J., Eds.; Taylor & Francis Group, LLC: Abingdon, UK, 2020; Chapter 4; pp. 52–117. [Google Scholar]
- Glushkova, M.; Zhyanski, M.; Velinova, K. Nut quality assessment of chestnut cultivars from ‘Ivanik’ clone collection. For. Sci. 2010, 1, 3–14. [Google Scholar]
- De Biaggi, M.; Rapalino, S.; Donno, D.; Mellano, M.G.; Beccaro, G.L. Genotype influence on chemical composition and sensory traits of chestnut in 18 cultivars grown on the same rootstock and at the same agronomic conditions. Acta Hortic. 2018, 1220, 215–220. [Google Scholar] [CrossRef]
- Míguez Bernárdez, M.; De la Montaña Miguélez, J.; García Queijeiro, J. HPLC determination of sugars in varieties of chestnut fruits from Galicia (Spain). J. Food Compost. Anal. 2004, 17, 63–67. [Google Scholar] [CrossRef]
- Beccaro, G.L.; Donno, D.; Lione, G.G.; De Biaggi, M.; Gamba, G.; Rapalino, S.; Riondato, I.; Gonthier, P.; Mellano, M.G. Castanea spp. agrobiodiversity conservation: Genotype influence on chemical and sensorial traits of cultivars grown on the same clonal rootstock. Foods 2020, 9, 1062. [Google Scholar] [CrossRef]
- Silvanini, A.; Dall’Asta, C.; Morrone, L.; Cirlini, M.; Beghè, D.; Fabbri, A.; Ganino, T. Altitude effects on fruit morphology and flour composition of two chestnut cultivars. Sci. Hortic. 2014, 176, 311–318. [Google Scholar] [CrossRef]
- Linhares, I.; Martins, A.; Borges, O.; Guedes, C.; Seixas Sousa, V. Effect of irrigation and soil management practices on fruit production and quality in chestnut orchards of northern Portugal. Acta Hortic. 2005, 693, 701–706. [Google Scholar] [CrossRef]
- Mota, M.; Pinto, T.; Vilela, A.; Marques, T.; Borges, A.; Caço, J.; Ferreira-Cardoso, J.; Raimundo, F.; Gomes-Laranjo, J. Irrigation positively affects the chestnut’s quality: The chemical composition, fruit size and sensory attributes. Sci. Hortic. 2018, 238, 177–186. [Google Scholar] [CrossRef]
- Ferreira-Cardoso, J.V.; Torres-Pereira, J.M.G.; Sequeira, C.A. Effect of year and cultivar on chemical composition of chestnuts from northeastern Portugal. Acta Hortic. 2005, 693, 271–278. [Google Scholar] [CrossRef]
- Ertürk, Ü.; Mert, C.; Soylu, A. Chemical composition of fruits of some important chestnut cultivars. Braz. Arch. Biol. Technol. 2006, 49, 183–188. [Google Scholar] [CrossRef]
- Borges, O.P.; Carvalho, J.S.; Correia, P.R.; Silva, A.P. Lipid and fatty acid profiles of Castanea sativa Mill. chestnuts of 17 native Portuguese cultivars. J. Food Compost. Anal. 2007, 20, 80–89. [Google Scholar] [CrossRef]
- Cristofori, V.; Muganu, M.; Graziosi, P.; Beratazza, G.; Bignami, C. Comparison of nut traits and quality evaluation of chestnut (Castanea sativa Mill.) germplasm in Latium Region (Central Italy). Acta Hortic. 2009, 815, 133–140. [Google Scholar] [CrossRef]
- Moradi, Y.; Khadivi, A.; Salehi-Arjmand, H. Morphological and pomological characterizations of cornelian cherry (Cornus mas L.) to select the superior accessions. Sci. Hortic. 2019, 249, 208–218. [Google Scholar] [CrossRef]
- Khadivi, A.; Rezaei, M.; Heidari, P.; Safari-Khuzani, A.; Sahebi, M. Morphological and fruit characterizations of common medlar (Mespilus germanica L.) germplasm. Sci. Hortic. 2019, 252, 38–47. [Google Scholar] [CrossRef]
- Norouzi, E.; Erfani-Moghadam, J.; Fazeli, A.; Khadivi, A. Morphological variability within and among three species of Ziziphus genus using multivariate analysis. Sci. Hortic. 2017, 222, 180–186. [Google Scholar] [CrossRef]
- Khadivi, A.; Anjam, R.; Anjam, K. Morphological and pomological characterization of edible fig (Ficus carica L.) to select the superior trees. Sci. Hortic. 2018, 238, 66–74. [Google Scholar] [CrossRef]
- Hashemi, S.; Khadivi, A. Morphological and pomological characteristics of white mulberry (Morus alba L.) accessions. Sci. Hortic. 2020, 259, 108827. [Google Scholar] [CrossRef]
- Khadivi, A.; Mirheidari, F.; Moradi, Y.; Paryan, S. Morphological variability of wild pomegranate (Punica granatum L.) accessions from natural habitats in the Northern parts of Iran. Sci. Hortic. 2020, 264, 109156. [Google Scholar] [CrossRef]
- Khadivi, A.; Mohammadi, M.; Asgari, K. Morphological and pomological characterizations of sweet cherry (Prunus avium L.), sour cherry (Prunus cerasus L.) and duke cherry (Prunus × gondouinii Rehd.) to choose the promising selections. Sci. Hortic. 2019, 257, 108719. [Google Scholar] [CrossRef]
- Valero Galván, J.; Jorrín Novo, J.V.; Gómez Cabrera, A.; Ariza, D.; Garcia-Olmo, J.; Navarro Cerillo, R.M. Population variability based on the morphometry and chemical composition of the acorn in Holm oak (Quercus ilex subsp. ballota [Desf.] Samp.). Eur. J. Forest Res. 2011, 131, 893–904. [Google Scholar] [CrossRef]
- Loewe-Muñoz, V.; Álvarez, A.; Navarro-Cerrillo, R. Morphometric and chemical fruit variability of selected stone pine trees (Pinus pinea L.) grown in non-native environments. Plant. Biosyst. 2018, 152, 547–555. [Google Scholar] [CrossRef]
- Izhaki, I.; Tsahar, E.; Paluy, I.; Friedman, J. Within population variation and interrelationships between morphology, nutritional content and secondary compounds of Rhamnus alaternus fruits. New Phytol. 2002, 156, 217–223. [Google Scholar] [CrossRef]
- Boublenzaa, I.; El haitoum, A.; Ghezlaoui, S.; Mahdad, M.; Vasaï, F.; Chemat, F. Algerian carob (Ceratonia siliqua L.) populations. Morphological and chemical variability of their fruits and seeds. Sci. Hortic. 2019, 256, 108537. [Google Scholar] [CrossRef]
- Lotan, A.; Izhaki, I. Could abiotic environment shape fleshy fruit traits? A field study of the desert shrub Ochradenus baccatus. J. Arid Environ. 2013, 92, 34–41. [Google Scholar] [CrossRef]
- Cecil, J.S.; Barth, G.E.; Maier, N.A.; Chvyl, W.L.; Bartetzko, M.N. Leaf chemical composition and nutrient removal by stems of Leucadendron cvv. Silvan Red and Safari Sunset. Aust. J. Exp. Agric. 1995, 35, 275–283. [Google Scholar] [CrossRef]

| Trait | Traditional Sweet Chestnut Varieties | Hybrid Varieties | ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ‘Lovran Marron’ | ‘Marradi’ | ‘Bouche de Bétizac’ | ‘Marsol’ | ||||||||
| Mean Value ± SD | CV/% | Mean Value ± SD | CV/% | Mean Value ± SD | CV/% | Mean Value ± SD | CV/% | T/H | T | H | |
| Nut mass/g | 12.9 ± 2.5 | 19.3 | 9.6 ± 1.4 | 14.6 | 27.8 ± 5.1 | 18.4 | 22.2 ± 3.7 | 16.8 | >0.01 | >0.01 | >0.01 |
| Nut height/mm | 28.4 ± 1.8 | 6.3 | 27.1 ± 1.6 | 6.0 | 39.5 ± 1.5 | 3.7 | 39.7 ± 1.6 | 4.0 | >0.01 | >0.01 | ns |
| Nut width/mm | 35.0 ± 2.8 | 8.1 | 33.6 ± 2.4 | 7.1 | 45.5 ± 3.3 | 7.3 | 39.8 ± 2.7 | 6.8 | >0.01 | ns | >0.01 |
| Nut thickness/mm | 21.9 ± 2.6 | 12.0 | 19.0 ± 1.6 | 8.4 | 26.0 ± 2.9 | 11.0 | 25.2 ± 2.4 | 9.7 | >0.01 | >0.01 | ns |
| Hilum length/mm | 23.4 ± 2.8 | 11.9 | 24.2 ± 1.7 | 7.1 | 34.0 ± 3.2 | 9.5 | 34.8 ± 4.1 | 11.9 | >0.01 | ns | ns |
| Hilum width/mm | 12,0 ± 1.7 | 13.8 | 11.8 ± 1.1 | 9.3 | 17.3 ± 2.0 | 11.3 | 18.6 ± 1.9 | 10.3 | >0.01 | ns | >0.01 |
| Seeds per nut | 1.0 ± 0.1 | 10.2 | 1.1 ± 0.2 | 20.1 | 1.6 ± 0.6 | 34.4 | 1.00 ± 0.0 | 0.0 | >0.01 | ns | >0.01 |
| Number of intrusions | 1.3 ± 1.3 | 95.1 | 0.9 ± 0.8 | 85.0 | 2.2 ± 1.3 | 58.5 | 1.8 ± 1.1 | 60.1 | >0.01 | ns | ns |
| Length of the longest intrusion of the seed coat into the kernel/mm | 2.3 ± 1.7 | 72.9 | 2.1 ± 1.7 | 82.2 | 3.4 ± 1.7 | 49.1 | 3.4 ± 1.7 | 50.4 | >0.01 | ns | ns |
| Nut height/nut width | 0.82 ± 0.06 | 7.24 | 0.81 ± 0.05 | 5.93 | 0.87 ± 0.05 | 5.65 | 1.00 ± 0.06 | 6.13 | >0.01 | ns | >0.01 |
| Nut thickness/nut height | 0.77 ± 0.09 | 11.63 | 0.70 ± 0.07 | 10.03 | 0.66 ± 0.06 | 9.68 | 0.64 ± 0.06 | 10.08 | >0.01 | >0.01 | >0.05 |
| Nut thickness/nut width | 0.63 ± 0.08 | 12.58 | 0.57 ± 0.05 | 9.03 | 0.57 ± 0.04 | 6.92 | 0.63 ± 0.06 | 9.47 | >0.01 | >0.01 | >0.01 |
| Hilum length/nut width | 0.67 ± 0.05 | 8.09 | 0.72 ± 0.04 | 5.57 | 0.75 ± 0.03 | 3.61 | 0.87 ± 0.07 | 7.84 | >0.01 | >0.01 | >0.01 |
| Hilum width/nut thickness | 0.55 ± 0.06 | 11.25 | 0.62 ± 0.04 | 6.75 | 0.67 ± 0.04 | 5.84 | 0.74 ± 0.08 | 11.21 | >0.01 | >0.01 | >0.01 |
| Hilum width/hilum length | 0.51 ± 0.06 | 12.25 | 0.49 ± 0.04 | 9.08 | 0.51 ± 0.03 | 6.60 | 0.54 ± 0.08 | 14.59 | >0.01 | >0.01 | >0.05 |
| Length of the longest intrusion of the seed coat into the kernel/nut thickness | 0.10 ± 0.08 | 72.96 | 0.11 ± 0.10 | 93.42 | 0.13 ± 0.07 | 51.04 | 0.13 ± 0.07 | 50.44 | >0.01 | ns | ns |
| Major Details | Name of Descriptor | Traditional Sweet Chestnut Varieties | Hybrid Varieties | ||
|---|---|---|---|---|---|
| ‘Lovran Marron’ | ‘Marradi’ | ‘Bouche de Bétizac’ | ‘Marsol’ | ||
| UPOV number 26 | Time of beginning of fruit ripening | late | late | very early | early |
| UPOV number 27 | Fruit: embryony | monoembryonyc | monoembryonyc | polyembryonyc | monoembryonyc |
| UPOV number 29 | Fruit: penetration of seed coat into the kernel | present | present | present | present |
| UPOV number 30 | Fruit: degree of penetration of seed coat into the kernel | low/medium | low/medium | low/medium | low/medium |
| UPOV number 31 | Fruit: shape | transverse ellipsoid | transverse ellipsoid | transverse broad ellipsoid | broad ovoid |
| UPOV number 32 | Fruit: size of hilum | small | small | medium | medium |
| UPOV number 34 | Fruit: glossiness (immediately after opening of cupule) | present | present | present | present |
| UPOV number 35 | Fruit: color | reddish brown | reddish brown | reddish brown, quickly turning dark brown | reddish brown |
| UPOV number 36 | Fruit: size | big | small/medium | very big | very big |
| UPOV number 38 | Kernel: color | cream | cream | cream | cream |
| Poljak et al. [35], Martin et al. [64] | Fruit: raised stripes | present | present | absent | absent |
| This study | Seed: seed coat peeling | easy | easy | easy | easy |
| Trait | Traditional Sweet Chestnut Varieties | Hybrid Varieties | ANOVA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ‘Lovran Marron’ | ‘Marradi’ | ‘Bouche de Bétizac’ | ‘Marsol’ | ||||||||
| Mean Value ± SD | CV/% | Mean Value ± SD | CV/% | Mean Value ± SD | CV/% | Mean Value ± SD | CV/% | T/H | T | H | |
| w(moisture)/(g/100 g) | 55.8 ± 2.2 | 4.0 | 52.9 ± 1.5 | 2.9 | 62.5 ± 1.3 | 2.1 | 64.5 ± 1.2 | 1.8 | >0.01 | >0.01 | >0.05 |
| w(fat)/(g/100 g dm) | 2.6 ± 0.5 | 19.3 | 1.7 ± 0.3 | 18.7 | 1.3 ± 0.2 | 10.9 | 1.4 ± 0.3 | 20.0 | >0.01 | >0.01 | ns |
| w(protein)/(g/100 g dm) | 3.7 ± 0.4 | 9.4 | 4.6 ± 0.3 | 5.6 | 5.6 ± 0.8 | 14.8 | 5.0 ± 0.7 | 14.2 | >0.01 | >0.01 | ns |
| w(ash)/(g/100 g dm) | 2.3 ± 0.3 | 11.3 | 2.6 ± 0.2 | 7.1 | 2.6 ± 0.2 | 7.4 | 2.8 ± 0.2 | 9.1 | >0.01 | >0.05 | ns |
| w(total carbohydrate)/(g/100 g dm) | 91.4 ± 0.5 | 0.5 | 91.1 ± 0.4 | 0.4 | 90.4 ± 0.8 | 0.9 | 91.0 ± 1.0 | 1.1 | >0.01 | ns | ns |
| w(K)/(mg/100 g dm) | 1282.5 ± 194.3 | 15.2 | 1218.9 ± 205.1 | 16.8 | 1554.6 ± 48.5 | 3.1 | 1383.9 ± 58.7 | 4.2 | >0.01 | ns | >0.01 |
| w(Mg)/(mg/100 g dm) | 74.7 ± 5.1 | 6.8 | 80.3 ± 10.5 | 13.0 | 91.2 ± 5.2 | 5.7 | 97.9 ± 6.8 | 7.0 | ns | ns | ns |
| w(Ca)/(mg/100 g dm) | 79.1 ± 7.8 | 9.8 | 79.4 ± 16.5 | 20.7 | 56.9 ± 3.8 | 6.7 | 100.7 ± 5.8 | 5.7 | ns | ns | >0.01 |
| w(Na)/(mg/100 g dm) | 28.1 ± 5.6 | 19.9 | 18.4 ± 2.6 | 14.0 | 31.8 ± 2.5 | 7.9 | 39.2 ± 0.4 | 1.1 | >0.01 | >0.01 | >0.01 |
| w(Mn)/(mg/100 g dm) | 2.1 ± 1.0 | 49.3 | 4.8 ± 0.7 | 15.4 | 2.2 ± 0.6 | 27.3 | 1.0 ± 0.4 | 38.3 | >0.01 | >0.01 | >0.01 |
| w(Fe)/(mg/100 g dm) | 1.4 ± 0.4 | 27.5 | 1.2 ± 0.4 | 31.3 | 1.6 ± 0.1 | 5.9 | 2.0 ± 0.1 | 6.3 | >0.01 | ns | >0.01 |
| w(Zn)/(mg/100 g dm) | 1.5 ± 0.2 | 12.7 | 1.3 ± 0.3 | 22.0 | 1.4 ± 0.04 | 3.3 | 1.5 ± 0.1 | 6.5 | ns | ns | >0.05 |
| w(Cu)/(mg/100 g dm) | 0.9 ± 0.2 | 23.9 | 1.0 ± 0.2 | 17.0 | 1.3 ± 0.04 | 2.8 | 1.6 ± 0.1 | 11.5 | >0.01 | ns | ns |
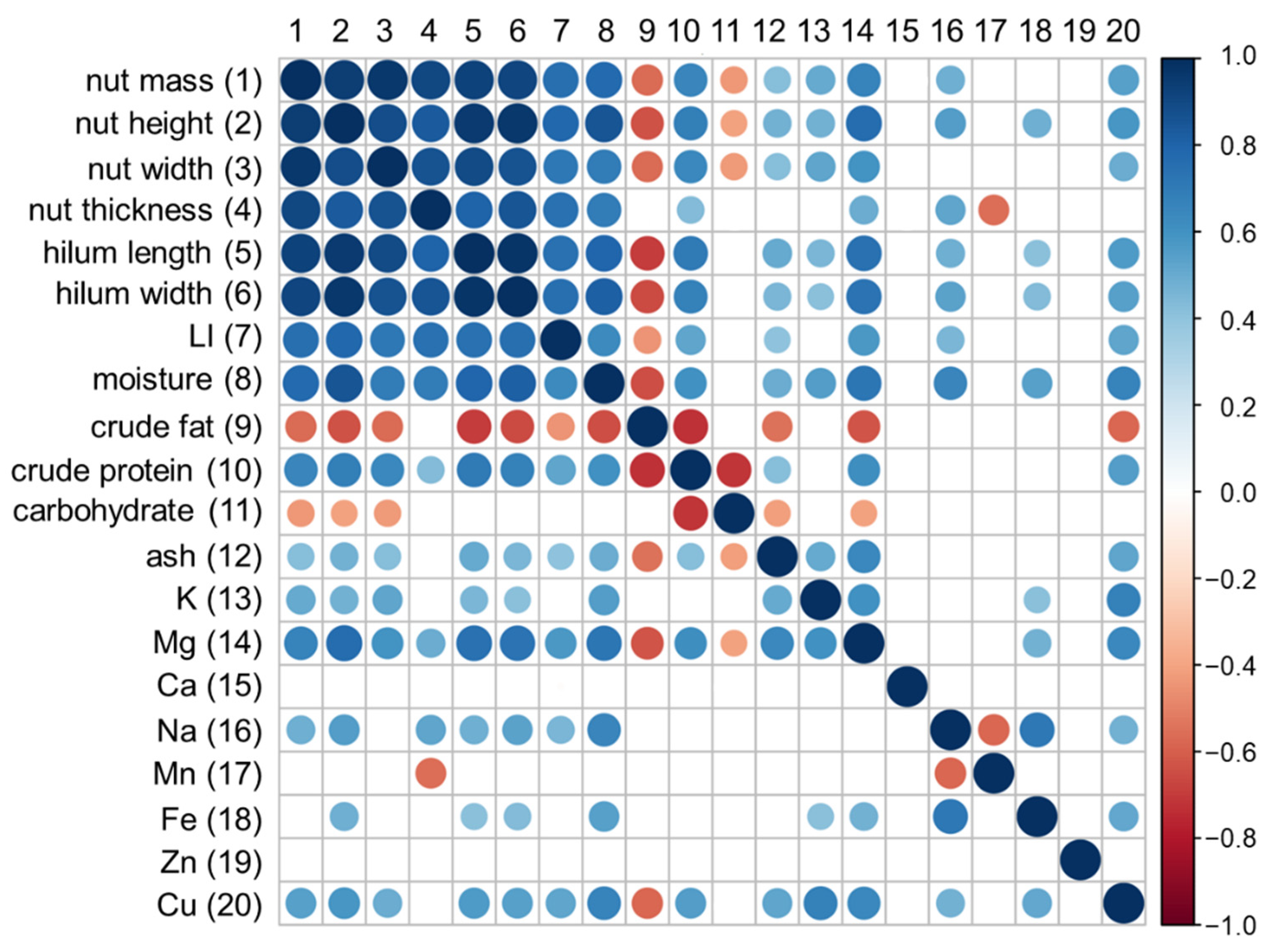
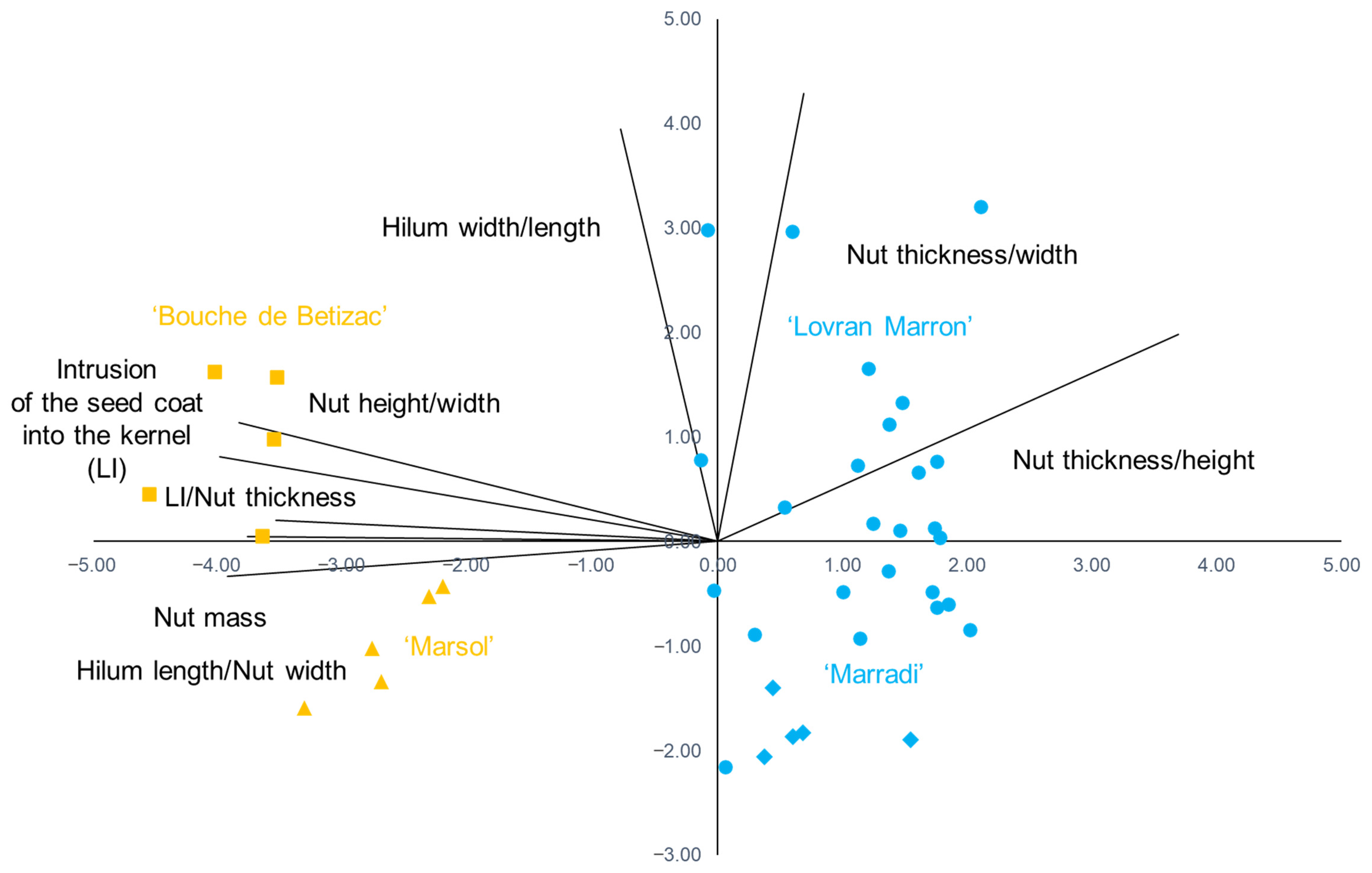
| Trait | PC—Principal Component | ||
|---|---|---|---|
| PC1 | PC2 | PC3 | |
| Nut mass | −0.817 | 0.011 | 0.093 |
| LI | −0.865 | 0.176 | 0.461 |
| Nut height/width | −0.831 | 0.247 | −0.407 |
| Nut thickness/height | 0.800 | 0.430 | 0.342 |
| Nut thickness/width | 0.150 | 0.929 | 0.010 |
| Hilum length/Nut width | −0.851 | −0.071 | −0.299 |
| Hilum width/length | −0.168 | 0.856 | −0.215 |
| LI/Nut thickness | −0.767 | 0.044 | 0.560 |
| Eigenvalue | 4.111 | 1.881 | 0.954 |
| % of variance | 51.39 | 23.51 | 11.93 |
| Trait | Principal Component | ||
|---|---|---|---|
| PC1 | PC2 | PC3 | |
| Moisture | −0.885 | 0.139 | −0.150 |
| Ash | −0.679 | −0.320 | 0.371 |
| Crude fat | 0.716 | 0.252 | −0.241 |
| Crude protein | −0.718 | −0.503 | −0.240 |
| Total carbohydrate | 0.462 | 0.523 | 0.408 |
| K | −0.694 | −0.025 | 0.059 |
| Mg | −0.870 | −0.046 | 0.133 |
| Ca | −0.175 | 0.462 | 0.547 |
| Na | −0.598 | 0.615 | −0.325 |
| Mn | 0.186 | −0.616 | 0.608 |
| Fe | −0.646 | 0.531 | 0.036 |
| Zn | −0.109 | 0.584 | 0.308 |
| Cu | −0.835 | −0.042 | 0.080 |
| Eigenvalue | 5.273 | 2.309 | 1.340 |
| % of variance | 40.56 | 17.76 | 10.31 |
| Trait | Wiks’ Lambda | Partial Lambda | F-Remove | p-Value |
|---|---|---|---|---|
| Hilum length/Nut width | 0.0026189 | 0.2781729 | 25.08390 | *** |
| Nut mass | 0.0026156 | 0.2785233 | 25.04018 | *** |
| Nut height/width | 0.0012205 | 0.5968889 | 6.528419 | ** |
| Nut thickness/height | 0.0009738 | 0.7480803 | 3.255298 | * |
| Nut thickness/width | 0.0009544 | 0.7632529 | 2.998424 | * |
| Hilum width/length | 0.0009509 | 0.7661024 | 2.951315 | * |
| LI | 0.0007851 | 0.9278293 | 0.751916 | ns |
| LI/Nut thickness | 0.0007623 | 0.9556403 | 0.448716 | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poljak, I.; Vahčić, N.; Vidaković, A.; Tumpa, K.; Žarković, I.; Idžojtić, M. Traditional Sweet Chestnut and Hybrid Varieties: Chemical Composition, Morphometric and Qualitative Nut Characteristics. Agronomy 2021, 11, 516. https://doi.org/10.3390/agronomy11030516
Poljak I, Vahčić N, Vidaković A, Tumpa K, Žarković I, Idžojtić M. Traditional Sweet Chestnut and Hybrid Varieties: Chemical Composition, Morphometric and Qualitative Nut Characteristics. Agronomy. 2021; 11(3):516. https://doi.org/10.3390/agronomy11030516
Chicago/Turabian StylePoljak, Igor, Nada Vahčić, Antonio Vidaković, Katarina Tumpa, Ivan Žarković, and Marilena Idžojtić. 2021. "Traditional Sweet Chestnut and Hybrid Varieties: Chemical Composition, Morphometric and Qualitative Nut Characteristics" Agronomy 11, no. 3: 516. https://doi.org/10.3390/agronomy11030516
APA StylePoljak, I., Vahčić, N., Vidaković, A., Tumpa, K., Žarković, I., & Idžojtić, M. (2021). Traditional Sweet Chestnut and Hybrid Varieties: Chemical Composition, Morphometric and Qualitative Nut Characteristics. Agronomy, 11(3), 516. https://doi.org/10.3390/agronomy11030516







