Novel Bacillus cereus Strain, ALT1, Enhance Growth and Strengthens the Antioxidant System of Soybean under Cadmium Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolation, Screening, and Identification
2.2. ALT1 Isolate Produces IAA and Organic Acids
2.3. Plant Growth Conditions
2.4. Endogenous Abscisic Acid and Salicylic Acid Quantification
2.5. Antioxidant Enzyme Activities
2.6. Determination of Cd and K Uptake by Plants
2.7. Statistical Analysis
3. Results
3.1. Bacterial Isolation, Screening, and Identification
3.2. In Vitro IAA and Organic Acid Production under Cd Stress
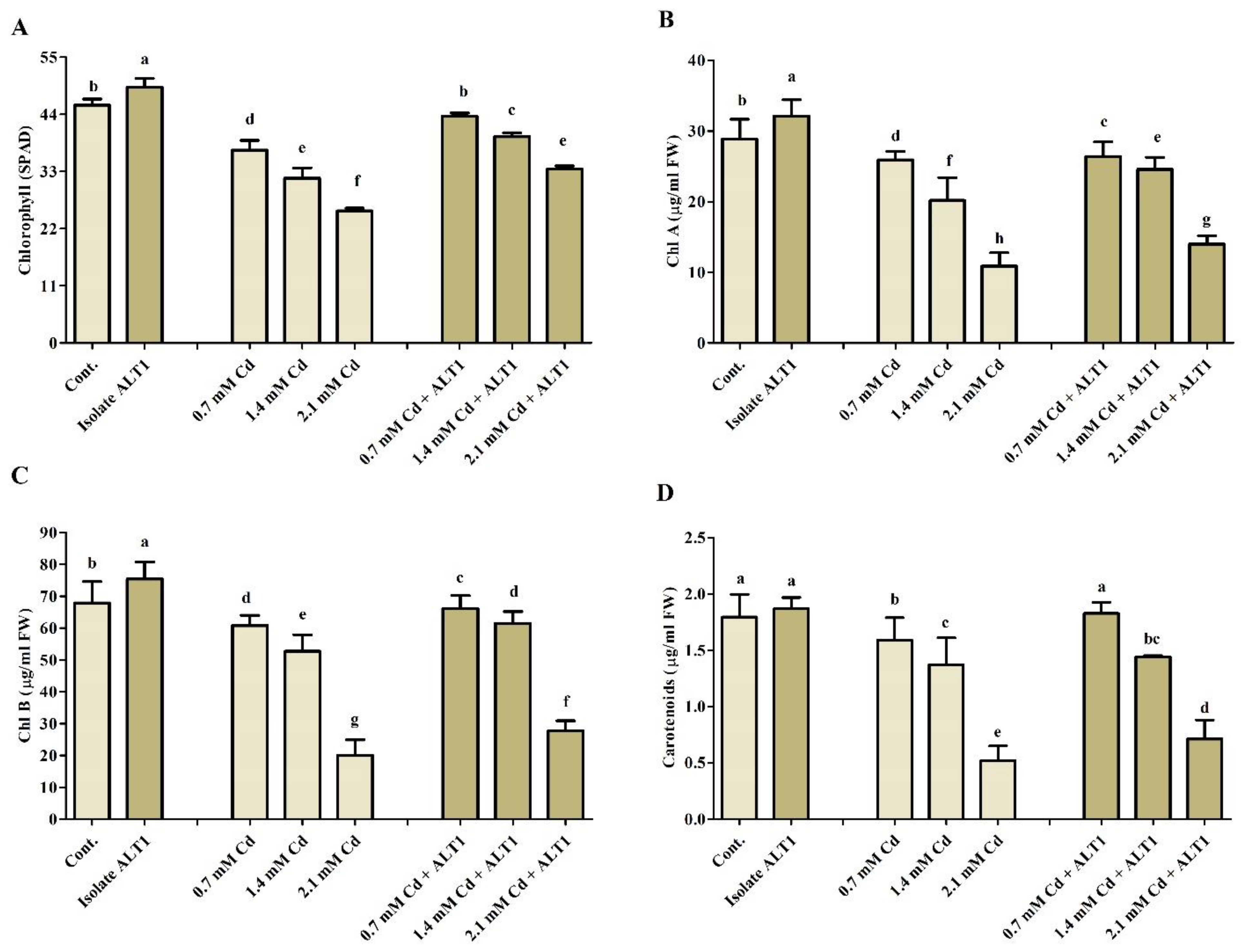
3.3. Bacterial Isolate ALT1 Regulates Soybean Growth under Cd Stress
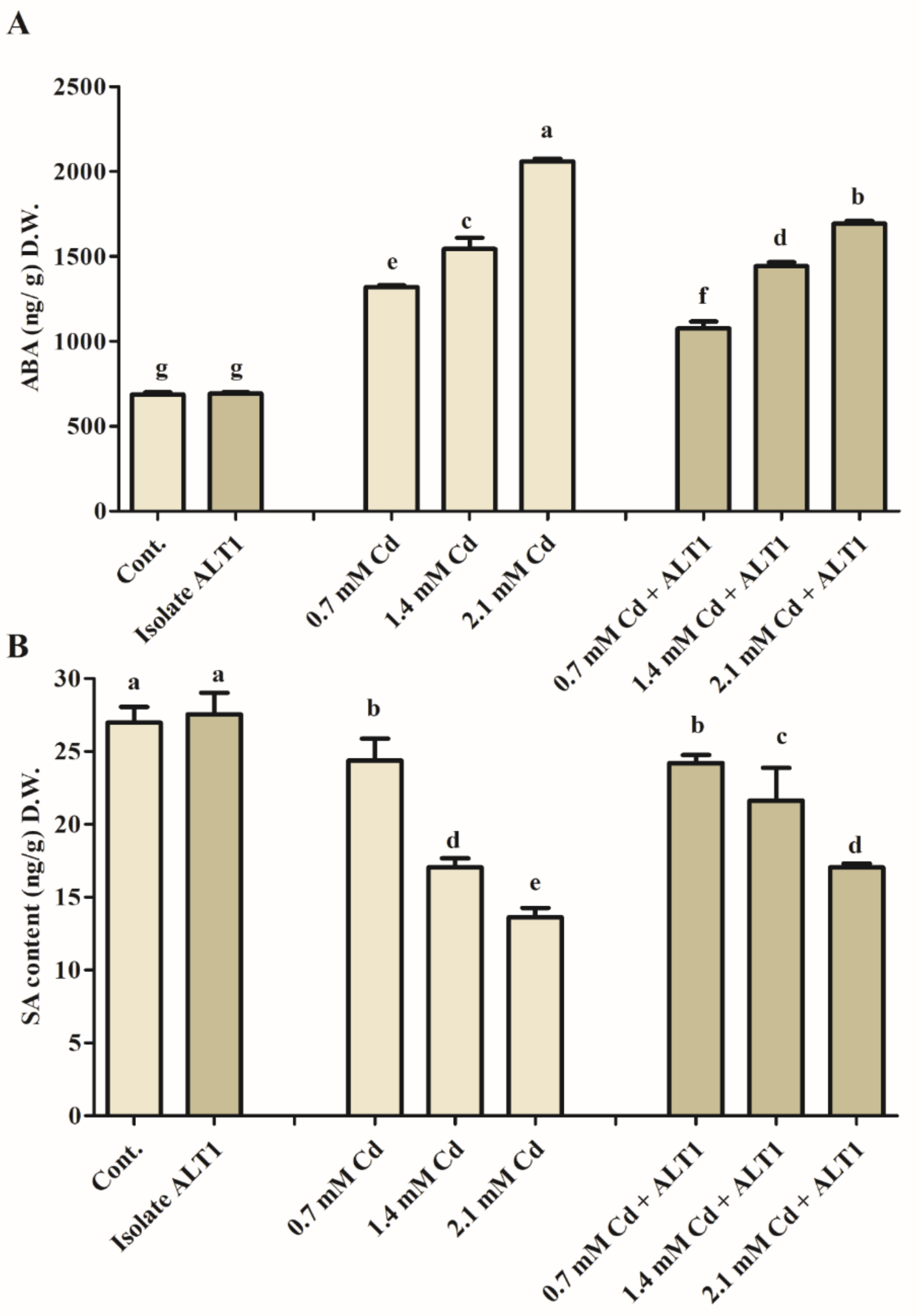
3.4. Effect of Cd Stress on Plant Endogenous ABA and SA Content
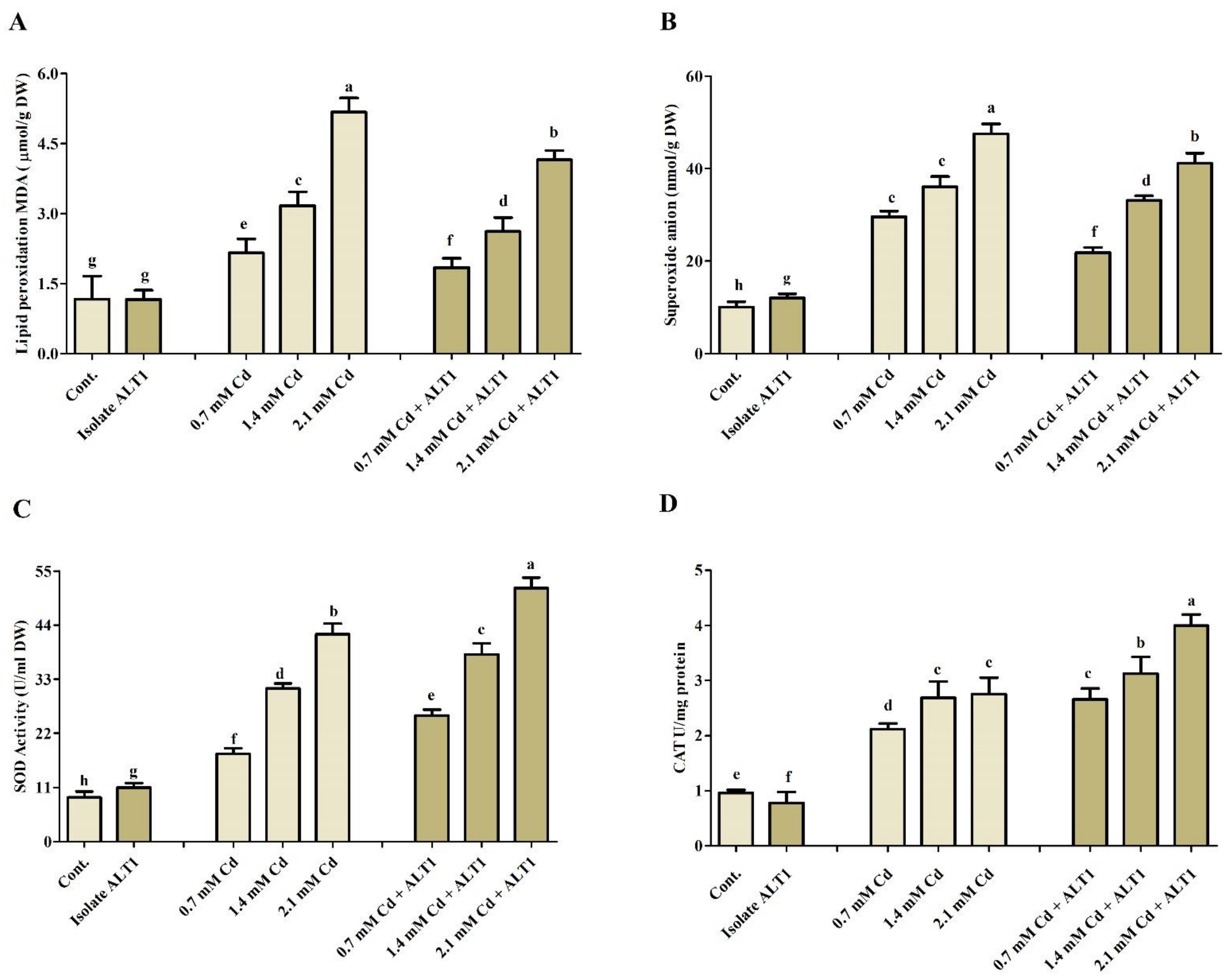
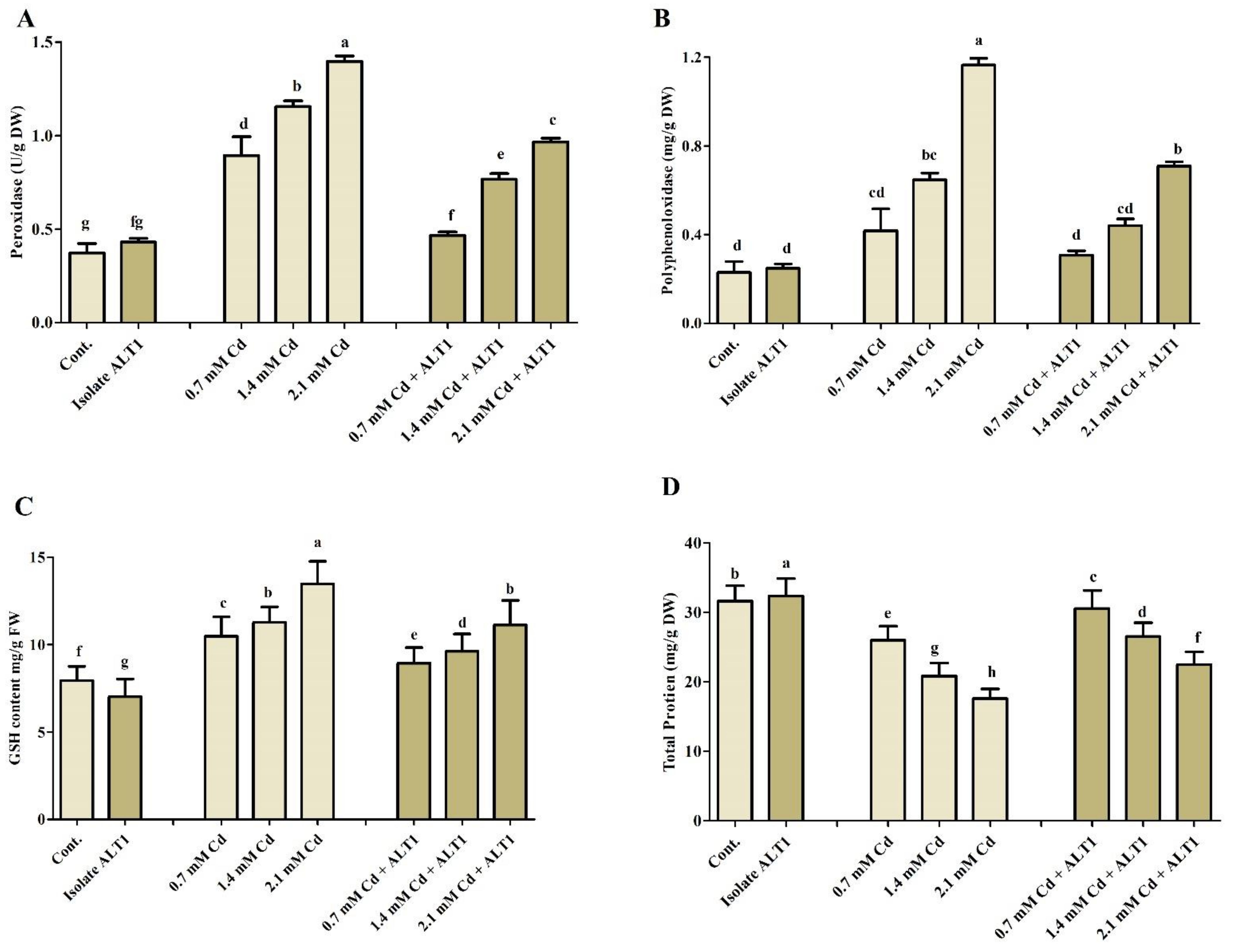
3.5. Effect of Cd Stress on Antioxidant Components in Soybean Plants
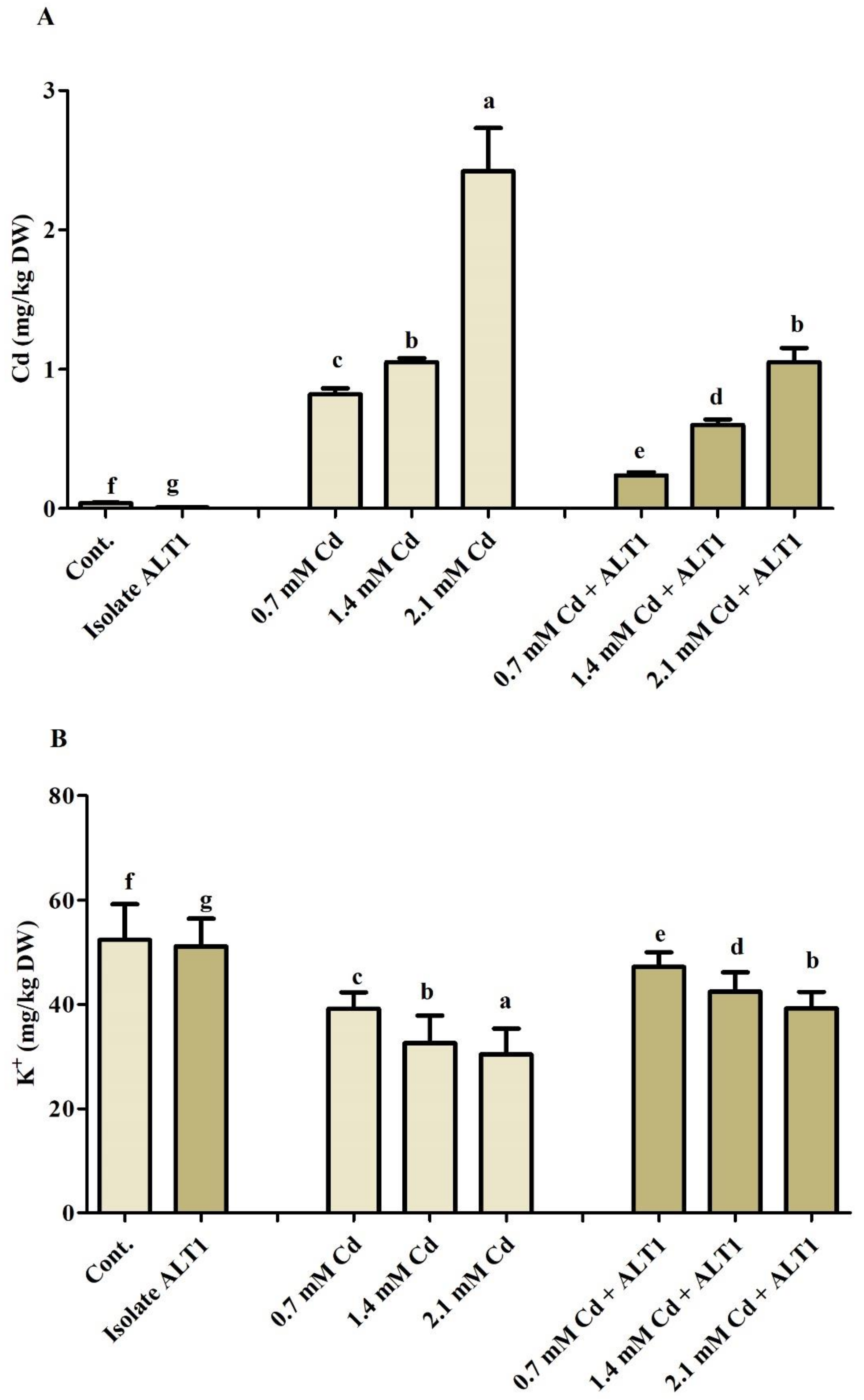
3.6. Role of Ion Uptake in Cd-Stressed Soybean Plants
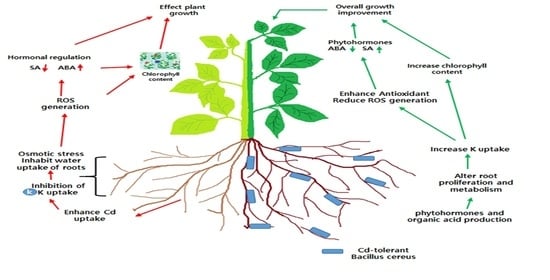
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Luo, S.; Li, X.; Wan, Y.; Chen, J.; Liu, C. Interaction of Cd-hyperaccumulator Solanum nigrum L. and functional endophyte Pseudomonas sp. Lk9 on soil heavy metals uptake. Soil Biol. Biochem. 2014, 68, 300–308. [Google Scholar] [CrossRef]
- Ahmad, I.; Akhtar, M.J.; Asghar, H.N.; Ghafoor, U.; Shahid, M. Differential Effects of Plant Growth-Promoting Rhizobacteria on Maize Growth and Cadmium Uptake. J. Plant Growth Regul. 2016, 35, 303–315. [Google Scholar] [CrossRef]
- Jan, M.; Shah, G.; Masood, S.; Shinwari, K.I.; Hameed, R.; Rha, E.S.; Jamil, M. Bacillus Cereus Enhanced Phytoremediation Ability of Rice Seedlings under Cadmium Toxicity. BioMed Res. Int. 2019, 2019, 8134651. [Google Scholar] [CrossRef]
- Shah, S.S.; Mohammad, F.; Shafi, M.; Bakht, J.; Zhou, W. Effects of cadmium and salinity on growth and photosynthesis parameters of Brassica species. Pak. J. Bot. 2011, 43, 333–340. [Google Scholar]
- Anjum, S.A.; Tanveer, M.; Hussain, S.; Ullah, E.; Wang, L.; Khan, I.; Samad, R.A.; Tung, S.A.; Anam, M.; Shahzad, B. Morpho-physiological growth and yield responses of two contrasting maize cultivars to cadmium exposure. Clean Soil Air Water 2016, 44, 29–36. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Rhizobacteria AK1 remediates the toxic effects of salinity stress via regulation of endogenous phytohormones and gene expression in soybean. Biochem. J. 2019, 476, 2393–2409. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Extending thermotolerance to tomato seedlings by inoculation with SA1 isolate of Bacillus cereus and comparison with exogenous humic acid application. PLoS ONE 2020, 15, e0232228. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Adhikari, A.; Jan, R.; Ali, S.; Imran, M.; Kim, K.-M.; Lee, I.-J. Halotolerant Rhizobacterial Strains Mitigate the Adverse Effects of NaCl Stress in Soybean Seedlings. BioMed Res. Int. 2019. [Google Scholar] [CrossRef]
- Chen, Y.; Chao, Y.; Li, Y.; Lin, Q.; Bai, J.; Tang, L.; Wang, S.; Ying, R.; Qiu, R. Survival Strategies of the Plant-Associated Bacterium Enterobacter sp. Strain EG16 under Cadmium Stress. Appl. Environ. Microbiol. 2016, 82, 1734–1744. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Jan, R.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Thermotolerance effect of plant growth-promoting Bacillus cereus SA1 on soybean during heat stress. BMC Microbiol. 2020, 20, 175. [Google Scholar] [CrossRef] [PubMed]
- Shamsi, I.H.; Jiang, L.; Wei, K.; Jilani, G.; Hua, S.; Zhang, G.-P. Alleviation of cadmium toxicity in soybean by potassium supplementation. J. Plant Nutr. 2010, 33, 1926–1938. [Google Scholar] [CrossRef]
- Wang, X.; Shi, M.; Hao, P.; Zheng, W.; Cao, F. Alleviation of cadmium toxicity by potassium supplementation involves various physiological and biochemical features in Nicotiana tabacum L. Acta Physiol. Plant. 2017, 39, 132. [Google Scholar] [CrossRef]
- Kamran, M.A.; Syed, J.H.; Eqani, S.A.M.A.S.; Munis, M.F.H.; Chaudhary, H.J. Effect of plant growth-promoting rhizobacteria inoculation on cadmium (Cd) uptake by Eruca sativa. Environ. Sci. Pollut. Res. 2015, 22, 9275–9283. [Google Scholar] [CrossRef]
- Evangelou, M.W.H.; Ebel, M.; Schaeffer, A. Chelate assisted phytoextraction of heavy metals from soil. Effect, mechanism, toxicity, and fate of chelating agents. Chemosphere 2007, 68, 989–1003. [Google Scholar] [CrossRef]
- Mitra, S.; Pramanik, K.; Ghosh, P.K.; Soren, T.; Sarkar, A.; Dey, R.S.; Pandey, S.; Maiti, T.K. Characterization of Cd-resistant Klebsiella michiganensis MCC3089 and its potential for rice seedling growth promotion under Cd stress. Microbiol. Res. 2018, 210, 12–25. [Google Scholar] [CrossRef]
- Pramanik, K.; Mitra, S.; Sarkar, A.; Soren, T.; Maiti, T.K. Characterization of cadmium-resistant Klebsiella pneumoniae MCC 3091 promoted rice seedling growth by alleviating phytotoxicity of cadmium. Environ. Sci. Pollut. Res. 2017, 24, 24419–24437. [Google Scholar] [CrossRef]
- Ahmad, I.; Akhtar, M.J.; Zahir, Z.A.; Naveed, M.; Mitter, B.; Sessitsch, A. Cadmium-tolerant bacteria induce metal stress tolerance in cereals. Environ. Sci. Pollut. Res. 2014, 21, 11054–11065. [Google Scholar] [CrossRef] [PubMed]
- Dell’Amico, E.; Cavalca, L.; Andreoni, V. Improvement of Brassica napus growth under cadmium stress by cadmium-resistant rhizobacteria. Soil Biol. Biochem. 2008, 40, 74–84. [Google Scholar] [CrossRef]
- Pandey, S.; Saha, P.; Barai, P.K.; Maiti, T.K. Characterization of a Cd2+-Resistant Strain of Ochrobactrum sp. Isolated from Slag Disposal Site of an Iron and Steel Factory. Curr. Microbiol. 2010, 61, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Belimov, A.A.; Hontzeas, N.; Safronova, V.I.; Demchinskaya, S.V.; Piluzza, G.; Bullitta, S.; Glick, B.R. Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biol. Biochem. 2005, 37, 241–250. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, J.; Wang, S.; Lin, Q.; Ruan, D.; Chi, H.; Zheng, M.; Chao, Y.; Qiu, R.; Yang, Y. Effects of cadmium-resistant plant growth-promoting rhizobacteria and Funneliformis mosseae on the cadmium tolerance of tomato (Lycopersicon esculentum L.). Int. J. Phytoremediation 2020, 22, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Hamayun, M.; Hussain, A.; Khan, S.A.; Kim, H.-Y.; Khan, A.L.; Waqas, M.; Irshad, M.; Iqbal, A.; Rehman, G.; Jan, S.; et al. Gibberellins Producing Endophytic Fungus Porostereum spadiceum AGH786 Rescues Growth of Salt Affected Soybean. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Khan, M.A.; Ullah, I.; Waqas, M.; Hamayun, M.; Khan, A.L.; Asaf, S.; Kang, S.-M.; Kim, K.-M.; Jan, R.; Lee, I.-J. Halo-tolerant rhizospheric Arthrobacter woluwensis AK1 mitigates salt stress and induces physio-hormonal changes and expression of GmST1 and GmLAX3 in soybean. Symbiosis 2019, 77, 9–21. [Google Scholar] [CrossRef]
- Noriega, G.O.; Balestrasse, K.B.; Batlle, A.; Tomaro, M.L. Cadmium induced oxidative stress in soybean plants also by the accumulation of delta-aminolevulinic acid. Biometals Int. J. Role Met. Ions Biol. Biochem. Med. 2007, 20, 841–851. [Google Scholar] [CrossRef] [PubMed]
- Kotoky, R.; Nath, S.; Maheshwari, D.K.; Pandey, P. Cadmium resistant plant growth promoting rhizobacteria Serratia marcescens S2I7 associated with the growth promotion of rice plant. Environ. Sustain. 2019, 2, 135–144. [Google Scholar] [CrossRef]
- Pramanik, K.; Mitra, S.; Sarkar, A.; Soren, T.; Maiti, T.K. Characterization of a Cd2+-resistant plant growth promoting rhizobacterium (Enterobacter sp.) and its effects on rice seedling growth promotion under Cd2+-stress in vitro. Agric. Nat. Resour. 2018, 52, 215–221. [Google Scholar] [CrossRef]
- Guo, J.; Chi, J. Effect of Cd-tolerant plant growth-promoting rhizobium on plant growth and Cd uptake by Lolium multiflorum Lam. and Glycine max (L.) Merr. in Cd-contaminated soil. Plant Soil 2014, 375, 205–214. [Google Scholar] [CrossRef]
- Begum, N.; Hu, Z.; Cai, Q.; Lou, L. Influence of PGPB Inoculation on HSP70 and HMA3 Gene Expression in Switchgrass under Cadmium Stress. Plants 2019, 8, 504. [Google Scholar] [CrossRef]
- Kim, Y.N.; Khan, M.A.; Kang, S.M.; Hamayun, M.; Lee, I.J. Enhancement of Drought-Stress Tolerance of Brassica oleracea var. italica L. by Newly Isolated Variovorax sp. YNA59. J. Microbiol. Biotechnol. 2020, 30, 1500–1509. [Google Scholar] [CrossRef]
- Khan, M.A.; Asaf, S.; Khan, A.L.; Adhikari, A.; Jan, R.; Ali, S.; Imran, M.; Kim, K.M.; Lee, I.J. Plant growth-promoting endophytic bacteria augment growth and salinity tolerance in rice plants. Plant Biol. 2020, 22, 850–862. [Google Scholar] [CrossRef]
- Khan, M.A.; Hamayun, M.; Iqbal, A.; Khan, S.A.; Hussain, A.; Asaf, S.; Khan, A.L.; Yun, B.-W.; Lee, I.-J. Gibberellin application ameliorates the adverse impact of short-term flooding on Glycine max L. Biochem. J. 2018. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Khan, M.A.; Asaf, S.; Lubna; Lee, I.-J.; Kim, K.M. Metal Resistant Endophytic Bacteria Reduces Cadmium, Nickel Toxicity, and Enhances Expression of Metal Stress Related Genes with Improved Growth of Oryza Sativa, via Regulating Its Antioxidant Machinery and Endogenous Hormones. Plants 2019, 8, 363. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Chaoui, A.; Mazhoudi, S.; Ghorbal, M.H.; El Ferjani, E. Cadmium and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in bean (Phaseolus vulgaris L.). Plant Sci. 1997, 127, 139–147. [Google Scholar] [CrossRef]
- Zhang, J.; Kirkham, M.B. Drought-Stress-Induced Changes in Activities of Superoxide Dismutase, Catalase, and Peroxidase in Wheat Species. Plant Cell Physiol. 1994, 35, 785–791. [Google Scholar] [CrossRef]
- Waqas, M.; Khan, A.L.; Kang, S.-M.; Kim, Y.-H.; Lee, I.-J. Phytohormone-producing fungal endophytes and hardwood-derived biochar interact to ameliorate heavy metal stress in soybeans. Biol. Fertil. Soils 2014, 50, 1155–1167. [Google Scholar] [CrossRef]
- Khanna, K.; Jamwal, V.L.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R. Metal resistant PGPR lowered Cd uptake and expression of metal transporter genes with improved growth and photosynthetic pigments in Lycopersicon esculentum under metal toxicity. Sci. Rep. 2019, 9, 5855. [Google Scholar] [CrossRef]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Chiboub, M.; Saadani, O.; Fatnassi, I.C.; Abdelkrim, S.; Abid, G.; Jebara, M.; Jebara, S.H. Characterization of efficient plant-growth-promoting bacteria isolated from Sulla coronaria resistant to cadmium and to other heavy metals. Comptes Rendus Biol. 2016, 339, 391–398. [Google Scholar] [CrossRef]
- Tran, T.A.; Popova, L.P. Functions and toxicity of cadmium in plants: Recent advances and future prospects. Turk. J. Bot. 2013, 37, 1–13. [Google Scholar]
- Chen, L.; Luo, S.; Xiao, X.; Guo, H.; Chen, J.; Wan, Y.; Li, B.; Xu, T.; Xi, Q.; Rao, C.; et al. Application of plant growth-promoting endophytes (PGPE) isolated from Solanum nigrum L. for phytoextraction of Cd-polluted soils. Appl. Soil Ecol. 2010, 46, 383–389. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, X.; Li, C.; Nan, Z. Effects of cadmium stress on seed germination, seedling growth and antioxidative enzymes in Achnatherum inebrians plants infected with a Neotyphodium endophyte. Plant Growth Regul. 2010, 60, 91–97. [Google Scholar] [CrossRef]
- Haneef, I.; Faizan, S.; Perveen, R.; Kausar, S. Impact of bio-fertilizers and different levels of cadmium on the growth, biochemical contents and lipid peroxidation of Plantago ovata Forsk. Saudi J. Biol. Sci. 2014, 21, 305–310. [Google Scholar] [CrossRef]
- Kunito, T.; Saeki, K.; Nagaoka, K.; Oyaizu, H.; Matsumoto, S. Characterization of copper-resistant bacterial community in rhizosphere of highly copper-contaminated soil. Eur. J. Soil Biol. 2001, 37, 95–102. [Google Scholar] [CrossRef]
- Glick, B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010, 28, 367–374. [Google Scholar] [CrossRef]
- Mukherjee, P.; Mitra, A.; Roy, M. Halomonas Rhizobacteria of Avicennia marina of Indian Sundarbans Promote Rice Growth under Saline and Heavy Metal Stresses through Exopolysaccharide Production. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Awasthi, S.; Srivastava, P.; Mishra, P.K. Application of EPS in agriculture: An important natural resource for crop improvement. Agric. Res. Technol. Open Access J. 2017, 8, 1–3. [Google Scholar] [CrossRef]
- Llamas, I.; Del Moral, A.; Martínez-Checa, F.; Arco, Y.; Arias, S.; Quesada, E. Halomonas maura is a physiologically versatile bacterium of both ecological and biotechnological interest. Antonie Van Leeuwenhoek 2006, 89, 395–403. [Google Scholar] [CrossRef]
- Chaca, M.V.P.; Vigliocco, A.; Reinoso, H.; Molina, A.; Abdala, G.; Zirulnik, F.; Pedranzani, H. Effects of cadmium stress on growth, anatomy and hormone contents in Glycine max (L.) Merr. Acta Physiol. Plant. 2014, 36, 2815–2826. [Google Scholar] [CrossRef]
- Dawuda, M.M.; Liao, W.; Hu, L.; Yu, J.; Xie, J.; Calderón-Urrea, A.; Wu, Y.; Tang, Z. Foliar application of abscisic acid mitigates cadmium stress and increases food safety of cadmium-sensitive lettuce (Lactuca sativa L.) genotype. PeerJ 2020, 8, e9270. [Google Scholar] [CrossRef]
- Hsu, Y.T.; Kao, C.H. Distinct roles of abscisic acid in rice seedlings during cadmium stress at high temperature. Bot. Stud. 2008, 49, 335–342. [Google Scholar]
- López-Climent, M.F.; Arbona, V.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Effects of cadmium on gas exchange and phytohormone contents in citrus. Biol. Plant. 2011, 55, 187–190. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.; Nan, Z. Effects of cadmium stress on growth and anti-oxidative systems in Achnatherum inebrians symbiotic with Neotyphodium gansuense. J. Hazard. Mater. 2010, 175, 703–709. [Google Scholar] [CrossRef]
- Ali, A.; Bilal, S.; Khan, A.L.; Mabood, F.; Al-Harrasi, A.; Lee, I.-J. Endophytic Aureobasidium pullulans BSS6 assisted developments in phytoremediation potentials of Cucumis sativus under Cd and Pb stress. J. Plant Interact. 2019, 14, 303–313. [Google Scholar] [CrossRef]

| RL (cm) | SL (cm) | FW (g) | DW (g) | |
|---|---|---|---|---|
| Control | 21 ± 1.2 b | 22.3 ± 0.6 b | 13.3 ± 1.5 b | 3.54 ± 0.01 b |
| Isolate ALT1 | 25 ± 1.1 a | 26.0 ± 1.4 a | 16.9 ± 1.5 a | 5.22 ± 0.25 a |
| 0.7 mM Cd | 15.2 ± 0.9 cd | 14.5 ± 1.3 d | 11.9 ± 0.8 b | 2.84 ± 0.10 d |
| 1.4 mM Cd | 12.1 ± 0.7 e | 11.1 ± 1.2 e | 9.2 ± 0.7 c | 2.25 ± 0.17 ef |
| 2.1 mM Cd | 9 ± 0.8 f | 10.1 ± 1.1 e | 7.4 ± 1.1 c | 1.88 ± 0.40 f |
| 0.7 mM + ALT1 | 17.38 ± 1.1 c | 18.3 ± 1.0 c | 12.5 ± 1.5 b | 4.16 ± 0.36 b |
| 1.4 mM + ALT1 | 15 ± 0.5 d | 17.2 ± 1.2 c | 12.4 ± 1.4 b | 3.87 ± 0.11 c |
| 2.1 mM + ALT1 | 14 ± 0.5 de | 13.4 ± 0.9 d | 9.5 ± 1.0 c | 2.56 ± 0.11 de |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahile, A.A.; Khan, M.A.; Hamayun, M.; Imran, M.; Kang, S.-M.; Lee, I.-J. Novel Bacillus cereus Strain, ALT1, Enhance Growth and Strengthens the Antioxidant System of Soybean under Cadmium Stress. Agronomy 2021, 11, 404. https://doi.org/10.3390/agronomy11020404
Sahile AA, Khan MA, Hamayun M, Imran M, Kang S-M, Lee I-J. Novel Bacillus cereus Strain, ALT1, Enhance Growth and Strengthens the Antioxidant System of Soybean under Cadmium Stress. Agronomy. 2021; 11(2):404. https://doi.org/10.3390/agronomy11020404
Chicago/Turabian StyleSahile, Atlaw Anbelu, Muhammad Aaqil Khan, Muhammad Hamayun, Muhammad Imran, Sang-Mo Kang, and In-Jung Lee. 2021. "Novel Bacillus cereus Strain, ALT1, Enhance Growth and Strengthens the Antioxidant System of Soybean under Cadmium Stress" Agronomy 11, no. 2: 404. https://doi.org/10.3390/agronomy11020404
APA StyleSahile, A. A., Khan, M. A., Hamayun, M., Imran, M., Kang, S.-M., & Lee, I.-J. (2021). Novel Bacillus cereus Strain, ALT1, Enhance Growth and Strengthens the Antioxidant System of Soybean under Cadmium Stress. Agronomy, 11(2), 404. https://doi.org/10.3390/agronomy11020404