Salinity Stress as an Elicitor for Phytochemicals and Minerals Accumulation in Selected Leafy Vegetables of Brassicaceae
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growing and Salt Treatments
2.2. Determination of Proline Content
2.3. Determination of Polyphenolic Compounds
2.4. Determination of Pigments Content
2.5. Determination of Glucosinolate Content
2.6. Determination of Elemental Content
2.7. Statistical Analysis
3. Results
3.1. Proline and K+/Na+ Ratio
3.2. Pigment Concentration
3.3. Specialized Metabolites Level
3.4. Element Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Lim, J.-H.; Park, K.-J.; Kim, B.-K.; Jeong, J.-W.; Kim, H.-J. Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chem. 2012, 135, 1065–1070. [Google Scholar] [CrossRef]
- Thakur, M.; Bhattacharya, S.; Khosla, P.K.; Puri, S. Improving production of plant secondary metabolites through biotic and abiotic elicitation. J. Appl. Res. Med. Aromat. Plants 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Response of nutrients, minerals, antioxidant leaf pigments, vitamins, polyphenol, flavonoid and antioxidant activity in selected vegetable amaranth under four soil water content. Food Chem. 2018, 252, 72–83. [Google Scholar] [CrossRef]
- Zia, M.; Zafar, H.; Gul, F.Z.; Abbasi, B.H.; Rizvi, Z.F.; Mannan, A. Synergistic influence of CuO nanoparticles and spectral lights transforms biomass, antioxidative response, and antioxidants in Brassica nigra. Plant. Cell Tiss. Organ. Cult 2021, in press. [Google Scholar] [CrossRef]
- Hassini, I.; Rios, J.J.; Garcia-Ibañez, P.; Baenas, N.; Carvajal, M.; Moreno, D.A. Comparative effect of elicitors on the physiology and secondary metabolites in broccoli plants. J. Plant Physiol. 2019, 239, 1–9. [Google Scholar] [CrossRef]
- Rouphael, Y.; Petropoulos, S.A.; Cardarelli, M.; Colla, G. Salinity as eustressor for enhancing quality of vegetables. Sci. Hortic. 2018, 234, 361–369. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Leaf pigmentation, its profiles and radical scavenging activity in selected Amaranthus tricolor leafy vegetables. Sci. Rep. 2020, 10, 18617. [Google Scholar] [CrossRef] [PubMed]
- Moyo, S.M.; Serem, J.C.; Bester, M.J.; Mavumengwana, V.; Kayitesi, E. African Green Leafy Vegetables Health Benefits Beyond Nutrition. Food Rev. Int. 2020, in press. [Google Scholar] [CrossRef]
- Šamec, D.; Pavlović, I.; Radojčić Redovniković, I.; Salopek-Sondi, B. Comparative analysis of phytochemicals and activity of endogenous enzymes associated with their stability, bioavailability and food quality in five Brassicaceae sprouts. Food Chem. 2018, 269, 96–102. [Google Scholar] [CrossRef]
- Punchay, K.; Inta, A.; Tiansawat, P.; Balslev, H.; Wangpakapattanawong, P. Nutrient and Mineral Compositions ofWild Leafy Vegetables of the Karen and Lawa Communities in Thailand. Foods 2020, 9, 1748. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Salopek-Sondi, B. Cruciferous (Brassicaceae) vegetables. In Nonvitamin and Nonmineral Nutritional Supplements; Nabavi, S.M., Sanches Silva, T., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 195–202. [Google Scholar]
- Šamec, D.; Kruk, V.; Ivanišević, P. Influence of seed origin on morphological characteristics and phytochemicals levels in Brassica oleracea var. acephala. Agronomy 2019, 9, 502. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Augmentation of leaf color parameters, pigments, vitamins, phenolic acids, flavonoids and antioxidant activity in selected Amaranthus tricolor under salinity stress. Sci. Rep. 2018, 8, 12349. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Salinity stress enhances color parameters, bioactive leaf pigments, vitamins, polyphenols, flavonoids and antioxidant activity in selected Amaranthus leafy vegetables. J. Sci. Food Agric. 2018, 99, 2275–2284. [Google Scholar] [CrossRef]
- Sarker, U.; Islam, T.; Oba, S. Salinity stress accelerates nutrients, dietary fiber, minerals, phytochemicals and antioxidant activity in Amaranthus tricolor leaves. PLoS ONE 2018, 13, e0206388. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, I.; Mlinarić, S.; Tarkowská, D.; Oklestkova, J.; Novák, O.; Lepeduš, H.; Vujčić Bok, V.; Radić Brkanac, S.; Strnad, M.; Salopek-Sondi, B. Early Brassica crops responses to salinity stress: A comparative analysis between Chinese cabbage, white cabbage and kale. Front. Plant Sci. 2019, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Linić, I.; Šamec, D.; Grúz, J.; Vujčić Bok, V.; Strnad, M.; Salopek Sondi, B. Involvement of Phenolic Acids in Short-Term Adaptation to Salinity Stress is Species-Specific among Brassicaceae. Plants 2019, 8, 155. [Google Scholar] [CrossRef]
- Falcinelli, B.; Sileoni, V.; Marconi, O.; Perretti, G.; Quinet, M.; Lutts, S.; Benincasa, P. Germination under Moderate Salinity Increases Phenolic Content and Antioxidant Activity in Rapeseed (Brassica napus var oleifera Del.) Sprouts. Molecules 2017, 22, 1377. [Google Scholar] [CrossRef]
- Guo, L.; Yang, R.; Wang, Z.; Guo, Q.; Gu, Z. Effect of NaCl stress on health-promoting compounds and antioxidant activity in the sprouts of three broccoli cultivars. Int. J. Food Sci. Nutr. 2014, 65, 476–481. [Google Scholar] [CrossRef]
- Yuan, G.; Wang, X.; Guo, R.; Wang, Q. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem. 2010, 121, 1014–1019. [Google Scholar] [CrossRef]
- Šamec, D.; Bogović, M.; Vincek, D.; Martinčić, J.; Salopek-Sondi, B. Assessing the authenticity of the white cabbage (Brassica oleracea var. capitata f. alba) cv. ‘Varaždinski’ by molecular and phytochemical markers. Food Res. Int. 2014, 60, 266–272. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV–VIS spectroscopy. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; p. 4. [Google Scholar]
- Aghajanzadeh, T.; Hawkesford, M.J.; Kok, L.J. The significance of glucosinolates for sulfur storage in Brassicaceae seedlings. Front. Plant Sci. 2014, 5, 704. [Google Scholar] [CrossRef]
- Fiket, Ž.; Mikac, N.; Kniewald, G. Mass fractions of forty-six major and trace elements, including rare earth elements, in sediment and soil reference materials used in environmental studies. Geostand. Geoanalytical. Res. 2016, 41, 123–135. [Google Scholar] [CrossRef]
- Keisham, M.; Mukherjee, S.; Bhatla, S.C. Mechanisms of sodium transport in plants—progresses and challenges. Int. J. Mol. Sci. 2018, 19, 647. [Google Scholar] [CrossRef] [PubMed]
- Negrao, S.; Schmockel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef]
- Boscaiu, M.; Lull, C.; Llinares, J.; Vicente, O.; Boira, H. Proline as a biochemical marker in relation to the ecology of two halophytic Juncus species. J. Plant Ecol. 2013, 6, 177–186. [Google Scholar] [CrossRef]
- Zaghdoud, C.; Alcaraz-Lopez, C.; Mota-Cadenas, C.; Martınez-Ballesta, M.C.; Moreno, D.A.; Ferchichi, A.; Carvajal, M. Differential Responses of Two Broccoli (Brassica oleracea L. var Italica) Cultivars to Salinity and Nutritional Quality Improvement. Sci. World J. 2012, 2012, 291435. [Google Scholar] [CrossRef]
- Sanoubar, R.; Cellini, A.; Veroni, A.M.; Spinelli, F.; Masia, A.; Antisari, L.V.; Orsini, F.; Gianquinto, G. Salinity thresholds and genotypic variability of cabbage (Brassica oleracea L.) grown under saline stress. J. Sci. Food Agric. 2016, 96, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić-Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef]
- Dominguez-Perles, R.; CarmenMartinez-Ballesta, M.; Riquelme, F.; Carvajal, M.; Garcia-Viguera, C.; Morenoa, D.A. Novel varieties of broccoli for optimal bioactive components under saline stress. J. Sci. Food Agric 2011, 9, 1638–1647. [Google Scholar] [CrossRef] [PubMed]
- Aghajanzadeh, T.A.; Reich, M.; Kopriva, S.; De Kok, L.J. Impact of chloride (NaCl, KCl) and sulphate (Na2SO4, K2SO4) salinity on glucosinolate metabolism in Brassica rapa. J. Agron Crop Sci. 2018, 204, 137–146. [Google Scholar] [CrossRef]
- Petretto, G.L.; Urgeghe, P.P.; Massa, D.; Melito, S. Effect of salinity (NaCl) on plant growth, nutrient content, and glucosinolates hydrolysis products trends in rocket genotypes. Plant Physiol. Biochem. 2019, 141, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wan, S.; Zhou, J.; Yang, Z.; Qin, P. Leaf chlorophyll fluorescence, hyperspectral reflectance, pigments content, malondialdehyde and proline accumulation responses of castor bean (Ricinus communis L.) seedlings to salt stress levels. Ind. Crop. Prod. 2010, 31, 13–19. [Google Scholar] [CrossRef]
- Gengmao, Z.; Yu, H.; Xing, S.; Shihui, L.; Quanmei, S.; Changhai, W. Salinity stress increases secondary metabolites and enzyme activity in safflower. Ind. Crop. Prod. 2015, 64, 175–181. [Google Scholar] [CrossRef]
- Akcin, A.; Yalcin, E. Effect of salinity stress on chlorophyll, carotenoid content, and proline in Salicornia prostrata Pall. and Suaeda prostrata Pall. subsp. prostrata (Amaranthaceae). Braz J. Bot. 2016, 39, 101–106. [Google Scholar] [CrossRef]
- Alam, A.; Juraimi, A.S.; Rafii, M.Y.; Hamid, A.A.; Aslani, F.; Alam, M.Z. Effects of salinity and salinity-induced augmented bioactive compounds in purslane (Portulaca oleracea L.) for possible economical use. Food Chem. 2015, 169, 439–447. [Google Scholar] [CrossRef]
- Pascale, S.D.; Maggio, A.; Fogliano, V.; Ambrosino, P.; Ritieni, A. Irrigation with saline water improves carotenoids content and antioxidant activity of tomato. J. Hortic. Sci. Biotechnol. 2001, 76, 447–453. [Google Scholar] [CrossRef]
- USDA, U.S. Department of Agriculture. Dietery guidelines for Americans 2015–2020, 8th ed. 2015. Available online: https://health.gov/dietaryguidelines/2015/resources/2015-2020_Dietary_Guidelines.pdf (accessed on 14 December 2020).
- Lucarini, M.; Canali, R.; Cappelloni, M.; Lullo, G.D.; Lombardi-Boccia, G. In vitro calcium availability from brassica vegetables (Brassica oleracea L.) and as consumed in composite dishes. Food Chem. 1999, 64, 519–523. [Google Scholar]
- Kamchan, A.; Puwastien, P.; Sirichakwal, P.P.; Kongkachuichai, R. In vitro calcium bioavailability of vegetables, legumes and seeds. J. Food Compos. Anal. 2004, 17, 311–320. [Google Scholar] [CrossRef]
- Armesto, J.; Gomez-Limia, L.; Carballo, J.; Martinez, S. Effects of different cooking methods on the antioxidant capacity and flavonoid, organic acid and mineral contents of Galega Kale (Brassica oleracea var. acephala cv. Galega). Int. J. Food Sci. Nutr. 2019, 70, 136–149. [Google Scholar] [CrossRef]
- Šamec, D.; Urlić, B.; Salopek-Sondi, B. Kale (Brassica oleracea var. acephala) as a superfood: Review of the scientific evidence behind the statement. Crit. Rev. Food Sci. Nutr. 2019, 59, 2411–2422. [Google Scholar] [CrossRef] [PubMed]
- U.S Department of Agriculture, Agricultural Research Service. FoodData Central. 2019. Available online: https://fdc.nal.usda.gov/ (accessed on 20 December 2020.).
- Grieve, C.M.; Shannon, M.C.; Poss, J.A. Mineral Nutrition of Leafy Vegetable Crops Irrigated with Saline Drainage Water. J. Veg. Crop. Prod. 2001, 7, 37–47. [Google Scholar] [CrossRef]
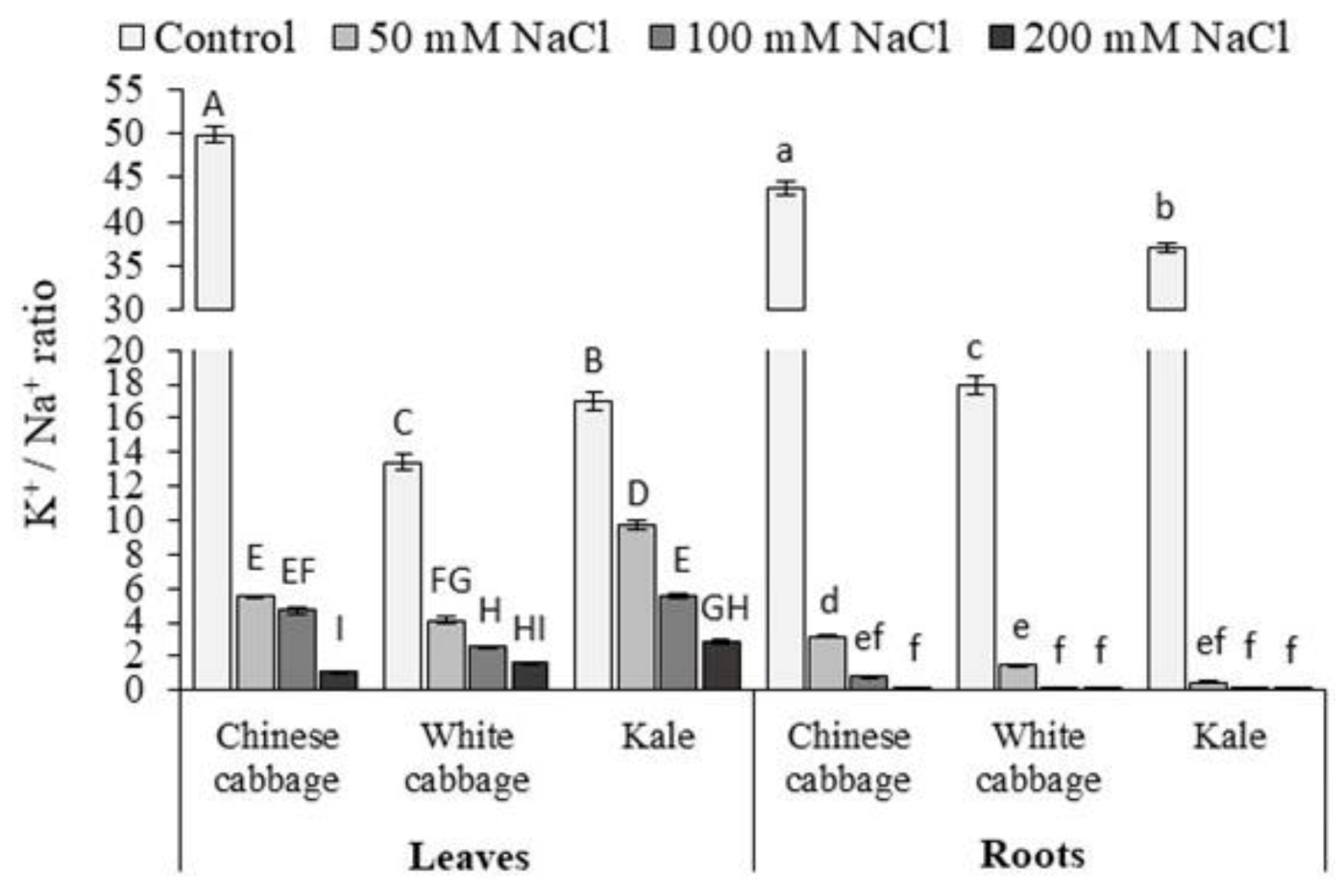
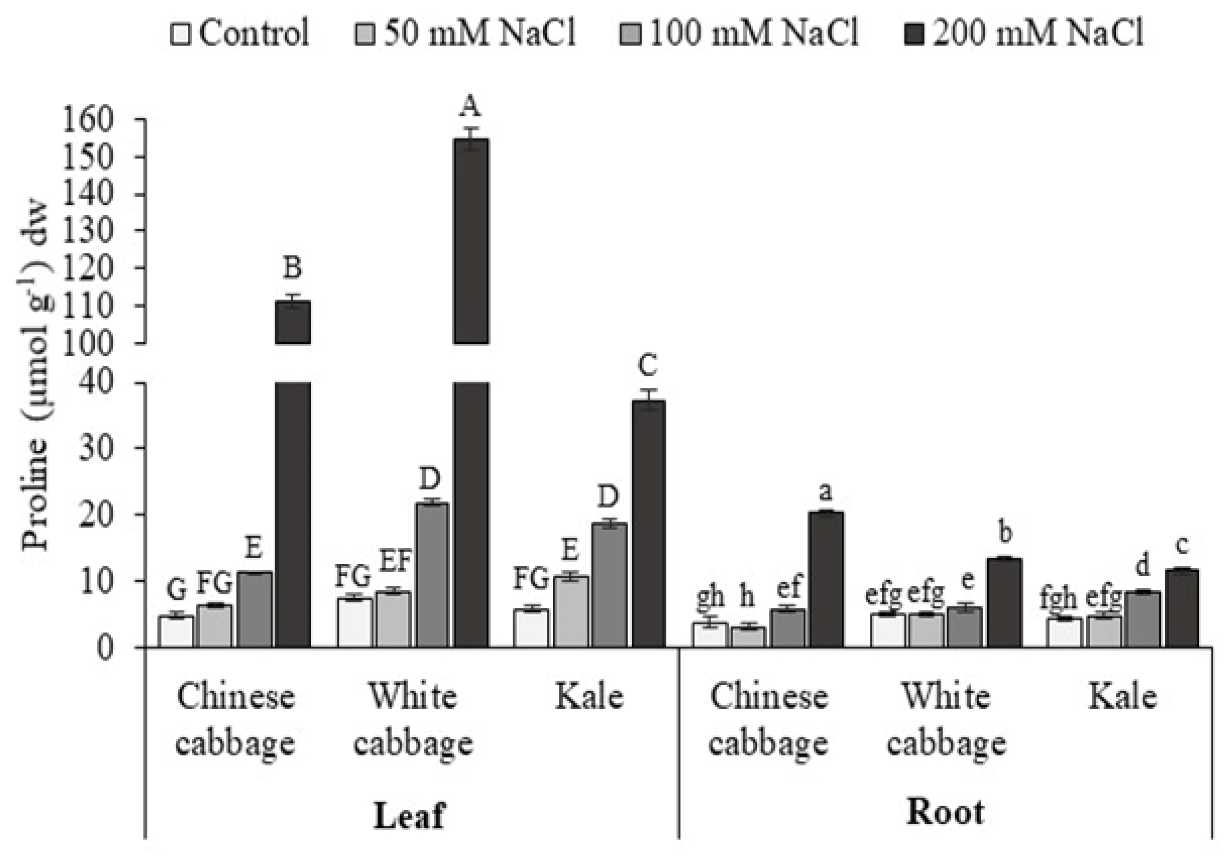
| Brassica Leafy Vegetables | NaCl (mM) | Chlorophyll a | Chlorophyll b | Total Chlorophylls |
|---|---|---|---|---|
| µg g−1 dw | µg g−1 dw | µg g−1 dw | ||
| Chinese cabbage | 0 | 4.67 ± 0.46 cd | 4.30 ± 0.53 abc | 8.97 ± 0.95 cd |
| 50 | 5.92 ± 0.25 bc | 4.91 ± 0.11 ab | 10.83 ± 0.30 abc | |
| 100 | 7.20 ± 0.20 ab | 5.04 ± 0.11 a | 12.24 ± 0.10 a | |
| 200 | 3.71 ± 024 d | 4.55 ± 0.56 abc | 8.26 ± 0.60 de | |
| White cabbage | 0 | 4.69 ± 0.40 cd | 2.17 ± 0.87 de | 6.86 ± 0.76 ef |
| 50 | 6.31 ± 0.40 ab | 3.22 ± 0.57 cd | 9.52 ± 0.20 cd | |
| 100 | 6.84 ± 0.45 ab | 3.71 ± 0.78 abc | 10.55 ± 0.34 abc | |
| 200 | 3.66 ± 0.54 d | 1.60 ± 0.33 e | 5.26 ± 0.86 f | |
| Kale | 0 | 7.21 ± 0.19 a | 3.50 ± 0.48 bcd | 10.71 ± 0.66 abc |
| 50 | 7.04 ± 0.45 ab | 3.51 ± 0.26 bcd | 10.54 ± 0.59 abc | |
| 100 | 6.77 ± 0.31 ab | 3.50 ± 0.26 bcd | 10.27 ± 0.20 bc | |
| 200 | 7.33 ± 0.88 a | 4.23 ± 0.41 abc | 11.56 ± 1.26 ab |
| NaCl mM | Total Phenols mg GAE g−1 dw | Total Phenolic Acids mg CAE g−1 dw | Total Flavonoids mg CE g−1 dw | Total Flavanols µg CE g−1 dw | Total Glucosinolates mg sin g−1 dw | Carotenoids µg g−1 dw | ||
|---|---|---|---|---|---|---|---|---|
| leaves | Chinese cabbage | 0 | 19.16 ± 0.63 G | 3.67 ± 0.05 BCD | 5.36 ± 0.08 C | 43.48 ± 2.84 EF | 41.79 ± 2.52 G | 0.35 ± 0.06 C |
| 50 | 22.83 ± 0.18 D | 3.86 ± 0.22 AB | 5.93 ± 0.16 AB | 53.17 ± 1.82 BC | 48.21 ± 1.34 F | 0.70 ± 0.05 BC | ||
| 100 | 24.93 ± 0.25 C | 4.17 ± 0.10 A | 5.09 ± 0.03 CD | 41.20 ± 2.03 F | 49.92 ± 1.88 EF | 0.74 ± 0.09 BC | ||
| 200 | 20.71 ± 0.34 F | 3.38 ± 0.07 DE | 5.74 ± 0.13 B | 50.44 ± 2.97 BCD | 60.77 ± 0.56 BC | 0.27 ± 0.07 C | ||
| White cabbage | 0 | 21.90 ± 0.31 E | 3.55 ± 0.10 BCD | 4.54 ± 0.07 E | 54.58 ± 1.83 AB | 54.20 ± 2.85 DE | 1.61 ± 0.38 A | |
| 50 | 18.83 ± 0.27 G | 3.15 ± 0.17 E | 3.96 ± 0.06 F | 52.88 ± 2.70 BC | 56.63 ± 1.21 CD | 1.65 ± 0.33 A | ||
| 100 | 24.34 ± 0.29 C | 3.73 ± 0.14 BC | 3.27 ± 0.07 G | 41.12 ± 2.20 F | 60.51 ± 1.52 BC | 1.60 ± 0.32 A | ||
| 200 | 22.97 ± 0.22 D | 3.13 ± 0.09 E | 4.97 ± 0.10 D | 48.75 ± 1.83 B-E | 70.23 ± 1.14 A | 1.28 ± 0.06 AB | ||
| Kale | 0 | 26.14 ± 0.22 B | 3.79 ± 0.084 B | 5.24 ± 0.10 CD | 60.56 ± 2.80 A | 46.61 ± 0.95 FG | 1.71 ± 0.30 A | |
| 50 | 30.50 ± 0.27 A | 3.58 ± 0.08 BCD | 6.15 ± 0.06 A | 53.30 ± 1.61 BC | 56.59 ± 2.18 CD | 1.75 ± 0.15 A | ||
| 100 | 24.63 ± 0.06 C | 3.85 ± 0.04 AB | 5.09 ± 0.13 CD | 47.68 ± 2.45 C-F | 60.64 ± 1.31 BC | 1.97 ± 0.25 A | ||
| 200 | 23.03 ± 0.30 D | 3.45 ± 0.04 CDE | 4.58 ± 0.13 E | 45.19 ± 2.44 DEF | 65.02 ± 0.71 B | 1.81 ± 0.36 A | ||
| roots | Chinese cabbage | 0 | 5.59 ± 0.30 g | 0.74 ± 0.06 abc | 0.79 ± 0.05 cde | 19.44 ± 2.49 ef | 27.08 ± 1.32 ef | ND |
| 50 | 8.13 ± 0.24 e | 0.74 ± 0.14 abc | 0.73 ± 0.12 de | 19.88 ± 2.54 ef | 29.25 ± 0.87 b-e | ND | ||
| 100 | 11.39 ± 0.05 bc | 0.85 ± 0.15 ab | 1.00 ± 0.15 bc | 18.14 ± 1.86 f | 28.68 ± 0.80 cde | ND | ||
| 200 | 9.53 ± 0.32 d | 0.91 ± 0.07 a | 0.65 ± 0.04 e | 16.75 ± 2.48 f | 30.14 ± 1.13 bcd | ND | ||
| White cabbage | 0 | 10.81 ± 0.19 c | 0.64 ± 0.08 bc | 1.36 ± 0.07 a | 65.04 ± 2.84 a | 27.84 ± 1.32 def | ND | |
| 50 | 11.40 ± 0.20 b | 0.53 ± 0.07 c | 0.92 ± 0.08 cd | 52.95 ± 1.82 b | 30.63 ± 0.78 bc | ND | ||
| 100 | 8.17 ± 0.10 e | 0.82 ± 0.04 ab | 1.38 ± 0.06 a | 33.85 ± 2.73 c | 31.82 ± 0.75 b | ND | ||
| 200 | 7.44 ± 0.12 f | 0.87 ± 0.05 ab | 0.76 ± 0.03 de | 26.02 ± 1.90 de | 35.12 ± 0.39 a | ND | ||
| Kale | 0 | 12.02 ± 0.17 a | 0.70 ± 0.07 abc | 1.16 ± 0.03 ab | 70.50 ± 1.59 a | 22.90 ± 0.67 g | ND | |
| 50 | 9.96 ± 0.12 d | 0.52 ± 0.09 c | 1.01 ± 0.06 bc | 49.59 ± 2.31 b | 25.93 ± 0.97 f | ND | ||
| 100 | 9.52 ± 0.26 d | 0.64 ± 0.05 bc | 0.76 ± 0.09 de | 27.34 ± 2.45 cd | 26.99 ± 0.70 ef | ND | ||
| 200 | 8.59 ± 0.08 e | 0.67 ± 0.10 abc | 0.88 ± 0.08 cde | 22.91 ± 2.98 def | 28.31 ± 0.96 c-f | ND |
| NaCl mM | Na mg g−1 dw | K mg g−1 dw | Ca mg g−1 dw | Mg mg g−1 dw | Mn µg g −1 dw | Fe µg g −1 dw | Cu µg g−1 dw | Zn µg g−1 dw | ||
|---|---|---|---|---|---|---|---|---|---|---|
| leaves | Chinese cabbage | 0 | 1.16 ± 0.03 G | 57.81 ± 0.72 A | 14.62 ± 0.17 BC | 12.29 ± 0.03 CDE | 244.96 ± 6.85 B | 121.06 ± 0.99 AB | 5.51 ± 0.06 B | 37.45 ± 0.50 B |
| 50 | 8.64 ± 0.39 D | 47.84 ± 2.40 B | 12.91 ± 0.80 CD | 10.85 ± 0.78 E | 188.64 ± 13.25 DE | 136.61 ± 10.12 A | 7.86 ± 0.47 A | 29.62 ± 4.81 BC | ||
| 100 | 9.71 ± 0.41 D | 45.90 ± 0.61 BC | 13.77 ± 0.29 CD | 12.35 ± 0.26 CDE | 206.55 ± 0.32 CD | 123.55 ± 3.89 AB | 5.55 ± 0.08 B | 25.67 ± 2.58 BCD | ||
| 200 | 33.14 ± 0.01 A | 38.10 ± 0.52 DE | 12.85 ± 0.01 CD | 13.30 ± 0.05 CD | 189.40 ± 1.80 DE | 108.14 ± 2.03 ABC | 4.95 ± 0.05 BC | 21.65 ±1.76 CD | ||
| White cabbage | 0 | 3.04 ± 0.07 F | 40.85 ± 1.94 CD | 13.48 ± 0.14 CD | 17.40 ± 0.34 A | 245.16 ± 4.31 B | 50.15 ± 2.34 D | 3.56 ± 0.08 EF | 21.96 ±1.90 CD | |
| 50 | 8.52 ± 0.26 D | 35.30 ± 1.97 DEF | 16.16 ± 0.62 AB | 15.99 ± 0.22 AB | 287.93 ± 3.88 A | 57.09 ± 5.07 D | 3.47 ± 0.11 EF | 24.13 ± 2.03 BCD | ||
| 100 | 16.06 ± 0.20 C | 41.96 ± 0.12 BCD | 9.79 ± 0.19 E | 15.89 ± 0.62 AB | 190.60 ± 1.41 DE | 65.17 ± 12.04 D | 3.98 ± 0.14 DE | 24.51 ± 2.22 BCD | ||
| 200 | 22.67 ± 0.47 B | 36.13 ± 0.84 DEF | 13.16 ± 0.36 CD | 13.98 ± 0.45 BC | 227.52 ± 8.09 BC | 64.78 ± 0.35 D | 3.02 ± 0.16 FG | 57.73 ± 3.50 A | ||
| Kale | 0 | 2.29 ± 0.04 FG | 39.12 ± 0.12 CDE | 17.78 ± 0.36 A | 13.80 ± 0.40 BC | 315.63 ± 9.12 A | 98.95 ± 2.29 BC | 4.92 ± 0.13 BCD | 26.01 ± 0.82 BCD | |
| 50 | 3.03 ± 0.14 F | 29.53 ± 0.76 FG | 9.38 ± 0.16 E | 12.33 ± 0.23 CDE | 150.53 ± 0.39 F | 58.62 ± 0.46 D | 2.65 ± 0.05 FG | 15.56 ± 0.76 D | ||
| 100 | 5.88 ± 0.26 E | 32.79 ± 0.81 EFG | 12.13 ± 0.47 D | 13.49 ± 0.44 C | 165.53 ± 2.57 EF | 78.55 ± 5.72 CD | 4.02 ± 0.15 CDE | 22.44 ±3.58 CD | ||
| 200 | 9.20 ± 0.08 D | 26.08 ± 1.18 G | 7.00 ± 0.05 F | 11.18 ± 0.14 DE | 104.61 ± 1.92 G | 59.16 ± 0.89 D | 2.41 ± 0.06 G | 15.95 ±1.43 CD | ||
| roots | Chinese cabbage | 0 | 1.467 ± 0.01 e | 64.02 ± 1.05 a | 3.36 ± 0.05 a | 3.68 ± 0.09 b | 680.35 ± 12.83 b | 244.52 ± 5.44 bc | 32.69 ± 0.56 de | 50.29 ± 4.49 a |
| 50 | 15.96 ± 0.03 de | 50.48 ± 0.83 c | 2.56 ± 0.09 b | 2.56 ± 0.00 d | 769.86 ± 5.37 a | 305.79 ± 1.27 ab | 44.27 ± 0.42 bcd | 49.12 ± 2.04 ab | ||
| 100 | 37.21 ± 0.45 bc | 32.15 ± 0.16 e | 2.27 ± 0.02 b | 2.46 ± 0.04 d | 92.09 ± 1.75 d | 236.26 ± 2.55 c | 48.23 ± 0.87 bc | 36.16 ± 1.46 cd | ||
| 200 | 79.30 ± 0.04 a | 13.62 ± 0.21 f | 1.26 ± 0.04 cde | 1.62 ± 0.02 fg | 21.63 ± 0.05 h | 168.60 ± 1.50 de | 39.49 ± 0.38 cd | 23.63 ± 0.17 e | ||
| White cabbage | 0 | 2.48 ± 0.02 e | 44.67 ± 1.38 d | 1.32 ± 0.03 cd | 4.48 ± 0.03 a | 48.32 ± 0.42 fg | 143.07 ± 1.76 e | 23.94 ± 0.13 e | 32.45 ± 1.48 cde | |
| 50 | 21.07 ± 0.34 d | 31.45 ± 1.26 e | 1.28 ± 0.05 cde | 2.40 ± 0.06 d | 75.77 ± 2.65 de | 135.04 ± 6.94 e | 29.74 ± 0.96 de | 36.19 ± 1.99 cd | ||
| 100 | 51.32 ± 0.21 b | 3.05 ± 0.04 g | 0.52 ± 0.05 g | 0.72 ± 0.01 h | 30.55 ± 0.20 gh | 267.33 ± 6.11 abc | 41.63 ± 0.56 bcd | 21.5 1 ± 1.60 e | ||
| 200 | 70.12 ± 0.61 a | 4.34 ± 0.06 g | 0.89 ± 0.04 efg | 0.89 ± 0.01 h | 14.83 ± 0.17 h | 328.99 ± 1.72 a | 79.34 ± 0.39 a | 38.38 ± 0.48 bc | ||
| Kale | 0 | 1.52 ± 0.04 e | 56.41 ± 0.98 b | 2.50 ± 0.04 b | 3.16 ± 0.09 c | 152.88 ± 3.98 c | 158.67 ± 1.35 de | 22.44 ± 0.56 e | 39.17 ± 0.18 abc | |
| 50 | 24.73 ± 0.72 cd | 12.56 ± 0.14 f | 1.35 ± 0.07 c | 2.26 ± 0.01 de | 38.92 ± 0.85 fgh | 136.11 ± 4.17 e | 34.87 ± 1.37 cde | 25.45 ± 0.12 de | ||
| 100 | 42.54 ± 1.65 b | 9.86 ± 0.42 f | 1.00 ± 0.03 def | 1.87 ± 0.05 ef | 57.75 ± 1.32 ef | 155.61 ± 2.81 de | 49.08± 0.92 bc | 26.08 ± 2.29 de | ||
| 200 | 68.80 ± 9.48 a | 4.35 ± 0.70 g | 0.73 ± 0.17 fg | 1.33 ± 0.20 g | 26.18 ± 4.07 gh | 213.47 ± 38.01 cd | 54.83± 8.64 b | 22.77 ± 3.34 e |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šamec, D.; Linić, I.; Salopek-Sondi, B. Salinity Stress as an Elicitor for Phytochemicals and Minerals Accumulation in Selected Leafy Vegetables of Brassicaceae. Agronomy 2021, 11, 361. https://doi.org/10.3390/agronomy11020361
Šamec D, Linić I, Salopek-Sondi B. Salinity Stress as an Elicitor for Phytochemicals and Minerals Accumulation in Selected Leafy Vegetables of Brassicaceae. Agronomy. 2021; 11(2):361. https://doi.org/10.3390/agronomy11020361
Chicago/Turabian StyleŠamec, Dunja, Ida Linić, and Branka Salopek-Sondi. 2021. "Salinity Stress as an Elicitor for Phytochemicals and Minerals Accumulation in Selected Leafy Vegetables of Brassicaceae" Agronomy 11, no. 2: 361. https://doi.org/10.3390/agronomy11020361
APA StyleŠamec, D., Linić, I., & Salopek-Sondi, B. (2021). Salinity Stress as an Elicitor for Phytochemicals and Minerals Accumulation in Selected Leafy Vegetables of Brassicaceae. Agronomy, 11(2), 361. https://doi.org/10.3390/agronomy11020361








