Abstract
Field trials were conducted to evaluate the effect of nitrogen level on wheat protein content and composition in 16 cultivars over two years at three locations. The nitrogen treatment comprised two nitrogen levels, 0 kg ha−1 as low and 100 kg ha−1 as high nitrogen, applied as top dressings of 50 kg nitrogen per ha at tillering and stem extension growth stages. Increased nitrogen level generally enhanced grain protein by 11.3% (11.5% vs. 12.8%). Considering protein composition determined by reversed phase–high-pressure liquid chromatography, higher nitrogen supply generally enhanced the proportion of total gliadins, α-gliadins, γ-gliadins and high-molecular-weight glutenin subunits by 1.1%, 2.0%, 3.7%, 0.6% and 0.9%, respectively, and reduced albumins and globulins, ω-gliadins, total glutenins and low-molecular-weight glutenin subunits by 1.1%, 1.7%, 1.9% and 3.2%. Under a high nitrogen level, the historical cultivars Libellula, San Pastore and U-1 had a higher protein content (13.1–15.2%) with significantly higher total gliadins, which resulted in a significantly higher gliadin/glutenin ratio (1.68–1.92). In the modern cultivars, protein content varied between 11.4% and 14.6% with a well-balanced gliadin/glutenin ratio (1.08–1.50), except for cultivar MV Nemere which had a high gliadin/glutenin ratio at both nitrogen levels (1.81 vs. 1.87). In summary, increasing nitrogen level enhanced grain protein content while the composition of gliadin and glutenin fractions was changed to a lesser extent and was largely cultivar specific and therefore should be considered for wheat baking quality assessment and breeding purposes.
1. Introduction
Among dominant grain cereals produced for human food consumption, common wheat (Triticum aestivum L.) is one of the most important. Yield and quality of wheat grain are dependent on genotype, soil fertility, growing conditions and crop nutrition. Bread wheat yield potential is largely genetically determined and varies significantly as a result of differences in climate and adequate production systems, which include mineral fertilization [1,2]. Utilization of nitrogen fertilization has many benefits. Nitrogen (N) is necessary for wheat growth, and crop yield and quality depend upon a sufficient input of N. A satisfactory supply of N is essential for the grain protein accumulation that is necessary for baking and processing quality [3,4]. Grain protein content is an important factor defining wheat end-use quality and usually varies between 6 and 19% and has an impact on the grading of flour quality for traditional bread making and other food products [5,6]. In Croatia, in accordance with the “Regulation on quality parameters and quality classes of wheat in the purchase of wheat (2018)”, in the formation of the market price, the final and decisive parameter is protein content (%) and, accordingly, wheat produced exclusively for purchase in the territory of the Republic of Croatia is classified into four qualitative classes: premium (>15%), I class (13.5–14.99%), II class (12–13.49%), III class (10.5–11.99) and IV class (<10.49%). According to their solubility characteristics, wheat grain proteins are classified as albumins and globulins (AGs), gliadins (GLIs) and glutenins (GLUs). GLIs and GLUs are the two main groups of gluten proteins, the endosperm storage proteins, accounting for 60~80% of the total grain proteins. Gluten proteins influence dough rheological properties that determine the processing quality of bread, noodles and cookies [7,8,9]. GLIs belong to the monomeric prolamin fraction, and are classified into three structural groups of polypeptides according to their electrophoretic mobility, α/β-, γ- and ω-GLI. The polymeric glutenin fraction is constituted of high-molecular-weight (HMW-GS) and low-molecular-weight glutenin subunits (LMW-GS) linked with intermolecular disulphide bonds. The gluten protein accumulation during the grain filling period is largely influenced by environmental growing conditions, particularly N availability. However, the type and distribution of gluten subunits have a strong genetic component [10,11]. It is reported that the composition of the protein fractions is more influenced by the N nutrition than by temperature [12].
Grain nitrogen content plays an important role affecting the ratio of storage to non-storage proteins, the percentage of the storage protein fractions and, thus, the GLI/GLU ratio and the ratio of HMW-GS/LMW-GS [8,11,13]. To obtain the maximum wheat potential in the field, N is the most limiting nutrient required by plants. The growth of wheat plants begins with protein reserves of the kernel, which serve to maintain the germination and further growth of the seedling until the first leaf appears. After rootlets are developed, additional N is obtained by the rootlet system and at this stage the N uptake is limited by root size. In the Northern Hemisphere, it is considered necessary to administer a first application of N fertilizer before the second leaf emerges. A second important time for N application is the tillering stage, when the N concentration in the tissue impacts the formation of the tillers in each plant. The third N application is the most important and it is critical for biomass formation during the extension stage and later for promoting protein accumulation [4,13].
In Croatia, the annual wheat production was 820,682 t in the last 10 years (2009–2018), with 5.12 t ha−1 [14]. Since producers in Croatia get a higher price for premium quality wheat (grain protein content above 15%), it is desirable to select high-quality cultivars that offer a competitive advantage by minimizing yield penalty in environments that are not classified as high yielding. A few studies regarding bread-making quality and environmental influence on gluten proteins are being done continuously. However, comprehensive research on nitrogen’s influence in different environments on protein content and composition has not been carried out so extensively. Therefore, the hypothesis and objective of this study is to determine the quantitative changes of quality-related proteins in historical and currently grown wheat cultivars grown in different agro-ecological conditions at two N fertilization levels in order to assess if there is a specific cultivar reaction with nitrogen treatment in terms of their quality potential for breeding and technological purposes.
2. Materials and Methods
2.1. Plant Materials and Field Experiments
Sixteen winter wheat cultivars were evaluated in field trials under two N fertilization levels during two consecutive seasons (2016/17 and 2017/18) at three locations (Osijek, Zagreb and Poreč) representing different agro-ecological conditions and soil types in Croatia. The panel was assembled to represent the historical and current significance of cultivars in production in Croatia and their parental contributions. The cultivar list, year of registration and their country of origin and breeding institution are listed in Table 1.

Table 1.
List of wheat cultivars, year of registration, country of origin and breeding institution.
Each of the six field experiments was set up in a split-plot factorial design in three replications with N fertilization levels as the main plots and 16 wheat cultivars as sub-plots. The harvested plot size was 7.56 m2 at Osijek and Poreč and 4.95 m2 at Zagreb. Seeding level was 350 kernels m−2 in all trials and for all cultivars. Buffer plots were planted between the main N treatment plots. Basic fertilization before sowing of 74 kg N ha−1, 80 kg P2O5 ha−1 and 120 kg K2O ha−1 was applied by adding 100 kg ha−1 of urea (46% N) and 400 kg ha−1 NPK (7:20:30).
The N treatment comprised two N fertilization levels, 0 kg N ha−1 as low N and 100 kg N ha−1 as high N, applied as top dressings of 50 kg N ha−1 at tillering (GS23-25 after Zadoks et al. (1974)) and stem extension (GS33-35) growth stages. All other cultural practices, including application of herbicides, insecticides and fungicides to control major weeds, insects and foliar diseases, were typical for commercial wheat production in Croatia. Total N fertilizer application in commercial wheat production varies from 120–180 kg of N ha−1. Descriptions of the soil type, soil N content and N fertilization levels for N0 and N100 treatments for the six location–season combinations are given in Table S1 (Supplementary Materials).
Osijek and Zagreb locations are classified as having a moderately warm and rainy oceanic climate (Cfb), while the Poreč location is classified as having humid subtropical climate (Cfa) by the Köppen and Geiger climate classification [15]. Basic meteorological data for all locations for 2016/17 and 2017/18 and multi-annual averages are provided in Supplementary File Table S5. On average, during both seasons, Osijek was the driest and coolest site, while Poreč was the warmest and had the highest amount of rainfall. Zagreb had the highest amount of precipitation and the lowest average temperature only in the 2017/18 season (Table S5, summary in Supplementary Materials).
2.2. Protein Analysis
2.2.1. Grain Protein Content
Replicate grain samples of 0.5 kg were collected after the harvest and were polled and homogenized and used further for protein analysis. Grain protein content (P) (N × 5.7, DM) was measured using an Infratec 1241 Grain Analyzer (Infratec 1241, Foss Tecator, Hillerød, Denmark).
2.2.2. Wet Gluten Content and Gluten Index
Wet gluten content (WG) and gluten index (GI) of approximately 70% extraction flour (Quadrumat Senior mill, Duisburg, Brabender, Germany) were analyzed using a Glutomatic 2200 (Perten Instruments, Hagersten, Sweden) according to ICC standard No. 155.
2.2.3. Extraction of Proteins
Prior to the reversed phase–high-pressure liquid chromatography (RP-HPLC) analysis, the single protein fractions were sequentially extracted according to Wieser et al. [12] with some modifications. Briefly, 50 mg of wholemeal flour (Retsch Centrifugal Mill ZM1, Haan, Germany, equipped with 1 mm sieve) were extracted by 1 mL of 0.4 M NaCl for 30 min at RT (albumin/globulin (AG) extract). From the remaining pellet, the gliadins (GLIs) were extracted with 1 mL of 50% 1-PrOH for 60 min at RT and, finally, the glutenins (GLUs) were extracted with 1 mL of a solution containing 50% (v/v) 1-PrOH, 2 M urea, 1% (w/v) dithioerythritol and 0.05 M Tris-HCl (pH 7.5) for 60 min at 60 °C. During all extractions, the suspensions were vortexed for 1 min every 10 min and then centrifuged for 15 min at 14,000 rpm and RT. The obtained supernatants were stored at −20 °C and filtered through a 0.45 μm PVDF syringe filter prior to HPLC analysis. Two extractions were performed for each sample.
2.2.4. HPLC Analysis of Proteins
Extracted wheat proteins were analysed according to the method of Wieser et al. [12] using high-performance liquid chromatography (HPLC) (Perkin Elmer Instruments, Waltham, MA, USA) coupled with Total-Chrom software and a photodiode array detector. Elution was performed with linear gradient of acetonitrile (ACN/0.1%TFA) in water (H2O/0.1%TFA) from 24–54% in 30 min at flow rate of 1 mL min−1 and column temperature of 50 °C. Proteins were separated on a C18 reverse phase column (5 μm 4.6 × 150 mm; Sigma-Aldrich Chemie GmbH, Steinheim, Germany). Quantification of protein fractions was based on measuring their peak area at 210 nm. The peak areas under AG, GLI and GLU chromatograms were used for further calculations of their relative proportion (%) in total extracted proteins.
2.3. Statistical Analysis
All statistical analysis was done in R (R Core Team 2013). Two linear models were employed as described by Cormier et al. [16]. Firstly, in all field trials (where N levels, year and location of testing were separated), least square means were calculated using cultivars as fixed factors. These adjusted means were used in the subsequent analyses using the following linear model:
Yijl is the phenotype of genotype i in location j and year k at N treatment level l (0 kg/ha and 100 kg/ha of N applied as top dressing), is the general mean, is the effect of N, is cultivar (genotype) effect, is the effect of environment (3 levels), is the year effect (2 levels), is the interaction term for all possible combinations and is residual error terms.
The differences among cultivar means were tested using Tukey’s HSD test (library emmeans). The statistical significance of the differences among main factors and treatments was tested using an LSD test at the level of probability p < 0.05.
3. Results and Discussion
The results of ANOVA analysis are shown in Table S2 (Supplementary Materials). For all analysed traits, statistically significant differences were found for the main factors (cultivars, N level, year and location) and their interactions, which is in accordance with the results of other authors [17,18,19]. Among the main sources of variation, cultivar was the main contributing factor for the majority of quality-related proteins, therefore, in the data presentation, we further emphasized the differences between cultivars grown under different N treatments. Grain and flour quality of wheat cultivars under low and high N rates are presented in Table 2. The mean values of the P and WG content for 16 wheat cultivars under low N were relatively low, with means of 11.5% and 22.5%, respectively. Under same low N application, the examined cultivars expressed relatively high gluten strength, with an average GI value of 95. The cultivars OS Olimpija and U-1 showed the highest grain P (13.4% and 13.0%, respectively), while cultivars Graindor, Solehio and Sofru had the lowest (9.9%, 10.2% and 10.3%, respectively). WG ranged from 17.2% (Isengrain) to 26.6% (OS Olimpija), while GI ranged from 89 (U-1) and 99 (Graindor and BC Ljepotica). The P and WG contents were significantly higher under high N levels and were cultivar dependent (Table 2 and Table S2). Generally, the P (11.5% vs. 12.8%) and WG (22.2% vs. 25.8%) increased by 11.3% and 16.2%, while GI (95 vs. 92) decreased by 3.2%, which is in agreement with Zheng et al. [20], who also found that processing quality parameters, except gluten index, were remarkably improved after increasing N application. These authors noted that protein content of the four cultivars and four N levels (N0–N75–N150–N225) increased during two consecutive growing seasons from 10.2% to 13.9% and 9.5% to 12.2%, respectively. Yu et al. [21], studying six Australian bread wheat cultivars grown under different N treatments over two years at several locations, noted that the genotype was dominant in grain P content in response to N availability, indicating the diversity of cultivars used in the experiment.

Table 2.
Mean 1 values of grain and flour quality in wheat under low and high N levels.
The modern cultivars (bred after 1960 in a phase of the Green Revolution) (Table 1), OS Olimpija, Viktorija and Kraljica, with the highest P, WG and GI, have shown the greatest bread-making quality potential and their increase in the P content under a high N level was 8.8%, 12.5% and 11.0% (Table 2) and, in accordance with Croatian regulations, belong to the quality class I. It is important to note that all cultivars, except Isengrain and BC Ljepotica, achieved a higher quality class due to the increase in N intake. It is interesting to note that old cultivars (cross-bred until the late 1960s), Libellula, San Pastore and U-1, with P content of 11.6–13.0%, showed a significantly (p < 0.05) lower GI (89–91) than the modern ones, with a GI from 93 to 99. These cultivars also showed a remarkable increase in P content with increasing N level by 14%, 13.2% and 17.1%, respectively. The old cultivar Bezostaja-1 with 13.7% P under higher N showed the largest decrease in GI (94 vs. 86) as well as cultivars MV Nemere (95 vs. 87) and Libellula (90 vs. 82), meaning that there was a significant decrease in gluten strength under higher N level, which could negatively affect the baking properties of flour. Guerrini et al. [22] noted that flour from the old wheat cultivars is typically characterized by high yield and poor technological properties and is not suitable for industrial baking but, recently [23], the old wheat cultivars have been reintroduced in wheat breeding programs as a contribution to biodiversity and nutritional and nutraceutical properties.
As we mentioned before, the protein accumulation during the grain filling period is largely influenced by N fertilization level, however, the composition of protein fractions has strong genetic background [19,24,25,26]. AG represents approximately 15–20% of the grain proteins and often has structural and metabolic functions with limited impact on the baking properties [27]. The AG proportion under low N ranged from 13.8% (OS Olimpija) to 22.0% (Isengrain). In relation to N increase (Figure 1), the AG mean generally decreased by 1.1%, but the N effect on cultivars was not consistent, so, in Bologna, Isengrain and San Pastore, the AG proportion decreased by 15.3%, 11.4% and 10.9%, while in Bezostaje-1, Ingenio and Solehio, it increased by 7.2%, 8.4% and 7.5%, respectively.
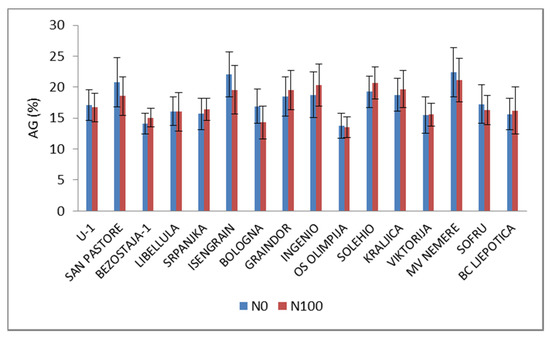
Figure 1.
Proportions of albumins and globulins (AG) in total wheat grain protein under low (N0) and high (N100) N levels. (Histograms are showing mean values ± standard deviation across years and locations).
Some authors [12,17,28] noted that AG remained nearly unaffected under the higher N level, while Rekowski et al. [29] reported that late N supply significantly increased AG by 8–9%.
Storage GLI and GLU fractions constitute 80–85% of total grain proteins and contribute to rheological, pasting and textural properties of dough [27]. Polymeric glutenins predominantly define the dough elasticity while monomeric gliadins are responsible for dough extensibility [30,31,32]. Significant variability among cultivars under a low N level was observed in the proportion of GLI fractions, with the cultivar Isengrain having the lowest proportion of total GLI (40.0%) and the cultivar U-1 having the highest (55%). The proportion of minor ω-GLI ranged from 4.0% (Sofru) to 9.4% (Kraljica), while the proportion of major α-GLI ranged from 20.4% (Isengrain) to 29.5% (U-1) (Table 3). All GLI fractions were affected by the higher N supply and, generally, the total GLI (46.1% vs. 47.0%), α-GLI (24.2% vs. 25.1%) and γ-GLI (16.0 vs. 16.1) increased by 1.9%, 3.7% and 1.3%, respectively, while ω-GLI (5.9 vs. 5.8) decreased by 1.7%, meaning that the dominant α-GLI reacted the most to the N level increase (Table 3). Hurkman et al. [33] and Rossmann et al. [26] found that α-GLI and ω-GLI proportions increased under higher N levels, while the γ-GLI proportion decreased. Variations in composition of GLI fractions were strongly cultivar dependent (Table 3 and Table S3 in Supplementary Materials), with the cultivars San Pastore and Sofru achieving the largest increase in total GLI (6.3% and 5.5%, respectively), while in cultivar U-1, the total GLI proportion decreased by 1% under a higher N level. A similar inconsistency was observed for other GLI factions.

Table 3.
Mean 1 values of GLI proportions in total grain protein of wheat under low and high N levels.
The old cultivars Libellula, San Pastore and U-1 under both N levels achieved a significantly higher total GLI and α-GLI compared to the modern ones (Table 3). De Santis et al. [34], who studied only gluten proteins, without AG, reported that the old and modern Italian cultivars showed similar α/γ-GLI expression (61% vs. 58%), while a marked decrease in ω-GLI expression was observed in the modern ones (11% vs. 4%), which is not so clear among our cultivars (Table 3). The composition and quantity of the GLU are the major determinants of wheat bread-making quality because a higher content of GLU is related to an increase in gluten strength that allows the dough to maintain the desired final shape and maintains the desired appearance of the final baking products [31]. The total GLU proportion under a low level ranged from 27.7% (MV Nemere) to 42.0% (Sofru). The proportion of LMW-GS as a major GLU fraction ranged from 18.4% (San Pastore) to 30.8% (Sofru) and for minor HMW-GS from 7.8% (U-1) to 13.2% (Bologna) (Table 4).

Table 4.
Mean 1 values of GLU proportions in total grain protein of wheat under low and high N levels.
Analysis of the data revealed significant effects of N levels on the GLU components and they were greatly cultivar dependent (Table 4 and Table S4 in Supplementary Materials). In relation to the N increase (Table 4), the HMW-GS mean increased by 0.9% (10.9% vs. 11.0%), while total GLU (36.2% vs. 35.5%) and LMW-GS (25.3% vs. 24.5%) decreased by 1.8% and 3.0%, respectively, meaning that major LMW-GS reacted most sensitively to increased nitrogen intake. These results are in agreement with others [26,33,35], who showed that proportions of low-S proteins (such as ω-GLI and HMW-GS) increase, while proportions of S-rich proteins (such as LMW-GS) decrease with increasing N fertilization rates. The largest decrease in the total GLU proportion under higher N was observed for the cultivars BC Ljepotica and Srpanjka (5.0% and 5.4%, respectively), while Bologna and U-1 showed an increase in GLU by 3.0%. It has been reported that HMW-GS have a crucial impact on wheat baking quality even though they constitute only 10% of the total grain P. Among cultivars, significant variations between low and high N levels were observed for HMW-GS, ranging from −6.6% (BC Ljepotica) to + 7.5% (U-1) (Table 4), which is consistent with others who found that the expression of storage proteins as well as HMW-GS is strongly associated with genotype [19,36]. Hurkman et al. [33] analyzed the effect of different temperature regimes with and without fertilizer on individual gluten proteins (quantitative 2-DE) of one cultivar and concluded that under moderate temperature, the majority of individual protein spots of HMW-GS, ω-GLI and some α-GLI increased in the range of 25.2–49.2%, 56.0–107.6% and 21.5–50.2%, respectively, while two LMW-GS spots and a minor γ-GLI spot decreased by 23.1%, 31.4% and −28%, respectively. Xue et al. [37] reported that globulins, LMW-GS and α- and γ-GLI were more sensitive to split N application, while Zhong et al. [38] noted that α-GLI and HMW-GS were commonly dependent on nitrogen top dressing timing.
The GLI/GLU ratio is related to the balance of dough strength and extensibility [32]. The negative influence of a higher GLI/GLU ratio on dough rheological properties is well known [31,32,39]. The GLI/GLU ratio under a low N level varied from 0.98 (Sofru) to 1.98 (U-1) and, under higher N supply, the GLI/GLU ratio was generally increased by 4.6% (1.32 vs. 1.38) (Table 4). The highest increase in the GLI/GLU ratio was noticed in the cultivars San Pastore, BC Ljepotica and Sofru (10.8%, 9.6% and 9.3%, respectively). In the old cultivars, the total P increase resulted in an increase in the GLI/GLU ratio (San Pastore: 1.72 vs. 1.91 and Libellula: 1.58 vs. 1.68), while the cultivar U-1 retained the highest GLI/GLU value despite its reduction by 3% under higher N (1.98 vs. 1.92). In the modern cultivars under a high N level, the GLI/GLU ratio ranged between 1.08 (Sofru) and 1.50 (Kraljica), except for cultivar MV Nemere which had a high GLI/GLU ratio at both N levels (1.81 vs. 1.87). De Santis et al. [34] reported that the better technological performance of modern Italian cultivars compared to old ones was found to be due to the presence of superior HMW-GS and LMW-GS alleles and to the differential expression of specific storage proteins and because the higher GI (55.3 vs. 9.6 for old cultivars in the first crop season and 46 vs. 7.5 in the second crop season) observed in modern genotypes was correlated with a decreased GLI/GLU ratio (1.6 vs. 2.6 for old cultivars in the first crop season and 1.8 vs. 2.9 in the second crop season). From a breeder’s perspective, in order to hold their seed market share, there are presently two major concerns: to extend the target regions and to increase the end-use quality [40]. To fulfill those requirements, there is a practical consequence of the higher cost of breeding programs, due to an increased number of field trials and an increased number of laboratory tests to assess. This means that breeders are faced with the dilemma that increasing the number of trials with additional N levels would increase their costs, but, on the other hand, there is a penalty in terms of missing information about the agronomic and quality performance and responsiveness of new genotypes. Sylvester-Bradley et al. [41] suggested that we should select for wheat cultivars showing a low N optimum and that are highly responsive to N at lower doses while, simultaneously, high N responsiveness is kept under high N conditions. In our study, N treatment had an effect on both wheat protein content and composition, but not equally on all protein fractions. The effect on GLI was more pronounced than on GLU, while AG were not as increased. Furthermore, there was a cultivar-specific reaction to N treatment. This could offer breeders a blueprint for selecting genotypes more responsive to N treatment by selecting for those protein fractions that contribute to the overall wheat quality under increased N levels, while, on the other side, selecting other protein fractions that could be more important under reduced N regimes. Ideally, such an approach could contribute to the selection of new genotypes with improved stability of quality under different soil fertility and N fertilization regimes.
4. Conclusions
Significant differences were found among analyzed wheat grain proteins depending on cultivars, nitrogen level and environment, as well as their interactions. Increasing N level enhanced grain P content and moved cultivars into a higher quality class, while the composition of quality-related GLI and GLU fractions was changed to a lesser extent and was strongly cultivar specific and therefore should be considered for wheat quality assessment and breeding purposes because higher grain protein concentrations are major targets of modern wheat breeding, together with well-balanced proportions of quality-related proteins.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4395/11/2/325/s1, Table S1: Descriptions of the soil type, soil N content and N fertilization levels for N0 and N100 treatments for the six location–season combinations, Table S2: ANOVA and the sources of variation for the P, WG and GI, Table S3: ANOVA and the sources of variation for the grain AG and GLI fractions, Table S4: ANOVA and the sources of variation for the grain GLU fractions. Table S5: Rainfalls (mm) and temperatures (°C) by years and months for locations Osijek, Poreč and Zagreb.
Author Contributions
Conceptualization, D.H. and G.Š.; methodology, D.N. and D.H.; software, D.N. and K.D.; validation, K.D.; formal analysis, M.I. and I.P.; investigation, M.I. and I.P.; resources, D.N.; writing—original draft preparation, D.H.; writing—review and editing, G.Š. and D.N.; visualization, K.D.; funding acquisition, D.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been financed by a grant of the Croatian Science Foundation IP-2016-06-2178 and the project KK.01.1.1.01.0005 Biodiversity and Molecular Plant Breeding, Centre of Excellence for Biodiversity and Molecular Plant Breeding (CoE CroP-BioDiv), Zagreb, Croatia.
Data Availability Statement
Restrictions apply to the availability of these data. The data presented in this study were obtained from the Agricultural Institute Osijek and are available on request [from the authors] with the permission of the Agricultural Institute Osijek.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| N | nitrogen |
| kg | kilogram |
| ha | hectare |
| P | protein |
| RP-HPLC | reversed phase–high-pressure liquid chromatography |
| GLI | gliadins |
| α-GLI | α-gliadins |
| γ-GLI | γ-gliadins |
| HMW-GS | high-molecular-weight glutenin subunits |
| AG | albumins/globulins |
| ω-GLI | ω-gliadins |
| GLU | glutenins |
| LMW-GS | low-molecular-weight glutenin subunits |
| GLI/GLU | gliadins/glutenins |
| α/β-GLI | α/β-gliadins |
| HMW-GS/LMW-GS | high-molecular-weight glutenin subunits/low-molecular-weight glutenin subunits |
| t | ton |
| m2 | square meter |
| P2O5 | phosphorus pentoxide |
| K2O | potassium oxide |
| NPK | nitrogen, phosphorus and potassium fertilizer |
| HR | Croatia |
| HU | Hungary |
| FR | France |
| RU | Russia |
| IT | Italy |
| GS23-25 | growth stages 23–25 |
| GS33-35 | growth stages 33–35 |
| DM | dry matter |
| WG | wet gluten |
| GI | gluten index |
| ICC | International Association for Cereal Science and Technology |
| mg | milligram |
| mm | millimeter |
| mL | milliliter |
| M | molar |
| NaCl | natrium chloride |
| min | minute |
| RT | room temperature |
| 1-PrOH | 1-propanol |
| v/v | volume/volume |
| w/v | weight/volume |
| Tris-HCl | Tris-hydrochloride |
| pH | lat. potentia hydrogenii |
| °C | degrees Celsius |
| rpm | rotations per minute |
| µm | micrometer |
| PVDF | polyvinylidene difluoride |
| HPLC | high-pressure liquid chromatography |
| USA | United States of America |
| ACN | acetonitrile |
| TFA | trifluoroacetic acid |
| H2O | water |
| C18 | carbon18 |
| nm | nanometer |
| GEN | genotype |
| LOC | location |
| YEAR | year |
| HSD | honestly significant difference in Tukey’s post hoc test |
| LSD | least significance difference |
| ANOVA | analysis of variance |
References
- Hristov, N.; Mladenov, N.; Kondić-Špika, A. Effect of environmental and genetic factors on the correlation and stability of grain yield components in wheat. Genetika 2011, 43, 141–152. [Google Scholar] [CrossRef]
- Rajičić, V.; Milivojević, J.; Popović, V.; Branković, S.; Đurić, N.; Perišić, V.; Terzić, D. Winter wheat yield and quality depending on the level of nitrogen, phosphorus and potassium fertilization. Agric. For. 2019, 65, 79–88. [Google Scholar] [CrossRef]
- Wilson, T.L.; Guttieri, M.J.; Nelson, N.O.; Fritz, A.; Tilley, M. Nitrogen and sulfur effects on hard winter wheat quality and asparagine concentration. J. Cereal Sci. 2020, 93, 102969. [Google Scholar] [CrossRef]
- Zörb, C.; Malcolm, U.L.; Hawkesford, J. Perspective on Wheat Yield and Quality with Reduced Nitrogen Supply. Trends Plant Sci. 2018, 23, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Grahmann, K.; Govaerts, B.; Fonteyne, S.; Guzmán, C.; Soto, A.P.G.; Buerkert, A.; Verhulst, N. Nitrogen fertilizer placement and timing affects bread wheat (Triticum aestivum) quality and yield in an irrigated bed planting system. Nutr. Cycl. Agroecosyst. 2016, 106, 185–199. [Google Scholar] [CrossRef]
- Kong, L.G.; Si, J.S.; Zhang, B.; Feng, B.; Li, S.D.; Wang, F.H. Environmental modification of wheat grain protein accumulation and associated processing quality: A case study of China. Aust. J. Crop Sci. 2013, 7, 173–181. [Google Scholar]
- Rasheed, A.; Xia, X.; Yan, Y.; Appels, R.; Mahmood, T.; He, Z. Wheat seed storage proteins: Advances in molecular genetics, diversity and breeding applications. J. Cereal Sci. 2014, 60, 11–24. [Google Scholar] [CrossRef]
- Cho, S.W.; Kang, C.S.; Kang, T.G.; Cho, K.M.; Park, C.S. Influence of different nitrogen application on flour properties, gluten properties by HPLC and end-use quality of Korean wheat. J. Integr. Agric. 2018, 17, 982–993. [Google Scholar] [CrossRef]
- Cappelli, A.; Oliva, N.; Cini, E. A Systematic Review of Gluten-Free Dough and Bread: Dough Rheology, Bread Characteristics, and Improvement Strategies. App. Sci. 2020, 10, 6559. [Google Scholar] [CrossRef]
- Malik, A.H.; Kuktaite, R.; Johansson, E. Combined effect of genetic and environmental factors on the accumulation of proteins in the wheat grain and their relationship to breadmaking quality. J. Cereal Sci. 2013, 57, 170–174. [Google Scholar] [CrossRef]
- García-Molina, M.D.; Barro, F. Characterization of Changes in Gluten Proteins in Low-GliadinTransgenic Wheat Lines in Response to Application of Different Nitrogen Regimes. Front. Plant Sci. 2017, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Wieser, H.; Seilmeier, W. The influence of nitrogen fertilization on quantities and proportions of different protein types in wheat flour. J. Sci. Food Agric. 1998, 76, 49–55. [Google Scholar] [CrossRef]
- Pechanek, U.; Karger, A.; Gröger, S.; Charvat, B.; Schöggl, G.; Lelley, T. Effect of Nitrogen Fertilization on Quantity of Flour Protein Components, Dough Properties, and Breadmaking Quality of Wheat. Cereal Chem. 1997, 74, 800–805. [Google Scholar] [CrossRef]
- Croatian Bureau of Statistics. Available online: https://www.dzs.hr/default_e.htm (accessed on 20 November 2020).
- Klimatski Atlas Hrvatske. Available online: http://klima.hr/razno/publikacije/klimatski_atlas_hrvatske.pdf (accessed on 29 January 2021).
- Cormier, F.; Faure, S.; Dubreuil, P.; Heumez, E.; Beauchêne, K.; Lafarge, S.; Praud, S.; Le Gouis, J. A multi-environmental study of recent breeding progress on nitrogen use efciency in wheat (Triticum aestivum L.). Theor. Appl. Genet. 2013, 126, 3035–3048. [Google Scholar] [CrossRef]
- Triboï, E.; Martre, P.; Triboï-Blondel, A. Environmentally-induced changes in protein composition in developing grains of wheat are related to changes in total protein content. J. Exp. Bot. 2003, 54, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Altenbach, S. New insights into the effects of high temperature, drought and post-anthesis fertilizer on wheat grain development. J. Cereal Sci. 2012, 56, 39–50. [Google Scholar] [CrossRef]
- Horvat, D.; Drezner, G.; Sudar, R.; Šimić, G.; Dvojković, K.; Španić, V.; Magdić, D. Distribution of wheat protein components under different genetic backgrounds and environments. Turk. J. Field Crop. 2015, 20, 150–154. [Google Scholar] [CrossRef][Green Version]
- Zheng, T.; Qi, P.F.; Cao, Y.L.; Han, Y.N.; Ma, H.L.; Guo, Z.R.; Wang, J.P. Mechanisms of wheat (Triticum aestivum) grain storage proteins in response to nitrogen application and its impacts on processing quality. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Yu, Z.; Islam, S.; She, M.; Diepeveen, D.; Zhang, Y.; Tang, G.; Zhang, J.; Juhasz, A.; Yang, R.; Ma, W. Wheat grain protein accumulation and polymerization mechanisms driven by nitrogen fertilization. Plant J. 2018, 96, 1160–1177. [Google Scholar] [CrossRef]
- Guerrini, L.; Parenti, O.; Angeloni, G.; Zanoni, B. The bread making process of ancient wheat: A semi-structured interview to bakers. J. Cereal Sci. 2019, 87, 9–17. [Google Scholar] [CrossRef]
- Dinu, M.; Whittaker, A.; Pagliai, G.; Benedettelli, S.; Sofi, F. Ancient wheat species and human health: Biochemical and clinical implications. J. Nutr. Biochem. 2018, 52, 1–9. [Google Scholar] [CrossRef]
- Wieser, H.; Kieffer, R. Correlations of the amount of gluten protein types to the technological properties of wheat flours determined on a micro-scale. J. Cereal Sci. 2001, 34, 19–27. [Google Scholar] [CrossRef]
- Gabriel, D.; Pfitzner, C.; Haase, N.U.; Hüsken, A.; Prüfer, H.; Greef, J.M.; Rühl, G. New strategies for a reliable assessment of baking quality of wheat–Rethinking the current indicator protein content. J. Cereal Sci. 2017, 77, 126–134. [Google Scholar] [CrossRef]
- Rossmann, A.; Pitann, B.; Mühling, K.H. Splitting nitrogen applications improves wheat storage protein composition under low N supply. J. Soil Sci. Plant Nutr. 2019, 182, 347–355. [Google Scholar] [CrossRef]
- Shewry, P.R.; Tatham, A.S.; Barro, F.; Barcelo, P.; Lazzeri, P. Biotechnology of Bread making: Unraveling and Manipulating the Multi-Protein Gluten Complex. Nat. Biotechnol. 1995, 13, 1185–1190. [Google Scholar] [CrossRef]
- Raymbek, A.; Saljnikov, E.; Kenenbayev, S.; Perovic, V.; Cakmak, D.; Ramazanova, S. Protein content changes in wheat grain as influenced by nitrogen fertilization. Agrochimica 2017, 61, 180–189. [Google Scholar] [CrossRef]
- Rekowski, A.; Wimmer, M.A.; Henkelmann, G.; Zörb, C. Is a change of protein composition after late application of nitrogen sufficient to improve the baking quality of winter wheat? Agriculture 2019, 9, 101. [Google Scholar] [CrossRef]
- Kurtanjek, Z.; Horvat, D.; Magdic, D.; Drezner, G. Factor analysis and modelling for rapid quality assessment of Croatian Wheat cultivars with different gluten characteristics. Food Tech. Biotechnol. 2008, 46, 270–277. [Google Scholar]
- Barak, S.; Mudgil, D.; Khatkar, B.S. Influence of gliadin and glutenin fractions on rheological, pasting, and textural properties of dough. Int. J. Food. Prop. 2014, 17, 1428–1438. [Google Scholar] [CrossRef]
- Marti, A.; Augst, E.; Cox, S.; Koehler, P. Correlations between gluten aggregation properties defined by the GlutoPeak test and content of quality-related protein fractions of winter wheat flour. J. Cereal Sci. 2015, 66, 89–95. [Google Scholar] [CrossRef]
- Hurkman, W.J.; Tanaka, C.K.; Vensel, W.H.; Thilmony, R.; Altenbach, S.B. Comparative proteomic analysis of the effect of temperature and fertilizer on gliadin and glutenin accumulation in the developing endosperm and flour from Triticum aestivum L. cv. Butte 86. Proteome Sci. 2013, 11, 8. [Google Scholar] [CrossRef]
- De Santis, M.A.; Giuliani, M.M.; Giuzio, L.; De Vita, P.; Lovegrove, A.; Shewry, P.R.; Flagella, Z. Differences in gluten protein composition between old and modern durum wheat genotypes in relation to 20th century breeding in Italy. Eur. J. Agron. 2017, 87, 19–29. [Google Scholar] [CrossRef]
- Daniel, C.; Triboı, E. Changes in wheat protein aggregation during grain development: Effects of temperatures and water stress. Eur. J. Agron. 2002, 16, 1–12. [Google Scholar] [CrossRef]
- Tóth, B.; van Biljon, A.; Moloi, M.J.; Labuschagne, M. Effects of different fertilization levels on the concentration of high molecular weight glutenin subunits of two spring, hard red bread wheat cultivars. Cereal Chem. 2019, 96, 1004–1010. [Google Scholar] [CrossRef]
- Xue, C.; Matros, A.; Mock, H.P.; Mühling, K.H. Protein composition and baking quality of wheat flour as affected by split nitrogen application. Front. Plant Sci. 2019, 10, 642. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Wang, W.; Huang, X.; Liu, M.; Hebelstrup, K.H.; Yang, D.; Cai, J.; Wang, X.; Zhou, Q.; Cao, W.; et al. Nitrogen topdressing timing modifies the gluten quality and grain hardness related protein levels as revealed by iTRAQ. Food Chem. 2019, 277, 135–144. [Google Scholar] [CrossRef]
- Tang, J.W.; Liu, J.J.; Zhang, P.P.; Zhang, Y.; Xiao, Y.G.; Qu, Y.Y.; Zhang, Y.; He, Z.H. Effects of gluten protein fractions on dough property and products quality in common wheat. Sci. Agric. Sin. Sci. 2008, 41, 2937–2946. [Google Scholar]
- Williams, R.M.; O’Brien, L.O.; Eagles, H.A.; Solah, V.A.; Jayasena, V. The influences of genotype, environment, and genotype x environment interaction on wheat quality. Aust. J. Agric. Res. 2008, 59, 95–111. [Google Scholar] [CrossRef]
- Sylvester-Bradley, R.; Kindred, D.; Berry, P.M.; Storer, K.; Kendall, S.; Welham, S. Development of Appropriate Testing Methodology for Assessing Nitrogen Requirements of Wheat and Oilseed Rape Varieties. Final Report to Defra Project IF01110; HMSO: London, UK, 2015. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).