Foliar Application of Ulva rigida Water Extracts Improves Salinity Tolerance in Wheat (Triticum durum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Algae Material and Seaweed Liquid Preparation
2.2. Seaweed Liquid Extract Analysis
2.2.1. Color, pH and Minerals Determination
2.2.2. Nitrogen Content
2.2.3. Protein Content
2.2.4. Lipid Content
2.2.5. Ascorbic Acid Content
2.2.6. Determination of Glycinebetaine (GB)
2.2.7. Estimating Concentration of Phenolic Compounds
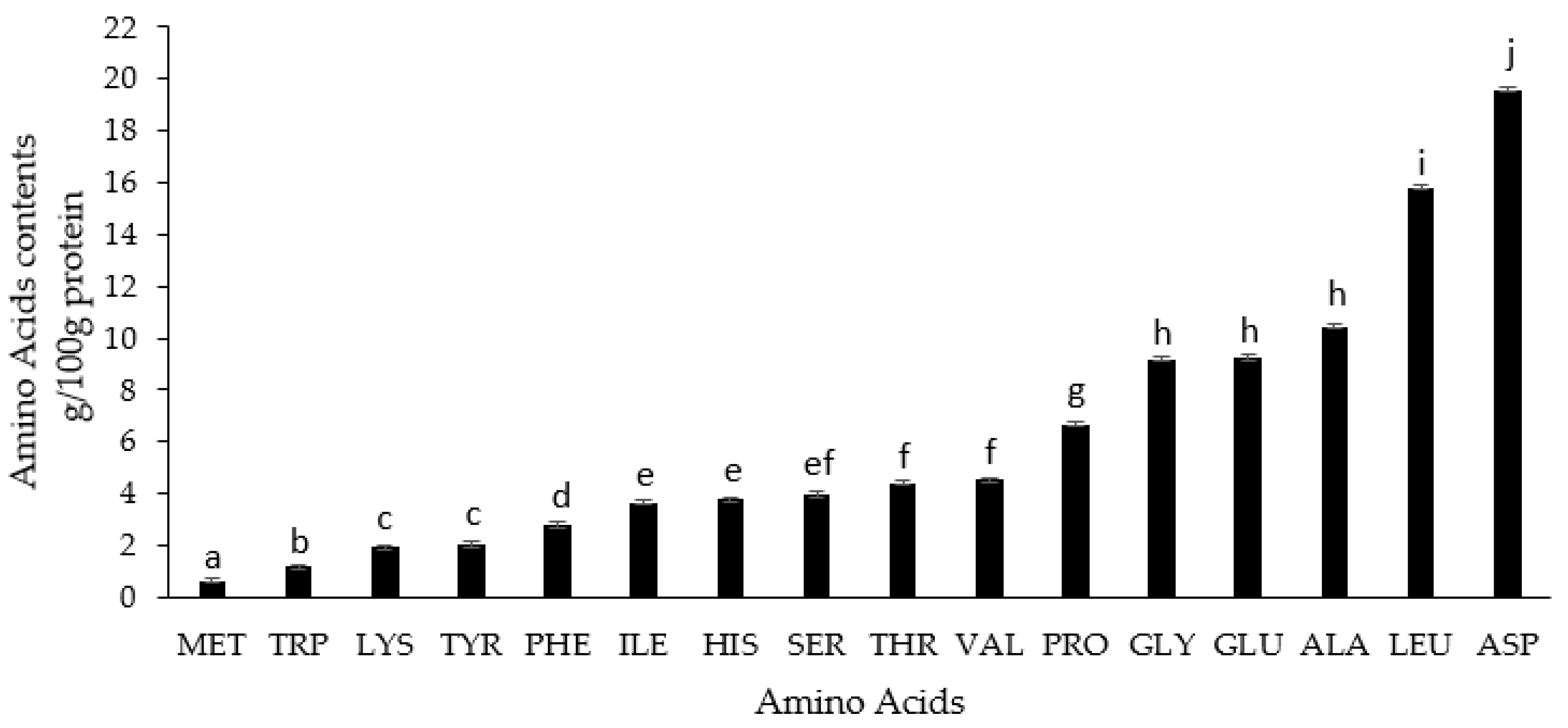
2.2.8. Amino Acids Analysis
2.3. Soil Characterization
2.4. Plant Material and Growth Conditions
2.5. Determination of Chlorophyll Content
2.6. Preparation of Plant Extracts for Enzyme Activity Assays
Enzyme Activity Assays
2.7. Statistical Analysis
3. Results
3.1. Physicochemical Proprieties and Biochemical Analysis of Seaweed Ulva rigida Extract
3.2. Biochemical Analysis of Ulva rigida Extract (SWE)
3.3. Soil Analysis
3.4. Effect of Algal Extracts on Shoot Length and Fresh Weight of Wheat Plants Cultivated under Salt Stress
3.5. Effect of Algal Extracts on Photosynthetic Pigment Contents of Wheat Plants Cultivated under Salt Stress
3.6. Effect of Algal Extracts on Antioxidant Enzymes of Wheat Plants Cultivated under Salt Stress
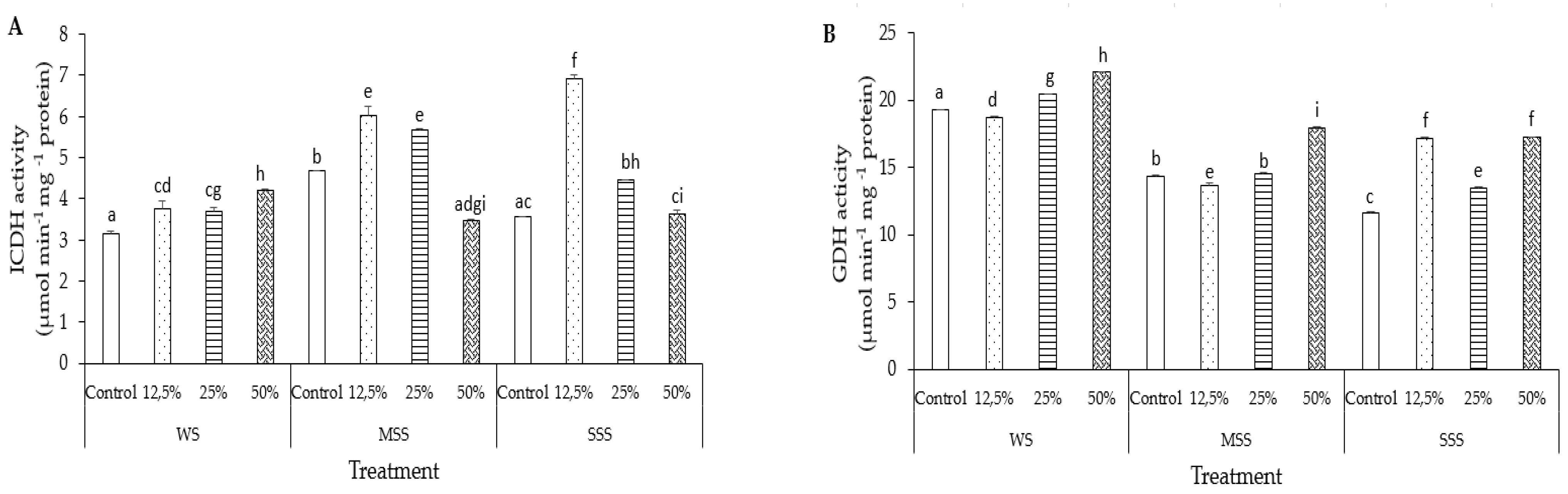
3.6.1. Isocitrate Dehydrogenase (ICDH)
3.6.2. Glutamate Dehydrogenase (GDH)
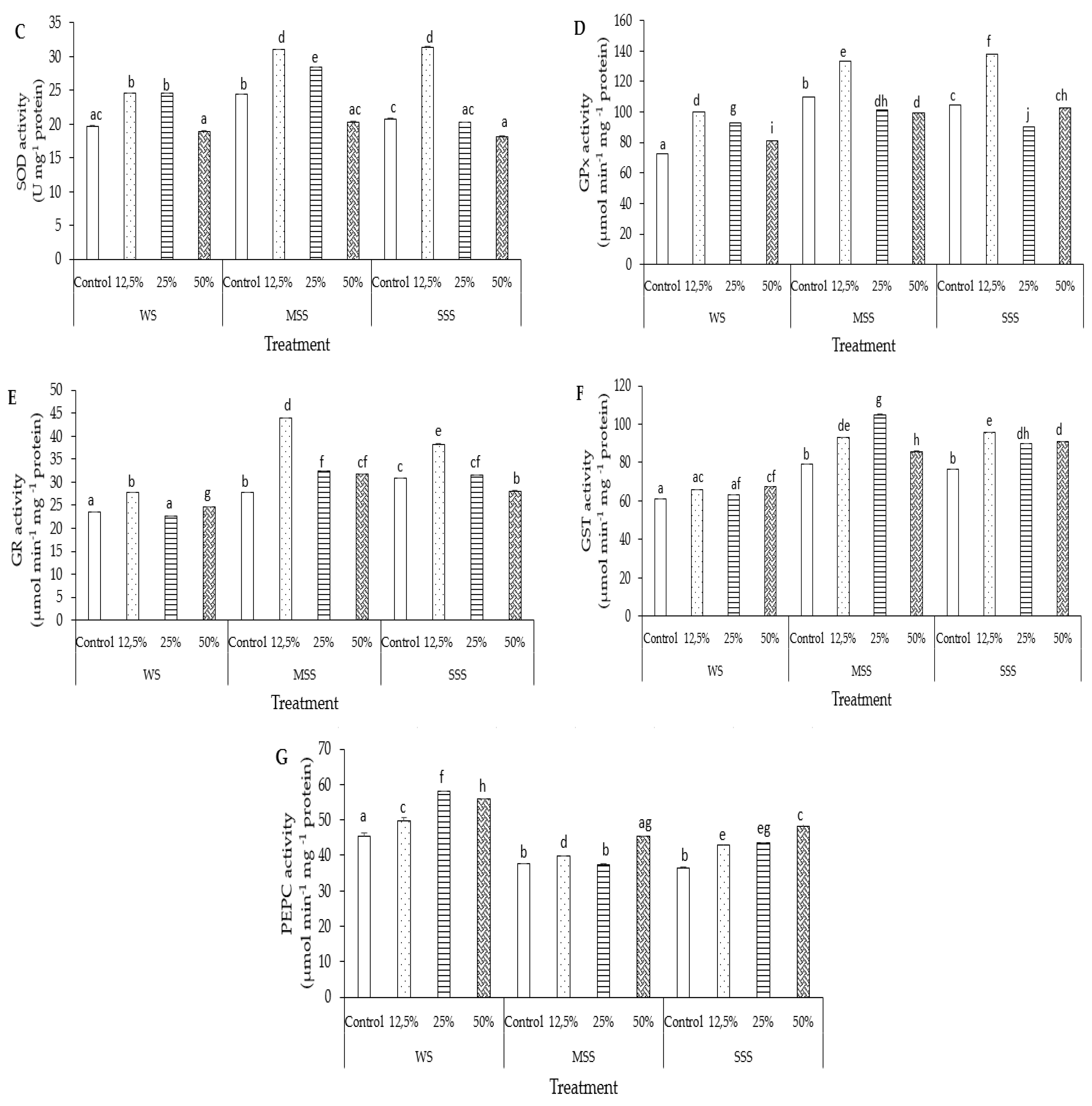
3.6.3. Superoxide Dismutase (SOD)
3.6.4. Glutathione Peroxidase (GPx)
3.6.5. Glutathione Reductase (GR)
3.6.6. Glutathione S-Transferase (GST)
3.6.7. Phosphoenolpyruvate Carboxylase (PECP)
3.7. Principle Component Analysis (PCA) Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farhoudi, R. Effect of salt stress on physiological andmorphological parameters of rapeseed cultivars. Adv. Environ. Biol. 2011, 5, 2501–2508. [Google Scholar]
- Liu, J.; Xia, J.; Fang, Y.; Li, T.; Liu, L. Effects of salt drought stress on growth and physiobiochemical characteristics of Tamarix chinensis seedlings. Sci. World J. 2014. [Google Scholar] [CrossRef]
- Gu, C.-S.; Liu, L.-Q.; Xu, C.; Zhao, Y.-H.; Zhu, X.-D.; Huang, S.-Z. Reference gene selection for quantitative real-time RT-PCR normalization in Iris. lactea var. chinensis roots under cadmium, lead, and salt stress conditions. Sci. World J. 2014. [Google Scholar] [CrossRef] [PubMed]
- Mostafazadeh-fard, B.; Heidarpour, M.; Aghakhani, Q.A.; Feizi, M. Effect of irrigation water salinity and leaching on soil chemical properties in an arid region. Int. J. Agric. Biol. 2007, 3, 166–469. [Google Scholar]
- Misra, S.C.; Randive, R.; Rao, V.S.; Sheshshayee, M.S.; Serraj, R.; Monneveux, P. Relationship between carbon isotope discrimination, ash content and grain yield in wheat in the Peninsular Zone of India. J. Agron. Crop Sci. 2006, 5, 352–362. [Google Scholar] [CrossRef]
- Polle, A. Dissecting the superoxide dismutase-ascorbate-glutathione-pathway in chloroplasts by metabolic modeling. Computer simulations as a step towards flux analysis. Plant Physiol. 2001, 126, 445–462. [Google Scholar] [CrossRef]
- Krumova, K.; Cosa, G. Overview of reactive oxygen species. Appl. Biosc. Nanosc. 2016, 1, 1–21. [Google Scholar]
- Gupta, M.; Cuypers, A.; Vangronsveld, J.; Clijsters, H. Copper affects the enzymes of the ascorbate-glutathione cycle and its related metabolites in the roots of Phaseolus vulgaris. Physiol. Plant. 1999, 106, 262–267. [Google Scholar] [CrossRef]
- Abd El-Baky, H.H.; El-Baroty, G.S. Characterization and bioactivity of phycocyanin isolated from Spirulina maxima grown under salt stress. Food Funct. 2012, 3, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. The water-water cycle in chloroplasts: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef] [PubMed]
- Kiddle, G.; Pastori, G.M.; Bernard, S.; Pignocchi, C.; Antoniw, J.; Verrier, P.J.; Foyer, C.H. Effects of leaf ascorbate content on defense and photosynthesis gene expression in Arabidopsis thaliana. Antioxid. Redox Signal. 2003, 5, 23–32. [Google Scholar] [CrossRef]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Bhaskar, N.; Kazuo, M. Lipid composition of Padina tetrastomatica (Dictyotales, Phaeophyta), a brown seaweed of the west coast of India. Indian J. Fish. 2005, 52, 263–268. [Google Scholar]
- Khan, M.S.; Zaidi, A.; Wani, P.A.; Oves, M. Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ. Chem. Lett. 2009, 7, 1–19. [Google Scholar] [CrossRef]
- Salim, B.B.M. Influence of biochar and seaweed extract applications on growth, yield and mineral composition of wheat (Triticum aestivum L.) under sandy soil conditions. Ann. Agric. Sci. 2016, 61, 257–265. [Google Scholar] [CrossRef]
- Rathore, S.S.; Chaudhary, D.R.; Boricha, G.N.; Ghosh, A.; Bhatt, B.P.; Zodape, S.T.; Patolia, J.S. Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. S. Afr. J. Bot. 2009, 75, 351–355. [Google Scholar] [CrossRef]
- Aziz, N.G.A.; Mahgoub, M.H.; Siam, H.S. Growth, flowering and chemical constituents performence of Amaranthus tricolor plants as influenced by seaweed (Ascophyllum nodosum) extract application under salt stress conditions. J. Appl. Sci. Res. 2011, 1472–1484. [Google Scholar]
- Mansori, M.; Farouk, I.A.; Hsissou, M.; Kaoua, E.I. Seaweed extract treatment enhances vegetative growth and antioxidant parameters in water stressed Salvia officinalis L. J. Mater. Environ. Sci 2019, 8, 756–766. [Google Scholar]
- Blunden, G. Agricultural uses of seaweeds and seaweed extracts. Seaweed Resour. Eur. 1991, 65–81. [Google Scholar]
- Washington, W.S.; Engleitner, S.; Boontjes, G.; Shanmuganathan, N. Effect of fungicides, seaweed extracts, tea tree oil, and fungal agents on fruit rot and yield in strawberry. Aust. J. Exp. Agric. 1999, 39, 487–494. [Google Scholar] [CrossRef]
- Basher, A.A.; Mohammed, A.J.; Teeb, A.I. Effect of seaweed and drainage water on germination and seedling growth of tomato (Lycopersicon spp.). Euphrates J. Agric. Sci. 2012, 4, 24–39. [Google Scholar]
- Latique, S.; Mohamed Aymen, E.; Halima, C.; Chérif, H.; Mimoun, E.K. Alleviation of salt stress in durum wheat (Triticum durum L.) seedlings through the application of liquid seaweed extracts of Fucus spiralis. Commun. Soil Sci. Plant Anal. 2017, 48, 2582–2593. [Google Scholar] [CrossRef]
- Lopes, R.D.; Rordorf, R.; De Ferrari, G.M.; Leonardi, S.; Thomas, L.; Wojdyla, D.M.; Ridefelt, P.; Lawrence, J.H.; De Caterina, R.; Vinereanu, D. Digoxin and mortality in patients with atrial fibrillation. J. Am. Coll. Cardiol. 2018, 71, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Berik, N.; Çankırılıgil, E.C. The Elemental Composition of Green Seaweed (Ulva rigida) Collected from Çanakkale, Turkey. Aquat. Sci. Eng. 2019, 34, 74–79. [Google Scholar]
- Irkin, L.C. Çanakkale Boğazında yayılış gösteren bazı makro alglerin kimyasal kompozisyonunun araştırılması. Inst. Appl. Sci. 2009. [Google Scholar] [CrossRef]
- Sivasankari, S.; Venkatesalu, V.; Anantharaj, M.; Chandrasekaran, M. Effect of seaweed extracts on the growth and biochemical constituents of Vigna sinensis. Bioresour. Technol. 2006, 97, 1745–1751. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for Examination of Water and Wastewater Analysis, 19th ed.; APHA: Washington, DC, USA, 1995. [Google Scholar]
- Sultana, S. Nutritional and functional properties of Moringa oleifera. Metab. Open 2020, 8, 100061. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Cunniff, P.; Washington, D.C. Official methods of analysis of aoac international. J. AOAC Int. 1997, 80, 127A. [Google Scholar]
- Omaye, S.T.; Turnbull, J.D.; Sauberlich, H.E. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1979; Volume 62, pp. 3–11. ISBN 0076-6879. [Google Scholar]
- Gorham, J. Separation of plant betaines and their sulphur analogues by cation-exchange high-performance liquid chromatography. J. Chromatogr. A 1984, 287, 345–351. [Google Scholar] [CrossRef]
- Sauvesty, A.; Page, F.; Huot, J. A simple method for extracting plant phenolic compounds. Can. J. For. Res. 1992, 22, 654–659. [Google Scholar] [CrossRef]
- OJEC, O. COMMISSION REGULATION (EC) No 2788/98 of 22 December 1998 Amending Council Directive 70/524/EEC Concerning Additives in Feeding Stuffs as Regards the Withdrawal of Authorisation for Certain Growth Promoters. 3 November 1998. Available online: http://extwprlegs1.fao.org/docs/pdf/eur18700.pdf (accessed on 15 November 2020).
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; US Department of Agriculture: Washington, DC, USA, 1954. [Google Scholar]
- Mountier, N.S.; Griggs, J.L.; Oomen, G.A.C. Sources of error in advisory soil tests: I. Laboratory sources. N. Z. J. Agric. Res. 1966, 9, 328–338. [Google Scholar] [CrossRef]
- Chernane, H.; Latique, S.; Mansori, M.; El Kaoua, M. Salt stress tolerance and antioxidative mechanisms in wheat plants (Triticum durum L.) by seaweed extracts application. IOSR J. Agric. Vet. Sci. 2015, 8, 36–44. [Google Scholar]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B-and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Magalhaes, J.R.; Huber, D.M. Response of ammonium assimilation enzymes to nitrogen form treatments in different plant species. J. Plant Nutr. 1991, 14, 175–185. [Google Scholar] [CrossRef]
- Mrid, R.B.; Bouargalne, Y.; El Omari, R.; El Mourabit, N.; Nhiri, M. Activities of Carbon and Nitrogen Metabolism Enzymes of Sorghum (Sorghum bicolor L. Moench) During Seed Development. J. Crop Sci. Biotechnol. 2018, 21, 283–289. [Google Scholar] [CrossRef]
- Mrid, R.B.; Omari, R.E.; Nhiri, M. Effect of nitrogen source and concentration on growth and activity of nitrogen assimilation enzymes in roots of a moroccan sorghum ecotype. Plant 2016, 4, 71. [Google Scholar] [CrossRef]
- Bouchmaa, N.; Ben Mrid, R.; Boukharsa, Y.; Nhiri, M.; Ait Mouse, H.; Taoufik, J.; Ansar, M.; Zyad, A. Cytotoxicity of new pyridazin-3 (2H)-one derivatives orchestrating oxidative stress in human triple-negative breast cancer (MDA-MB-468). Arch. Der Pharm. 2018, 351, 1800128. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nomura, M.; Muramoto, Y.; Yasuda, S.; Takabe, T.; Kishitani, S. The accumulation of glycinebetaine during cold acclimation in early and late cultivars of barley. Euphytica 1995, 83, 247–250. [Google Scholar] [CrossRef]
- Gressler, V.; Yokoya, N.S.; Fujii, M.T.; Colepicolo, P.; Filho, J.M.; Torres, R.P.; Pinto, E. Lipid, fatty acid, protein, amino acid and ash contents in four Brazilian red algae species. Food Chem. 2010, 120, 585–590. [Google Scholar] [CrossRef]
- Wong, K.H.; Cheung, P.C. Nutritional evaluation of some subtropical red and green seaweeds: Part I—proximate composition, amino acid profiles and some physico-chemical properties. Food Chem. 2000, 71, 475–482. [Google Scholar] [CrossRef]
- Shuuluka, D.; Bolton, J.J.; Anderson, R.J. Protein content, amino acid composition and nitrogen-to-protein conversion factors of Ulva rigida and Ulva capensis from natural populations and Ulva lactuca from an aquaculture system, in South Africa. J. Appl. Phycol. 2013, 25, 677–685. [Google Scholar] [CrossRef]
- Popova, O.V.; Ismailov, S.F.; Popova, T.N.; Dietz, K.J.; Golldack, D. Salt-induced expression of NADP-dependent isocitrate dehydrogenase and ferredoxin-dependent glutamate synthase in Mesembryanthemum Cryst. Planta 2002, 215, 906–913. [Google Scholar] [CrossRef] [PubMed]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Noctor, G.; Gomez, L.; Vanacker, H.; Foyer, C.H. Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling. J. Exp. Bot. 2002, 53, 1283–1304. [Google Scholar] [CrossRef]
- Frendo, P.; Matamoros, M.A.; Alloing, G.; Becana, M. Thiol-based redox signaling in the nitrogen-fixing symbiosis. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef]
- Pang, J.; Wang, A.; Zheng, M.; Zhang, T. Hydrolysis of cellulose into glucose over carbons sulfonated at elevated temperatures. Chem. Commun. 2010, 46, 6935–6937. [Google Scholar] [CrossRef] [PubMed]
- El Omari, R.; Ben Mrid, R.; Chibi, F.; Nhiri, M. Involvement of phosphoenolpyruvate carboxylase and antioxydants enzymes in nitrogen nutrition tolerance in Sorghum bicolor plants. Russ. J. Plant Physiol. 2016, 63, 719–726. [Google Scholar] [CrossRef]
- Iannelli, F.; Oggioni, M.R.; Pozzi, G. Allelic variation in the highly polymorphic locus pspC of Streptococcus pneumoniae. Gene 2002, 284, 63–71. [Google Scholar] [CrossRef]
- Chollet, R.; Vidal, J.; O’Leary, M.H. Phospho enol pyruvate carboxylase: A ubiquitous, highly regulated enzyme in plants. Annu. Rev. Plant Biol. 1996, 47, 273–298. [Google Scholar] [CrossRef]
- González, M.C.; Sanchez, R.; Cejudo, F.J. Abiotic stresses affecting water balance induce phosphoenolpyruvate carboxylase expression in roots of wheat seedlings. Planta 2003, 216, 985–992. [Google Scholar] [CrossRef]
- Ibrahim, W.M.; Ali, R.M.; Hemida, K.A.; Sayed, M.A. Role of Ulva lactuca extract in alleviation of salinity stress on wheat seedlings. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Gollan, J.R.; Wright, J.T. Limited grazing pressure by native herbivores on the invasive seaweed Caulerpa taxifolia in a temperate Australian estuary. Mar. Freshw. Res. 2006, 57, 685–694. [Google Scholar] [CrossRef]
- Attememe, J.Y.A. The effect of humic acid and sea weed extracts on the growth, chemical characteristics and oil characteristics of Rosmarinus officinalis L. In Proceedings of the The 6th Scientific Conference; Biology Department, College of Education, University of Tikrit: Tikrit, Iraq, 2009; pp. 1–17. [Google Scholar]
- Whapham, C.A.; Blunden, G.; Jenkins, T.; Hankins, S.D. Significance of betaines in the increased chlorophyll content of plants treated with seaweed extract. J. Appl. Phycol. 1993, 5, 231. [Google Scholar] [CrossRef]
- Genard, H.; Le Saos, J.; Billard, J.-P.; Tremolieres, A.; Boucaud, J. Effect of salinity on lipid composition, glycine betaine content and photosynthetic activity in chloroplasts of Suaeda maritima. Plant Physiol. Biochem. 1991, 29, 421–427. [Google Scholar]
- Ashraf, M.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Anjum, A.S.; Ashraf, U.; Zohaib, A.; Tanveer, M.; Naeem, M.; Ali, I.; Tabassum, T.; Nazir, U. Growth and developmental responses of crop plants under drought stress: A review. Zemdirb. Agric. 2017, 104, 267–276. [Google Scholar] [CrossRef]
- Chen, R.D.; Gadal, P. Do the mitochondria provide the 2-oxoglutarate needed for glutamate synthesis in higher plant chloroplasts? Plant Physiol. Biochem. 1990, 28, 141–145. [Google Scholar]
- Bouthour, D.; Hajaji-Nasraoui, A.; Saafi, L.; Gouia, H.; Chaffei-Haouari, C. Reversibility effects of NaCl in growth and activity of enzymes involved in carbon metabolism in leaves of tobacco (Nicotiana rustica). Afr. J. Biotech. 2012, 11, 12619–12629. [Google Scholar] [CrossRef]
- Hemida, M.G.; Chu, D.K.; Poon, L.L.; Perera, R.A.; Alhammadi, M.A.; Ng, H.; Siu, L.Y.; Guan, Y.; Alnaeem, A.; Peiris, M. MERS coronavirus in dromedary camel herd, Saudi Arabia. Emerg. Infect. Dis. 2014, 20, 1231. [Google Scholar] [CrossRef]
- Candan, N.; Tarhan, L. The correlation between antioxidant enzyme activities and lipid peroxidation levels in Mentha pulegium organs grown in Ca2+, Mg2+, Cu2+, Zn2+ and Mn2+ stress conditions. Plant Sci. 2003, 165, 769–776. [Google Scholar] [CrossRef]
- Fike, J.H.; Allen, V.G.; Schmidt, R.E.; Zhang, X.; Fontenot, J.P.; Bagley, C.P.; Ivy, R.L.; Evans, R.R.; Coelho, R.W.; Wester, D.B. Tasco-Forage: I. Influence of a seaweed extract on antioxidant activity in tall fescue and in ruminants. J. Anim. Sci. 2001, 79, 1011–1021. [Google Scholar] [CrossRef]
- Hoque, M.A.; Okuma, E.; Banu, M.N.A.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Exogenous proline mitigates the detrimental effects of salt stress more than exogenous betaine by increasing antioxidant enzyme activities. J. Plant Physiol. 2007, 164, 553–561. [Google Scholar] [CrossRef] [PubMed]


| Algal Species | Physical Property | Minerals Content (mg/g) | Biochemicals Content (mg/g) | Total Nitrogen (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Color | pH | K | Mg | Ca | Na | Cl | Protein | Lipid | Ascorbic Acid | Phenolic Compound | Betain | ||
| Ulva rigida | Green | 6.58 ± 0.1 | 12.33 ± 0.49 | 35.10 ± 0.68 | 0.68 ±0.031 | 0.42 ± 0.015 | 0.15 ± 0.012 | 9.2 ± 0.3 | 2.8 ± 0.1 | 35 ± 1 | 0.99 ± 0.006 | 8.03 ± 0.093 | 1.28 |
| Chemical Compound | P2O5 (ppm) | K2O (ppm) | N (%) |
|---|---|---|---|
| Concentration | 13.16 ± 0.056 | 147.575 ± 0.134 | 0.146 ± 0.004 |
| Soil characteristics | Moderately poor 1 | Poor 2 | Moderately poor 3 |
| Treatment | Growth Conditions | ||||
|---|---|---|---|---|---|
| WS | MSS | SSS | |||
| Shoot length (cm) | Control | 0% | 41.3 ± 5.91 a | 34.1 ± 2.83 b | 33.14 ± 1.56 b |
| Ulva rigida extract | 12.5% | 39 ± 5.65 ab | 38.9 ± 2.3 ab | 38.7 ± 4.08 ab | |
| 25% | 38 ± 1.27 ab | 37.4 ± 1.14 ab | 35.8 ± 1.79 ab | ||
| 50% | 38.8 ± 0.76 ab | 37.5 ± 4.47 ab | 37.5 ± 1.87 ab | ||
| Fresh weight (g plant−1) | Control | 0% | 2.01 ± 0.45 a | 1.50 ± 0.25 ab | 1.05 ± 0.03 b |
| Ulva rigida extract | 12.5% | 2.09 ± 0.33 a | 1.96 ± 0.14 a | 1.51 ± 0.27 ab | |
| 25% | 1.98 ± 0.01 a | 1.89 ± 0.35 a | 1.89 ± 0.47 a | ||
| 50% | 1.97 ± 0.22 a | 1.62 ± 0.32 ab | 1.42 ± 0.16 ab | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Latique, S.; Mrid, R.B.; Kabach, I.; Kchikich, A.; Sammama, H.; Yasri, A.; Nhiri, M.; El Kaoua, M.; Douira, A.; Selmaoui, K. Foliar Application of Ulva rigida Water Extracts Improves Salinity Tolerance in Wheat (Triticum durum L.). Agronomy 2021, 11, 265. https://doi.org/10.3390/agronomy11020265
Latique S, Mrid RB, Kabach I, Kchikich A, Sammama H, Yasri A, Nhiri M, El Kaoua M, Douira A, Selmaoui K. Foliar Application of Ulva rigida Water Extracts Improves Salinity Tolerance in Wheat (Triticum durum L.). Agronomy. 2021; 11(2):265. https://doi.org/10.3390/agronomy11020265
Chicago/Turabian StyleLatique, Salma, Reda Ben Mrid, Imad Kabach, Anass Kchikich, Hasnaa Sammama, Abdelaziz Yasri, Mohamed Nhiri, Mimoun El Kaoua, Allal Douira, and Karima Selmaoui. 2021. "Foliar Application of Ulva rigida Water Extracts Improves Salinity Tolerance in Wheat (Triticum durum L.)" Agronomy 11, no. 2: 265. https://doi.org/10.3390/agronomy11020265
APA StyleLatique, S., Mrid, R. B., Kabach, I., Kchikich, A., Sammama, H., Yasri, A., Nhiri, M., El Kaoua, M., Douira, A., & Selmaoui, K. (2021). Foliar Application of Ulva rigida Water Extracts Improves Salinity Tolerance in Wheat (Triticum durum L.). Agronomy, 11(2), 265. https://doi.org/10.3390/agronomy11020265






