Chemical and Microbial Characteristics of Blackening Disease in Lotus (Nelumbo nucifera Gaertn.) Caused by Hirschmanniella diversa Sher
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Damage Index Evaluation
2.2. Chemical and Microscopic Analyses
2.2.1. Cultivation Fields
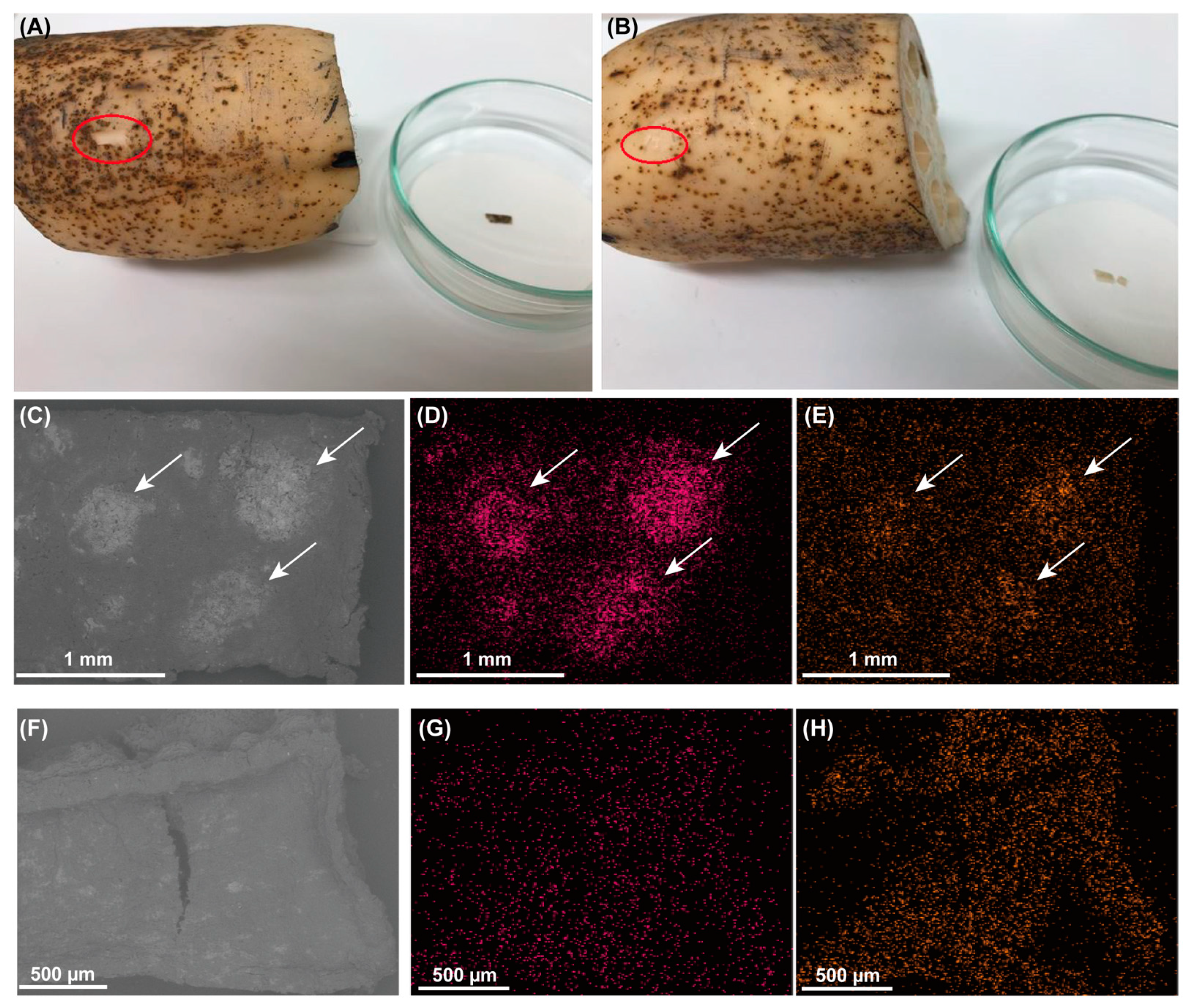
2.2.2. Lotus Tubers and Black Spots
2.3. 16S rRNA Gene Analysis
2.3.1. DNA Extraction, PCR Amplification, and 16S rRNA Gene Sequencing Analysis
2.3.2. Deposition of 16S rRNA Gene Sequence Data
3. Results
3.1. Survey and Chemical Analysis of the Lotus Cultivation Field
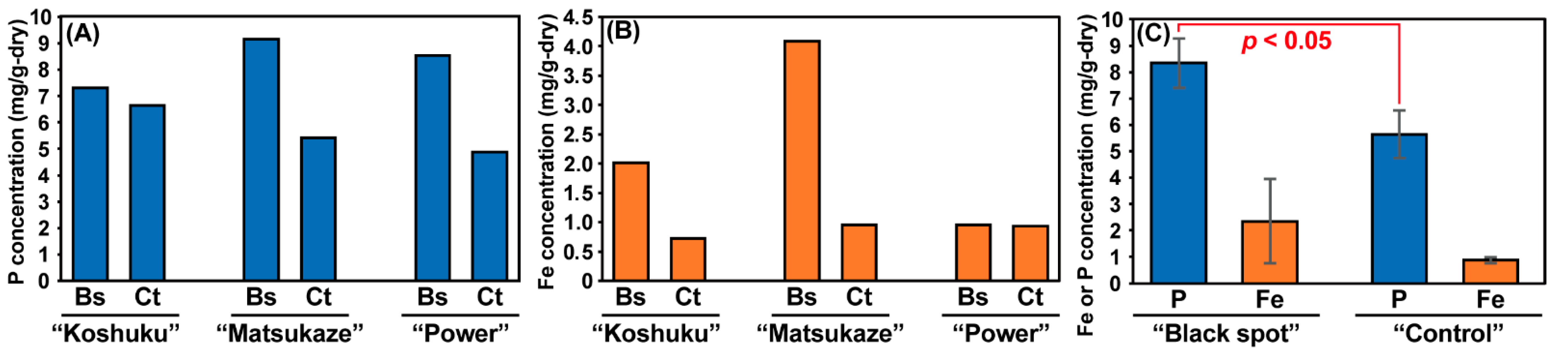
3.2. Chemical Analysis of Lotus Tubers Damaged by Hirschmanniella Diversa
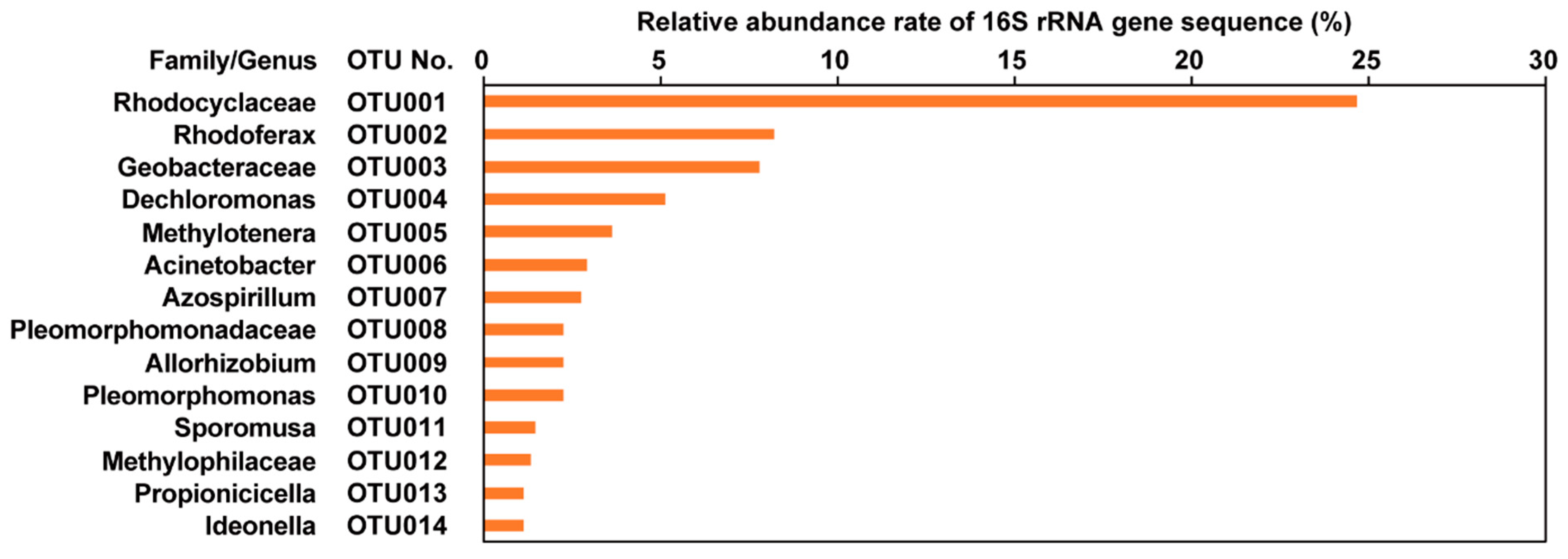
3.3. Microbial Community Analysis of Black Spots on the Lotus Tubers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurashita, H.; Kuroda, K.; Narihiro, T.; Takagi, M.; Goto, M.; Ikeda, S.; Hirakata, Y.; Hatamoto, M.; Maki, S.; Yamaguchi, T.; et al. Accurate evaluation of blackening disease in lotus (Nelumbo nucifera Gaertn.) using a quantitative PCR-based assay for Hirschmanniella diversa Sher and H. imamuri Sher. Crop Prot. 2021, 139, 105380. [Google Scholar] [CrossRef]
- Lu, H.-F.; Tan, Y.-W.; Zhang, W.-S.; Qiao, Y.-C.; Campbell, D.E.; Zhou, L.; Ren, H. Integrated emergy and economic evaluation of lotus-root production systems on reclaimed wetlands surrounding the Pearl River Estuary, China. J. Clean. Prod. 2017, 158, 367–379. [Google Scholar] [CrossRef]
- Mihira, T. Browning tuber disease of Indian lotus Nelumboi nucifera, Kurokawa-senchu-byo caused by rice root nematode, Hirschmanniella imamuri. Bull. Chiba Prefect. Agric. Res. Cent. 2002, 1, 121–124. [Google Scholar]
- Takagi, M.; Goto, M.; Wari, D.; Kashima, T.; Toyota, K. Seasonal occurrence and life cycle of lotus root nematode Hirschmanniella diversa (Tylenchida: Pratylenchidae) in lotus roots in paddy fields. Appl. Entomol. Zool. 2019, 54, 465–471. [Google Scholar] [CrossRef]
- Jeger, M.; Bragard, C.; Caffier, D.; Candresse, T.; Chatzivassiliou, E.; Dehnen-Schmutz, K.; Gilioli, G.; Grégoire, J.C.; Anton, J.; Miret, J.; et al. Pest categorisation of Hirschmanniella spp. EFSA J. 2018, 16, e05297. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Thar, S.P.; Kizaki, C.; Toyota, K.; Sawada, E.; Abe, N. Development of specific primers to Hirschmanniella spp. causing damage to lotus and their economic threshold level in Tokushima prefecture in Japan. Nematology 2013, 15, 851–858. [Google Scholar] [CrossRef]
- Takagi, M.; Goto, M.; Hisatsune, K.; Kashima, T. Evaluation of weeds and wild lotus in and around lotus paddy fields in Ibaraki prefecture as possible alternative host plants of the lotus root nematode, Hirschmanniella diversa (Tylenchida: Pratylenchidae) causing damages to lotus, Nelumbo nucifera. Annu. Rep. Kanto-Tosan Plant Prot. Soc. 2016, 63, 98–101. [Google Scholar] [CrossRef]
- Uematsu, S.; Yabu, T.; Mitsuyoshi, Y.; Kurihara, T.; Koga, H. Light and scanning electron microscopy of the Indian lotus roots invaded by Hirschmanniella diversa. Nematol. Res. 2016, 46, 79–82. [Google Scholar] [CrossRef][Green Version]
- Takagi, M.; Goto, M.; Wari, D.; Saito, M.; Perry, R.; Toyota, K. Screening of Nematicides against the Lotus Root Nematode, Hirschmanniella diversa Sher (Tylenchida: Pratylenchidae) and the Efficacy of a Selected Nematicide under Lotus Micro-Field Conditions. Agronomy 2020, 10, 373. [Google Scholar] [CrossRef]
- Takagi, M.; Ogawara, T.; Toyota, K. The new integrated pest management system incorporating benfuracarb granule against lotus root nematode Hirschmanniella diversa. Plant Prot. 2020, 74, 22–26. [Google Scholar]
- Uematsu, S.; Yabu, T.; Yao, M.; Kurihara, T.; Koga, H. Ultrastructure of Hirschmanniella diversa early-stage infection in browning rhizomes of Indian lotus. J. Nematol. 2020, 52, e2020-55. [Google Scholar] [CrossRef]
- Nagashima, T.; Toda, A.; Higashi, T. Relationship between the occurrence of epidermic blackening and browning of lotus rhizome, and redox state in the plow layer at lotus field–Fundamental studies on the land consolidation of lotus field (I). Trans.-Jpn. Soc. Irrig. Drain. Reclam. Eng. 1997, 189, 81–88. [Google Scholar] [CrossRef]
- Nagashima, T.; Toda, A.; Higashi, T. A Study on the Properties of Soil in the Plow Layer of Lotus Field, and Effect of some Conditions to its Redox State-Fundamental studies on the land consolidation of lotus field (II). Trans. Jpn. Soc. Irrig. Drain. Reclam. Eng. 1997, 189, 89–96. [Google Scholar] [CrossRef]
- Kozich, J.J.; Westcott, S.L.; Baxter, N.T.; Highlander, S.K.; Schloss, P.D. Development of a Dual-Index Sequencing Strategy and Curation Pipeline for Analyzing Amplicon Sequence Data on the MiSeq Illumina Sequencing Platform. Appl. Environ. Microbiol. 2013, 79, 5112–5120. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, K.; Kurashita, H.; Arata, T.; Miyata, A.; Kawazoe, M.; Nobu, M.K.; Narihiro, T.; Ohike, T.; Hatamoto, M.; Maki, S.; et al. Influence of Green Tuff Fertilizer Application on Soil Microorganisms, Plant Growth, and Soil Chemical Parameters in Green Onion (Allium fistulosum L.) Cultivation. Agronomy 2020, 10, 929. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glöckner, F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef]
- Weelink, S.A.B.; van Doesburg, W.; Saia, F.T.; Rijpstra, W.I.C.; Röling, W.F.M.; Smidt, H.; Stams, A.J.M. A strictly anaerobic betaproteobacterium Georgfuchsia toluolica gen. nov., sp. nov. degrades aromatic compounds with Fe(III), Mn(IV) or nitrate as an electron acceptor. FEMS Microbiol. Ecol. 2009, 70, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Finneran, K.T.; Johnsen, C.V.; Lovley, D.R. Rhodoferax ferrireducens sp. nov., a psychrotolerant, facultatively anaerobic bacterium that oxidizes acetate with the reduction of Fe(III). Int. J. Syst. Evol. Microbiol. 2003, 53, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, D.J.; Jenter, H.L.; Coates, J.D.; Phillips, E.J.P.; Schmidt, T.M.; Lovley, D.R. Phylogenetic analysis of dissimilatory Fe(III)-reducing bacteria. J. Bacteriol. 1996, 178, 2402–2408. [Google Scholar] [CrossRef] [PubMed]
- Schink, B. Fermentation of 2,3-butanediol by Pelobacter carbinolicus sp. nov. and Pelobacter propionicus sp. nov., and evidence for propionate formation from C2 compounds. Arch. Microbiol. 1984, 137, 33–41. [Google Scholar] [CrossRef]
- Wolterink, A.; Kim, S.; Muusse, M.; Kim, I.S.; Roholl, P.J.M.; van Ginkel, C.G.; Stams, A.J.M.; Kengen, S.W.M. Dechloromonas hortensis sp. nov. and strain ASK-1, two novel (per)chlorate-reducing bacteria, and taxonomic description of strain GR-1. Int. J. Syst. Evol. Microbiol. 2005, 55, 2063–2068. [Google Scholar] [CrossRef] [PubMed]
- Rothe, M.; Kleeberg, A.; Hupfer, M. The occurrence, identification and environmental relevance of vivianite in waterlogged soils and aquatic sediments. Earth-Sci. Rev. 2016, 158, 51–64. [Google Scholar] [CrossRef]
- Nanzyo, M.; Kanno, H. Inorganic Constituents in Soil: Basics and Visuals; Springer Nature: Basingstoke, UK, 2018; ISBN 9789811312144. [Google Scholar]
- Nriagu, J.O. Stability of vivianite and ion-pair formation in the system fe3(PO4)2-H3PO4H3PO4-H2o. Geochim. Cosmochim. Acta 1972, 36, 459–470. [Google Scholar] [CrossRef]
- Viulu, S.; Nakamura, K.; Okada, Y.; Saitou, S.; Takamizawa, K. Geobacter luticola sp. nov., an Fe(III)-reducing bacterium isolated from lotus field mud. Int. J. Syst. Evol. Microbiol. 2013, 63, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Wang, S.; Wang, X.; Li, N. Biosynthesis of vivianite from microbial extracellular electron transfer and environmental application. Sci. Total Environ. 2021, 762, 143076. [Google Scholar] [CrossRef] [PubMed]
- Nanzyo, M.; Onodera, H.; Hasegawa, E.; Ito, K.; Kanno, H. Formation and Dissolution of Vivianite in Paddy Field Soil. Soil Sci. Soc. Am. J. 2013, 77, 1452–1459. [Google Scholar] [CrossRef]
- Glasauer, S.; Weidler, P.G.; Langley, S.; Beveridge, T.J. Controls on Fe reduction and mineral formation by a subsurface bacterium. Geochim. Cosmochim. Acta 2003, 67, 1277–1288. [Google Scholar] [CrossRef]
- O’Loughlin, E.J.; Boyanov, M.I.; Flynn, T.M.; Gorski, C.A.; Hofmann, S.M.; McCormick, M.L.; Scherer, M.M.; Kemner, K.M. Effects of bound phosphate on the bioreduction of lepidocrocite (γ-FeOOH) and maghemite (γ-Fe2O3) and formation of secondary minerals. Environ. Sci. Technol. 2013, 47, 9157–9166. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wilfert, P.; Dugulan, I.; Goubitz, K.; Korving, L.; Witkamp, G.J.; van Loosdrecht, M.C.M. Fe(III) reduction and vivianite formation in activated sludge. Sep. Purif. Technol. 2019, 220, 126–135. [Google Scholar] [CrossRef]
- Wang, S.; An, J.; Wan, Y.; Du, Q.; Wang, X.; Cheng, X.; Li, N. Phosphorus Competition in Bioinduced Vivianite Recovery from Wastewater. Environ. Sci. Technol. 2018, 52, 13863–13870. [Google Scholar] [CrossRef] [PubMed]
- You, J.S.; Lee, Y.J.; Kim, K.S.; Kim, S.H.; Chang, K.J. Ethanol extract of lotus (Nelumbo nucifera) root exhibits an anti-adipogenic effect in human pre-adipocytes and anti-obesity and anti-oxidant effects in rats fed a high-fat diet. Nutr. Res. 2014, 34, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Li, R.H.; Cui, J.L.; Li, X.D.; Li, X.Y. Phosphorus Removal and Recovery from Wastewater using Fe-Dosing Bioreactor and Cofermentation: Investigation by X-ray Absorption Near-Edge Structure Spectroscopy. Environ. Sci. Technol. 2018, 52, 14119–14128. [Google Scholar] [CrossRef] [PubMed]
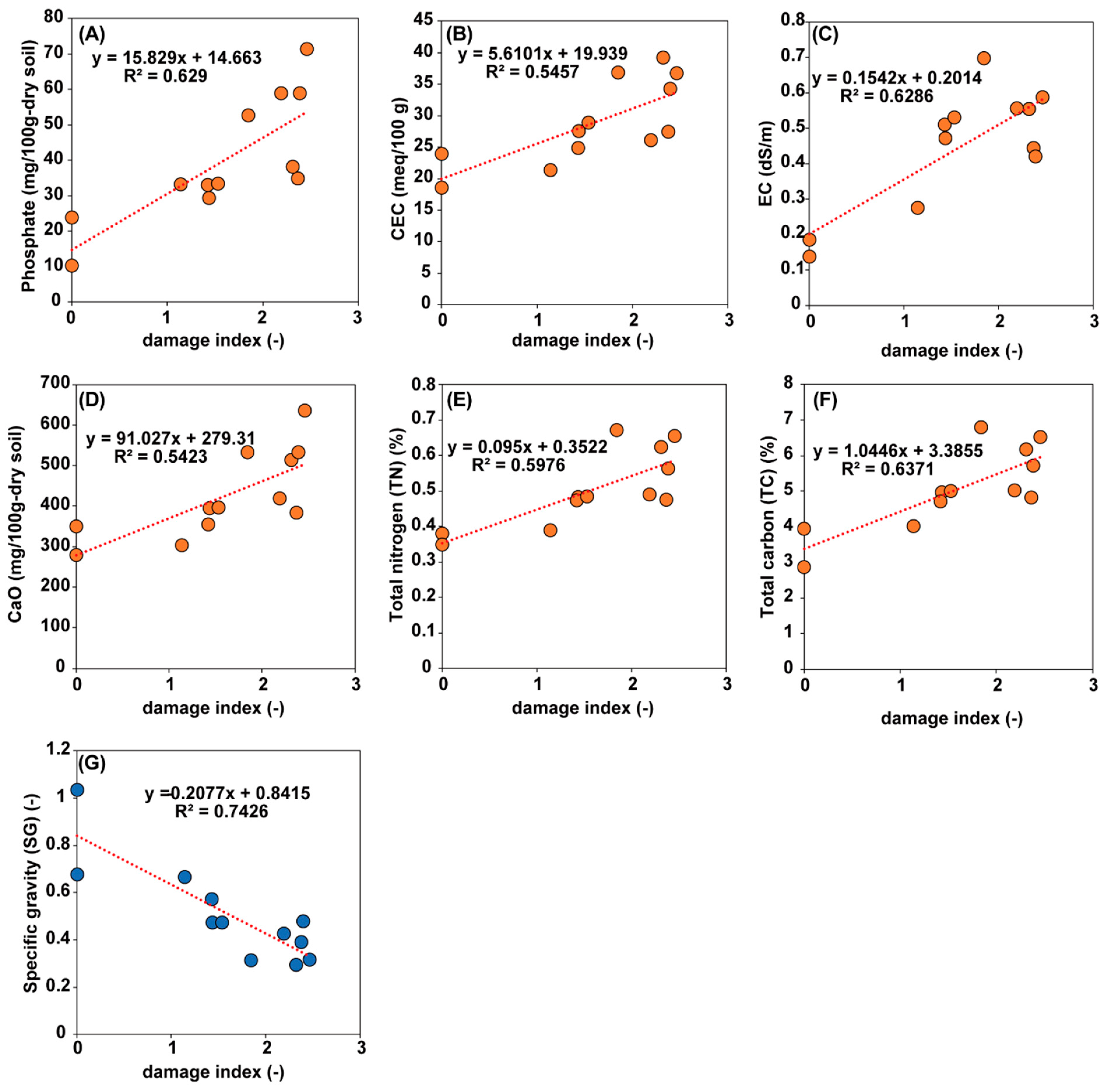

| Field | Field Size | Lotus | Damage Index | pH | EC | NH4-N | P2O5 | K2O | CaO | MgO | SG c | TN | TC | C/N | CEC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name | (m2) | Cultivar | (0,1,2,3,4) | (-) | (dS/m) | (mg/100 g-Dry Soil) | (-) | (%) | (%) | (-) | (meq/100 g) | ||||
| I aA-1 b | ca. 1000 | “Koshuku” | 2.42 ± 0.99 (31) d | 5.8 | 0.44 | 5.3 | 34.8 | 28.8 | 383.6 | 110.2 | 0.39 | 0.48 | 4.81 | 10.1 | 27.5 |
| IA-2 | ca. 1000 | 2.60 ± 0.82 (25) | 6.0 | 0.55 | 39.3 | 38.1 | 66.2 | 513.0 | 146.5 | 0.30 | 0.62 | 6.17 | 9.9 | 39.3 | |
| IB-1 | ca. 1000 | 2.34 ± 1.29 (32) | 6.0 | 0.56 | 13.7 | 58.9 | 54.6 | 418.8 | 133.1 | 0.43 | 0.49 | 5.03 | 10.3 | 26.1 | |
| IB-2 | ca. 1000 | 2.07 ± 0.83 (27) | 6.1 | 0.70 | 22.2 | 52.8 | 65.7 | 532.8 | 187.6 | 0.31 | 0.67 | 6.80 | 10.1 | 36.9 | |
| IC-1 | ca. 1100 | “Kanasumi No. 24” | 0 (20) | 6.3 | 0.18 | 20.7 | 10.2 | 56.1 | 349.0 | 73.4 | 0.68 | 0.38 | 3.94 | 10.4 | 24.0 |
| IC-2 | ca. 1100 | 0 (19) | 6.8 | 0.14 | 23.4 | 23.9 | 65.4 | 278.0 | 71.2 | 1.04 | 0.35 | 2.88 | 8.3 | 18.6 | |
| ID-1 | ca. 1000 | “Power” | 2.41 ± 0.94 (17) | 6.7 | 0.59 | 68.1 | 71.4 | 66.5 | 635.1 | 104.3 | 0.32 | 0.66 | 6.53 | 10.0 | 36.8 |
| ID-2 | ca. 1000 | 2.42 ± 0.90 (12) | 6.5 | 0.42 | 21.1 | 59.0 | 47.8 | 533.1 | 99.5 | 0.48 | 0.56 | 5.71 | 10.2 | 34.3 | |
| IE-1 | ca. 1000 | “Koshuku” | 1.75 ± 0.79 (20) | 5.8 | 0.47 | 13.7 | 29.4 | 42.1 | 394.0 | 69.4 | 0.48 | 0.48 | 4.96 | 10.3 | 27.6 |
| IE-2 | ca. 1000 | 1.52 ± 0.64 (27) | 5.6 | 0.51 | 12.0 | 33.0 | 33.5 | 353.8 | 61.6 | 0.57 | 0.47 | 4.71 | 9.9 | 24.9 | |
| IF-1 | ca. 1000 | “Koshuku” | 1.18 ± 0.90 (28) | 6.0 | 0.28 | 9.6 | 33.3 | 46.0 | 302.4 | 53.4 | 0.67 | 0.39 | 4.01 | 10.3 | 21.4 |
| IF-2 | ca. 1000 | 1.90 ± 0.91 (20) | 5.9 | 0.53 | 17.8 | 33.4 | 47.7 | 395.9 | 71.9 | 0.47 | 0.48 | 5.00 | 10.3 | 28.9 | |
| IG | ca. 2000 | “Power” | 2.67 ± 0.58 (3) | – e | – | – | – | – | – | – | – | – | – | – | – |
| IH | ca. 1000 | “Matsukaze” | 3.25 ± 0.50 (4) | – | – | – | – | – | – | – | – | – | – | – | – |
| NI | ca. 4000 | “Daruma” | 3.25 ± 0.47 (20) | – | – | – | – | – | – | – | – | – | – | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurashita, H.; Kuroda, K.; Maki, S.; Sato, T.; Takagi, M.; Goto, M.; Kariya, T.; Hatamoto, M.; Yamaguchi, T.; Tomita, S.; et al. Chemical and Microbial Characteristics of Blackening Disease in Lotus (Nelumbo nucifera Gaertn.) Caused by Hirschmanniella diversa Sher. Agronomy 2021, 11, 2517. https://doi.org/10.3390/agronomy11122517
Kurashita H, Kuroda K, Maki S, Sato T, Takagi M, Goto M, Kariya T, Hatamoto M, Yamaguchi T, Tomita S, et al. Chemical and Microbial Characteristics of Blackening Disease in Lotus (Nelumbo nucifera Gaertn.) Caused by Hirschmanniella diversa Sher. Agronomy. 2021; 11(12):2517. https://doi.org/10.3390/agronomy11122517
Chicago/Turabian StyleKurashita, Hazuki, Kyohei Kuroda, Shinya Maki, Takeshi Sato, Motonori Takagi, Maki Goto, Tetsuro Kariya, Masashi Hatamoto, Takashi Yamaguchi, Shun Tomita, and et al. 2021. "Chemical and Microbial Characteristics of Blackening Disease in Lotus (Nelumbo nucifera Gaertn.) Caused by Hirschmanniella diversa Sher" Agronomy 11, no. 12: 2517. https://doi.org/10.3390/agronomy11122517
APA StyleKurashita, H., Kuroda, K., Maki, S., Sato, T., Takagi, M., Goto, M., Kariya, T., Hatamoto, M., Yamaguchi, T., Tomita, S., & Narihiro, T. (2021). Chemical and Microbial Characteristics of Blackening Disease in Lotus (Nelumbo nucifera Gaertn.) Caused by Hirschmanniella diversa Sher. Agronomy, 11(12), 2517. https://doi.org/10.3390/agronomy11122517






