Sensor-Based Irrigation Reduces Water Consumption without Compromising Yield and Postharvest Quality of Soilless Green Bean
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growing Conditions
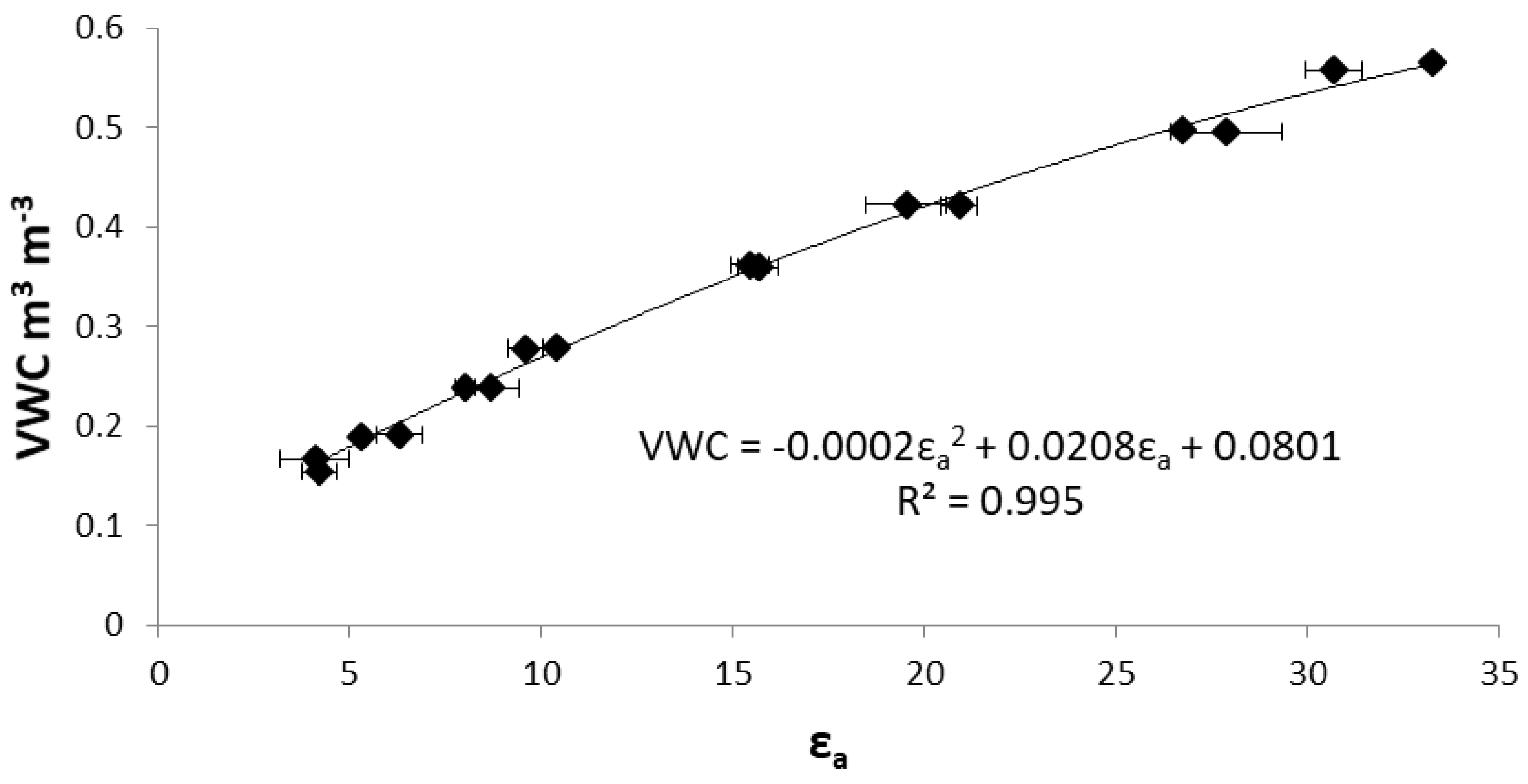
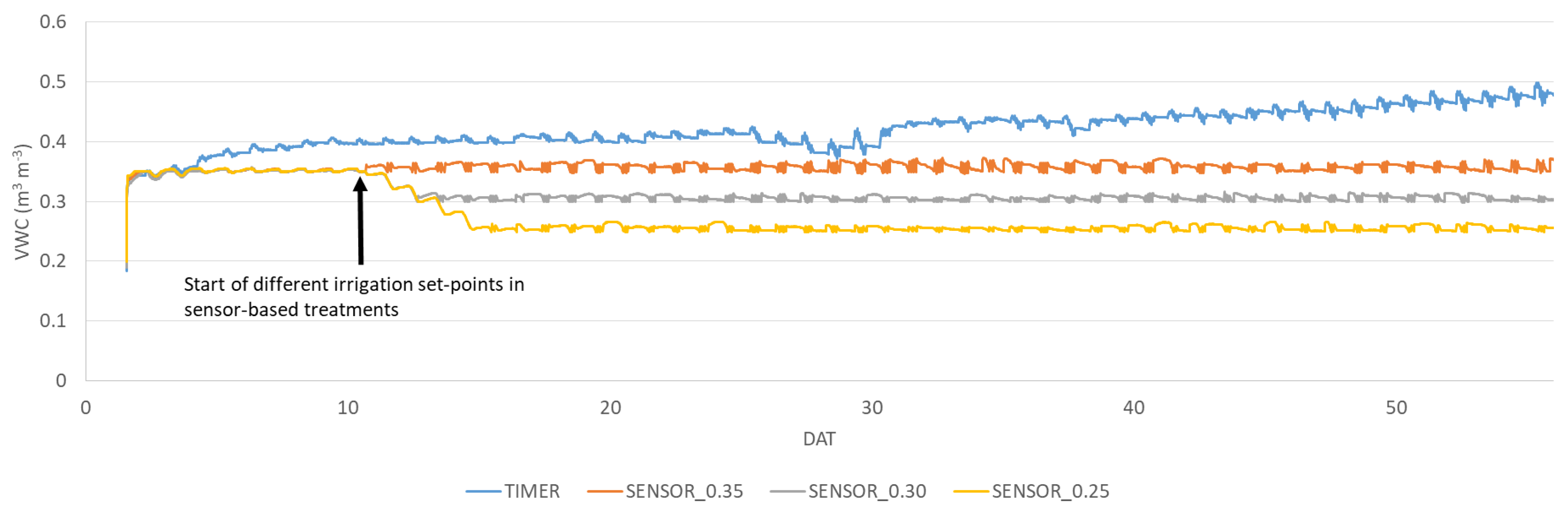
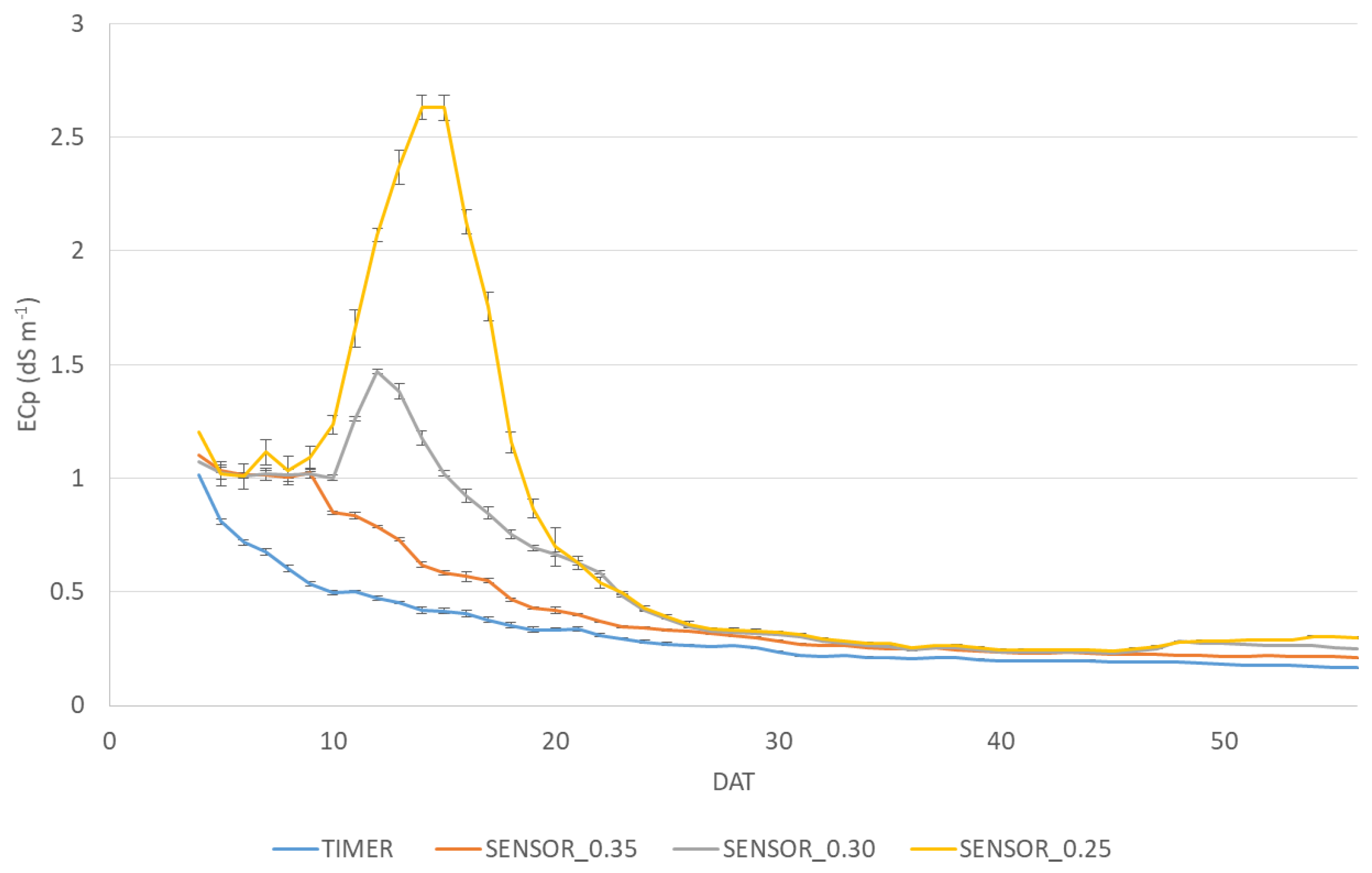
2.2. Irrigation Treatments Setup
2.3. Crop Performance, Plant Physiology and Chemical Composition
2.3.1. Water Consumption
2.3.2. Plant Growth, Physiological Parameters and Crop Performance
2.3.3. Plant Tissue and Fruit Chemical Analysis
2.4. Postharvest Quality Parameters
2.4.1. Respiration Rate
2.4.2. Sensory Analysis and Color Parameters
2.4.3. Texture, Electrolyte Leakage, Weight Loss and Dry Matter
2.4.4. Antioxidant Activity, Total Phenol Content and Total Chlorophyll Content
2.5. Statistical Analysis
3. Results and Discussion
3.1. Water Consumption, Crop Performance, Plant Physiology and Chemical Composition
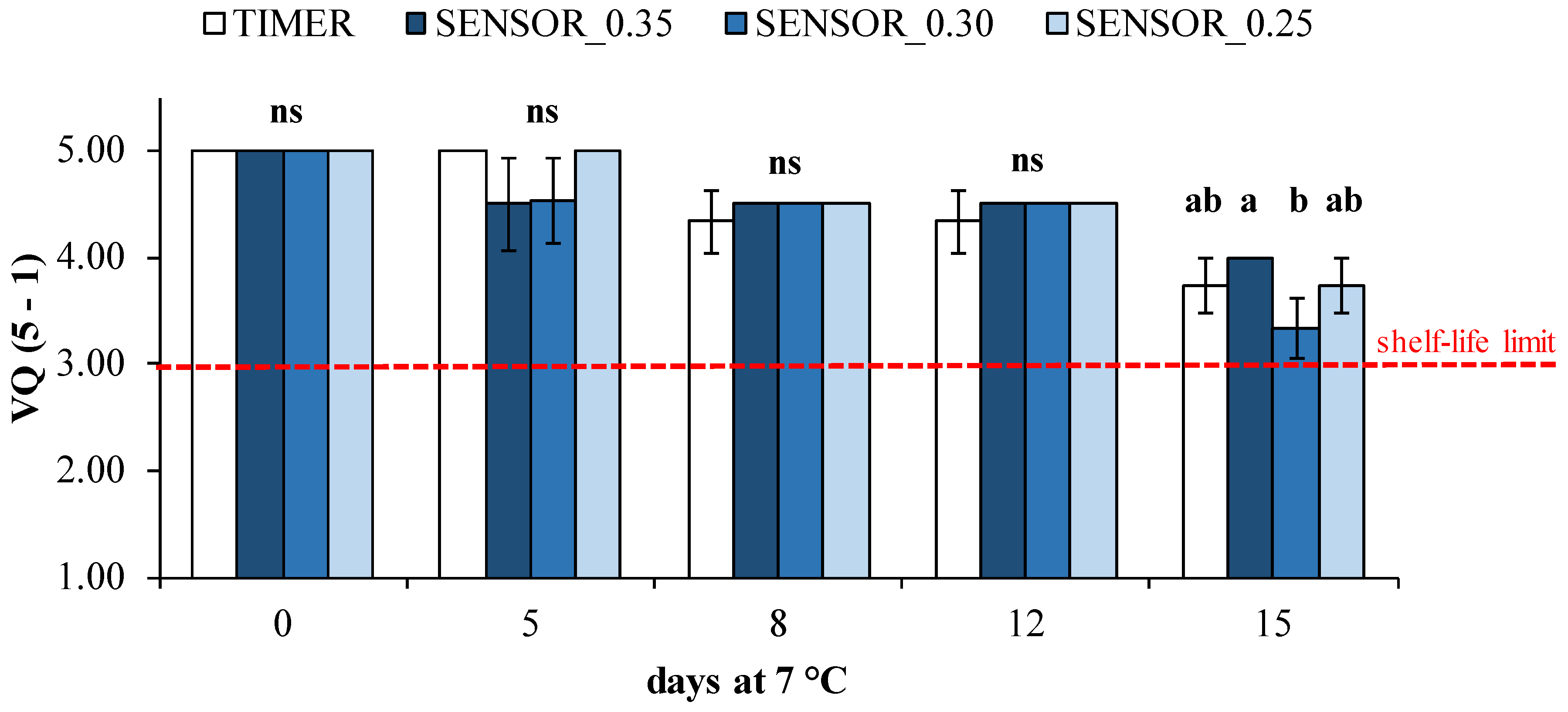
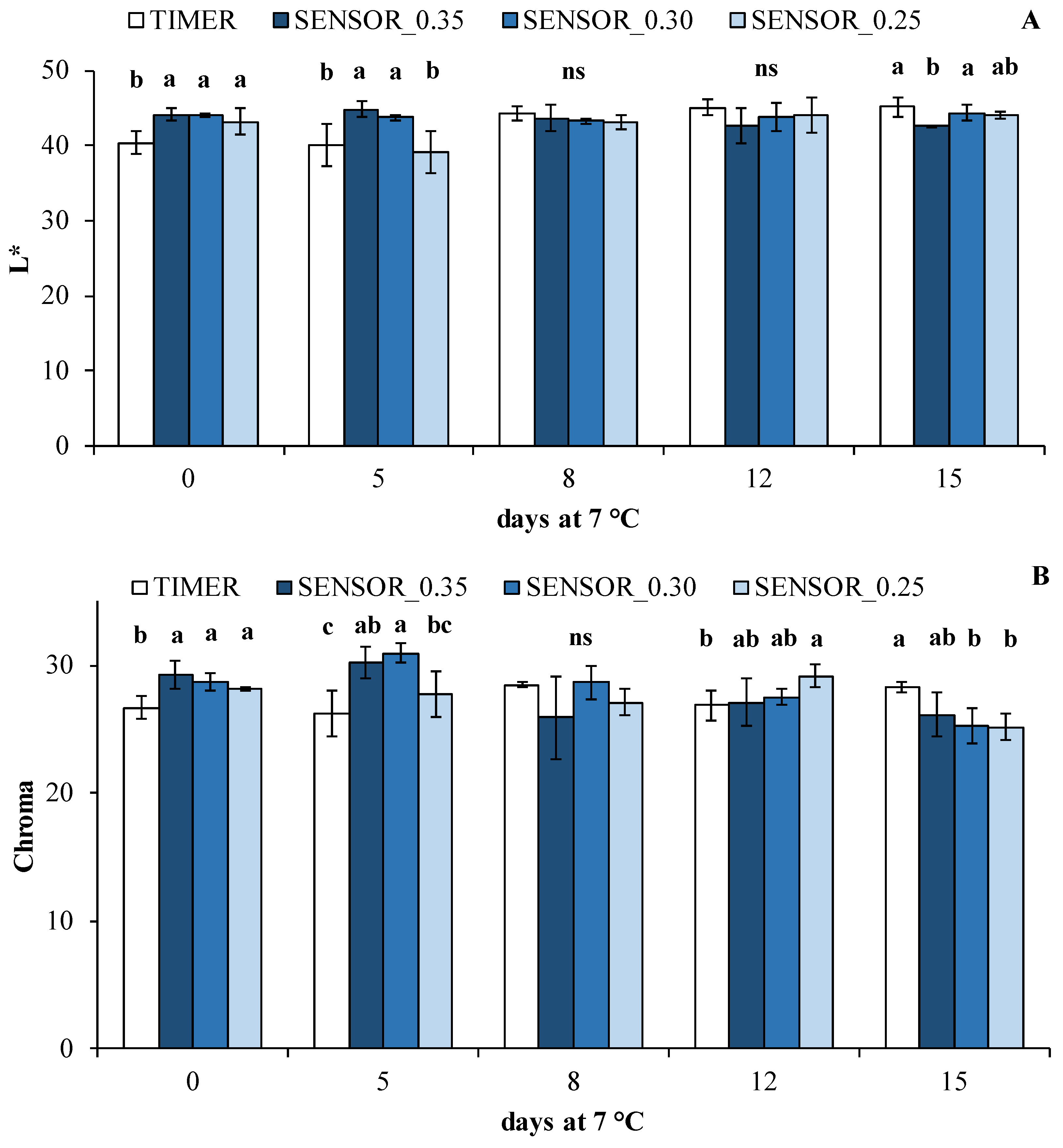
3.2. Postharvest Quality Parameters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, R.B.; Incrocci, L.; van Ruijven, J.; Massa, D. Reducing contamination of water bodies from European vegetable production systems. Agric. Water Manag. 2020, 240, 106258. [Google Scholar] [CrossRef]
- Verain, M.C.; Sijtsema, S.J.; Antonides, G. Consumer segmentation based on food-category attribute importance: The relation with healthiness and sustainability perceptions. Food Qual. Prefer. 2016, 48, 99–106. [Google Scholar] [CrossRef]
- Massa, D.; Magán, J.J.; Montesano, F.F.; Tzortzakis, N. Minimizing water and nutrient losses from soilless cropping in southern Europe. Agric. Water Manag. 2020, 241, 106395. [Google Scholar] [CrossRef]
- Lieth, J.H.; Oki, L.K. Irrigation in soilless production. In Soilless Culture; Elsevier: Amsterdam, The Netherlands, 2019; pp. 381–423. [Google Scholar]
- Sambo, P.; Nicoletto, C.; Giro, A.; Pii, Y.; Valentinuzzi, F.; Mimmo, T.; Lugli, P.; Orzes, G.; Mazzetto, F.; Astolfi, S.; et al. Hydroponic solutions for soilless production systems: Issues and opportunities in a smart agriculture perspective. Front. Plant Sci. 2019, 10, 923. [Google Scholar] [CrossRef]
- van Iersel, M.W.; Chappell, M.; Lea-Cox, J.D. Sensors for improved efficiency of irrigation in greenhouse and nursery production. Hort. Technol. 2013, 12, 735–746. [Google Scholar] [CrossRef] [Green Version]
- Raviv, M.; Blom, T.J. The effect of water availability and quality on photosynthesis and productivity of soilless-grown cut roses. Sci. Hortic. 2001, 88, 257–276. [Google Scholar] [CrossRef]
- Montesano, F.F.; Van Iersel, M.W.; Parente, A. Timer versus moisture sensor-based irrigation control of soilless lettuce: Effects on yield, quality and water use efficiency. Hortic. Sci. 2016, 43, 67–75. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Xu, Q. Precision irrigation scheduling using ECH2O moisture sensors for lettuce cultivated in a soilless substrate culture. Water 2018, 10, 549. [Google Scholar] [CrossRef] [Green Version]
- Palumbo, M.; Pace, B.; Cefola, M.; Montesano, F.F.; Serio, F.; Colelli, G.; Attolico, G. Self-Configuring CVS to Discriminate Rocket Leaves According to Cultivation Practices and to Correctly Attribute Visual Quality Level. Agronomy 2021, 11, 1353. [Google Scholar] [CrossRef]
- Choi, E.Y.; Choi, K.Y.; Lee, Y.B. Non-drainage irrigation scheduling in coir substrate hydroponic system for tomato cultivation by a frequency domain reflectometry sensor. Eur. J. Hortic. Sci. 2013, 56, 166–177. [Google Scholar]
- Montesano, F.F.; Van Iersel, M.W.; Boari, F.; Cantore, V.; D’Amato, G.; Parente, A. Sensor-based irrigation management of soilless basil using a new smart irrigation system: Effects of set-point on plant physiological responses and crop performance. Agric. Water Manag. 2018, 203, 20–29. [Google Scholar] [CrossRef]
- Montesano, F.F.; Serio, F.; Mininni, C.; Signore, A.; Parente, A.; Santamaria, P. Tensiometer-based irrigation management of subirrigated soilless tomato: Effects of substrate matric potential control on crop performance. Front. Plant Sci. 2015, 6, 1150. [Google Scholar] [CrossRef]
- Pardossi, A.; Incrocci, L.; Incrocci, G.; Malorgio, F.; Battista, P.; Bacci, L.; Rapi, B.; Marzialetti, P.; Hemming, J.; Balendonck, J. Root Zone Sensors for Irrigation Management in Intensive Agriculture. Sensors 2009, 9, 2809–2835. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hortic. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Brasil, I.M.; Siddiqui, M.W. Postharvest quality of fruits and vegetables: An overview. In Preharvest Modulation of Postharvest Fruit and Vegetable Quality, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–40. [Google Scholar]
- Weston, L.A.; Barth, M.M. Preharvest factors affecting postharvest quality of vegetables. Hortic. Sci. 1997, 32, 812–816. [Google Scholar] [CrossRef] [Green Version]
- Nemali, K.S.; Montesano, F.F.; Dove, S.K.; van Iersel, M.W. Calibration and performance of moisture sensors in soilless substrates: ECH2O and Theta probes. Sci. Hortic. 2007, 112, 227–234. [Google Scholar] [CrossRef]
- Hilhorst, M.A. A Pore Water Conductivity Sensor. Soil Sci. Soc. Am. J. 2000, 64, 1922–1925. [Google Scholar] [CrossRef] [Green Version]
- Hagen, E.; Nambuthiri, S.; Fulcher, A.; Geneve, R. Comparing substrate moisture-based daily water use and on-demand irrigation regimes for oakleaf hydrangea grown in two container sizes. Sci. Hortic. 2014, 179, 132–139. [Google Scholar] [CrossRef]
- Valdés, R.; Ochoa, J.; Franco, J.A.; Sánchez-Blanco, M.J.; Bañón, S. Saline irrigation scheduling for potted geranium based on soil electrical conductivity and moisture sensors. Agric. Water Manag. 2015, 149, 123–130. [Google Scholar] [CrossRef]
- Eastin, E.F. Use of ammonia electrode for total nitrogen determination in plants. Commun. Soil Sci. Plan. 1976, 7, 477–481. [Google Scholar] [CrossRef]
- D’Imperio, M.; Parente, A.; Montesano, F.F.; Renna, M.; Logrieco, A.F.; Serio, F. Boron Biofortification of Portulaca oleracea L. through Soilless Cultivation for a New Tailored Crop. Agronomy 2020, 10, 999. [Google Scholar] [CrossRef]
- Cantwell, M.; Suslow, T. Bean, Snap: Recommendations for Maintaining Postharvest Quality. Available online: http://postharvest.ucdavis.edu/Commodity_Resources/Fact_Sheets/Datastores/Vegetables_English/?uid=3&ds=799 (accessed on 11 February 2021).
- Kader, A.A. (Ed.) Methods of gas mixing, sampling and analysis. In Postharvest Technology of Horticultural Crops; University of California Agriculture and Natural Resources: Oakland, CA, USA, 2002; pp. 145–148. [Google Scholar]
- Proulx, E.; Yagiz, Y.; Cecilia, M.; Nunes, N.; Emond, J.P. Quality attributes limiting snap bean (Phaseolus vulgaris L.) postharvest life at chilling and non-chilling temperatures. Hortic. Sci. 2010, 45, 1238–1249. [Google Scholar] [CrossRef] [Green Version]
- Cáceres, D.; Díaz, M.; Shinya, P.; Infante, R. Assessment of peach internal flesh browning through colorimetric measures. Postharvest Biol. Technol. 2016, 111, 48–52. [Google Scholar] [CrossRef]
- Singh, A.; Singh, A.P.; Ramaswamy, H.S. Effect of processing conditions on quality of green beans subjected to reciprocating agitation thermal processing. Food Res. Int. 2015, 78, 424–432. [Google Scholar] [CrossRef]
- Kim, J.G.; Luo, Y.; Tao, Y.; Saftner, R.A.; Gross, K.C. Effect of initial oxygen concentration and film oxygen transmission rate on the quality of fresh-cut romaine lettuce. J. Sci. Food Agric. 2005, 85, 1622–1630. [Google Scholar] [CrossRef]
- Cefola, M.; Pace, B. Application of oxalic acid to preserve the overall quality of rocket and baby spinach leaves during storage. J. Food Process. Preserv. 2015, 39, 2523–2532. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Cefola, M.; Pace, B.; Sergio, L.; Baruzzi, F.; Gatto, M.A.; Carito, A.; Linsalata, V.; Cascarano, N.A.; Di Venere, D. Postharvest performance of fresh-cut ‘Big Top’nectarine as affected by dipping in chemical preservatives and packaging in modified atmosphere. Int. J. Food Sci. Technol. 2014, 49, 1184–1195. [Google Scholar] [CrossRef]
- Fadda, A.; Pace, B.; Angioni, A.; Barberis, A.; Cefola, M. Suitability for ready-to-eat processing and preservation of six green and red baby leaves cultivars and evaluation of their antioxidant value during storage and after the expiration date. J. Food Process. Preserv. 2016, 40, 550–558. [Google Scholar] [CrossRef]
- Qi, G.; Michel, J.C.; Charpentier, S.; Boivin, P. A laboratory method for measuring physical properties of growing media and their evolution during drying/wetting cycles: Preliminary results. Acta Hortic. 2011, 891, 181–185. [Google Scholar]
- Michel, J.C.; Rivie‘re, L.M.; Bellon-Fontaine, M.N. Measurement of the wettability of organic materials in relation to water content by the capillary rise method. Eur. J. Soil Sci. 2001, 52, 459–467. [Google Scholar] [CrossRef]
- Naasz, R.; Michel, J.C.; Charpentier, S. Water repellency of organic growing mediarelated to hysteretic water retention properties. Eur. J. Soil Sci. 2008, 59, 156–165. [Google Scholar] [CrossRef]
- Allaire-Leung, S.E.; Caron, J.; Parent, J.L.E. Changes in physical properties of peat substrates during plants growth. Can. J. Soil Sci. 1999, 79, 137–139. [Google Scholar] [CrossRef]
- Cannavo, P.; Hafdhi, H.; Michel, J.C. Impact of root growth on the physical properties of peat substrate under a constant water regimen. HortScience 2011, 46, 1394–1399. [Google Scholar] [CrossRef] [Green Version]
- Fonteno, W.C. Growing media: Types and physical/chemical properties. In Water, Media, and Nutrition for GreenHouse Crops; Reed, D.W., Ed.; Ball Publishing: Batavia, IL, USA, 1996; pp. 93–122. [Google Scholar]
- Bayer, A.; Ruter, J.; van Iersel, M.W. Optimizing irrigation and fertilization of Gardenia jasminoides for good growth and minimal leaching. HortScience 2015, 50, 994–1001. [Google Scholar] [CrossRef] [Green Version]
- Bittelli, M. Measuring Soil Water Content: A Review. HortTechnology 2011, 21, 293–300. [Google Scholar] [CrossRef]
- Bañón, S.; Ochoa, J.; Bañón, D.; Ortuño, M.F.; Sánchez-Blanco, M.J. Assessment of the Combined Effect of Temperature and Salinity on the Outputs of Soil Dielectric Sensors in Coconut Fiber. Sustainability 2020, 12, 6577. [Google Scholar] [CrossRef]
- Bañón, S.; Álvarez, S.; Bañón, D.; Ortuño, M.F.; Sánchez-Blanco, M.J. Assessment of soil salinity indexes using electrical conductivity sensors. Sci. Hortic. 2021, 285, 110171. [Google Scholar] [CrossRef]
- Fields, J.S.; Owen, J.S.; Altland, J.E.; van Iersel, M.W.; Jackson, B.E. Soilless substrate hydrology can be engineered to influence plant water status for an ornamental containerized crop grown within optimal water potentials. J. Am. Soc. Hortic. Sci. 2018, 143, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Zhen, S.; Burnett, S.E.; Day, M.E.; van Iersel, M.W. Effects of substrate water content on morphology and physiology of rosemary, canadian columbine, and cheddar pink. HortScience 2014, 49, 486–492. [Google Scholar] [CrossRef]
- Nam, S.; Kang, S.; Kim, J. Maintaining a constant soil moisture level can enhance the growth and phenolic content of sweet basil better than fluctuating irrigation. Agric. Water Manag. 2020, 238, 106203. [Google Scholar] [CrossRef]
- Flexas, J.; Ribas-Carbo, M.; Diaz-Espejo, A.; Galmes, J.; Medrano, H. Mesophyll conductance to CO2: Current knowledge and future prospects. Plant Cell Environ. 2008, 31, 602–621. [Google Scholar] [CrossRef]
- Garland, K.F.; Burnett, S.E.; Day, M.E.; van Iersel, M.W. Influence of substrate water content and daily light integral on photosynthesis, water use efficiency, and morphology of Heuchera americana. J. Am. Soc. Hortic. Sci. 2012, 137, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Kader, A.A. (Ed.) Postharvest biology and technology: An overview. In Postharvest Technology of Horticultural Crops; University of California Agriculture and Natural Resources: Oakland, CA, USA, 2002; pp. 39–47. [Google Scholar]
- Cho, M.; Hurr, B.M.; Jeong, J.; Lim, C.; Huber, D.J. Postharvest senescence and deterioration of ‘Thoroughbred’ and ‘Carlo’ green beans (Phaseolus vulgaris L.) in response to 1-methylcyclopropene. HortScience 2008, 43, 427–430. [Google Scholar] [CrossRef]
- Duan, Y.Q.; Tong, S.S.; Feng, S.Q.; Zhao, Y.M.; Rong, R.F.; Wang, P. Study on Snap Bean for Freshness Protection. Storage Process 2001, 2, 13–16. [Google Scholar]
- El-tahan, I.M.; El-hamahmy, M.A.; Elewa, A.E.; Baz, A.I. Biochemical and Anatomical Characters of Snap Bean (Phaseolus vulgaris L.) Pods under Furrow and Drip Irrigation System at Harvest and during Postharvest. J. Plant Prod. Sci. 2016, 5, 12–22. [Google Scholar]
- Colelli, G.; Elia, A. I prodotti ortofrutticoli di IV gamma: Aspetti fisiologici e tecnologici. Italus Hortus 2009, 16, 55–78. [Google Scholar]
- Nunes, M.C.N.; Emond, J.P.; Brecht, J.K.; Dea, S.; Proulx, E. Quality curves for mango fruit (cv. Tommy Atkins and Palmer) stored at chilling and nonchilling temperatures. J. Food Qual. 2007, 30, 104–120. [Google Scholar] [CrossRef]
- Kasim, R.; Kasim, M.U. Biochemical changes and color properties of fresh-cut green bean (Phaseolus vulgaris L. cv. ginaGina) treated with calcium chloride during storage. Food Sci. Technol. 2015, 35, 266–272. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Sánchez, A.; Tudela, J.A.; Luna, C.; Allende, A.; Gil, M.I. Low oxygen levels and light exposure affect quality of fresh-cut Romaine lettuce. Postharvest Biol. Technol. 2011, 59, 34–42. [Google Scholar] [CrossRef]
- Pevicharova, G.; Sofkova-Bobcheva, S.; Zsivanovits, G. Sensory and Instrumental Texture of Snap Bean (Phaseolus vulgaris L.). Int. J. Food Prop. 2015, 18, 1169–1180. [Google Scholar] [CrossRef] [Green Version]
- Chaurasia, S.; Saxena, R. Evaluation of total phenol and flavonoid content, antioxidant and iron chelation activities of ethanolic extracts of green beans. Am. J. PharmTech Res. 2014, 4, 614–624. [Google Scholar]
- Cefola, M.; Carbone, V.; Minasi, P.; Pace, B. Phenolic profiles and postharvest quality changes of fresh-cut radicchio (Cichorium intybus L.): Nutrient value in fresh vs. stored leaves. J. Food Compos. Anal. 2016, 51, 76–84. [Google Scholar] [CrossRef]
- Capotorto, I.; Innamorato, V.; Cefola, M.; Cervellieri, S.; Lippolis, V.; Longobardi, F.; Pace, B. High CO2 short-term treatment to preserve quality and volatiles profile of fresh-cut artichokes during cold storage. Postharvest Biol. Technol. 2020, 160, 111056. [Google Scholar] [CrossRef]


| Sensory Parameters | Scores and Descriptions | ||||
|---|---|---|---|---|---|
| 5 | 4 | 3 | 2 | 1 | |
| Visual quality | excellent | good | acceptable | poor | unusable |
| Chilling injury | no pitting | small pits or rusty brown spots | slight pitting and small rusty brown spots | moderate pitting, medium rusty brown spots and discoloration | severe pitting, large rusty brown spots and discoloration of the whole pod |
| Browning | no browning | slight browning | moderate browning | severe browning | extreme browning |
| Firmness | extremely tender and firm on touch, snap very easily | tender and firm on touch, snap easily | tender but less firm on touch, not snap easily | soft on touch, not snap, bent easily | extremely soft on touch, not snap |
| Total Water Consumption | Leaching | Shoot Fresh Weight | Root Fresh Weight | Total Yield | Leaf Area | Total Fruit Number | WUE | |
|---|---|---|---|---|---|---|---|---|
| (L·pot−1) | (g·pot−1) | (cm2) | (n·pot−1) | (g·L−1) | ||||
| TIMER | 23.6 | 7.4 a | 153 a | 48.0 a | 148 ab | 5457 a | 52.3 ab | 6.3 c |
| SENSOR_0.35 | 15.1 | 1.3 b | 149 a | 45.7 a | 160 a | 4945 ab | 56.0 a | 10.7 ab |
| SENSOR_0.30 | 13.9 | 1.2 b | 136 ab | 35.9 ab | 138 b | 4608 ab | 47.8 b | 9.9 b |
| SENSOR_0.25 | 12.4 | 1.2 b | 108 b | 31.3 b | 135 b | 3771 b | 47.2 b | 10.9 a |
| Significance | -§ | <0.0001 | 0.023 | 0.011 | 0.004 | 0.014 | 0.007 | <0.0001 |
| Parameters | Irrigation Treatments (A) | Storage Time (B) | A × B |
|---|---|---|---|
| Respiration rate (mL CO2 kg−1·h) | <0.0001 **** | <0.0001 **** | <0.0001 **** |
| Visual quality (5-1) | 0.054 ns | <0.0001 **** | 0.009 ** |
| Chilling injury (5-1) | 0.129 ns | 0.004 ** | 0.192 ns |
| Browning (5-1) | 0.218 ns | <0.0001 **** | 0.860 ns |
| Firmness (5-1) | <0.0001 **** | <0.0001 **** | 0.004 ** |
| Dry matter (%) | 0.002 ** | <0.0001 **** | 0.068 ns |
| Weight loss (%) | 0.650 ns | 0.0003 *** | 0.748 ns |
| L* | 0.184 ns | 0.011 * | 0.0008 *** |
| Chroma | 0.274 ns | 0.0005 *** | 0.0003 *** |
| Hue angle (°) | 0.229 ns | <0.0001 **** | 0.583 ns |
| Electrolyte leakage (%) | 0.027 * | <0.0001 **** | 0.130 ns |
| Texture (N mm−2) | 0.299 ns | 0.006 ** | 0.773 ns |
| Total chlorophyll content (mg 100 g−1·fw) | 0.629 ns | 0.265 ns | 0.374 ns |
| Antioxidant activity (mg Trolox 100 g−1·fw) | 0.445 ns | <0.0001 **** | 0.061 ns |
| Total phenol content (mg gallic acid 100 g−1·fw) | 0.410 ns | <0.0001 **** | 0.074 ns |
| Parameters | Irrigation Treatments | Storage Time (Days) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| TIMER | SENSOR 0.35 | SENSOR 0.30 | SENSOR 0.25 | 0 | 5 | 8 | 12 | 15 | |
| Chilling injury (5-1) | 4.80 ab | 4.80 ab | 4.70 b | 4.96 a | 5.00 a | 4.83 a | 4.87 a | 4.87 a | 4.50 b |
| Browning (5-1) | 4.67 | 4.70 | 4.80 | 4.80 | 5.00 a | 4.96 a | 4.87 a | 4.87 a | 4.00 b |
| Dry matter (%) | 8.08 b | 8.10 b | 8.17 ab | 8.26 a | 8.38 a | 8.36 a | 8.18 b | 8.05 c | 7.78 b |
| Weight loss (%) | 0.18 | 0.18 | 0.24 | 0.18 | 0 c | 0.13 a | 0.16 b | 0.28 ab | 0.23 ab |
| Hue angle (°) | 122 b | 122 a | 125 ab | 122 ab | 122 a | 121 a | 121 a | 121 b | 121 c |
| Electrolyte leakage (%) | 11.16 ab | 12.26 a | 11.33 ab | 10.42 b | 8.96 b | 11.98 a | 12.60 a | 11.38 a | 11.41 a |
| Texture (N mm−2) | 3.34 | 3.15 | 3.10 | 3.10 | 3.50 a | 3.24 ab | 3.21 ab | 3.03 bc | 2.89 c |
| Total chlorophyll content (mg 100 g−1 fw) | 9.96 | 9.97 | 9.64 | 10.20 | 10.10 ab | 10.18 ab | 10.34 a | 9.37 b | 9.73 ab |
| Antioxidant activity (mg Trolox 100 g−1 fw) | 5.50 | 4.81 | 4.80 | 4.92 | 6.20 b | 2.62 d | 2.95 d | 4.34 c | 8.94 a |
| Total phenol content (mg 100 g−1 fw) | 13.49 | 14.43 | 13.43 | 13.55 | 12.90 b | 11.37 c | 10.97 c | 13.94 b | 19.46 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palumbo, M.; D’Imperio, M.; Tucci, V.; Cefola, M.; Pace, B.; Santamaria, P.; Parente, A.; Montesano, F.F. Sensor-Based Irrigation Reduces Water Consumption without Compromising Yield and Postharvest Quality of Soilless Green Bean. Agronomy 2021, 11, 2485. https://doi.org/10.3390/agronomy11122485
Palumbo M, D’Imperio M, Tucci V, Cefola M, Pace B, Santamaria P, Parente A, Montesano FF. Sensor-Based Irrigation Reduces Water Consumption without Compromising Yield and Postharvest Quality of Soilless Green Bean. Agronomy. 2021; 11(12):2485. https://doi.org/10.3390/agronomy11122485
Chicago/Turabian StylePalumbo, Michela, Massimiliano D’Imperio, Vincenzo Tucci, Maria Cefola, Bernardo Pace, Pietro Santamaria, Angelo Parente, and Francesco Fabiano Montesano. 2021. "Sensor-Based Irrigation Reduces Water Consumption without Compromising Yield and Postharvest Quality of Soilless Green Bean" Agronomy 11, no. 12: 2485. https://doi.org/10.3390/agronomy11122485
APA StylePalumbo, M., D’Imperio, M., Tucci, V., Cefola, M., Pace, B., Santamaria, P., Parente, A., & Montesano, F. F. (2021). Sensor-Based Irrigation Reduces Water Consumption without Compromising Yield and Postharvest Quality of Soilless Green Bean. Agronomy, 11(12), 2485. https://doi.org/10.3390/agronomy11122485












