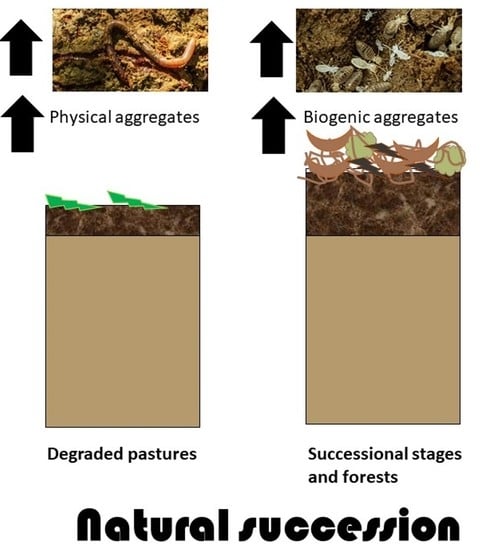
Soil Quality Restoration during the Natural Succession of Abandoned Cattle Pastures in Deforested Landscapes in the Colombian Amazon
Abstract
1. Introduction
2. Materials and Methods
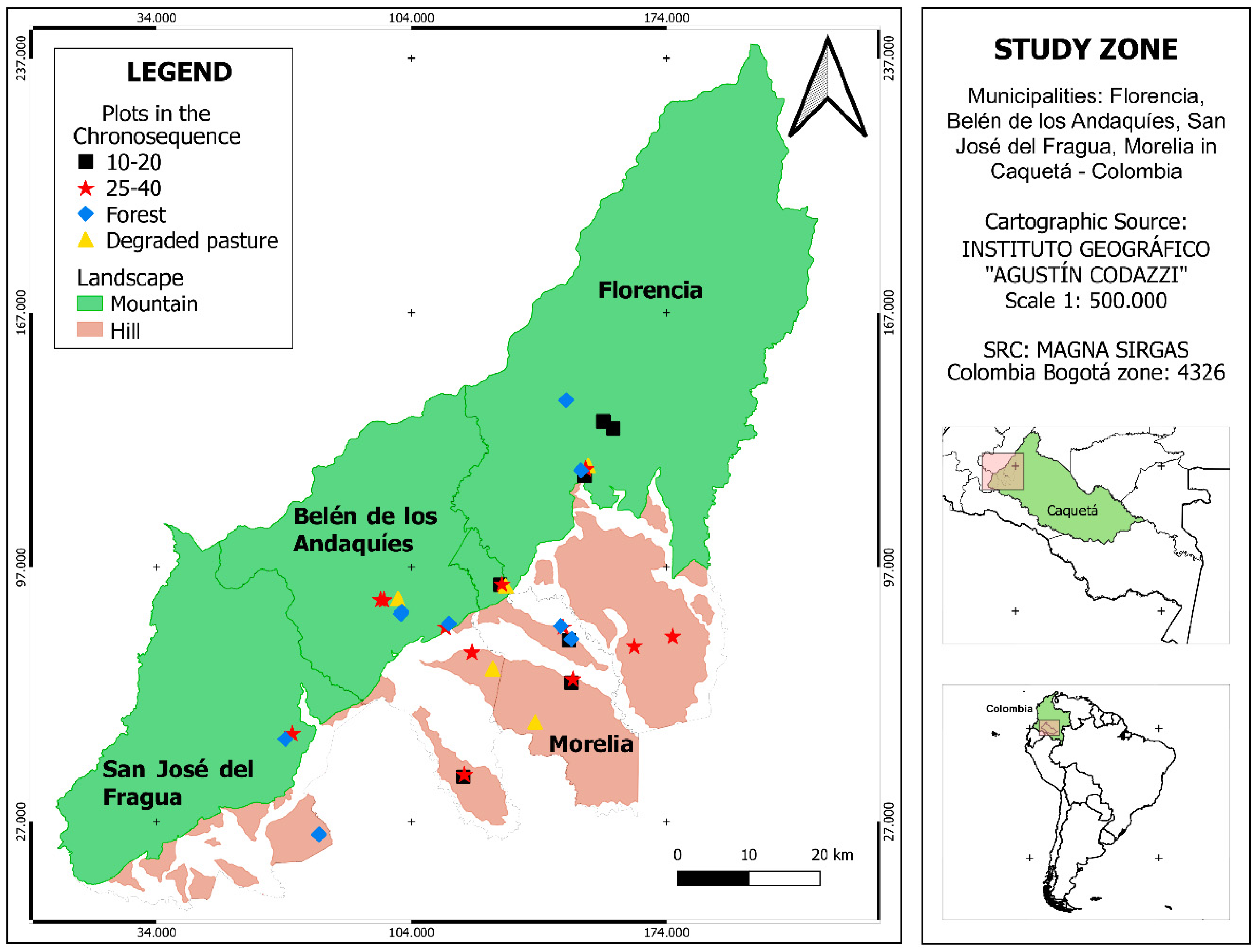
2.1. Study Area and Sampling Design
2.2. Soil Sampling and Evaluation of Soil Quality
2.2.1. Soil Macrofauna
2.2.2. Soil Macroaggregation
2.2.3. Soil Physico–Chemical Properties
2.2.4. Soil Quality Assessment
2.3. Statistical Analysis
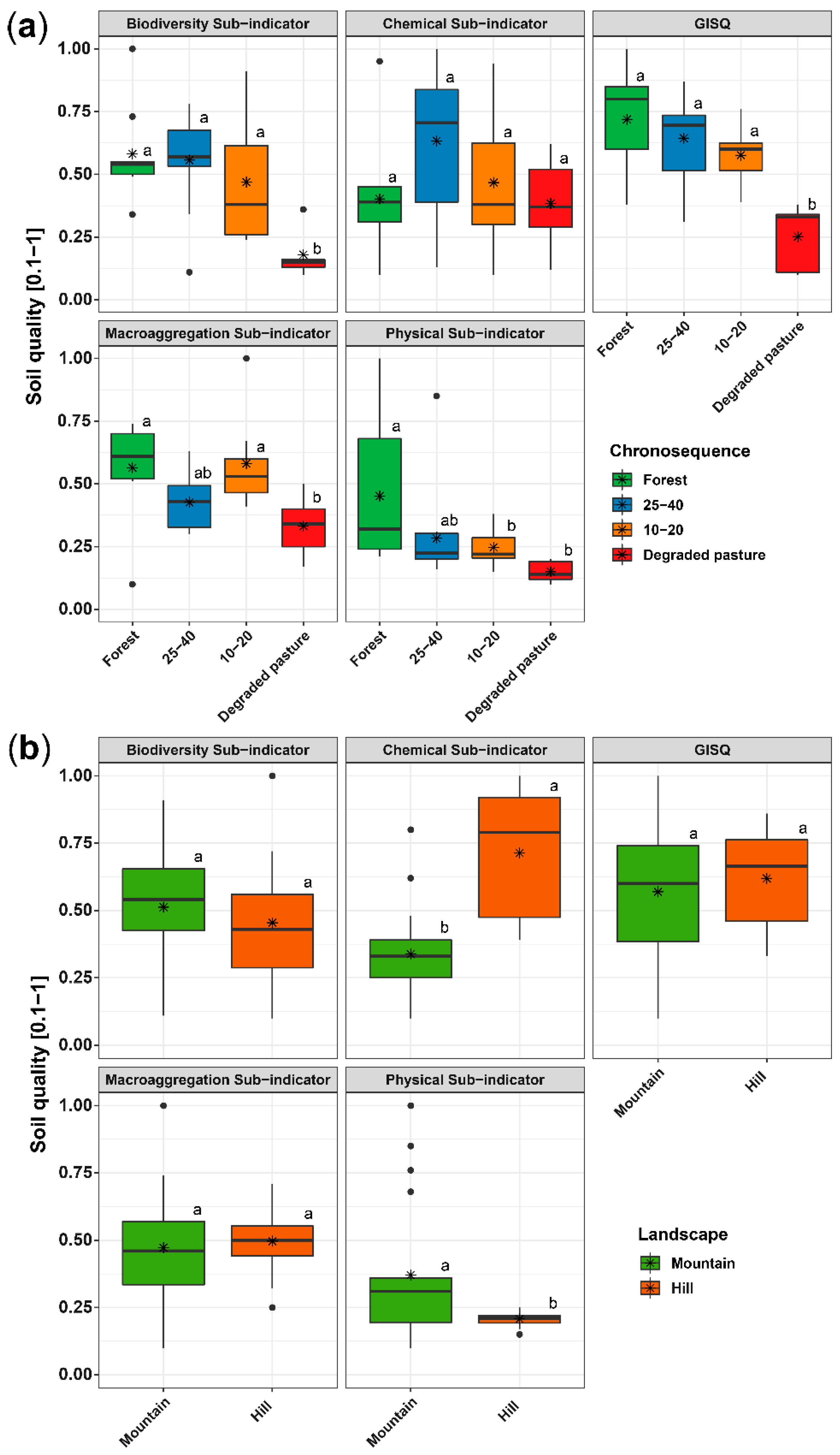
3. Results
3.1. Changes in Soil Physico–Chemical Properties
3.2. Responses of Soil Macrofauna Communities to Narural Succesion
3.3. Soil Macroaggregation
3.4. Soil Quality Indicator (GISQ)
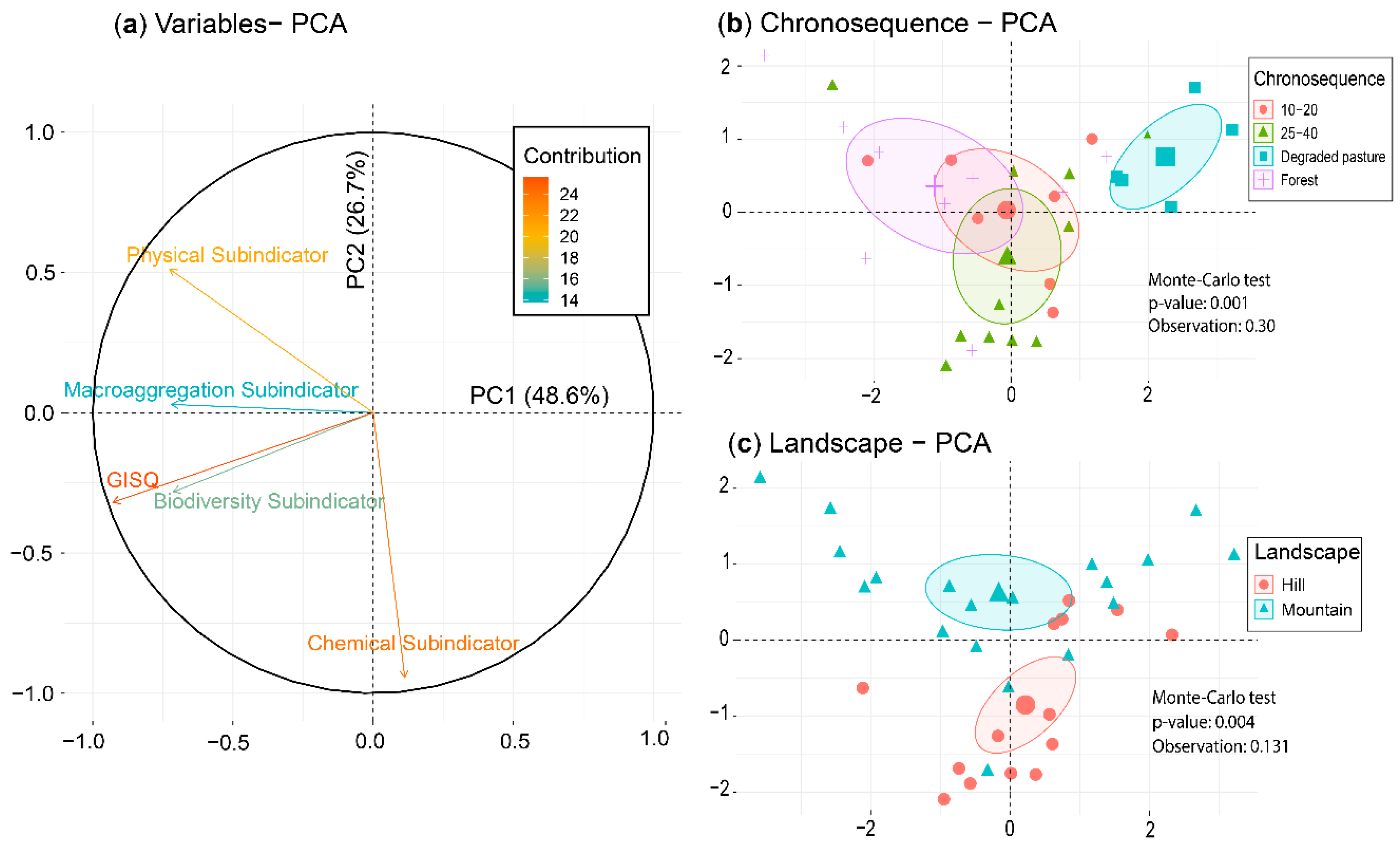
3.5. Relationship between Data Matrices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dirzo, R.; Raven, P.H. Global State of Biodiversity and Loss. Annu. Rev. Environ. Resour. 2003, 28, 137–167. [Google Scholar] [CrossRef]
- Figueiredo, F.O.G.; Zuquim, G.; Tuomisto, H.; Moulatlet, G.M.; Balslev, H.; Costa, F.R.C. Beyond climate control on species range: The importance of soil data to predict distribution of Amazonian plant species. J. Biogeogr. 2017, 45, 190–200. [Google Scholar] [CrossRef]
- Pan, Y.; Birdsey, R.A.; Fang, J.; Houghton, R.; Kauppi, P.E.; Kurz, W.A.; Phillips, O.L.; Shvidenko, A.; Lewis, S.L.; Canadell, J.G.; et al. A Large and Persistent Carbon Sink in the World’s Forests. Science 2011, 333, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ziv, G.; Adami, M.; de Almeida, C.A.; Antunes, J.F.G.; Coutinho, A.C.; Esquerdo, J.C.D.M.; Gomes, A.R.; Galbraith, D. Upturn in secondary forest clearing buffers primary forest loss in the Brazilian Amazon. Nat. Sustain. 2020, 3, 290–295. [Google Scholar] [CrossRef]
- Finer, M.; Mamani, N. Deforestación En La Amazonía 2020 (Final) MAAP #136. Canada. 2020. Available online: https://maaproject.org/2021/amazon-hotspots-2020-final/ (accessed on 22 November 2021).
- Murad, C.A.; Pearse, J. Landsat study of deforestation in the Amazon region of Colombia: Departments of Caquetá and Putumayo. Remote. Sens. Appl. Soc. Environ. 2018, 11, 161–171. [Google Scholar] [CrossRef]
- Melo, V.F.; Orrutéa, A.G.; Motta, A.C.V.; Testoni, S.A. Land Use and Changes in Soil Morphology and Physical-Chemical Properties in Southern Amazon. Rev. Bras. Cienc. Solo 2017, 41, 1–14. [Google Scholar] [CrossRef]
- Nóbrega, R.L.B.; Guzha, A.C.; Torres, G.N.; Kovacs, K.; Lamparter, G.; Amorim, R.S.S.; Couto, E.; Gerold, G. Correction: Effects of conversion of native cerrado vegetation to pasture on soil hydro-physical properties, evapotranspiration and streamflow on the Amazonian agricultural frontier. PLoS ONE 2020, 15, e0236236. [Google Scholar] [CrossRef]
- Olaya-Montes, A.; Llanos-Cabrera, M.P.; Cherubin, M.R.; Herrera-Valencia, W.; Ortiz-Morea, F.A.; Silva-Olaya, A.M. Restoring soil carbon and chemical properties through silvopastoral adoption in the Colombian Amazon region. Land Degrad. Dev. 2021, 32, 3720–3730. [Google Scholar] [CrossRef]
- Polanía-Hincapié, K.L.; Olaya-Montes, A.; Cherubin, M.R.; Herrera-Valencia, W.; Ortiz-Morea, F.A.; Silva-Olaya, A.M. Soil physical quality responses to silvopastoral implementation in Colombian Amazon. Geoderma 2021, 386, 114900. [Google Scholar] [CrossRef]
- Davidson, E.A.; de Araüjo, A.C.; Artaxo, P.; Balch, J.K.; Brown, I.F.; Mercedes, M.M.; Coe, M.T.; Defries, R.S.; Keller, M.; Longo, M.; et al. The Amazon basin in transition. Nature 2012, 481, 321–328, Erratum in Nature 2012, 483, 232. [Google Scholar] [CrossRef]
- Mesquita, R.D.; Massoca, P.E.; Jakovac, C.C.; Bentos, T.V.; Williamson, G.B. Amazon Rain Forest Succession: Stochasticity or Land-Use Legacy? BioScience 2015, 65, 849–861. [Google Scholar] [CrossRef]
- Jakovac, C.C.; Peña-Claros, M.; Kuyper, T.W.; Bongers, F. Loss of secondary-forest resilience by land-use intensification in the Amazon. J. Ecol. 2015, 103, 67–77. [Google Scholar] [CrossRef]
- Höfer, H.; Hanagarth, W.; Garcia, M.; Martius, C.; Franklin, E.; Römbke, J.; Beck, L. Structure and function of soil fauna communities in Amazonian anthropogenic and natural ecosystems. Eur. J. Soil Biol. 2001, 37, 229–235. [Google Scholar] [CrossRef]
- Van Hall, R.L.; Cammeraat, L.H.; Keesstra, S.D.; Zorn, M. Impact of secondary vegetation succession on soil quality in a humid Mediterranean landscape. Catena 2017, 149, 836–843. [Google Scholar] [CrossRef]
- Nadal-Romero, E.; Cammeraat, E.; Pérez-Cardiel, E.; Lasanta, T. Effects of secondary succession and afforestation practices on soil properties after cropland abandonment in humid Mediterranean mountain areas. Agric. Ecosyst. Environ. 2016, 228, 91–100. [Google Scholar] [CrossRef]
- Teixeira, H.M.; Cardoso, I.M.; Bianchi, F.J.J.A.; da Cruz Silva, A.; Jamme, D.; Peña-Claros, M. Linking vegetation and soil functions during secondary forest succession in the Atlantic forest. For. Ecol. Manag. 2019, 457, 117696. [Google Scholar] [CrossRef]
- Robinson, S.J.B.; van den Berg, E.; Meirelles, G.S.; Ostle, N. Factors influencing early secondary succession and ecosystem carbon stocks in Brazilian Atlantic Forest. Biodivers. Conserv. 2015, 24, 2273–2291. [Google Scholar] [CrossRef]
- Deng, L.; Shangguan, Z.P.; Sweeney, S. “Grain for Green” driven land use change and carbon sequestration on the Loess Plateau, China. Sci. Rep. 2014, 4, 7039. [Google Scholar] [CrossRef]
- Lesschen, J.P.; Cammeraat, L.H.; Kooijman, A.M.; van Wesemael, B. Development of spatial heterogeneity in vegetation and soil properties after land abandonment in a semi-arid ecosystem. J. Arid. Environ. 2008, 72, 2082–2092. [Google Scholar] [CrossRef]
- Guerra, C.A.; Heintz-Buschart, A.; Sikorski, J.; Chatzinotas, A.; Guerrero-Ramírez, N.; Cesarz, S.; Beaumelle, L.; Rillig, M.C.; Maestre, F.T.; Delgado-Baquerizo, M.; et al. Blind spots in global soil biodiversity and ecosystem function research. Nat. Commun. 2020, 11, 3870. [Google Scholar] [CrossRef]
- Fengler, F.H.; Bressane, A.; Carvalho, M.M.; Longo, R.M.; De Medeiros, G.A.; De Melo, W.J.; Jakovac, C.C.; Ribeiro, A.I. Forest restoration assessment in Brazilian Amazonia: A new clustering-based methodology considering the reference ecosystem. Ecol. Eng. 2017, 108, 93–99. [Google Scholar] [CrossRef]
- Uhl, C.; Buschbacher, R.; Serrao, E.A.S. Abandoned Pastures in Eastern Amazonia. I. Patterns of Plant Succession. J. Ecol. 1988, 76, 663. [Google Scholar] [CrossRef]
- Nortcliff, S. Standardisation of soil quality attributes. Agric. Ecosyst. Environ. 2002, 88, 161–168. [Google Scholar] [CrossRef]
- Jakovac, C.C.; Junqueira, A.B.; Crouzeilles, R.; Peña-Claros, M.; Mesquita, R.C.G.; Bongers, F. The role of land-use history in driving successional pathways and its implications for the restoration of tropical forests. Biol. Rev. 2021, 96, 1114–1134. [Google Scholar] [CrossRef] [PubMed]
- Onet, A.; Dincă, L.C.; Grenni, P.; Laslo, V.; Teusdea, A.C.; Vasile, D.L.; Enescu, R.E.; Crisan, V.E. Biological indicators for evaluating soil quality improvement in a soil degraded by erosion processes. J. Soils Sediments 2019, 19, 2393–2404. [Google Scholar] [CrossRef]
- Al-Maliki, S.; Al-Taey, D.K.; Al-Mammori, H.Z. Earthworms and eco-consequences: Considerations to soil biological indicators and plant function: A review. Acta Ecol. Sin. 2021, 41, 512–523. [Google Scholar] [CrossRef]
- Viana, R.M.; Ferraz, J.B.S.; Neves, A.F.; Vieira, G.; Pereira, B.F. Soil quality indicators for different restoration stages on Amazon rainforest. Soil Tillage Res. 2014, 140, 1–7. [Google Scholar] [CrossRef]
- Brussaard, L. Soil fauna, guilds, functional groups and ecosystem processes. Appl. Soil Ecol. 1998, 9, 123–135. [Google Scholar] [CrossRef]
- Lavelle, P.; Blanchart, E.; Martin, A.; Martin, S. Impact of Soil Fauna on the Properties of Soils in the Humid Tropics. In Myths and Science of Soils of the Tropics; Lal, R., Sanchez, P., Eds.; Soil Science Society of America and American Society of Agronomy: Madison, WI, USA, 1992; pp. 157–185. [Google Scholar]
- Lavelle, P. Faunal Activities and Soil Processes: Adaptative Strategy That Determine Ecosystem Function. Adv. Ecol. Res. 1997, 24, 93–132. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; van Bodegom, P.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nat. Cell Biol. 2018, 560, 233–237. [Google Scholar] [CrossRef]
- Velasquez, E.; Lavelle, P. Soil macrofauna as an indicator for evaluating soil based ecosystem services in agricultural landscapes. Acta Oecol. 2019, 100, 103446. [Google Scholar] [CrossRef]
- FAO; ITPS; GSBI; CBD; EC. State of Knowledge of Soil Biodiversity—Status, Challenges and Potentialities; Food and Agriculture Organization of the United Nations: Rome, Italy, 2020; ISBN 9789251335826. [Google Scholar]
- Cole, R.J.; Holl, K.D.; Zahawi, R.A.; Wickey, P.; Townsend, A.R. Leaf litter arthropod responses to tropical forest restoration. Ecol. Evol. 2016, 6, 5158–5168. [Google Scholar] [CrossRef] [PubMed]
- Vieira, D.L.M.; Rodrigues, S.B.; Jakovac, C.C.; da Rocha, G.P.E.; Reis, F.; Borges, A. Active Restoration Initiates High Quality Forest Succession in a Deforested Landscape in Amazonia. Forests 2021, 12, 1022. [Google Scholar] [CrossRef]
- Decaëns, T.; Jiménez, J.J.; Barros, E.; Chauvel, A.; Blanchart, E.; Fragoso, C.; Lavelle, P. Soil macrofaunal communities in permanent pastures derived from tropical forest or savanna. Agric. Ecosyst. Environ. 2004, 103, 301–312. [Google Scholar] [CrossRef]
- Serra, R.T.; Santos, C.D.; Rousseau, G.X.; Triana, S.P.; Muñoz Gutiérrez, J.A.; Burgos Guerrero, J.E. Fast recovery of soil macrofauna in regenerating forests of the Amazon. J. Anim. Ecol. 2021, 90, 2094–2108. [Google Scholar] [CrossRef]
- Sofo, A.; Mininni, A.N.; Ricciuti, P. Soil Macrofauna: A Key Factor for Increasing Soil Fertility and Promoting Sustainable Soil Use in Fruit Orchard Agrosystems. Agronomy 2020, 10, 456. [Google Scholar] [CrossRef]
- Brussaard, L.; Behan-Pelletier, V.M.; Bignell, D.E.; Brown, V.K.; Didden, W.; Folgarait, P.; Fragoso, C.; Freckman, D.W.; Gupta, V.V.S.R.; Hattori’S, T.; et al. Biodiversity and Ecosystem Functioning in Soil. Ambio 1997, 26, 563–570. [Google Scholar] [CrossRef]
- Marichal, R.; Grimaldi, M.; Feijoo, M.A.; Oszwald, J.; Praxedes, C.; Ruiz Cobo, D.H.; del Pilar Hurtado, M.; Desjardins, T.; da Silva Junior, M.L.; da Silva Costa, L.G.; et al. Soil macroinvertebrate communities and ecosystem services in deforested landscapes of Amazonia. Appl. Soil Ecol. 2014, 83, 177–185. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Bongiorno, G.; Bai, Z.; Creamer, R.E.; De Deyn, G.; de Goede, R.; Fleskens, L.; Geissen, V.; Kuyper, T.W.; Mäder, P.; et al. Soil quality—A critical review. Soil Biol. Biochem. 2018, 120, 105–125. [Google Scholar] [CrossRef]
- Grimaldi, M.; Oszwald, J.; Dolédec, S.; Hurtado, M.D.P.; Miranda, I.D.S.; De Sartre, X.A.; De Assis, W.S.; Castañeda, E.; Desjardins, T.; Dubs, F.; et al. Ecosystem services of regulation and support in Amazonian pioneer fronts: Searching for landscape drivers. Landsc. Ecol. 2014, 29, 311–328. [Google Scholar] [CrossRef]
- Bush, M.B.; Nascimento, M.N.; Åkesson, C.M.; Cárdenes-Sandí, G.M.; Maezumi, S.Y.; Behling, H.; Correa-Metrio, A.; Church, W.; Huisman, S.N.; Kelly, T.; et al. Widespread reforestation before European influence on Amazonia. Science 2021, 372, 484–487. [Google Scholar] [CrossRef] [PubMed]
- Suárez, L.R.; Josa, Y.T.P.; Samboni, E.J.A.; Cifuentes, K.D.L.; Bautista, E.H.D.; Salazar, J.C.S. Soil macrofauna under different land uses in the Colombian Amazon. Pesqui. Agropecuária Bras. 2018, 53, 1383–1391. [Google Scholar] [CrossRef]
- Amazonas, N.T.; Viani, R.A.G.; Rego, M.G.A.; Camargo, F.F.; Fujihara, R.T.; Valsechi, O.A. Soil macrofauna density and diversity across a chronosequence of tropical forest restoration in Southeastern Brazil. Braz. J. Biol. 2017, 78, 449–456. [Google Scholar] [CrossRef]
- Franco, A.L.C.; Sobral, B.W.; Silva, A.L.C.; Wall, D.H. Amazonian deforestation and soil biodiversity. Conserv. Biol. 2018, 33, 590–600. [Google Scholar] [CrossRef]
- Rodríguez, L.; Suárez, J.C.; Rodriguez, W.; Artunduaga, K.J.; Lavelle, P. Agroforestry systems impact soil macroaggregation and enhance carbon storage in Colombian deforested Amazonia. Geoderma 2020, 384, 114810. [Google Scholar] [CrossRef]
- Rodríguez, L.; Suárez Salazar, J.C.; Casanoves, F.; Ngo Bieng, M.A. Cacao Agroforestry Systems Improve Soil Fertility: Comparison of Soil Properties between Forest, Cacao Agroforestry Systems, and Pasture in the Colombian Amazon. Agric. Ecosyst. Environ. 2021, 314, 107349. [Google Scholar] [CrossRef]
- Pedrinho, A.; Mendes, L.W.; Merloti, L.F.; de Cassia Da Fonseca, M.; de Souza Cannavan, F.; Tsai, S.M. Forest-to-pasture conversion and recovery based on assessment of microbial communities in Eastern Amazon rainforest. FEMS Microbiol. Ecol. 2019, 95, fiy236. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.D.; Barajas-Aceves, M.; Araújo, A.S.; Ara´ujo, F.F.; Melo, W.J. Soil Microbial Biomass After Three-Year Consecutive Composted Tannery Sludge Amendment. Pedosphere 2014, 24, 469–475. [Google Scholar] [CrossRef]
- Rousseau, G.X.; dos Santos Silva, P.R.; Celentano, D.; de Carvalho, C.J.R. Macrofauna do solo em uma cronosequência de capoeiras, florestas e pastos no Centro de Endemismo Belém, Amazônia Oriental. Acta Amaz. 2014, 44, 499–512. [Google Scholar] [CrossRef]
- Huera-Lucero, T.; Labrador-Moreno, J.; Blanco-Salas, J.; Ruiz-Téllez, T. A Framework to Incorporate Biological Soil Quality Indicators into Assessing the Sustainability of Territories in the Ecuadorian Amazon. Sustainability 2020, 12, 3007. [Google Scholar] [CrossRef]
- Lehmann, J.; Bossio, D.A.; Kögel-Knabner, I.; Rillig, M.C. The concept and future prospects of soil health. Nat. Rev. Earth Environ. 2020, 1, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Rinot, O.; Levy, G.J.; Steinberger, Y.; Svoray, T.; Eshel, G. Soil health assessment: A critical review of current methodologies and a proposed new approach. Sci. Total. Environ. 2019, 648, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S.S.; Karlen, D.L.; Cambardella, C.A. The Soil Management Assessment Framework. Soil Sci. Soc. Am. J. 2004, 68, 1945–1962. [Google Scholar] [CrossRef]
- Thoumazeau, A.; Bustany, C.; Rodrigues, J.; Bessou, C. Using the LANCA® Model to Account for Soil Quality Within LCA: First Application and Approach Comparison in Two Contrasted Tropical Case Studies. Indones. J. Life Cycle Assess. Sustain. 2019, 3, 1. [Google Scholar] [CrossRef]
- Cherubin, M.R.; Karlen, D.L.; Cerri, C.E.P.; Franco, A.L.C.; Tormena, C.A.; Davies, C.A.; Cerri, C.C. Soil Quality Indexing Strategies for Evaluating Sugarcane Expansion in Brazil. PLoS ONE 2016, 11, e0150860. [Google Scholar] [CrossRef] [PubMed]
- Purakayastha, T.J.; Pathak, H.; Kumari, S.; Biswas, S.; Chakrabarty, B.; Padaria, R.N.; Kamble, K.; Pandey, M.; Sasmal, S.; Singh, A. Soil health card development for efficient soil management in Haryana, India. Soil Tillage Res. 2019, 191, 294–305. [Google Scholar] [CrossRef]
- Velasquez, E.; Lavelle, P.; Andrade, M. GISQ, a multifunctional indicator of soil quality. Soil Biol. Biochem. 2007, 39, 3066–3080. [Google Scholar] [CrossRef]
- Velásquez, E.; Fonte, S.J.; Barot, S.; Grimaldi, M.; Desjardins, T.; Lavelle, P. Soil macrofauna-mediated impacts of plant species composition on soil functioning in Amazonian pastures. Appl. Soil Ecol. 2012, 56, 43–50. [Google Scholar] [CrossRef]
- Cherubin, M.R.; Chavarro-Bermeo, J.P.; Silva-Olaya, A.M. Agroforestry Systems Improve Soil Physical Quality in Northwestern Colombian Amazon. Agrofor. Syst. 2019, 93, 1741–1753. [Google Scholar] [CrossRef]
- Duran-Bautista, E.H.; Muñoz, Y.; Galindo, J.D.; Ortiz, T.; Bermúdez, M. Soil Physical Quality and Relationship to Changes in Termite Community in Northwestern Colombian Amazon. Front. Ecol. Evol. 2020, 8, 419. [Google Scholar] [CrossRef]
- IGAC. Estudio General de Suelos y Zonificación de Tierras Departamento de Caquetá; IGAC: Boulder, CO, USA, 2014; ISBN 978958832373-2. [Google Scholar]
- Instituto Geográfico Agustin Codazzi (IGAC). Caquetá, Características Geográficas; Imprenta nacional de Colombia: Bogotá, Colombia, 2010. [Google Scholar]
- Norden, N.; Angarita, H.A.; Bongers, F.; Martínez-Ramos, M.; La Cerda, I.G.D.; Van Breugel, M.; Lebrija-Trejos, E.; Meave, J.A.; Vandermeer, J.; Williamson, G.B.; et al. Successional dynamics in Neotropical forests are as uncertain as they are predictable. Proc. Natl. Acad. Sci. USA 2015, 112, 8013–8018. [Google Scholar] [CrossRef]
- Blanco, J.C.; Rojas, A.C.; Rodrìguez, C.H.; Malagon, R. Relación Entre Índices de Vegetación y Estados de Degradación de Pasturas En Zonas de Lomerío Del Departamento de Caquetá. Momentos Cienc. 2014, 11, 35–41. [Google Scholar]
- Lavelle, P.; Rodríguez, N.; Arguello, O.; Bernal, J.; Botero, C.; Chaparro, P.; Gómez, Y.; Gutiérrez, A.; del Pilar Hurtado, M.; Loaiza, S.; et al. Soil ecosystem services and land use in the rapidly changing Orinoco River Basin of Colombia. Agric. Ecosyst. Environ. 2014, 185, 106–117. [Google Scholar] [CrossRef]
- ISO, I.O. for S. ISO 23611-5 Soil Quality: Sampling of Soil Invertebrates. Part 5: Sampling and Extraction of Soil Macro-Invertebrates; ISO: Geneva, Switzerland, 2011; p. 12. [Google Scholar]
- Anderson, J.M.; Ingram, J. Tropical Soil Biology and Fertility: A Handbook of Methods, 2nd ed.; CAB International: Wallingford, UK, 1993. [Google Scholar]
- Ruiz, N.; Lavelle, P.; Jiménez, J. Soil Macrofauna Field Manual; FAO: Rome, Italy, 2008. [Google Scholar]
- Sarkar, D.; Haldar, A. Physical and Chemical Methods in Soil Analysis; New Age International Pvt Ltd. Publishers: New Dehli, India, 2005. [Google Scholar]
- Blake, G.R.; Hartge, K.H. Bulk Density. Methods of Soil Analysis: Part 1—Physical and Mineralogical Methods; Encyclopedia of Earth Sciences Series; Klute, A., Ed.; American Society of Agronomy—Soil Science Society of America: Madison, WI, USA, 1986; pp. 363–375. [Google Scholar]
- Zamudio, A.M.; Carrascal Carrascal, M.L.; Pulido Roa, C.E.; Gallardo, J.F.; Gómez Guzmán, I.D. Métodos Analíticos del Laboratorio de Suelos; Instituto Geografico Agustin Codazzi (IGAC): Bogotá, Colombia, 2006; p. 6. ISBN 9789589067987. [Google Scholar]
- Pieri, C.J.M.G. Fertility of Soils: A Future for Farming in the West African Savannah; Springer: Berlin/Heidelberg, Germany, 1992; Volume 10, ISBN 978-3-642-84322-8. [Google Scholar]
- Thioulouse, J.; Dray, S.; Dufour, A.-B.; Siberchicot, A.; Jombart, T.; Pavoine, S. Multivariate Analysis of Ecological Data with Ade4; Springer: Berlin/Heidelberg, Germany, 2018; ISBN 978-1-4939-8848-8. [Google Scholar]
- Dolédec, S.; Chessel, D. Co-inertia analysis: An alternative method for studying species-environment relationships. Freshw. Biol. 1994, 31, 277–294. [Google Scholar] [CrossRef]
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D. Nlme: Linear and Nonlinear Mixed Effects Models. R Package Version 3.1-131.1; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S.; Bojesen, R.H.; Singmann, H.; Dai, B.; Scheipl, F.; Grothendieck, G.; Green, P.; et al. Package Lme4:Linear Mixed-Effects Models Using “Eigen” and S4 Package Version: 1.1-27; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Di Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat; Grupo InfoStat, FCA, Universidad Nacional de Córdoba: Córdoba, Argentina, 2020. [Google Scholar]
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D. Package ‘ggplot2’: Create Elegant Data Visualisations Using the Grammar of Graphics Version 3.3.3; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Dray, S.; Dufour, A.B. The Ade4 Package: Implementing the Duality Diagram for Ecologists. J. Stat. Softw. 2007, 22, 1–20. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7; R Foundation for Statistical Computing: Vienna, Austria, 2020; p. 84. [Google Scholar]
- Schoenholtz, S.H.; Van Miegroet, H.; Burger, J.A. A review of chemical and physical properties as indicators of forest soil quality: Challenges and opportunities. For. Ecol. Manag. 2000, 138, 335–356. [Google Scholar] [CrossRef]
- Mathieu, J.; Grimaldi, M.; Jouquet, P.; Rouland, C.; Lavelle, P.; Desjardins, T.; Rossi, J.-P. Spatial patterns of grasses influence soil macrofauna biodiversity in Amazonian pastures. Soil Biol. Biochem. 2009, 41, 586–593. [Google Scholar] [CrossRef]
- McGrath, D.A.; Smith, C.K.; Gholz, H.L.; Oliveira, F. de A. Effects of Land-Use Change on Soil Nutrient Dynamics in Amazônia. Ecosystems 2001, 4, 625–645. [Google Scholar] [CrossRef]
- Pereira, P.; Francos, M.; Brevik, E.C.; Ubeda, X.; Bogunovic, I. Post-fire soil management. Curr. Opin. Environ. Sci. Health 2018, 5, 26–32. [Google Scholar] [CrossRef]
- San Emeterio, L.; Múgica, L.; Ugarte, M.D.; Goicoa, T.; Canals, R.M. Sustainability of traditional pastoral fires in highlands under global change: Effects on soil function and nutrient cycling. Agric. Ecosyst. Environ. 2016, 235, 155–163. [Google Scholar] [CrossRef]
- Alcañiz, M.; Outeiro, L.; Francos, M.; Úbeda, X. Effects of prescribed fires on soil properties: A review. Sci. Total Environ. 2018, 613–614, 944–957. [Google Scholar] [CrossRef] [PubMed]
- Girona-García, A.; Ortiz-Perpiñá, O.; Badía-Villas, D. Dynamics of topsoil carbon stocks after prescribed burning for pasture restoration in shrublands of the Central Pyrenees (NE-Spain). J. Environ. Manag. 2019, 233, 695–705. [Google Scholar] [CrossRef]
- Navarrete, D.; Sitch, S.; Aragão, L.E.O.C.; Pedroni, L.; Duque, A. Conversion from forests to pastures in the Colombian Amazon leads to differences in dead wood dynamics depending on land management practices. J. Environ. Manag. 2016, 171, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, H.L. Effects of forest disturbance on the structure of ground-foraging ant communities in central Amazonia. Biodivers. Conserv. 1999, 8, 407–418. [Google Scholar] [CrossRef]
- Marchão, R.L.; Lavelle, P.; Leonide, C.; Balbino, C.; Vilela, L.; Becquer, T. Soil macrofauna under integrated crop-livestock systems in a Brazilian Cerrado Ferralsol. Pesq. Agropec. Bras. 2009, 44, 1011–1020. [Google Scholar] [CrossRef]
- Castro, D.; Fernández, F.; Meneses, A.D.; Tocora, M.C.; Sanchez, S.; Peña-Venegas, C.P. A preliminary checklist of soil ants (Hymenoptera: Formicidae) of Colombian Amazon. Biodivers. Data J. 2018, 6, e29278. [Google Scholar] [CrossRef]
- Marichal, R.; Martinez, A.F.; Praxedes, C.; Ruiz, D.; Carvajal, A.F.; Oszwald, J.; del Pilar Hurtado, M.; Brown, G.G.; Grimaldi, M.; Desjardins, T.; et al. Invasion of Pontoscolex corethrurus (Glossoscolecidae, Oligochaeta) in landscapes of the Amazonian deforestation arc. Appl. Soil Ecol. 2010, 46, 443–449. [Google Scholar] [CrossRef]
- Jones, D.T.; Susilo, F.X.; Bignell, D.E.; Hardiwinoto, S.; Gillison, A.N.; Eggleton, P. Termite assemblage collapse along a land-use intensification gradient in lowland central Sumatra, Indonesia. J. Appl. Ecol. 2003, 40, 380–391. [Google Scholar] [CrossRef]
- Bandeira, A.G.; Vasconcellos, A.; Silva, M.P.; Constantino, R. Effects of Habitat Disturbance on the Termite Fauna in a Highland Humid Forest in the Caatinga Domain, Brazil. Sociobiology 2003, 42, 117–128. [Google Scholar]
- Bourguignon, T.; Dahlsjö, C.A.L.; Salim, K.A.; Evans, T.A. Termite diversity and species composition in heath forests, mixed dipterocarp forests, and pristine and selectively logged tropical peat swamp forests in Brunei. Insectes Sociaux 2018, 65, 439–444. [Google Scholar] [CrossRef]
- Castro, D.; Carrijo, T.F.; Serna, F.J.; Peña-Venegas, C.P. Can Rubber Crop Systems Recover Termite Diversity in Previously Degraded Pastures in the Colombian Amazon Region? Neotrop. Entomol. 2021, 50, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Liu, W.; Jiang, X.; Wu, J. Effects of rubber-based agroforestry systems on soil aggregation and associated soil organic carbon: Implications for land use. Geoderma 2017, 299, 13–24. [Google Scholar] [CrossRef]
- Baumert, V.L.; Vasilyeva, N.A.; Vladimirov, A.A.; Meier, I.C.; Kögel-Knabner, I.; Mueller, C.W. Root Exudates Induce Soil Macroaggregation Facilitated by Fungi in Subsoil. Front. Environ. Sci. 2018, 6, 140. [Google Scholar] [CrossRef]
- Rodríguez, L.; Audor, L.C.U.; Salazar, J.C.S. Formation of Macroaggregates and Organic Carbon in Cocoa Agroforestry Systems. Floresta Ambient. 2019, 26, 1–12. [Google Scholar] [CrossRef]
- Gehring, C.A. Introduction: Mycorrhizas and Soil Structure, Moisture, and Salinity; Johnson, N.C., Gehring, C., Jansa, J.B.T.-M.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 235–240. ISBN 978-0-12-804312-7. [Google Scholar]
- Sandoval, M.A.; Celis, J.E.; Morales, P. Structural remediation of an alfisol by means of sewage sludge amendments in association with yellow serradela (Ornithopus compressus L.). J. Soil Sci. Plant Nutr. 2011, 11, 68–78. [Google Scholar] [CrossRef]
- Vial, A.M.; Sandoval, E.M. Soil structural condition and its relationship with pastures under different conditions in the Simpson Valley (Humid western Patagonia, Chile). Idesia 2015, 33, 31–40. [Google Scholar] [CrossRef][Green Version]
- Baptistella, J.L.C.; de Andrade, S.A.L.; Favarin, J.L.; Mazzafera, P. Urochloa in Tropical Agroecosystems. Front. Sustain. Food Syst. 2020, 4, 119. [Google Scholar] [CrossRef]
- Barros, E.; Pashanasi, B.; Constantino, R.; Lavelle, P. Effects of land-use system on the soil macrofauna in western Brazilian Amazonia. Biol. Fertil. Soils 2002, 35, 338–347. [Google Scholar] [CrossRef]
- Decaëns, T.; Mariani, L.; Lavelle, P. Soil surface macrofaunal communities associated with earthworm casts in grasslands of the Eastern Plains of Colombia. Appl. Soil Ecol. 1999, 13, 87–100. [Google Scholar] [CrossRef]
- Barros, E.; Neves, A.; Blanchart, E.; Fernandes, E.C.M.; Wandelli, E.; Lavelle, P. Development of the soil macrofauna community under silvopastoral and agrosilvicultural systems in Amazonia. Pedobiologia 2003, 47, 273–280. [Google Scholar] [CrossRef]
- Barros, E.; Grimaldi, M.; Sarrazin, M.; Chauvel, A.; Mitja, D.; Desjardins, T.; Thierry, D.; Lavelle, P. Soil physical degradation and changes in macrofaunal communities in Central Amazon. Appl. Soil Ecol. 2004, 26, 157–168. [Google Scholar] [CrossRef]
- Ramírez Pisco, R.; Guzmán Álvarez, M.E.; Leiva Rojas, E.I. Population Dynamics of Earthworms in an Andisol under Different Soil Use Systems. Rev. Fac. Nac. Agr. Medellín 2013, 66, 7045–7055. [Google Scholar]
- Meloni, F.; Varanda, E.M. Litter and soil arthropod colonization in reforested semi-deciduous seasonal Atlantic forests. Restor. Ecol. 2015, 23, 690–697. [Google Scholar] [CrossRef]
- Rocha, G.P.E.; Vieira, D.L.M.; Simon, M.F. Fast natural regeneration in abandoned pastures in southern Amazonia. For. Ecol. Manag. 2016, 370, 93–101. [Google Scholar] [CrossRef]
- Domínguez, J.; Bohlen, P.J.; Parmelee, R.W. Earthworms Increase Nitrogen Leaching to Greater Soil Depths in Row Crop Agroecosystems. Ecosystems 2004, 7, 672–685. [Google Scholar] [CrossRef]
- Mora, P.; Miambi, E.; Jiménez, J.J.; Decaëns, T.; Rouland, C. Functional complement of biogenic structures produced by earthworms, termites and ants in the neotropical savannas. Soil Biol. Biochem. 2005, 37, 1043–1048. [Google Scholar] [CrossRef]
- Bottinelli, N.; Jouquet, P.; Capowiez, Y.; Podwojewski, P.; Grimaldi, M.; Peng, X. Why is the influence of soil macrofauna on soil structure only considered by soil ecologists? Soil Tillage Res. 2014, 146, 118–124. [Google Scholar] [CrossRef]
- Snyder, B.A.; Hendrix, P.F. Current and Potential Roles of Soil Macroinvertebrates (Earthworms, Millipedes, and Isopods) in Ecological Restoration. Restor. Ecol. 2008, 16, 629–636. [Google Scholar] [CrossRef]
- Pollierer, M.M.; Klarner, B.; Ott, D.; Digel, C.; Ehnes, R.B.; Eitzinger, B.; Erdmann, G.; Brose, U.; Maraun, M.; Scheu, S. Diversity and functional structure of soil animal communities suggest soil animal food webs to be buffered against changes in forest land use. Oecologia 2021, 196, 195–209. [Google Scholar] [CrossRef]
- Menta, C.; Remelli, S. Soil Health and Arthropods: From Complex System to Worthwhile Investigation. Insects 2020, 11, 54. [Google Scholar] [CrossRef]
- Rousseau, G.X.; Rogério, P.; Silva, S.; Reis De Carvalho, C.J. Earthworms, Ants and Other Arthropods as Soil Health Indicators in Traditional and No-Fire Agro-Ecosystems from Eastern Brazilian Amazonia. Acta Zool. Mex. 2010, 2, 117–134. [Google Scholar]
- Menta, C.; Conti, F.D.; Pinto, S.; Bodini, A. Soil Biological Quality index (QBS-ar): 15 years of application at global scale. Ecol. Indic. 2018, 85, 773–780. [Google Scholar] [CrossRef]
- Domínguez-Haydar, Y.; Velásquez, E.; Carmona, J.; Lavelle, P.; Chavez, L.F.; Jiménez, J.J. Evaluation of reclamation success in an open-pit coal mine using integrated soil physical, chemical and biological quality indicators. Ecol. Indic. 2019, 103, 182–193. [Google Scholar] [CrossRef]



| Chronosequence | Landscape | Disturbed vs. Undisturbed | Chronosequence vs. Landscape | |||||
|---|---|---|---|---|---|---|---|---|
| Forest | 25–40 | 10–20 | Degraded Pasture | Hill | Mountain | p-Value | p-Value | |
| Physical Properties | ||||||||
| Bulk density (g cm−3) | 1.32 ± 0.02 b | 1.35 ± 0.02 b | 1.38 ± 0.03 ab | 1.45 ± 0.03 a | 1.35 ± 0.01 b | 1.40 ± 0.02 a | 0.0125 | 0.8855 |
| Clay (%) | 35.56 ± 0.33 a | 39.44 ± 2.93 a | 37.47 ± 3.76 a | 29.50 ± 4.44 a | 47.44 ± 2.25 a | 23.54 ± 2.88 b | 0.9834 | 0.6259 |
| Sand (%) | 55.17 ± 3.62 ab | 51.08 ± 3.25 b | 53.08 ± 4.14 ab | 64.17 ± 4.87 a | 43.93 ± 2.24 b | 67.82 ± 3.33 a | 0.8294 | 0.4442 |
| Silt (%) | 9.28 ± 1.10 a | 9.47 ± 1.02 a | 9.50 ± 1.28 a | 6.33 ± 1.50 a | 8.65 ± 0.56 a | 8.64 ± 1.11 a | 0.5304 | 0.4606 |
| Total porosity (%) | 44.77 ± 0.93 a | 43.62 ± 0.84 a | 42.37 ± 1.07 ab | 39.30 ± 1.26 b | 42.62 ± 0.57 a | 41.41 ± 0.87 b | 0.0126 | 0.8836 |
| Soil moisture (%) | 25.34 ± 1.59 a | 23.38 ± 1.44 a | 21.24 ± 1.82 ab | 16.01 ± 2.14 b | 23.3 ± 0.97 a | 19.60 ± 1.48 b | 0.0126 | 0.8837 |
| SI (%) | 7.13 ± 0.70 a | 6.26 ± 0.98 a | 5.95 ± 0.69 a | 5.47 ± 0.85 a | 4.33 ± 0.17 b | 8.08 ± 0.79 a | 0.1584 | 0.5881 |
| Chemical properties | ||||||||
| pH | 4.27 ± 0.07 a | 4.50 ± 0.07 a | 4.49 ± 0.09 a | 4.50 ± 0.10 a | 4.54 ± 0.03 a | 4.34 ± 0.08 b | 0.0186 | 0.4958 |
| CEC (meq 100 g−1) | 5.48 ± 0.72 a | 7.00 ± 0.57 a | 6.98 ± 0.77 a | 5.25 ± 0.92 a | 7.48 ± 0.61 a | 4.87 ± 0.45 b | 0.2826 | 0.9651 |
| N (%) | 0.13 ± 0.01 a | 0.12 ± 0.01 a | 0.12 ± 0.01 ab | 0.09 ± 0.01 b | 0.12 ± 0.004 a | 0.11 ± 0.01 a | 0.1291 | 0.7007 |
| K (mg kg−1) | 55.26 ± 6.54 a | 54.52 ± 5.12 a | 55.26 ± 6.54 a | 34.81 ± 7.70 a | 45.59 ± 3.71 a | 49.37 ± 5.15 a | 0.6806 | 0.6354 |
| P (mg kg−1) | 3.26 ± 0.27 a | 3.13 ± 0.25 a | 3.49 ± 0.31 a | 2.80 ± 0.37 a | 3.03 ± 0.17 a | 3.30 ± 0.25 a | 0.7077 | 0.5883 |
| EC (dS m−1) | 0.40 ± 10.04 a | 0.25 ± 0.03 bc | 0.32 ± 0.04 ab | 0.17 ± 0.05 c | 0.15 ± 0.01 b | 0.42 ± 0.04 a | 0.0011 | 0.0511 |
| SOC (%) | 1.49 ± 0.14 a | 1.47 ± 0.08 a | 1.41 ± 0.14 ab | 1.06 ± 0.10 b | 1.40 ± 0.09 a | 1.32 ± 0.08 a | 0.2628 | 0.6628 |
| Ca (mg kg−1) | 226.22 ± 6.35 a | 236.89 ± 10.76 a | 244.61 ± 8.94 a | 232.42 ± 8.53 a | 234.74 ± 6.60 a | 235.33 ± 5.80 a | 0.1731 | 0.2061 |
| Mg (mg kg−1) | 39.03 ± 1.23 a | 42.97 ± 3.12 a | 42.11 ± 1.53 a | 36.81 ± 2.02 a | 39.15 ± 1.55 a | 41.31 ± 1.41 a | 0.3862 | 0.4765 |
| EA (mg kg−1) | 342.40 ± 51.37 ab | 478.25 ±49.77 a | 377.54 ± 52.92 ab | 315.89 ± 38.35 b | 511.79 ± 37.02 a | 245.25 ± 31.25 b | 0.4159 | 0.8071 |
| Taxonomic Group | Chronosequence | Landscape | Disturbed vs. Undisturbed | Chronosequence vs. Landscape | ||||
|---|---|---|---|---|---|---|---|---|
| Forest | 25–40 | 10–20 | Degraded Pasture | Hill | Mountain | p-Value | p-Value | |
| Araneae | 107.93 ± 18.46 a | 98.95 ± 13.86 a | 96.55 ± 17.88 a | 24.87 ± 6.27 b | 62.18 ± 9.45 a | 81.45 ± 9.50 a | 0.0069 | 0.0003 |
| Blattodea | 37.17 ± 17.07 a | 29.99 ± 11.27 a | 29.21 ± 14.54 a | 9.24 ± 5.61 a | 20.79 ± 7.89 a | 26.38 ± 8.26 a | 0.2570 | 0.9621 |
| Chilopoda | 131.99 ± 24.86 a | 99.12 ± 15.32 ab | 110.22 ± 22.48 ab | 60.22 ± 15.02 b | 86.52 ± 13.52 a | 107.70 ± 13.78 a | 0.0613 | 0.0776 |
| Coleoptera adults | 98.34 ± 10.83 a | 74.02 ± 6.79 a | 94.26 ± 11.26 a | 50.60 ± 7.56 b | 80.25 ± 7.38 a | 73.42 ± 5.60 a | 0.0116 | 0.3860 |
| Coleoptera-larvae | 104.92 ± 20.41 a | 86.16 ± 13.73 a | 69.04 ± 14.61 ab | 41.31 ± 10.90 b | 55.52 ± 9.12 b | 91.46 ± 12.08 a | 0.0256 | 0.0235 |
| Dermaptera | 11.83 ± 4.63 a | 2.67 ± 3.78 a | 0.00 ± 5.00 a | 8.00 ± 5.97 a | 0.00 ± 3.78 b | 11.25 ± 3.13 a | 0.1415 | 0.3063 |
| Diplopoda | 98.34 ± 26.47 a | 118.65 ± 26.05 a | 126.21 ± 36.64 a | 32.66 ± 11.54 b | 90.49 ± 19.97 a | 76.64 ± 14.08 a | 0.4856 | 0.7487 |
| Diplura | 71.55 ± 31.77 a | 55.23 ± 20.05 a | 26.83 ± 13.02 a | 16.65 ± 9.77 a | 28.58 ± 10.55 a | 46.49 ± 14.02 a | 0.0871 | 0.2728 |
| Diptera-larvae | 6.67 ± 7.87 a | 17.33 ± 6.43 a | 8.67 ± 8.50 a | 5.33 ± 10.16 a | 4.00 ± 6.43 a | 15.00 ± 5.33 a | 0.6873 | 0.2184 |
| Formicidae | 1411.66 ± 139.70 a | 1287.51 ± 104.08 ab | 977.15 ± 104.68 b | 656.26 ± 84.28 c | 999.79 ± 81.01 a | 1079.82 ± 72.51 a | 0.0005 | 0.6544 |
| Hemiptera | 23.85 ± 7.70 b | 42.86 ± 11.16 ab | 95.20 ± 32.55 a | 20.66 ± 8.66 b | 39.07 ± 10.28 a | 36.29 ± 7.87 a | 0.1091 | 0.5139 |
| Isopoda | 40.00 ± 12.11 a | 25.78 ± 9.89 a | 30.67 ± 13.08 a | 5.33 ± 15.64 a | 28.22 ± 9.89 a | 22.67 ± 8.20 a | 0.1862 | 0.2600 |
| Isoptera | 4867.72 ± 1187.70 a | 4806.44 ± 957.57 a | 3648.65 ± 961.68 a | 728.25 ± 229.81 b | 3032.86 ± 604.81 a | 2599.73 ± 429.56 a | 0.0108 | 0.0369 |
| Lepidoptera-larvae | 15.92 ± 46.32 a | 10.67 ± 37.82 a | 140.33 ± 50.03 a | 5.33 ± 59.79 a | 72.67 ± 37.82 a | 13.46 ± 31.36 a | 0.5133 | 0.1868 |
| Oligochaeta | 214.00 ± 47.61 a | 115.44 ± 21.08 b | 276.09 ± 66.27 a | 416.60 ± 120.18 a | 255.54 ± 46.37 a | 209.35 ± 31.54 a | 0.6926 | 0.9039 |
| Opiliones | 39.19 ± 12.20 ab | 53.18 ± 13.47 a | 19.60 ± 6.71 b | 23.55 ± 9.80 ab | 23.66 ± 6.19 a | 41.46 ± 8.74 a | 0.4179 | 0.1224 |
| Orthoptera | 9.33 ± 3.84 a | 10.67 ± 3.13 a | 8.00 ± 4.14 a | 2.67 ± 4.95 a | 5.33 ± 3.13 a | 10.00 ± 2.60 a | 0.6274 | 0.3721 |
| Pseudoscorpionida | 18.50 ± 1.52 b | 34.82 ± 1.72 a | 22.63 ± 1.83 b | 22.63 ± 2.16 b | 27.36 ± 1.52 a | 20.99 ± 1.17 b | 0.0002 | <0.0001 |
| Symphyla | 1.33 ± 3.86 b | 6.67 ± 3.16 ab | 0.00 ± 4.17 b | 16.00 ± 4.99 a | 7.33 ± 3.16 a | 4.67 ± 2.62 a | 0.1840 | 0.4342 |
| Rychness † | 14.99 ± 1.36 a | 14.16 ± 1.09 a | 8.08 ± 1.08 b | 5.92 ± 1.14 b | 10.06 ± 1.04 a | 10.02 ± 0.81 a | <0.0001 | 0.6995 |
| Density | 7324.98 ± 937.89 a | 7079.25 ± 740.13 a | 5995.62 ± 829.30 a | 2333.66 ± 386.24 b | 5743.31 ± 600.78 a | 4689.98 ± 406.72 a | 0.0023 | 0.0609 |
| Soil Macroaggregates | Chronosequence | Landscape | Disturbed vs. Undisturbed | Chronosequence vs. Landscape | ||||
|---|---|---|---|---|---|---|---|---|
| Forest | 25–40 | 10–20 | Degraded Pasture | Lomerío | Mountain | p-Value | p-Value | |
| Biogenic macroaggregates | 19.72 ± 4.14 b | 44.11 ± 3.38 a | 33.40 ± 4.47 a | 15.41 ± 5.35 b | 24.52 ± 3.38 a | 31.80 ± 2.80 a | 0.0297 | 0.9865 |
| Non-macroaggregated | 49.86 ± 4.67 a | 27.02 ± 3.82 b | 41.26 ± 5.05 a | 22.18 ± 6.03 b | 36.50 ± 3.82 a | 33.66 ± 3.16 a | 0.0014 | 0.8059 |
| Organic material | 0.97 ± 0.15 a | 0.65 ± 0.14 ab | 0.44 ± 0.18 bc | 0.04 ± 0.20 c | 0.32 ± 0.05 b | 0.73 ± 0.16 a | 0.0029 | 0.2140 |
| Physicogenic macroaggregates | 19.64 ± 3.51 b | 20.41 ± 2.87 b | 16.55 ± 3.80 b | 33.80 ± 4.54 a | 27.05 ± 2.87 a | 18.15 ± 2.38 b | 0.3491 | 0.6347 |
| Root macroaggregates | 9.81 ± 2.60 b | 7.81 ± 2.12 b | 8.35 ± 2.80 b | 28.58 ± 3.35 a | 11.62 ± 2.12 a | 15.65 ± 1.76 a | 0.1079 | 0.8565 |
| Coinertia Analysis | Projected Inertia | RV | p-Value | |
|---|---|---|---|---|
| Axis 1 | Axis 2 | |||
| Macrofauna vs. Chemical | 52.3 | 21.1 | 0.24 | 0.035 |
| Macrofauna vs. Physical | 51.6 | 39.1 | 0.23 | 0.036 |
| Macrofauna vs. Macroaggregation | 68.3 | 22.1 | 0.21 | 0.040 |
| Chemical vs. Physical | 79.6 | 16.7 | 0.53 | 0.001 |
| Chemical vs. Macroaggregation | 51.2 | 39.9 | 0.17 | 0.043 |
| Physical vs. Macroaggregation | 73.9 | 22.8 | 0.14 | 0.044 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-León, C.H.; Peña-Venegas, C.P.; Sterling, A.; Castro, D.; Mahecha-Virguez, L.K.; Virguez-Díaz, Y.R.; Silva-Olaya, A.M. Soil Quality Restoration during the Natural Succession of Abandoned Cattle Pastures in Deforested Landscapes in the Colombian Amazon. Agronomy 2021, 11, 2484. https://doi.org/10.3390/agronomy11122484
Rodríguez-León CH, Peña-Venegas CP, Sterling A, Castro D, Mahecha-Virguez LK, Virguez-Díaz YR, Silva-Olaya AM. Soil Quality Restoration during the Natural Succession of Abandoned Cattle Pastures in Deforested Landscapes in the Colombian Amazon. Agronomy. 2021; 11(12):2484. https://doi.org/10.3390/agronomy11122484
Chicago/Turabian StyleRodríguez-León, Carlos H., Clara P. Peña-Venegas, Armando Sterling, Daniel Castro, Lizeth K. Mahecha-Virguez, Yeny R. Virguez-Díaz, and Adriana M. Silva-Olaya. 2021. "Soil Quality Restoration during the Natural Succession of Abandoned Cattle Pastures in Deforested Landscapes in the Colombian Amazon" Agronomy 11, no. 12: 2484. https://doi.org/10.3390/agronomy11122484
APA StyleRodríguez-León, C. H., Peña-Venegas, C. P., Sterling, A., Castro, D., Mahecha-Virguez, L. K., Virguez-Díaz, Y. R., & Silva-Olaya, A. M. (2021). Soil Quality Restoration during the Natural Succession of Abandoned Cattle Pastures in Deforested Landscapes in the Colombian Amazon. Agronomy, 11(12), 2484. https://doi.org/10.3390/agronomy11122484