Abstract
Black Sigatoka, a disease caused by the fungus Pseudocercospora fijiensis, can lead to the complete loss of banana and plantain production in the absence of chemical control. The development of resistant cultivars is the focus of many banana breeding programs and is an alternative to the use of fungicides. In order to define a refined method of selection in genetic breeding programs, this study evaluated 23 improved diploids, seven tetraploids, and two commercial cultivars in the presence of P. fijiensis. Four selection criteria were considered: means of the disease severity index (ID) and area under the disease progress curve (AUDPC) estimated over the total period of the experiment, only in summer, only in winter, and the emission and harvesting of bunches. The selection of genotypes was more effective in the winter, and the evaluation of four leaves no. 3 emitted after six months of growth was efficient at differentiating the resistant and susceptible genotypes. For the improved diploids and tetraploid hybrids, DI varied from 0.0 to 48.8 and from 15.1 to 63.5, respectively, and the AACPD for the improved hybrids and tetraploid hybrids varied from 0.0 to 2439.5 and 1000.2 to 3717.7, respectively. The tetraploid hybrid of the Prata-type CNPMF0906 and the commercial cultivar, which is a hybrid of the BRS Princesa Silk type, showed quantitative resistance and can be used by banana producers. Results suggest that the guidelines adopted for the selection of genotypes resistant to black Sigatoka may include methodologies that reduce the evaluation time. In addition, new sources of resistance to the disease and the influence of its genetic inheritance in future crosses were found.
1. Introduction
Bananas and plantains are cultivated in tropical and subtropical regions of 150 countries, and are among the four most important foods in the world [1]. The world’s banana production was more than 114 million tons and plantains exceeded 40 million in 2018, cultivated in a total area of approximately 11 million hectares. Banana production is estimated to reach 154 megatons (Mt) and the 10 largest banana producers are India (30.5 Mt), China (11.4 Mt), the Philippines (9.1 Mt), Indonesia (7.2 Mt), Colombia (7.1 Mt), Ecuador (6.9 Mt), Brazil (6.7 Mt), Cameroon (5.7 Mt), Democratic Republic of the Congo (5.1 Mt), and Uganda (3.8 Mt) [2]. Together with rice, wheat, and corn, these fruits are considered some of the most important sources of food in the world, serving as basic foods and sources of income for numerous families, especially in Africa and Asia [3,4].
Some diseases are responsible for significant losses in the production of bananas and plantains, notably black Sigatoka, caused by Pseudocercospora fijiensis (sexual form: Mycosphaerella fijiensis) [5]. This fungus was described in 1963 on the islands of Fiji [6]. The disease was first reported in Brazil in 1998, near Colombia and Peru, when it was introduced in the municipalities of Tabatinga and Benjamin Constant, in the State of Amazonas [7]. The fungus propagates from two types of spores, conidia, which are asexual, and ascospores, which are sexual [8].
The first symptoms of the disease occur between 7 and 14 days after infection and manifest themselves as striae, which at more advanced stages lead to leaf necrosis, which limits photosynthesis. This condition can result in production losses of up 100%, depending on the environmental conditions, due to a reduction in the weight of the fruits and bunches, early fruit maturation, reduction in green growth, and changes in pulp color [9,10,11]. Black Sigatoka is routinely controlled using a combination of contact and systemic fungicides. However, this practice can increase the production costs by up to 30%, which is estimated to be 550 million dollars at the global level [8]. In addition, it can potentially impact health and contaminate the environment. Biological control of the pathogen is still very limited, due to the effective efficiency of some biological agents and production and application costs. Other management strategies are based on cultural practices, such as surgery or defoliation, which consists in partial or total elimination of the affected plant tissue and furthermore, the use of adequate fertilizers to reduce the predisposition of plants to the pathogen [12].
The development of cultivars resistant to black Sigatoka is the focus of various genetic breeding programs around the world, including at Empresa Brasileira de Pesquisa Agropecuária–Embrapa, in Brazil [13,14]. The main commercial cultivars of bananas and plantains are susceptible to black Sigatoka, and although chemical control is considered efficient, its indiscriminate use is harmful to human health and the environment [8].
It is inferred that genetic resistance to P. fijiensis is associated with a recessive allele (bs1) and two independent alleles with additive effects (bsr2 and bsr3) [15]. There are also intralocus interactions at the bs1 locus that are apparently associated with the appearance of the first symptoms in the leaves and with intraloci interactions at the bsr locus, which influence the development of the disease [16]. Therefore, it is necessary to study cultivars that present in their genealogy these resistance genes for P. fijiensis in bananas.
Since 1982, Embrapa has made crosses involving improved diploids and triploid commercial cultivars susceptible to black Sigatoka, Pseudocercospora musicola and Fusarium oxysporum f. sp. cubense race 1 [17]. Eleven tetraploid hybrids have been developed and recommended to Brazilian farmers using this strategy.
Developing improved diploids is a routine activity at Embrapa, which annually generates hybrids resistant to yellow Sigatoka and Fusarium wilt race 1. Some of these are also resistant to black Sigatoka, tolerant to weevil borer and nematodes, and present other agronomic characteristics of interest for improvement. It is a dynamic process, since new improved diploids with superior characteristics are routinely used in crosses and others are excluded for technical reasons [17]. The best strategy to develop new improved diploids is to cross genotypes that exhibit qualitative resistance with those that exhibit quantitative response to the pathogen. This strategy aims to develop hybrids with two types of resistance, increasing the durability of the resistance level in the field [13]. In the future, the improved diploids developed, after a complete agronomic evaluation, will be crossed with susceptible commercial cultivars, aiming for the transfer of resistant alleles. In this context, the new commercial cultivar could make use of two defense strategies: qualitative resistance, which could possibly be overcome by the pathogen, and quantitative resistance. Another strategy is to intercross the three diploids with complete resistance with the goal of gene pyramiding for resistance in a new hybrid, increasing the possibility of durable resistance to black Sigatoka in the field.
Properly identifying genotypes that are partially or moderately resistant to black Sigatoka is fundamental when conducting works that have the goal of selecting genotypes for crosses (improved diploids). Thus, in this study, new diploid and tetraploid banana hybrids developed by Embrapa were evaluated in the presence of P. fijiensis, with the goal of selecting resistant genotypes to use in crosses or to be recommended to farmers. In addition, using early selection criteria accelerates the development time of cultivars that will be made available to farmers. In this context, we tested four selection strategies: early yearly evaluation, during the entire period of the experiment, only in the summer months, only in the winter months, and a classical analysis during the emission and collection of bunches aiming to select resistant genotypes for use in the banana genetic breeding program.
2. Materials and Methods
2.1. Plant Material and Experimental Design
The experiment was conducted from May 2016 to January 2018 in the experimental field of Embrapa in Cruz das Almas, Bahia, Brazil (12°40′19″ S and 39°06′22″ W, 220 m a.s.l.). The climate is tropical hot and humid (Aw to Am) according to the classification by Köppen, with an average annual temperature of 24.5 °C, relative humidity of 80%, and average annual precipitation of 1.250 mm [18]. Evaluations were carried out in the first cycle, which comprises the period between plantation and the first harvest of bunches. Thirty-two genotypes with unknown profile responses to P. fijiensis were selected for the evaluation, including 23 improved diploids, seven tetraploids, and one commercial cultivar (BRS Princesa). As a susceptible control, the commercial cultivar Grand Naine was used, whose susceptibility to the disease is already known. The diploid and tetraploid hybrids were selected according to their genealogy since at least one of the parents has some level of qualitative or quantitative resistance to black Sigatoka, such as Malaccensis, Tjau Lagada, Pahang, Calcutta 4, and Tuugia (Table 1).

Table 1.
Banana hybrids evaluated for resistance to Pseudocercospora fijiensis in a naturally infested area in Brazil, Embrapa 2021.
The experimental design was completely randomized, with 10 replicates per genotype, spaced 4 m × 2 m × 2 m apart and irrigated with a micro sprinkler according to the needs of the crop. This management method avoids plant stress due to a water deficit, since the experiment was conducted in a region with dry periods.
The experiment was established in an area naturally infested by Pseudocercospora fijiensis, for more than 5 years, when the pathogen was first reported in the region of the State of Bahia in farms in regions close to the experimental field [19]. Since then, this environment has been used for studies for efficient selection of genotypes resistant to the disease [20]. A similar evaluation method was used by Kimunye et al. [21], evaluating 93 banana accessions in Uganda, Africa.
2.2. Disease Measurement and Selection Criteria
Four selection strategies for genotypes resistant to black Sigatoka were evaluated: (1) evaluation of symptoms during the summer months; (2) evaluation of symptoms during the winter months; (3) evaluation of symptoms of the disease during the entire period of the experiment, and (4) classical evaluation of symptoms at the emission and harvest of bunches. These methods are the most used for the evaluation of the disease in the field, and to our general knowledge, there are no reports in the literature of studies that used more than one method, in the greenhouse and in the field.
In the experimental condition of the location of the study, winter comprises the months from May to August and is characterized by an average temperature of 22.6 °C, relative humidity of 86.4%, and average rainfall index of 4.75 mm/day, with average light hours of 11.4 h/day. Summer comprises the months from November to February and is characterized by an average temperature of 25.6 °C, relative humidity of 73.8%, rainfall index of 1.20 mm/day, and average 12.8 h of light per day. These data were collected during the study by a weather station located 50 m from the experiment.
For evaluations carried out in winter, summer, and all year round, the genotypes were evaluated for their behavior in the presence of black Sigatoka using a scale of scores of symptoms of the disease in the leaves, which was proposed by Stover [22] and modified by Gauhl [23], where: (1) up to 1% of the leaf blade with symptoms; (2) 1 to 5% of the leaf blade with symptoms; (3) 6 to 15% of the leaf blade with symptoms; (4) 16 to 33% of the leaf blade with symptoms; (5) 34 to 50% of the leaf blade with symptoms; and (6) 51 to 100% of the leaf blade with symptoms.
In the classical evaluation method performed using the evaluation scale described by Fouré [24], a single evaluation was performed at the time of the bunch emission and another at the harvest period. This scale of notes ranges from 0 (absence of symptoms) to 6 (center of the spots become dry, gray-whitish and the edges become depressed, where a black ring is often surrounded by a yellow halo surrounding the gray center, and these spots remain visible after the leaf dries completely). The evaluation was performed by analyzing all leaves of plants that presented more than 50% of the active leaf area and still in a position parallel to the soil. At the end, the averages of the grades of each leaf were used to estimate the disease index (ID) in the emission and harvest of bunches and it was not possible to generate an AUDPC, since the evaluations were not performed over time.
The evaluations started in the sixth month after planting when symptoms of black Sigatoka on leaf number 3 were observed until senescence. At this stage, the next leaf number 3 was identified, and a new evaluation of the symptoms began (Table S1). This same methodology was used by Kimunye et al. [21] in studies of selection of black Sigatoka-resistant diploids for use in crosses at International Institute of Tropical Agriculture (IITA) and National Research Organization (NARO).
This process was standardized to four number 3 leaves, considering that more than 80% of the plants had emitted this quantity of leaves at harvest. Additional details about the duration of the n° 3 leaves are provided in Table S2. It is important to note that the plants emitted a variable number of leaves, but due to the criterion for evaluating the symptoms until senescence, some of these leaves were not evaluated. These issues were also reported by Kimunye et al. [21]. The scores were recorded at intervals of 15 days and transformed into a disease severity index (DI, %) based on the formula described by Mckinney [25]:
For each hybrid, the average DI was estimated based on the evaluations made every 15 days and the number of replicates per genotype.
The area under the disease progress curve (AUDPC) was estimated with the formula proposed by Madden et al. [26], where n is the number of evaluations, DI is the disease severity index, and T1–T2 is the time interval between two consecutive evaluations, as follows:
The AUDPC was standardized by the number of evaluations made every 15 days until the harvesting of the bunches, considering that the duration of the number 3 leaves varied among the genotypes.
Evaluations to select the hybrids in the summer (December to March) and winter (June to September) and estimates of the DI and AUDPC for these intervals (seasons of the year) were based on evaluations of the symptoms at 15-day intervals and the number of replicates per genotype.
2.3. Data Analysis
Data from internal disease index (ID) and area below the disease progress curve (AUDPC) were transformed using the sine arc function [root(Y/100)] to achieve normality of the data. The analysis of variance with ID and AUDPC for each of the evaluation methods was carried out separately, whereas only the effect of genotypes, and the different methods as replicates of the experiment over time were observed. In the classical method, the evaluation was carried out only with IDs. The genotypes were compared in each method by the Scott–Knott test (p < 0.05). These analyses were performed with the ‘ExpDes.pt’ package in the software in R [27].
Using the same DIs and AUDPCs, a heat map analysis was conducted that produced a graphic interpretation, where data referring to each genotype are represented by colors; shades of green in the scale are associated with levels of genetic resistance and those of red in the scale indicate levels of susceptibility. This same analysis is often used in studies aimed at the evaluation of diseases both in the field and in a greenhouse, as it is an analysis of easy interpretation when compared to the classical methods of grouping, which are based on dendograms [20,28,29]. The statistical packages used for the analyses were devtools, NbClust, and gplots, executed in the R software.
The genotypes + genotypes vs. environments (GGE) interaction model was used, considering a GGE biplot (i.e., G + GE), which is constructed by the first two symmetrically scaled main components (PC1 and PC2), derived from the decomposition of a singular value of data from multiple environments. For this study, the different selection criteria were considered as environments. Detailed formulas of the models used are provided in Yan and Tinker [30] and Yan et al. [31]. From the ID values in the different selection criteria, for environmental evaluation, singular value partitioning with a focus on the environment (SVP = 2) was used. Was used the ‘Which-won-where ‘testers’ relationship option in order to identify which genotype was the winner in a given selection criterion (here taken as environments). These analyses were performed in the R software with the package “metan” [32].
3. Results
From the analysis of heat maps, based on the four strategies used to select genotypes resistant to P. fijiensis in summer (A), winter (B), annual (C), and harvest of clusters (D), it was possible to undo three clusters of genotypes, based on their similarity, classifying them as susceptible (S), moderately resistant (MR), and resistant (R) (Figure 1). However, it can be inferred that the most efficient way to differentiate genotypes in the resistant, moderately resistant, and susceptible classes is to evaluate the symptoms of P. fijiensis during the winter season. This is because this criterion seems to be more rigorous than the others, since a smaller number of genotypes are classified as resistant and/or moderately resistant to the disease, when compared to the other time periods (Figure 1B).
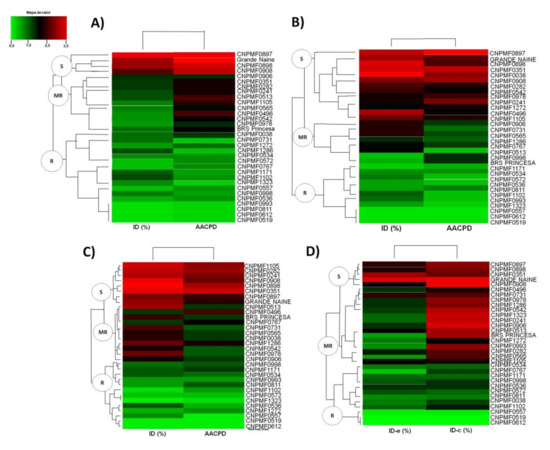
Figure 1.
Clustering of banana genotypes for resistance to black Sigatoka from the disease severity index (DI) and area under the disease progress curve (AUDPC), estimated by evaluating the symptoms of four n° 3 leaves: (A) evaluation in the summer, (B) winter, (C) year, and (D) classical. DI-e: bunch emission DI; DI-h: bunch harvest DI. S: susceptible, MR: moderately resistant, R: resistant. Embrapa 2021.
Absolute values for the DI and AUDPC are shown in Table 2. In the table, there are some inversions in the classification of the genotypes, depending on the evaluation strategy adopted. The improved diploids CNPMF0496, CNPMF0542, CNPMF0978, CNPMF1105, and CNPMF1272 were classified as susceptible to black Sigatoka in the evaluations made only in the winter; however, for the other analyses, the same genotypes were classified as resistant or moderately resistant. The same observation was made for some of the tetraploid hybrids (CNPMF0241, CNPMF0282, and CNPMF0351). Higher mean values were observed in the DI and AUDPC for the evaluations made in the winter.

Table 2.
Levels of resistance to black Sigatoka among the improved diploids and tetraploids hybrids. Embrapa 2021.
This period benefits the development of the disease, because there are environmental conditions that favor key points in the life cycle of the pathogen, such as dispersion, germination, penetration, colonization, and survival, increasing the severity of the disease and allowing the selection of resistant hybrids with greater rigor. In fact, with this criterion, fewer hybrids were classified as resistant and/or moderately resistant, when compared to the other evaluation times. It is also important to point out that this strategy is faster, since it does not require the evaluation of plants until the harvest of bunches, a classical methodology adopted in breeding routines to date, including at Embrapa.
One of the final objectives of genetic breeding programs is to develop cultivars resistant to black Sigatoka, and an incorrect evaluation of the behavior of a genotype against the disease can delay this for many years. For all selection criteria used, genotypes CNPMF0897, Grand Naine, CNPMF0038, and CNPMF0908 were classified as susceptible with the highest ID and AUDPC scores and cnpmf1171 genotypes, CNPMF0572, CNPMF0811, CNPMF0536, CNPMF1323, CNPMF0519, CNPMF0557, and CNPMF0612 were classified as resistant (Table 2).
For the analysis based on GGE Biplot, considering the 32 genotypes and the four selection methods (here taken as environments), the two main components (PC1 and PC2) represented approximately 95.0% of the genotypes vs. environments interaction, regarding the type of analysis “which-won-where”, which, therefore, was significant (Figure 2). From the lines perpendicular to the sides of the polygon that divide the biplot into sectors, it was possible to distinguish two environments, one composed of the criteria of annual selection, summer, emission, and harvest and the other composed of only the selection criterion in winter. For the first case, the cultivar Grand Naine was considered the most responsive, and for the second, the genotype CNPMF0897 (Figure 2). Regarding susceptibility, the genotypes allocated at the vertex of the polygon are more distant from the origin than the other genotypes and are classified as more responsive, being CNPMF0897, Grand Naine, CNPMF0038, and CNPMF0908 in relation to susceptibility and CNPMF0572, CNPMF0811, CNPMF0536, CNPMF1323, CNPMF0519, CNPMF0557, and CNPMF0612 in association with resistance, as they are arranged on the opposite side to the susceptible ones and in a quadrant where a selection method was inserted (Figure 2). Arranged in the central region of the biplot, there are genotypes with a median response in both environments, such as CNPMF0998, CNPMF0906, CNPMF0542, CNPMF0565, CNPMF0496, CNPMF0513, CNPMF1102, CNPMF0731, CNPMF0978, and BRS Princesa, classified as moderately resistant. In general, in the cluster harvesting environment, the genotypes considered susceptible respond better to the evaluations, while the other environments, especially winter, include genotypes with different responses to P. fijiensis as susceptible and moderately resistant, demonstrating that this method can be more efficient for selection in a set of genotypes with different responses to the disease.
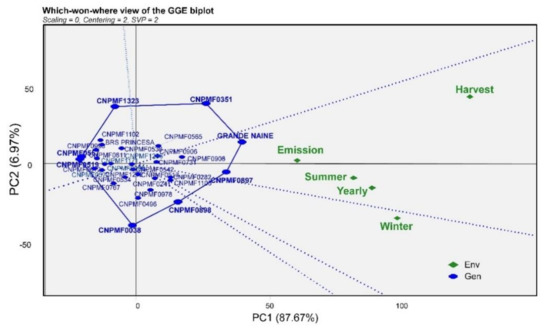
Figure 2.
Interaction of the genotypes × environments biplot showing the performance of 32 banana genotypes based on the severity of Pseudocercospora fijiensis evaluated by different selection criteria according to visualization “who won where”. The biplot was based on the decomposition of a singular value (Scaling = 1, Centering = 2). The partition of the singular value focused on the dotted line (SVP = 2) was used. The analysis was performed based on the data from the internal disease index (ID). Code: Env: environment; Gen: genotype, using the GGEBiplotGUI package in the R statistic software.
From the biplot of ranking of the environments, where the “ideal” environment is used as the center of a set of concentric lines that serve as a ruler to measure the distance between any environment and the ideal environment, it can be inferred that the selection criteria in winter, annual, summer, and emission of the bunches approach in the same way the ideal environment represented by the arrow with the small circle (Figure 3A). Thus, all test environments are desirable for selection of P. fijiensis under the study conditions, except for the evaluation performed in the collection of the bunch, which most distanced itself from the ideal environment. However, in the analysis of the relationship between environments, although all methods are correlated, the evaluation methods performed in the collection of the bunch and in winter have greater discriminating power of the genotypes, considering that they have the longest vectors, in their respective directions. Conversely, the method of analysis performed in the emission of the bunch has low discriminating power of genotypes (Figure 3B) and this characterizes a measure of the response capacity of genotypes for these environments [31]. These data are evidenced by the progress of the disease, since after flowering, banana trees do not produce new leaves and the severity of the disease increases as the plant ages; therefore, the performance of disease assessments at emission and harvest represents two extremes of genotype responses to the disease.
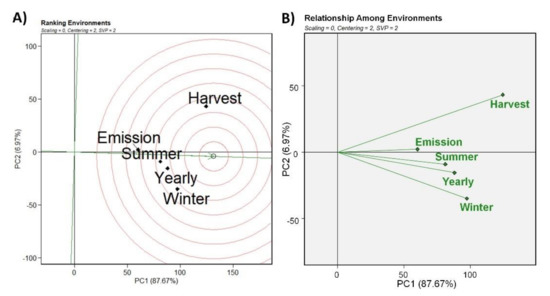
Figure 3.
Classification of different selection criteria (environments) based on their performance close to the ideal method of selection of banana genotypes for resistance against Pseudocercospora fijiensis (A) and the relationship between the selection criteria of banana genotypes resistant to Pseudocercospora fijiensis (B). The data were generated from the values of the internal index of the disease.
4. Discussion
This study compared different selection strategies of diploid and tetraploid hybrids and noticed that there are differences in the grouping of genotypes as resistant, moderately resistant, or susceptible, according to the selection criterion adopted (Figure 1, Table 2). It is known that the symptoms of P. fijiensis may vary according to the phenological stage of the plant, leaf age, period of infection of the pathogen, pressure of the inoculum, and cultivar used. Thus, the response to black Sigatoka differs in the evaluation of young and adult plants, suggesting the need to standardize the period and stage of symptom evaluation in the selection of resistant genotypes [33].
Many genotypes were considered susceptible with the evaluation criterion performed in winter. In Cruz das Almas (Bahia, Brazil), this period takes place between June and September in the southern hemisphere, favoring the development of the disease because it has environmental conditions adequate for key points of the life cycle of the pathogen, such as dispersal, germination, penetration, and survival. This increases the severity of the disease, allowing for a more rigorous selection of resistant genotypes. It is known that environmental conditions with high rainfall, humid climate, and temperatures ranging from 25 to 28 °C provide a favorable environment for the development of P. fijiensis and the establishment of its sexual and asexual phases continuously [34]. Regardless of the strategy adopted, the results presented corroborate those in the international literature, which classify the Grand Naine cultivar as susceptible to the disease, reinforcing the accuracy of the evaluations. Furthermore, some genotypes kept their profile regardless of the type of evaluation [8,35].
The summer and winter seasons have distinct average temperatures, relative humidity, and total and daily precipitation. The average data for summer was 26 °C, 73% humidity, and 105 mm of rain (1.2 mm/day) and the data for winter was 22 °C, 86% humidity, and 460 mm of rain (4.7 mm/day). Some environmental conditions are required for black Sigatoka to develop, such as temperatures around 27 °C and moisture on the leaf surface, whereas for the conidia formation in the cycle of the pathogen, high relative humidity is necessary (RH) and for the formation and expansion of the leaf lesions, high temperatures are necessary [36]. Although, according to these authors, in environments with high humidity, water on the leaf surface is not always necessary, especially when conidia are the main source of inoculum of the disease, whose germination is not affected by leaf wetting [37]. The response evaluation of 22 NARITA hybrids to P. fijiensis, under natural field conditions, in four sites in Africa, considered environments with high pressure of the disease as ideal. Additionally, the evaluation can be performed in cycle 2 or cycle 1 of the crop, besides using highly susceptible cultivars as propagators, to act as sources of inoculum, before establishing a test [37]. In this study, this environment was provided by the presence of inoculum in the area for long periods and the optimal conditions for the development of the disease were increased in the winter period. In addition, it was shown that evaluations performed every 15 days are adequate to obtain satisfactory results, regardless of the crop cycle in which the evaluations are conducted (Supplementary Materials Table S2).
Considering the efficient discrimination of the genotypes for the analysis of the symptoms of black Sigatoka during winter under the climatic conditions in the study area (Figure 2), more detail is provided about the results of the genotypes for this period. It is important to note that this strategy is faster, since it eliminates the evaluation of plants until the fruits are harvested, a classic methodology that is still routinely used by banana breeding programs, including Embrapa, as cited previously. Therefore, the strategy adopted in this study was mainly due to the difficulty in evaluating the large amount of plants developed by the breeding program in different locations so that selection is viable in a single environment from replicated measurements in time and considering the effect of the environment on the development of the disease. This methodology can be replicated in selection cycles initially with hybrids originating from seeds and then with clones of plants selected that likewise will be evaluated from the measurements in time and with a number of replicates. Therefore, at the end of two production cycles, it will be possible to perform evaluations with many measurements in time, which will provide greater reliability in the identification of resistant genotypes.
For the set of improved diploids, the DI varied from 0.0 to 48.8 and the AUDPC varied from 0.0 to 2439.5 (Table 2). Three improved diploids presented high resistance to black Sigatoka: CNPMF0519, CNPMF 0557, and CNPMF0612. Another nine exhibited quantitative resistance to the pathogen, notably CNPMF0811 and CNPMF0993, which had the lowest DI and AUDPC among the genotypes. These five genotypes have potential for use in crosses with susceptible cultivars, with the goal of developing commercial cultivars resistant to black Sigatoka, because sources of alleles are likely to be favorable to the combination of qualitative and quantitative resistance, presenting potential for use as parents in breeding programs to increase the frequency of these alleles. All these improved diploids that presented some kind of resistance have Calcutta 4 or Malaccensis in their genealogy, corroborating the studies by Nascimento et al. [20] and Kimunye et al. [21].
Considering the genealogies of the three improved diploids that exhibited complete resistance in our study, it is possible to speculate that the genetic control of their resistance to black Sigatoka is monogenic, while for the genotypes with quantitative resistance, it is inferred that the control is polygenic. However, new studies are needed to confirm these hypotheses. It is reported that the resistance to black Sigatoka in the diploid CNPMF0557 comes from the alleles of the wild diploid Pisang Lilin, which is resistant to the pathogen [35]. In the case of CNPMF0612, the SH3263 hybrid probably transferred alleles of resistance during crossing. CNPMF0811 and CNPMF0993 have Calcutta 4 and Tuu Gia in their genealogies, which are both resistant to black Sigatoka [20,38,39]. Embrapa considers disease resistance in the diploid germplasm, especially to Fusarium wilt race 1 and black Sigatoka, as a criterion for planning crosses. Other agronomic characteristics are also considered, such as a short height, production of over 100 fruits per bunch, male fertility, and peduncle architecture of the bunch [40]. Thus, these data are in accordance with the recently reviewed literature, which indicates a large number of studies where wild diploids of M. acuminata, such as Calcutta 4 and other diploid cultivars, may harbor sources of resistance genes, serving as parents for the generation of improved diploids and subsequent introgression of genes in new cultivars [14].
Embrapa has been developing improved diploids for use in crosses since 1982. All of the hybrids selected, until now, are resistant to yellow Sigatoka and Fusarium wilt race 1, and some are also resistant to black Sigatoka, tolerant of weevil borer and nematodes, and have other agronomic characteristics of interest for improvement. It should be noted that developing improved diploids is a dynamic process, since new genotypes with superior characteristics are developed and selected each year [17].
For the tetraploid hybrids evaluated for resistance against black Sigatoka, the DI varied from 15.1 and 63.5 and the AUDPC varied from 1000.2 to 3717.7 (Table 2). The genotype CNPMF0906 (Prata type) and the cultivar BRS Princesa (Silk type) exhibited moderate resistance to the pathogen. This is the first work to report the level of resistance of BRS Princesa to black Sigatoka, which also exhibits resistance to Fusarium wilt race 1 and yellow Sigatoka [28].
The development of cultivars resistant to black Sigatoka is an excellent alternative to overcome problems caused by the susceptibility of commercial cultivars, since depending on the cultivar, environmental conditions, and level of infection, losses caused by the disease can vary from 30–100% [8]. These losses are not necessarily associated with the death of the plant but are reflected in the reduced weight of the bunch and early ripening of the fruits [40,41]. Furthermore, when using fungicides, there is the risk of contamination of the environment and humans, especially people living near banana plantations [42].
In the context of global climate change, according to probabilistic models associated with an increase in the average temperature, a 50% increase is estimated in areas on the planet cultivated with bananas, especially in subtropical regions, such as southern Brazil, and areas at higher elevations. In this scenario of higher temperatures, water will be supplied by irrigation, which will also increase the severity and incidence of black Sigatoka, since the fungal spores will be in more favorable conditions for germination, because the use of certain irrigation systems may increase disease severity [43,44]. Therefore, cultivars adapted to this new condition will be in increasing demand by farmers, especially those associated with genetic resistance to pests and diseases.
5. Conclusions
This study demonstrated that the selection of genotypes resistant to Pseudocercospora fijiensis in the condition of the Recôncavo Region of Bahia, Brazil, is more effective during the winter, given the better conditions for the development of the pathogen. Additionally, the evaluation of symptoms in four number 3 leaves emitted six months after planting until complete senescence was efficient at discriminating between susceptible and resistant banana genotypes. The improved diploids, CNPMF 0519, CNPMF 0557, and CNPMF 0612, showed complete resistance to black Sigatoka and have potential for use in crosses with susceptible commercial cultivars, with the objective of transferring resistance alleles to commercial germplasm. A tetraploid hybrid of the Prata type exhibited quantitative resistance to black Sigatoka with potential for tests in production areas, with the objective of quantifying its potential for commercial use. Likewise, the commercial cultivar BRS Princesa, a Silk-type hybrid developed by Embrapa, displayed quantitative resistance to black Sigatoka. This same hybrid has resistance to Fusarium wilt, race 1, and tolerance to low temperatures, characteristics that surpass the traditional Silk cultivar. In order to reduce the screening for resistance using replicated measurements in time, the selection of a single environment is suggested, considering the effect of different environments in the development of the disease as shown here.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agronomy11122483/s1, Table S1. Raw grade data for symptom assessments of black Sigatoka of diploid and tetraploid banana hybrids in an area naturally infested. Embrapa 2019. Table S2: Life span (days) of four n° 3 leaves among the diploid and tetraploid banana hybrids in an area naturally infested with black Sigatoka. Embrapa 2021.
Author Contributions
Conceptualization of work and field evaluations, Z.S.G.; Statistical analysis, A.d.J.R.; methodology, Z.S.G., E.P.A., F.H.; formal data analysis, C.F.F., E.P.A., V.B.d.O.A.; writing of the work, Z.S.G., A.d.J.R., E.P.A.; revision, C.F.F., V.B.d.O.A.; Supervision, E.P.A. All authors read and agree with the published version of the manuscript.
Funding
This research was funded by IITA/Bill and the Melinda Gates Foundation—Accelerated Breeding of Better Bananas, grant number IITA 20600.15/0008-8—Phase II..
Acknowledgments
The authors thank the financial support for the accomplishment of this work: The National Council for Scientific and Technological Development (CNPq), the Brazilian Agricultural Research Company (Embrapa) for the availability of the area and the entire structure and the Accelerated breeding of better bananas (Bill and Melinda Gates Foundation grant led by IITA).
Conflicts of Interest
All authors declare no conflict of interests.
References
- Weber, O.B.; Garruti, D.D.S.; Norões, N.P. Performance of banana genotypes with resistance to black leaf streak disease in Northeastern Brazil. Pesquisa Agropecuária Brasileira 2017, 52, 161–169. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Data. Available online: http://faostat.fao.org/ (accessed on 4 August 2021).
- Perrier, X.; De Langhe, E.; Donohue, M.; Lentfer, C.; Vrydaghs, L.; Bakry, F.; Carrel, F.; Hippolyte, I.; Horry, J.P.; Jenny, C.; et al. Multidisciplinary perspectives on banana (Musa spp.) domestication. Proc. Natl. Acad. Sci. USA 2011, 108, 11311–11318. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Qi, Y.X.; Zhang, H.; Zhang, H.Q.; Pu, J.J.; Xie, Y.X. Separation and identification of Musa acuminata Colla (banana) leaf proteins by two-dimensional gel electrophoresis and mass spectrometry. Genet. Mol. Res. 2013, 12, 6871–6881. [Google Scholar] [CrossRef]
- Isaza, R.E.A.; Dias-Trujillo, C.; Dhillon, B.; Aerts, A.; Carlier, J.; Crane, C.F.; De Jong, T.V.; De Vries, I.; Dietrich, R.; Farmer, A.D.; et al. Combating a global threat to a clonal crop: Banana black sigatoka pathogen Pseudocercospora fijiensis (Synonym Mycosphaerella fijiensis) genomes reveal clues for disease control. PLoS Genet. 2016, 12, 1–36. [Google Scholar] [CrossRef]
- Crous, P.W.; Tanaka, K.; Summerell, B.A.; Groenewald, J.Z. Additions to Mycosphaerella complex. IMA Fungus 2010, 2, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Gasparotto, L.; Pereira, J.C.R.; Trindade, D.R. Situação atual da Sigatoka negra da bananeira. Fitopatologia Brasileira 2001, 26, 449. [Google Scholar]
- Churchill, A.C.L. Mycosphaerella fijiensis, the black leaf streak pathogen of banana: Progress towards understanding pathogen biology and detection, disease development, and the challenges of control. Mol. Plant Pathol. 2011, 12, 307–328. [Google Scholar] [CrossRef] [PubMed]
- Castelana, F.P.; Saraiva, L.A.; Lange, F.; de Bellaire, L.D.L.; Cordenunsi, B.R.; Chillet, M. Effects of black leaf streak disease and Sigatoka disease on fruit quality and maturation process of bananas produced in the subtropical conditions of Southern Brazil. J. Crop. Prot. 2012, 35, 127–131. [Google Scholar] [CrossRef]
- Gagné, E.S.; Cluzet, S.; Merillon, J.M.; Geny, L. ABA initiates anthocyanin production in grape cell cultures. Plant Growth Regul. 2011, 30, 1–10. [Google Scholar] [CrossRef]
- Chillet, M.; Abadie, C.; Hubert, O.; Chilin-Charles, Y.; De Bellaire, L.D.L. Sigatoka disease reduces the greenlife of bananas. Crop Prot. 2009, 28, 41–45. [Google Scholar] [CrossRef]
- Jimenez, M.I.; Van Der Veken, L.; Neirynck, H.; Rodriguez, H.; Ruiz, O.; Swennen, R. Organic banana production in Ecuador: Its implications on black Sigatoka development and plant-soil nutritional status. Renew. Agric. Food Syst. 2007, 22, 297–306. [Google Scholar] [CrossRef]
- Alakonya, A.E.; Kimunye, J.; Mahuku, G.; Amah, D.; Uwimana, B.; Brown, A.; Swennen, R. Progress in understanding Pseudocercospora banana pathogens and the development of resistant Musa germplasm. Plant Pathol. 2018, 67, 59–770. [Google Scholar] [CrossRef]
- Soares, J.M.; Rocha, A.J.; Nascimento, F.S.; Santos, A.S.; Miller, R.N.; Ferreira, C.F.; Haddad, F.; Amorim, V.B.O.; Amorim, E.P. Genetic improvement for resistance to black Sigatoka in bananas: A systematic review. Front. Plant Sci. 2021, 12, 657916. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.; Vuylsteke, D. Inheritance of black Sigatoka resistance in plantain and banana (Musa spp.) hybrids. Theor. Appl. Genet. 1994, 89, 46–52. [Google Scholar] [CrossRef]
- Craenen, K.; Ortiz, R. Effect of the bs1 gene in plantain-banana hybrids on response to black Sigatoka. Theor. Appl. Genet. 1997, 95, 497–505. [Google Scholar] [CrossRef]
- Amorim, E.P.; Amorim, V.B.O.; Silva, M.S.; Haddad, F.; Ferreira, C.F.; Santos-Serejo, J.A. Developing hybrid banana varieties with improved properties. In Achieving Sustainable Cultivation of Bananas: Germplasm and Genetic Improvement; Kema, G.H.J., Drenth, A., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2021; Volume 2, pp. 1–17. ISBN 978 1 78676 344 0. [Google Scholar]
- Agritempo. Sistema de Monitoramento Agrometeorológico. Available online: http://www.agritempo.gov.br/agroclima/sumario (accessed on 4 August 2021).
- Ramos, J.B.; Bragança, C.A.D.; Oliveira, A.D.S.; Cordeiro, Z.J.M.; Haddad, F. First report of black Sigatoka of banana caused by Mycosphaerella fijiensis in Bahia, Brazil. Plant Dis. 2018, 102, 2035–2045. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.S.; Sousa, Y.M.; Rocha, A.J.; Ferreira, C.F.; Haddad, F.; Amorim, E.P. Sources of black Sigatoka resistance in wild banana diploids. Rev. Bras. Frutic. 2020, 42, 1–14. [Google Scholar] [CrossRef]
- Kimunye, J.; Were, E.; Swennen, R.; Viljoen, A.; Mahuku, G. Sources of resistence to Pseudocercospora fijiensis, the cause of black Sigatoka in banana. J. Plant Pathol. 2021, 71, 1651–1664. [Google Scholar] [CrossRef] [PubMed]
- Stover, R.H. Banana Plantain and Abaca Diseases; Commonwelth Mycological Institute: Richmond, UK, 1972; Volume 316. [Google Scholar]
- Gauhl, F.; Pasberg-Gauhl, C.; Vuylsteke, D.; Ortiz, R. Multilocational Evaluation of Black Sigatoka Resistance in Banana and Plantain; International Institute of Tropical Agriculture: Abuja, Nigeria, 1989; pp. 47–59. [Google Scholar]
- Fouré, E. Varietal reactions of bananas and plantains to black leaf streak disease. Banana Plantain Breed. Strateg. 1987, 21, 110–113. [Google Scholar]
- Mckinney, H.H. Influence of soil, temperature and moisture on infection of wheat seedlings by Helminthosporium sativum. J. Agric. Res. 1923, 26, 195–217. [Google Scholar]
- Madden, L.V.; Hugles, G.; Bosch, F.V.D. The study of plant disease epidemics. Am. Phytopathol. Soc. 2007, 32, 31–63. [Google Scholar]
- R Core Development Team. R: A Language and Environment for Statistical Computing, Version 2.12.1.; R Foundation for Statistical Computing: Vienna, Austria, 2011; ISBN 3-900051-07-0. [Google Scholar]
- Rebouças, T.A.; Haddad, F.; Ferreira, C.F.; Oliveira, S.A.S.; Ledo, C.A.S.; Amorim, E.P. Identification of banana genotypes resistant to Fusarium wilt race 1 under field and greenhouse conditions. Sci. Hortic. 2018, 239, 308–313. [Google Scholar] [CrossRef]
- Gonçalves, Z.S.; Haddad, F.; Amorim, V.B.O.; Ferreira, C.F.; Oliveira, S.A.S.; Amorim, E.P. Agronomic characterization and identification of banana genotypes resistant to Fusarium wilt race 1. Eur. J. Plant Pathol. 2019, 155, 1093–1103. [Google Scholar] [CrossRef]
- Yan, W.; Tinker, N.A. Biplot analysis of multi-environment trial data: Principles and applications. Can. J. Plant Sci. 2006, 86, 623–645. [Google Scholar] [CrossRef]
- Yan, W.; Kang, M.S.; Ma, B.; Woods, S.; Cornelius, P.L. GGE biplot vs. AMMI analysis of genotype-by-environment data. Crop Sci. 2007, 47, 641–653. [Google Scholar] [CrossRef]
- Olivoto, T.; Lúcio, A.D.C. Metan: An R package for multi-environment trial analysis. Methods Ecol. Evol. 2020, 11, 783–789. [Google Scholar] [CrossRef]
- Jones, D.R. Sigatoka. In Diseases of Banana, Abacá and Enset; CABI Publishing: New York, NY, USA, 2000; pp. 79–92. [Google Scholar]
- Cordeiro, Z.J.M.; Matos, A.P.; Haddad, F. Doenças fúngicas e bacterianas. In O Agronegórcio da Banana; Ferreira, C.F., Silva, S.O., Amorim, E.P., Santos-Serejo, J.A., Eds.; Embrapa: Brasília, Brasil, 2016; Volume 1, pp. 111–136. [Google Scholar]
- Barekye, A.; Tongoona, P.; Derera, J.; Laing, M.; Tushermereirwe, W.K. Appraisal of methods for assessing black Sigatoka resistance in diploid banana populations. Afr. J. Plant Sci. 2011, 5, 900–908. [Google Scholar] [CrossRef]
- Gauhl, F.; Pasberg-Gauhl, C.; Jones, D.R. Black leaf streak: Disease cycle and epidemiology. In Diseases of Banana, Abacá and Enset; CABI Publishing: New York, NY, USA, 2000; pp. 56–62. [Google Scholar]
- Kimunye, J.; Jomanga, K.; Tazuba, A.F.; Were, E.; Viljoen, A.; Swennen, R.; Mahuku, G. Genotype X environment response of ‘Matooke’ hybrids (Naritas) to Pseudocercospora fijiensis, the cause of black Sigatoka in banana. Agronomy 2021, 11, 1145. [Google Scholar] [CrossRef]
- Ortiz-Vasquez, E.; Kaemmer, D.; Zhang, H.B.; Muth, J.; Rodriguez-Mendiola, M.; Arias-Castro, C.; James, A. Construction and characterization of a plant transformation-competent BIBAC library of the black Sigatoka-resistant banana Musa acuminata cv. Tuu Gia (AA). Theor. Appl. Genet. 2005, 110, 706–713. [Google Scholar] [CrossRef]
- Timm, E.S.; Pardo, H.L.; Coello, R.P.; Navarrete, T.C.; Villegas, O.N.; Ordonez, E.S. Identification of differentially-expressed genes in response to Mycosphaerella fijiensis in the resistant Musa accession ‘Calcutta-4’ using suppression subtractive hybridization. PLoS ONE 2016, 11, 1–17. [Google Scholar] [CrossRef][Green Version]
- Amorim, E.P.; Santos-Serejo, J.A.; Amorim, V.B.O.; Ferreira, C.F.; Silva, S. Banana breeding at Embrapa cassava and fruits. Acta Hortic. 2013, 986, 171–176. [Google Scholar] [CrossRef]
- Chillet, M.; Castelana, F.P.; Abadie, C.; Hubert, O.; Chilin-Charles, Y.; De Bellaire, L.L. Effect of different levels of Sigatoka disease severity on banana pulp colour and early ripening. Can. J. Plant Pathol. 2014, 36, 48–53. [Google Scholar] [CrossRef]
- De Bellaire, L.L.; Four, E.; Abadie, C.; Carlier, J. Black leaf streak disease is challenging the banana industry. Fruits 2010, 65, 327–342. [Google Scholar] [CrossRef]
- Calberto, G.G.; Staver, C.; Siles, P. An assessment of global banana production and suitability under climate change scenarios. In Climate Change and Food Systems: Global Assessments and Implications for Food Security and Trade; Elbehri, A., Ed.; Food Agriculture Organization of the United Nations (FAO): Rome, Italy, 2015; pp. 266–291. [Google Scholar]
- Yonow, T.; Ramirez-Villegas, J.; Abadie, C.; Darnell, R.E.; Ota, N.; Kriticos, D.J. Black Sigatoka in bananas: Ecoclimatic suitability and disease pressure assessments. PLoS ONE 2019, 14, 1–25. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).