Response of Pear Trees’ (Pyrus bretschneideri ‘Sinkiangensis’) Fine Roots to a Soil Water Regime of Regulated Deficit Irrigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material and Location
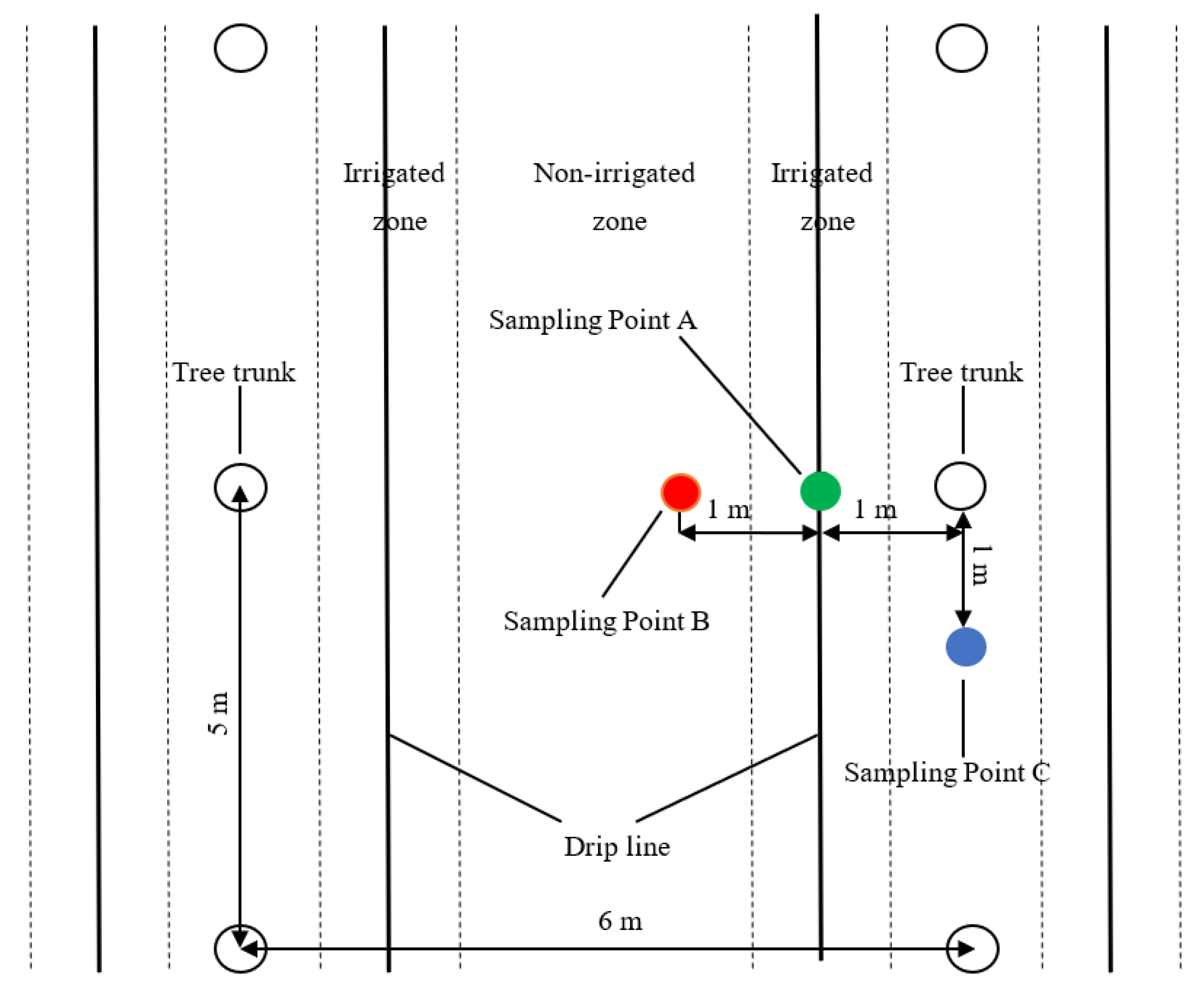
2.2. Experimental Design
2.3. Measurements
2.3.1. Soil Moisture and Evaporation Measurements
2.3.2. Tree Measurements
2.4. Data Analysis
3. Results
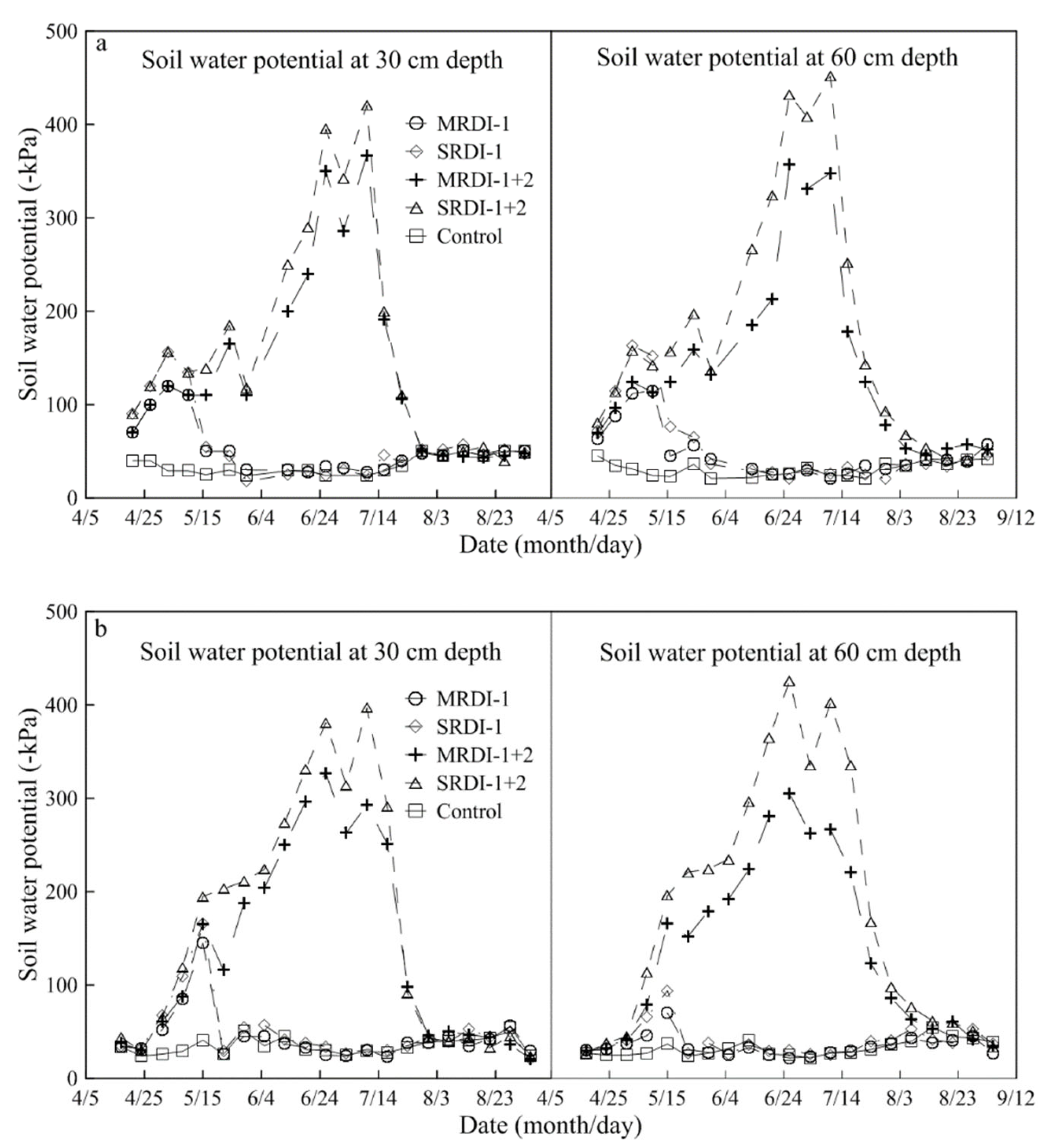
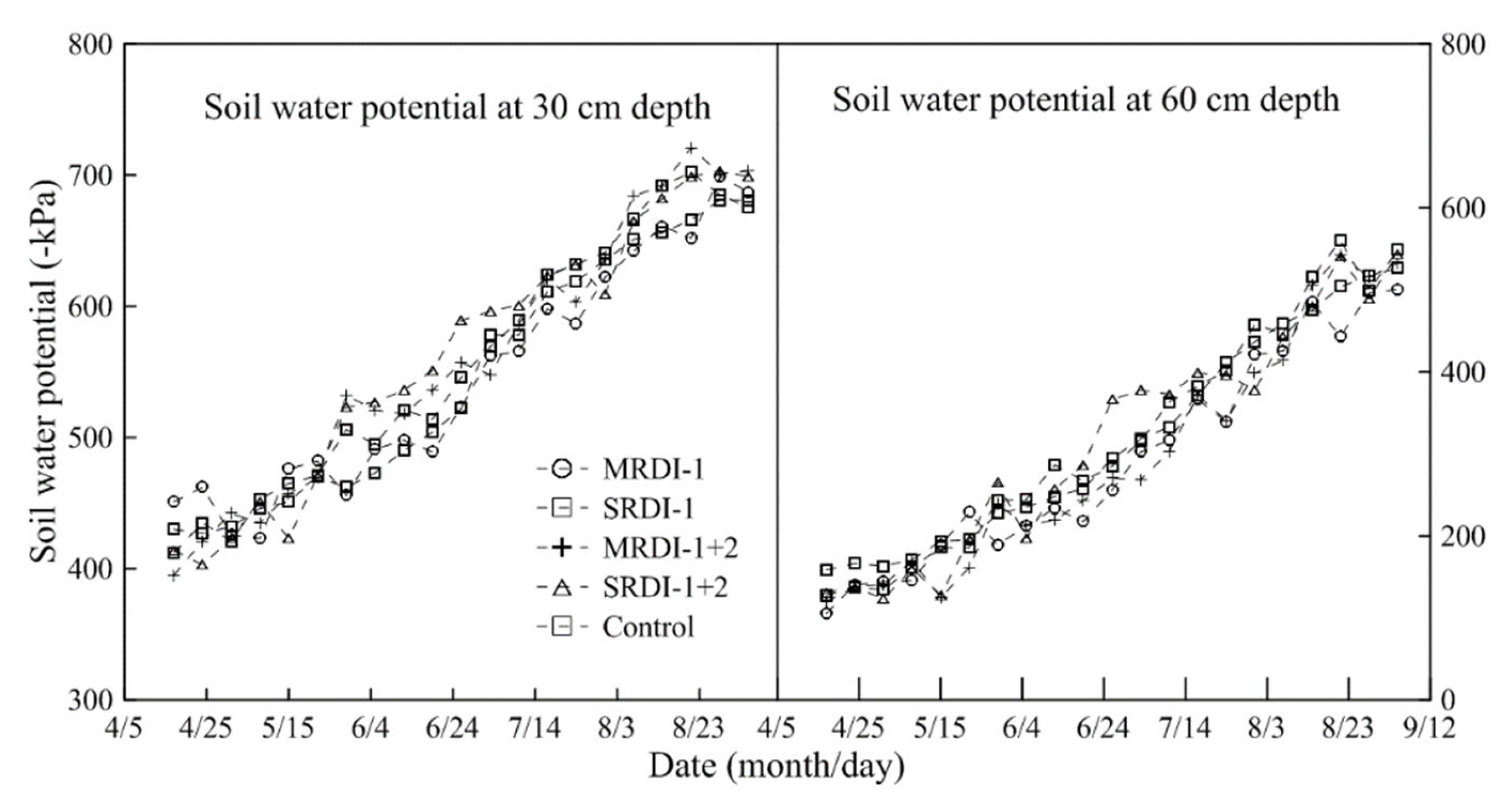
3.1. Soil Water Status Affected by RDI
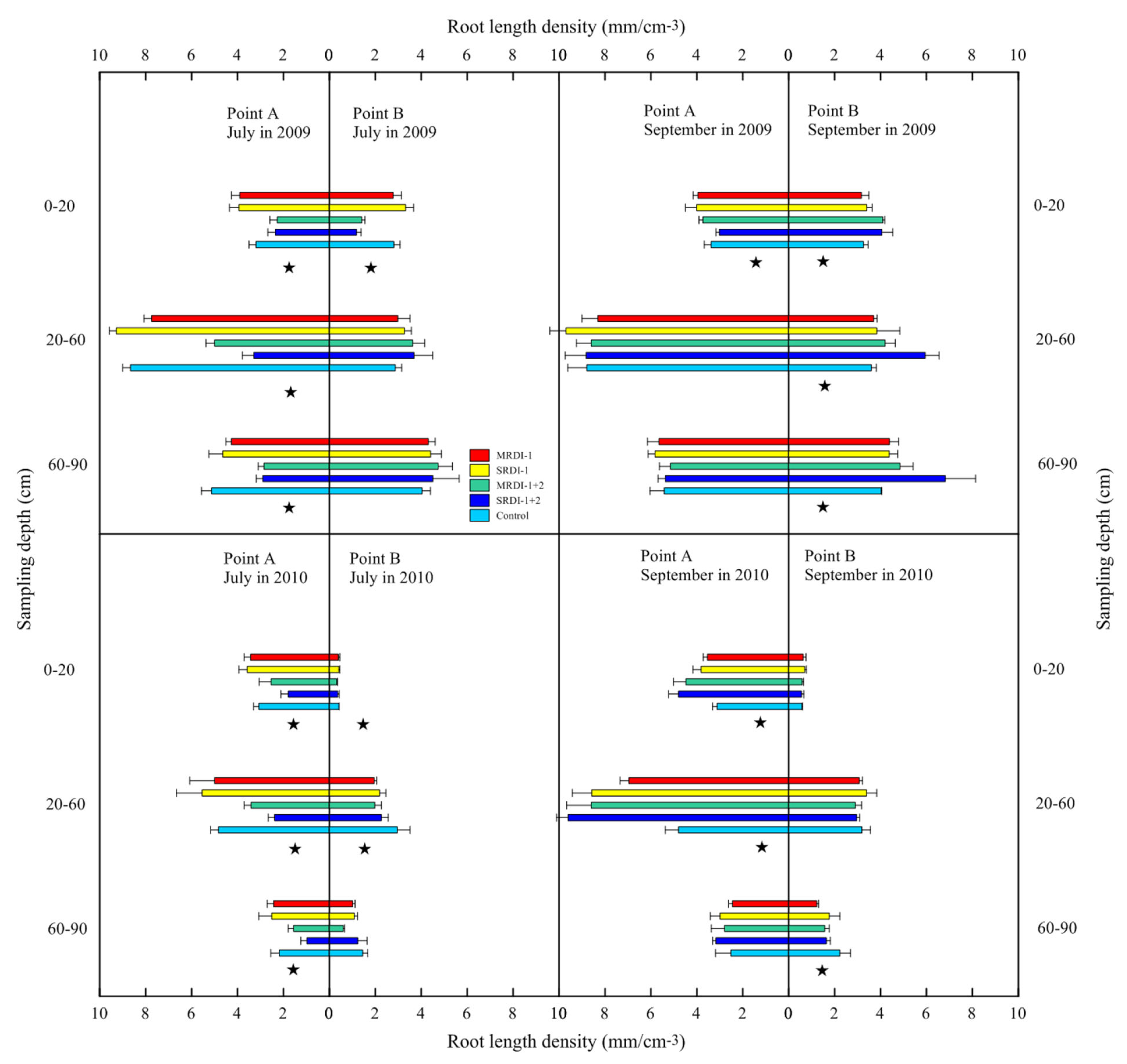
3.2. Responses of Fine Root Distribution to RDI Treatments
3.3. Leaf Photosynthetic Rate, Shoot Growth, and Fruit Yield of Each Treatment
4. Discussion
4.1. Fine Root Distribution in the Irrigated Zone Affected by RDI
4.2. Fine Root Distribution in a Non-Irrigated Zone Affected by RDI
4.3. Leaf Photosynthetic Rate, Shoot Growth, and Fruit Yield Affected by RDI
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mia, M.J.; Furmanczyk, E.M.; Golian, J.; Kwiatkowska, J.; Malusá, E.; Neri, D. Living Mulch with Selected Herbs for Soil Management in Organic Apple Orchards. Horticulturae 2021, 7, 59. [Google Scholar] [CrossRef]
- Wang, J.; Du, G.; Tian, J.; Zhang, Y.; Jiang, C.; Zhang, W. Effect of irrigation methods on root growth, root-shoot ratio and yield components of cotton by regulating the growth redundancy of root and shoot. Agric. Water Manag. 2020, 234, 106120. [Google Scholar] [CrossRef]
- Ong, C.K.; Corlett, J.E.; Singh, R.P.; Black, C.R. Above and below ground interactions in agroforestry systems. For. Ecol. Manag. 1991, 45, 45–57. [Google Scholar] [CrossRef] [Green Version]
- Oppelt, A.L.; Kurth, W.; Jentschke, G.; Godbold, D.L. Contrasting rooting patterns of some arid-zone fruit tree species from Botswana—I. Fine root distribution. Agrofor. Syst. 2005, 64, 1–11. [Google Scholar] [CrossRef]
- Sansavini, S.; Ancarani, V.; Neri, D. Overview of intensive pear culture: Planting density, rootstocks, orchard management, soil-water relations and fruit quality. Acta Hortic. 2008, 800, 35–49. [Google Scholar] [CrossRef]
- Steinberg, S.L.; Miller, J.C.; Mcfarland, M.J. Dry matter partitioning and vegetative growth of young peach trees under water stress. Aust. J. Plant Physiol. 1990, 17, 23–36. [Google Scholar] [CrossRef]
- Abrisqueta, J.M.; Mounzer, O.; Álvarez, S.; Conejero, W.; García-Orellana, Y.; Tapia, L.M.; Vera, J.; Abrisqueta, I.; Ruiz-Sánchez, M.C. Root dynamics of peach trees submitted to partial rootzone drying and continuous deficit irrigation. Agric. Water Manag. 2008, 95, 959–967. [Google Scholar] [CrossRef]
- Musacchi, S.; Iglesias, I.; Neri, D. Training Systems and Sustainable Orchard Management for European Pear (Pyrus communis L.) in the Mediterranean Area: A Review. Agronomy 2021, 11, 1765. [Google Scholar] [CrossRef]
- Ahmadi, S.H.; Plauborg, F.; Andersen, M.N.; Sepaskhah, A.R.; Jensen, C.R.; Hansen, S. Effects of irrigation strategies and soils on field grown potatoes: Root distribution. Agric. Water Manag. 2011, 98, 1280–1290. [Google Scholar] [CrossRef]
- Li, F.; Liu, X.; Li, S. Effects of early soil water distribution on the dry matter partition between roots and shoots of winter wheat. Agric. Water Manag. 2001, 49, 163–171. [Google Scholar] [CrossRef]
- Kulkarni, M.; Phalke, S. Evaluating variability of root size system and its constitutive traits in hot pepper (Capsicum annum L.) under water stress. Sci. Hortic. 2009, 120, 159–166. [Google Scholar] [CrossRef]
- Kramer, P.J.; Boyer, J.S. Water Relations of Plants and Soils; Academic Press: San Diego, CA, USA, 1995; pp. 145–147. [Google Scholar]
- Chalmers, D.J.; Mitchell, P.D.; Van Heek, L. Control of peach tree growth and productivity by regulated water supply, tree density, and summer pruning. J. Am. Soc. Hortic. Sci. 1981, 106, 307–312. [Google Scholar]
- Tognetti, R.; d’Andria, R.; Morelli, G.; Alvino, A. The effect of deficit irrigation on seasonal variations of plant water use in Olea europaea L. Plant Soil 2005, 273, 139–155. [Google Scholar] [CrossRef]
- Mitchell, P.D.; Jerie, P.H.; Chalmers, D.J. The effects of regulated water deficits on pear tree growth, flowering, fruit growth, and yield. J. Am. Soc. Hortic. Sci. 1984, 109, 604–606. [Google Scholar]
- Ahumada-Orellana, L.E.; Ortega-Farías, S.; Searles, P.S.; Retamales, J.B. Yield and water productivity responses to irrigation cut-off strategies after fruit set using stem water potential thresholds in a super-high density olive orchard. Front. Plant Sci. 2017, 8, 1280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, P.D.; Chalmers, D.J.; Jerie, P.H.; Burge, G. The use of initial withholding of irrigation and tree spacing to enhance the effect of regulated deficit irrigation on pear trees. J. Am. Soc. Hortic. Sci. 1986, 111, 858–861. [Google Scholar]
- Mitchell, P.D.; van den Ende, B.; Jerie, P.H.; Chalmers, D.J. Responses of ‘Bartlett’ pear to withholding irrigation, regulated deficit irrigation, and tree spacing. J. Am. Soc. Hortic. Sci. 1989, 114, 15–19. [Google Scholar]
- Caspari, H.W.; Behboudian, M.H.; Chalmers, D.J. Water use, growth, and fruit yield of ‘Hosui’ Asian pears under deficit irrigation. J. Am. Soc. Hortic. Sci. 1994, 119, 383–388. [Google Scholar] [CrossRef]
- Chalmers, D.J.; Burge, G.; Jerie, P.H.; Mitchell, P.D. The mechanism of regulation of ‘Bartlett’ pear fruit and vegetative growth by irrigation withholding and regulated deficit irrigation. J. Am. Soc. Hortic. Sci. 1986, 111, 904–907. [Google Scholar]
- Behboudian, M.H.; Lawes, G.S.; Griffiths, K.M. The influence of water deficit on water relations, photosynthesis and fruit growth in Asian pear (Pyrus serotina Rehd.). Sci. Hortic. 1994, 60, 89–99. [Google Scholar] [CrossRef]
- Marsal, J.; Rapoport, H.F.; Manrique, T.; Girona, J. Pear fruit growth under regulated deficit irrigation in container-grown trees. Sci. Hortic. 2000, 85, 243–259. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, Z.; Wang, W.; Ma, Y.; Huang, X. Yield and growth of mature pear trees under water deficit during slow fruit growth stages in sparse planting orchard. Sci. Hortic. 2013, 164, 189–195. [Google Scholar] [CrossRef]
- Çolak, Y.B.; Yazar, A. Evaluation of crop water stress index on Royal table grape variety under partial root drying and conventional deficit irrigation regimes in the Mediterranean Region. Sci. Hortic. 2017, 224, 384–394. [Google Scholar] [CrossRef]
- De la Hera, M.L.; Romero, P.; Gómez-Plaza, E.; Martinez, A. Is partial root-zone drying an effective irrigation technique to improve water use efficiency and fruit quality in field-grown wine grapes under semiarid conditions? Agric. Water Manag. 2007, 87, 261–274. [Google Scholar] [CrossRef]
- Egea, G.; González-Real, M.M.; Baille, A.; Nortes, P.A.; Sánchez-Bel, P.; Domingo, R. The effects of contrasted deficit irrigation strategies on the fruit growth and kernel quality of mature almond trees. Agric. Water Manag. 2009, 96, 1605–1614. [Google Scholar] [CrossRef]
- Egea, G.; Nortes, P.A.; González-Real, M.M.; Baille, A.; Domingo, R. Agronomic response and water productivity of almond trees under contrasted deficit irrigation regimes. Agric. Water Manag. 2010, 97, 171–181. [Google Scholar] [CrossRef]
- Blanco, V.; Torres-Sánchez, R.; Blaya-Ros, P.J.; Pérez-Pastor, A.; Domingo, R. Vegetative and reproductive response of ‘Prime Giant’ sweet cherry trees to regulated deficit irrigation. Sci. Hortic. 2019, 249, 478–489. [Google Scholar] [CrossRef]
- Cuevas, J.; Cañete, M.L.; Pinillos, V.; Zapata, A.J.; Fernández, M.D.; González, M.; Hueso, J.J. Optimal dates for regulated deficit irrigation in ‘Algerie’ loquat (Eriobotrya japonica Lindl.) cultivated in Southeast Spain. Agric. Water Manag. 2007, 89, 131–136. [Google Scholar] [CrossRef]
- Hueso, J.J.; Cuevas, J. Loquat as a crop model for successful deficit irrigation. Irrig. Sci. 2008, 26, 269–276. [Google Scholar] [CrossRef]
- Hueso, J.J.; Cuevas, J. Ten consecutive years of regulated deficit irrigation probe the sustainability and profitability of this water saving strategy in loquat. Agric. Water Manag. 2010, 97, 645–650. [Google Scholar] [CrossRef]
- Tejero, I.G.; Zuazo, V.H.D.; Bocanegra, J.A.J.; Fernández, J.L.M. Improved water-use efficiency by deficit-irrigation programmes: Implications for saving water in citrus orchards. Sci. Hortic. 2011, 128, 274–282. [Google Scholar] [CrossRef]
- Yan, Q.; Wang, W.; Ren, D.; Wu, Y.; Ma, Y.; Huang, X. Effects of wetted percentage on the root redistribution of mature Korla fragrant pear trees under drip irrigation. J. Irrig. Drain. 2011, 30, 63–67. [Google Scholar]
- Sokalska, D.I.; Haman, D.Z.; Szewczuk, A.; Sobota, J.; Dereń, D. Spatial root distribution of mature apple trees under drip irrigation system. Agric. Water Manag. 2009, 96, 917–924. [Google Scholar] [CrossRef]
- Persson, H.; Fircks, Y.V.; Majdi, H.; Nilsson, L.O. Root distribution in a Norway spruce (picea abies (L.) Karst.) stand subjected to drought and ammonium-sulphate application. Plant Soil 1995, 168, 161–165. [Google Scholar] [CrossRef]
- Wonisch, A.; Tausz, M.; Müller, M.; Weidner, W.; Dekok, L.J.; Grill, D. Treatment of young spruce shoots with SO2 and H2S: Effects on fine root chromosomes in relation to changes in the thiol content and redox state. Water Air Soil Pollut. 1999, 116, 423–428. [Google Scholar] [CrossRef]
- Ma, X.; Sanguinet, K.A.; Jacoby, P.W. Direct root-zone irrigation outperforms surface drip irrigation for grape yield and crop water use efficiency while restricting root growth. Agric. Water Manag. 2020, 231, 105993. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, M.; Yao, C.; Liu, Y.; Wang, Z.; Zhang, Y. Increasing seeding density under limited irrigation improves crop yield and water productivity of winter wheat by constructing a reasonable population architecture. Agric. Water Manag. 2021, 253, 106951. [Google Scholar] [CrossRef]
- Ahmed, A.; Alfalahi, H.M.; Al-Abodi, K.; Bassam, K.; Jabbar, A.; Amer, M.M.; Khiadher, A.S. Scheduling irrigation as a water saving practice for corn (Zea mays L.) production in Iraq. Int. J. Appl. Agric. Sci. 2015, 1, 55–59. [Google Scholar]
- Ullah, I.; Mao, H.; Rasool, G.; Gao, H.; Javed, Q.; Sarwar, A.; Khan, M.I. Effect of Deficit Irrigation and Reduced N Fertilization on Plant Growth, Root Morphology and Water Use Efficiency of Tomato Grown in Soilless Culture. Agronomy 2021, 11, 228. [Google Scholar] [CrossRef]
- Asseng, S.; Ritchie, J.T.; Smucker, A.J.M.; Robertson, M.J. Root growth and water uptake during water deficit and recovering in wheat. Plant Soil 1998, 201, 265–273. [Google Scholar] [CrossRef]
- Mossad, A.; Scalisi, A.; Lo Bianco, R. Growth and water relations of field-grown‘Valencia’ orange trees under long-term partial rootzone drying. Irrig. Sci. 2018, 36, 9–24. [Google Scholar] [CrossRef] [Green Version]
- Romero, P.; Botía, P.; Garcia, F. Effects of regulated deficit irrigation under subsurface drip irrigation conditions on water relations of mature almond trees. Plant Soil 2004, 260, 155–168. [Google Scholar] [CrossRef]
- McCully, M. Roots in soil: Unearthing the complexities of roots and their rhizospheres. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 695–718. [Google Scholar] [CrossRef] [PubMed]
- Levin, I.; Assaf, R.; Bravdo, B. Soil moisture and root distribution and an apple orchard irrigated by tricklers. Plant Soil 1979, 52, 31–40. [Google Scholar] [CrossRef]
- Fernández, J.E.; Moreno, F.; Cabrera, F.; Arrue, J.L.; Martín-Aranda, J. Drip irrigation, soil characteristics and the root distribution and root activity of olive trees. Plant Soil 1991, 133, 239–251. [Google Scholar] [CrossRef]
- Stevens, R.M.; Douglas, T. Distribution of grapevine roots and salt under drip and full-ground cover microjet irrigation systems. Irrig. Sci. 1994, 15, 147–152. [Google Scholar] [CrossRef]
- Ruiz-Sánchez, M.C.; Plana, V.; Ortuño, M.F.; Tapia, L.M.; Abrisqueta, J.M. Spatial root distribution of apricot trees in different soil tillage practices. Plant Soil 2005, 272, 211–221. [Google Scholar] [CrossRef]
- Ramos, A.F.; Santos, F.L. Yield and olive oil characteristics of a low-density orchard (cv. Cordovil) subjected to different irrigation regimes. Agric. Water Manag. 2010, 97, 363–373. [Google Scholar] [CrossRef]
- Fereres, E.; Cruz-Romero, G.; Hoffman, G.J.; Rawlins, S.L. Recovery of orange trees following severe water stress. J. Appl. Ecol. 1979, 16, 833–842. [Google Scholar] [CrossRef]
- Huang, B.; Fry, J.D. Root anatomical, physiological and morphological responses to drought stress for tall Fescue cultivars. Crop Sci. 1998, 38, 1017–1022. [Google Scholar] [CrossRef]
- Tanasescu, N.; Paltineanu, C. Root distribution of apple tree under various irrigation systems within the hilly region of Romania. Int. Agrophysics 2004, 18, 175–180. [Google Scholar]
- Moreno, F.; Fernández, J.E.; Clothier, B.E.; Green, S.R. Transpiration and root water uptake by olive trees. Plant Soil 1996, 184, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Lodolini, E.M.; Polverigiani, S.; Ali, S.; Mutawea, M.; Qutub, M.; Pierini, F.; Neri, D. Effect of complementary irrigation on yield components and alternate bearing of a traditional olive orchard in semi-arid conditions. Span. J. Agric. Res. 2016, 14, e1203. [Google Scholar] [CrossRef] [Green Version]

| Year | Stages | Precipitation (mm) | Evaporation (mm) | Irrigation Amount (mm) | ||||
|---|---|---|---|---|---|---|---|---|
| MRDI-1 | SRDI-1 | MRDI-1+2 | SRDI-1+2 | Control | ||||
| 2009 | Stage 1 | 2.4 | 171.5 | 94 | 58 | 94 | 59 | 122 |
| Stage 2 | 9.2 | 364.8 | 306 | 308 | 222 | 144 | 310 | |
| Stage 3 | 9.6 | 264.0 | 226 | 229 | 224 | 227 | 227 | |
| Total | 21.2 | 800.3 | 626 | 595 | 540 | 430 | 659 | |
| 2010 | Stage 1 | 9.2 | 136.5 | 74 | 44 | 73 | 44 | 97 |
| Stage 2 | 12.6 | 319.3 | 261 | 261 | 187 | 135 | 260 | |
| Stage 3 | 2.2 | 317.5 | 271 | 275 | 274 | 273 | 274 | |
| Total | 24.0 | 773.3 | 606 | 580 | 534 | 452 | 631 | |
| Year | Treatment | Yield (t/ha) | Total Soluble Solid Content (%) | Soluble Sugar Content (%) | Fruit Volume (cm3) | Final Shoot Length (cm) | Shoot Length in Late May (cm) |
|---|---|---|---|---|---|---|---|
| 2009 | MRDI-1 | 18.9 ab | 12.3 b | 8.14 c | 94 a | 27.5 b | 25.6 b |
| SRDI-1 | 21.5 a | 12.5 ab | 8.82 a | 100 a | 25.9 bc | 23.6 bc | |
| MRDI-1+2 | 18.1 bc | 12.0 bc | 7.97 c | 103 a | 26.0 b | 24.9 bc | |
| SRDI-1+2 | 15.8 c | 13.1 a | 8.61 b | 96 a | 24.0 c | 22.3 c | |
| Control | 18.6 b | 11.5 c | 6.93 d | 98 a | 32.4 a | 30.2 a | |
| 2010 | MRDI-1 | 21.2 ab | 12.8 b | 8.08 a | 116 a | 25.4 b | 23.1 b |
| SRDI-1 | 23.6 a | 13.8 a | 8.05 a | 123 a | 23.9 c | 21.9 b | |
| MRDI-1+2 | 20.6 b | 13.6 a | 7.71 bc | 113 a | 24.3 bc | 22.6 b | |
| SRDI-1+2 | 17.2 c | 13.9 a | 7.99 ab | 122 a | 22.5 c | 21.0 b | |
| Control | 19.8 b | 13.6 a | 7.63 c | 114 a | 31.3 a | 29.1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zhao, Z.; Zhao, F.; Cheng, X.; Zhao, P.; Liu, S. Response of Pear Trees’ (Pyrus bretschneideri ‘Sinkiangensis’) Fine Roots to a Soil Water Regime of Regulated Deficit Irrigation. Agronomy 2021, 11, 2316. https://doi.org/10.3390/agronomy11112316
Wu Y, Zhao Z, Zhao F, Cheng X, Zhao P, Liu S. Response of Pear Trees’ (Pyrus bretschneideri ‘Sinkiangensis’) Fine Roots to a Soil Water Regime of Regulated Deficit Irrigation. Agronomy. 2021; 11(11):2316. https://doi.org/10.3390/agronomy11112316
Chicago/Turabian StyleWu, Yang, Zhi Zhao, Feng Zhao, Xiaolei Cheng, Pingping Zhao, and Songzhong Liu. 2021. "Response of Pear Trees’ (Pyrus bretschneideri ‘Sinkiangensis’) Fine Roots to a Soil Water Regime of Regulated Deficit Irrigation" Agronomy 11, no. 11: 2316. https://doi.org/10.3390/agronomy11112316
APA StyleWu, Y., Zhao, Z., Zhao, F., Cheng, X., Zhao, P., & Liu, S. (2021). Response of Pear Trees’ (Pyrus bretschneideri ‘Sinkiangensis’) Fine Roots to a Soil Water Regime of Regulated Deficit Irrigation. Agronomy, 11(11), 2316. https://doi.org/10.3390/agronomy11112316





