Evaluation of Seed Dormancy, One of the Key Domestication Traits in Chickpea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Germination Testing
2.2. Germination Data Analysis
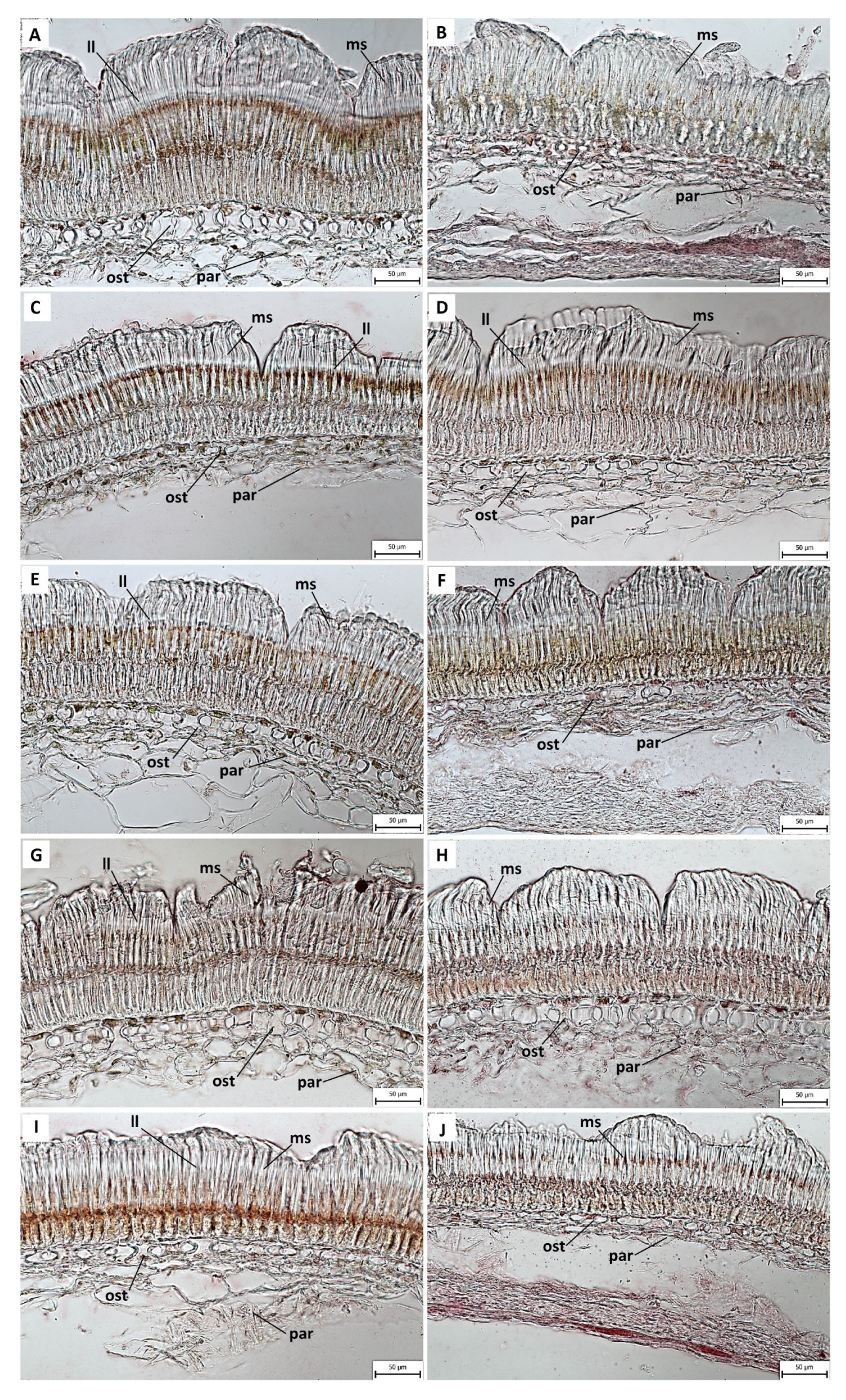
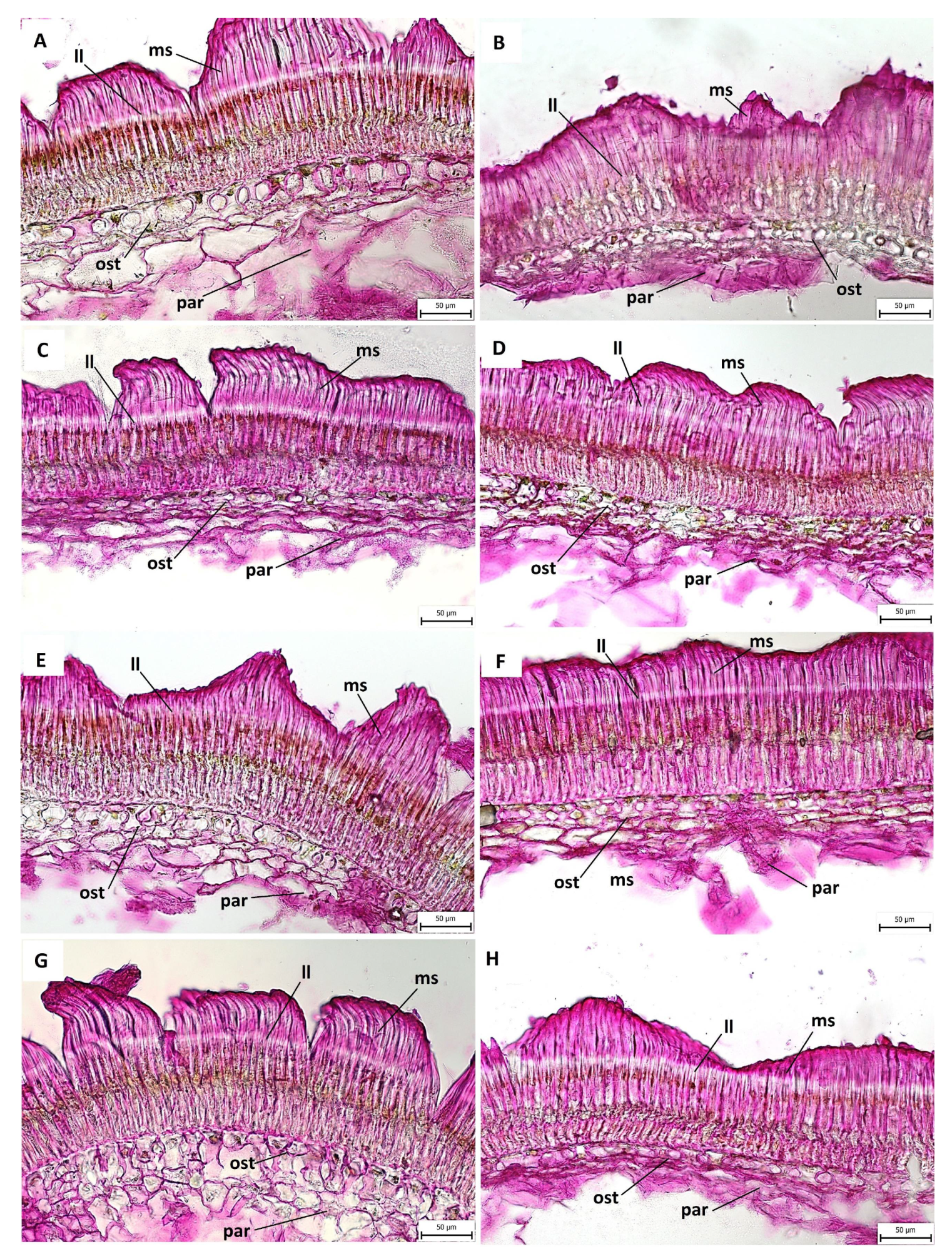
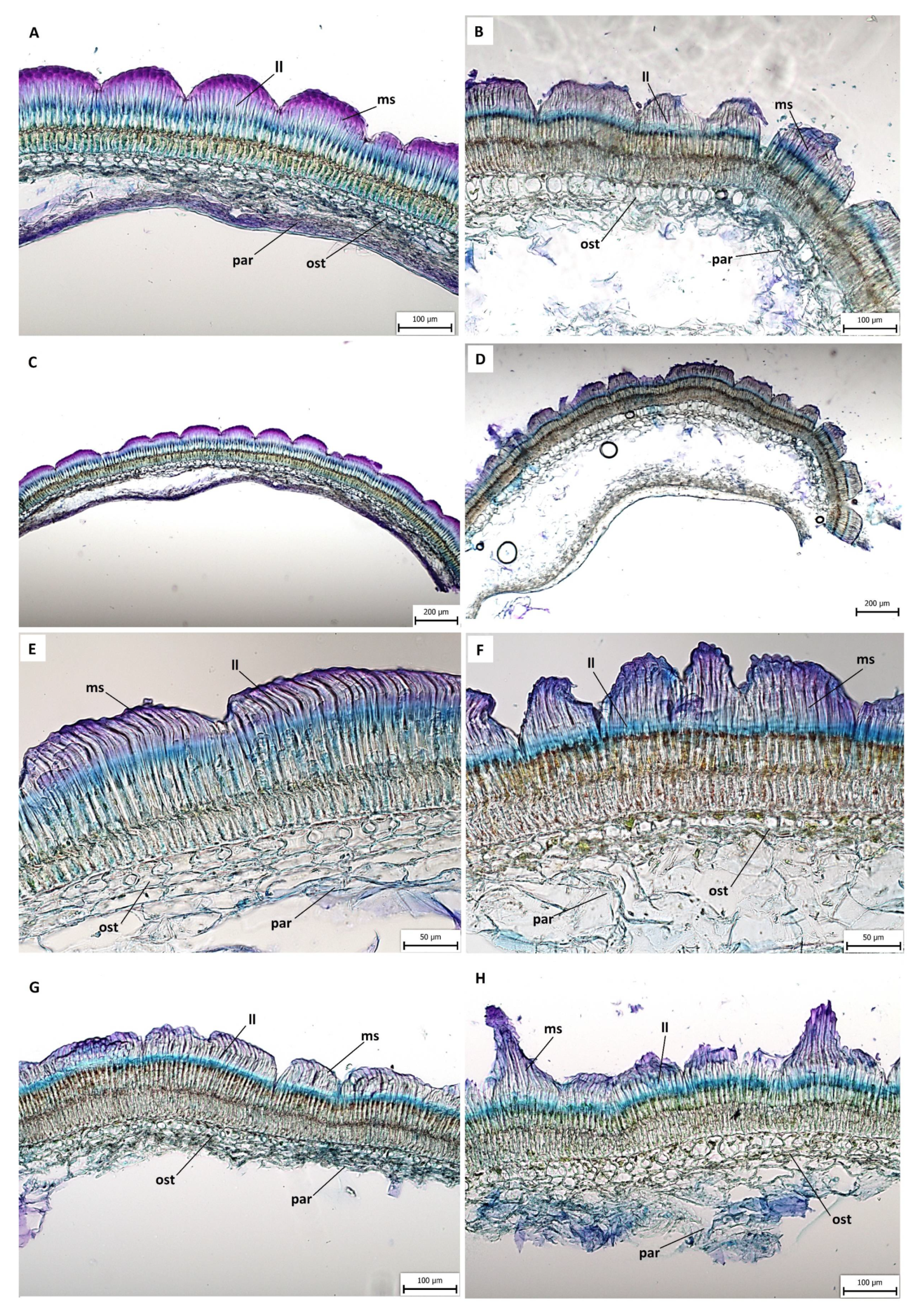
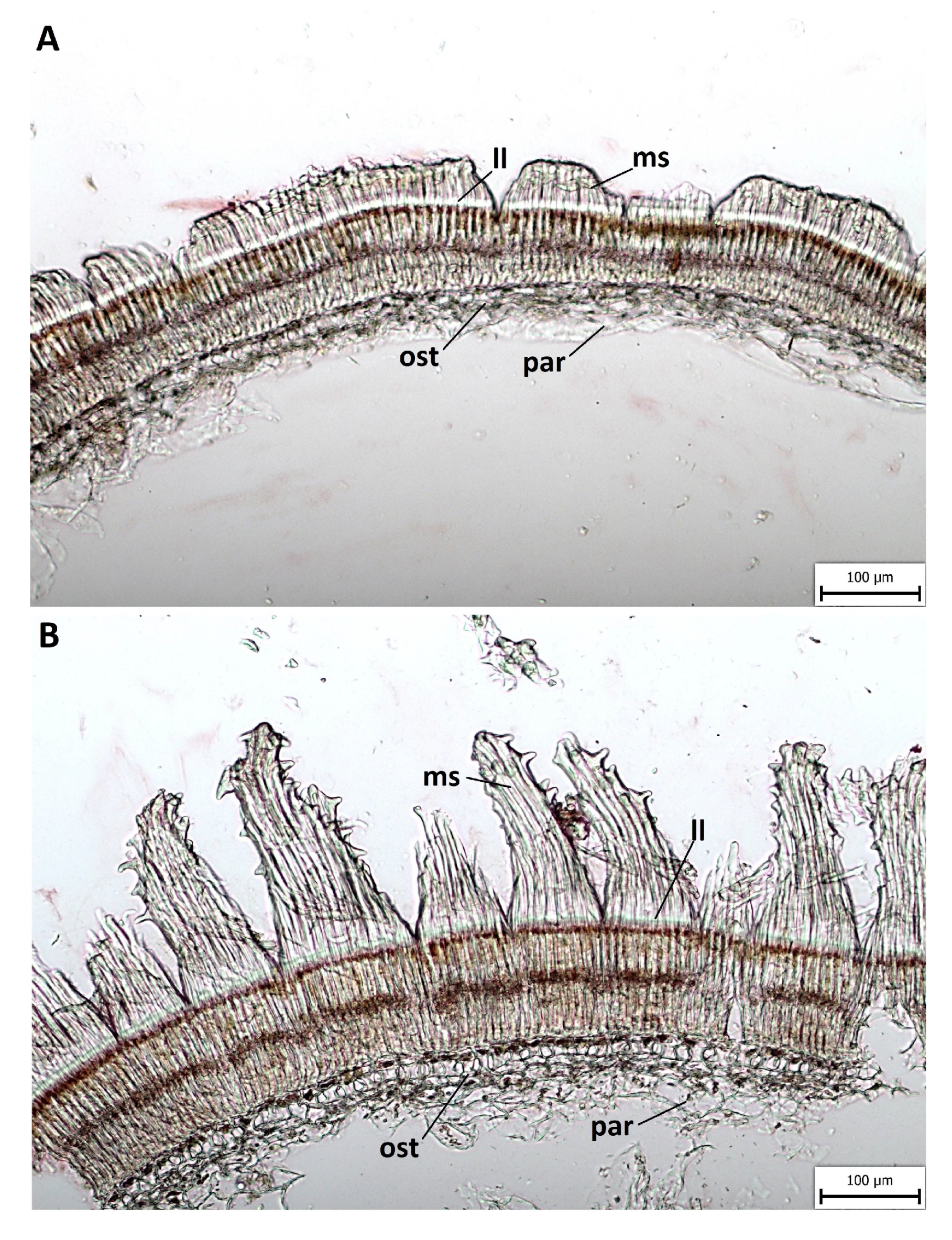
2.3. Anatomical and Histochemical Analysis
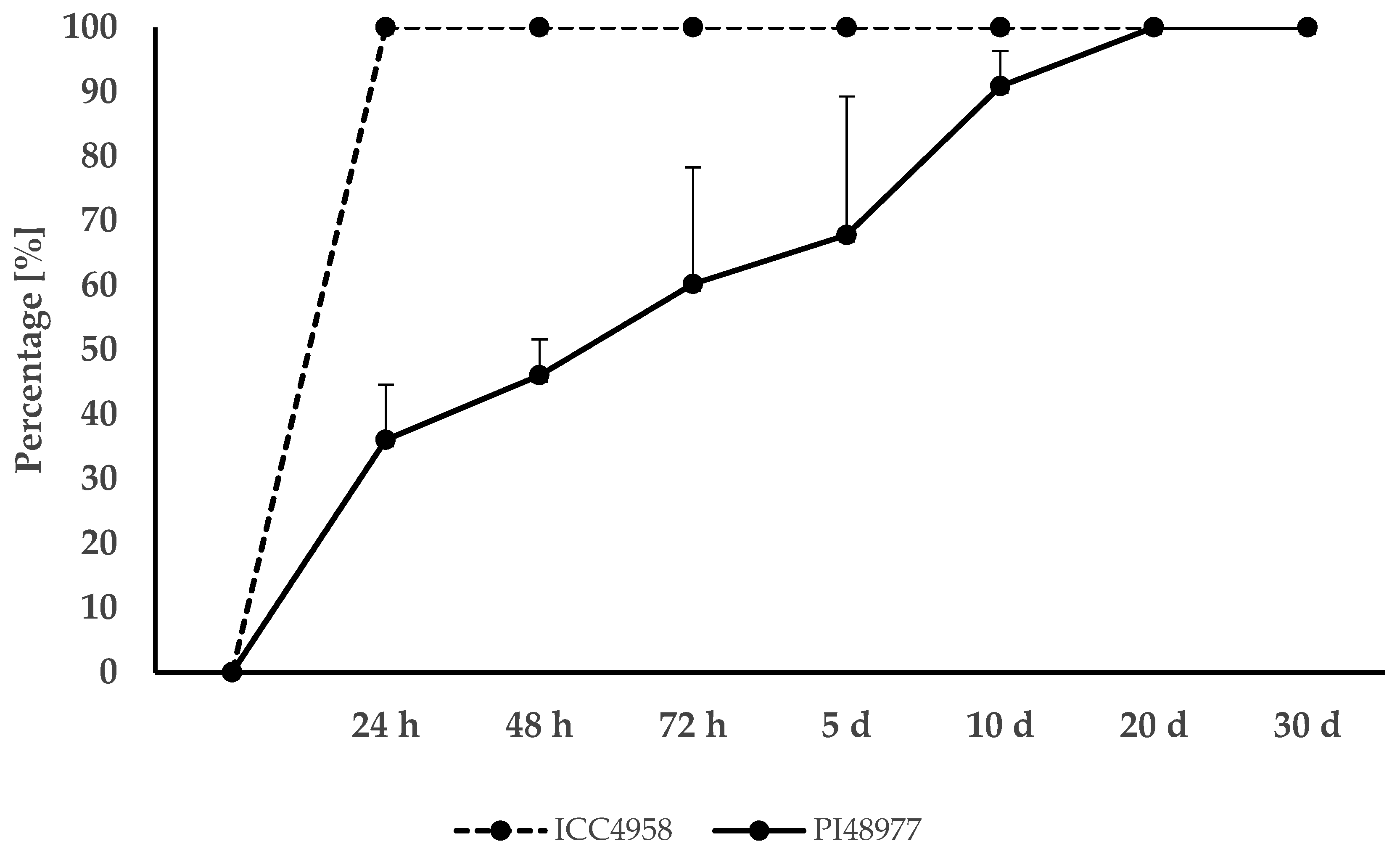
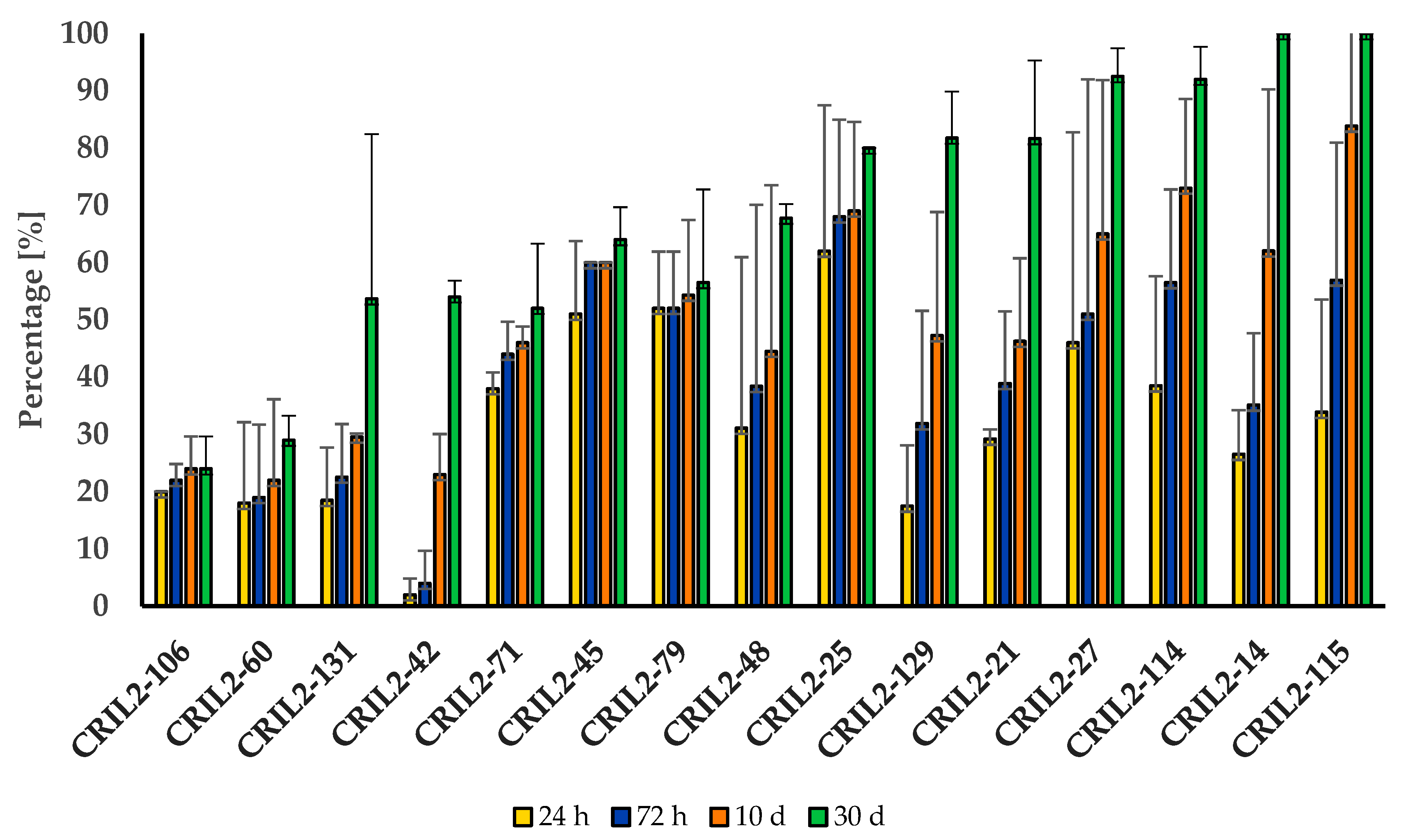
3. Results
3.1. Germination and Dormancy Testing
3.2. Anatomical and Histochemical Analysis
3.3. Length of Macroslereid
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vance, C.P.; Graham, P.H.; Allan, D.L. Biological Nitrogen Fixation: Phosphorus—A Critical Future Need? In Nitrogen Fixation: From Molecules to Crop Productivity, Current Plant Science and Biotechnology in Agriculture; Pedrosa, F.O., Hungria, M., Yates, G., Newton, W.E., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 509–514. [Google Scholar] [CrossRef]
- Merga, B.; Haji, J. Economic importance of chickpea: Production, value, and world trade. Cogent Food Agric. 2019, 5, 1615718. [Google Scholar] [CrossRef]
- Wang, R.; Gangola, M.P.; Irvine, C.; Gaur, P.M.; Båga, M.; Chibbar, R.N. Co-localization of genomic regions associated with seed morphology and composition in a desi chickpea (Cicer arietinum L.) population varying in seed protein concentration. Theor. Appl. Genet. 2019, 132, 1263–1281. [Google Scholar] [CrossRef]
- Kudapa, H.; Garg, V.; Chitikineni, A.; Varshney, R.K. The RNA-Seq-based high resolution gene expression atlas of chickpea (Cicer arietinum L.) reveals dynamic spatio-temporal changes associated with growth and development: RNA-Seq based chickpea gene expression atlas. Plant Cell Environ. 2018, 41, 2209–2225. [Google Scholar] [CrossRef] [Green Version]
- Afshin, A.; Micha, R.; Khatibzadeh, S.; Mozaffarian, D. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2014, 100, 278–288. [Google Scholar] [CrossRef]
- Shin, M.-G.; Bulyntsev, S.V.; Chang, P.L.; Korbu, L.B.; Carrasquila-Garcia, N.; Vishnyakova, M.A.; Samsonova, M.G.; Cook, D.R.; Nuzhdin, S.V. Multi-trait analysis of domestication genes in Cicer arietinum—Cicer reticulatum hybrids with a multidimensional approach: Modelling wide crosses for crop improvement. Plant Sci. 2019, 285, 122–131. [Google Scholar] [CrossRef]
- Coyne, C.J.; Kumar, S.; Wettberg, E.J.B.; Marques, E.; Berger, J.D.; Redden, R.J.; Ellis, T.H.N.; Brus, J.; Zablatzká, L.; Smýkal, P. Potential and limits of exploitation of crop wild relatives for pea, lentil, and chickpea improvement. Legume Sci. 2020, 2, e36. [Google Scholar] [CrossRef] [Green Version]
- Ladizinsky, G.; Adler, A. The origin of chickpea Cicer arietinum L. Euphytica 1976, 25, 211–217. [Google Scholar] [CrossRef]
- Ladizinsky, G. Plant Evolution under Domestication; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998. [Google Scholar]
- Redden, R.J.; Berger, J.D. History and origin of chickpea. In Chickpea Breeding and Management; Yadav, S.S., Redden, R.J., Chen, W., Sharma, B., Eds.; CABI: Wallingford, UK, 2007; pp. 1–13. [Google Scholar] [CrossRef]
- Hammer, K. Das Domestikationssyndrom. Die Kult. 1984, 32, 11–34. [Google Scholar] [CrossRef]
- Ladizinsky, G. Pulse domestication before cultivation. Econ. Bot. 1987, 41, 60–65. [Google Scholar] [CrossRef]
- Varma Penmetsa, R.; Carrasquilla-Garcia, N.; Bergmann, E.M.; Vance, L.; Castro, B.; Kassa, M.T.; Sarma, B.K.; Datta, S.; Farmer, A.D.; Baek, J.; et al. Multiple post-domestication origins of kabuli chickpea through allelic variation in a diversification-associated transcription factor. New Phytol. 2016, 211, 1440–1451. [Google Scholar] [CrossRef] [Green Version]
- Abbo, S.; Berger, J.; Turner, N.C. Viewpoint: Evolution of cultivated chickpea: Four bottlenecks limit diversity and constrain adaptation. Funct. Plant Biol. 2003, 30, 1081. [Google Scholar] [CrossRef] [Green Version]
- Gil, J.; Cubero, J.I. Inheritance of Seed Coat Thickness in Chickpea (Cicer arietinum L.) and its Evolutionary Implications. Plant Breed. 1993, 111, 257–260. [Google Scholar] [CrossRef]
- Iruela, M.; Rubio, J.; Cubero, J.I.; Gil, J.; Millán, T. Phylogenetic analysis in the genus Cicer and cultivated chickpea using RAPD and ISSR markers. Theor. Appl. Genet. 2002, 104, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.-T.; Cubero, J.I. Variation in Cicer arietinum L. Euphytica 1978, 27, 465–485. [Google Scholar] [CrossRef]
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.L.; Chibbar, R.N. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. Br. J. Nutr. 2012, 108, S11–S26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sastry, D.; Upadhyaya, H.D.; Srinivas, T.R. Variation for seed physical and hydration properties of chickpea (Cicer arietinum L.) mini core collection and their relevance to conservation and utilization. Plant Genet. Resour. 2019, 17, 311–324. [Google Scholar] [CrossRef]
- Smýkal, P.; Vernoud, V.; Blair, M.W.; Soukup, A.; Thompson, R.D. The role of the testa during development and in establishment of dormancy of the legume seed. Front. Plant Sci. 2014, 5, 351. [Google Scholar] [CrossRef] [Green Version]
- Smýkal, P.; Nelson, M.; Berger, J.; Von Wettberg, E. The Impact of Genetic Changes during Crop Domestication. Agronomy 2018, 8, 119. [Google Scholar] [CrossRef] [Green Version]
- Donohue, K.; Rubio de Casas, R.; Burghardt, L.; Kovach, K.; Willis, C.G. Germination, Postgermination Adaptation, and Species Ecological Ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C.; Li, X. Taxonomy, anatomy and evolution of physical dormancy in seeds. Plant Species Biol. 2000, 15, 139–152. [Google Scholar] [CrossRef]
- Souza, F.H.; Marcos-Filho, J. The seed coat as a modulator of seed-environment relationships in Fabaceae. Rev. Bras. Bot. 2001, 24, 365–375. [Google Scholar] [CrossRef] [Green Version]
- Janská, A.; Pecková, E.; Sczepaniak, B.; Smýkal, P.; Soukup, A. The role of the testa during the establishment of physical dormancy in the pea seed. Ann. Bot. 2019, 123, 815–829. [Google Scholar] [CrossRef]
- Cechová, M.; Válková, M.; Hradilová, I.; Janská, A.; Soukup, A.; Smýkal, P.; Bednář, P. Towards Better Understanding of Pea Seed Dormancy Using Laser Desorption/Ionization Mass Spectrometry. Int. J. Mol. Sci. 2017, 18, 2196. [Google Scholar] [CrossRef] [Green Version]
- Hradilová, I.; Trněný, O.; Válková, M.; Cechová, M.; Janská, A.; Prokešová, L.; Aamir, K.; Krezdorn, N.; Rotter, B.; Winter, P.; et al. A Combined Comparative Transcriptomic, Metabolomic, and Anatomical Analyses of Two Key Domestication Traits: Pod Dehiscence and Seed Dormancy in Pea (Pisum sp.). Front. Plant Sci. 2017, 8, 542. [Google Scholar] [CrossRef] [Green Version]
- Marbach, I.; Mayer, A.M. Permeability of Seed Coats to Water as Related to Drying Conditions and Metabolism of Phenolics. Plant Physiol. 1974, 54, 817–820. [Google Scholar] [CrossRef]
- Liu, C.; Jun, J.H.; Dixon, R.A. MYB5 and MYB14 Play Pivotal Roles in Seed Coat Polymer Biosynthesis in Medicago truncatula. Plant Physiol. 2014, 165, 1424–1439. [Google Scholar] [CrossRef] [Green Version]
- Renzi, J.P.; Duchoslav, M.; Brus, J.; Hradilová, I.; Pechanec, V.; Václavek, T.; Machalová, J.; Hron, K.; Verdier, J.; Smýkal, P. Plasticity of Medicago truncatula seed dormancy relates to large-scale environment variation (preprint). Plant Biol. 2019. [CrossRef]
- Renzi, J.P.; Duchoslav, M.; Brus, J.; Hradilová, I.; Pechanec, V.; Václavek, T.; Machalová, J.; Hron, K.; Verdier, J.; Smýkal, P. Physical Dormancy Release in Medicago truncatula Seeds Is Related to Environmental Variations. Plants 2020, 9, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weeden, N.F. Genetic Changes Accompanying the Domestication of Pisum sativum: Is there a Common Genetic Basis to the “Domestication Syndrome” for Legumes? Ann. Bot. 2007, 100, 1017–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, S.-J.; Sato, M.; Sato, K.; Jitsuyama, Y.; Fujino, K.; Mori, H.; Takahashi, R.; Benitez, E.R.; Liu, B.; Yamada, T.; et al. A Single-Nucleotide Polymorphism in an Endo-1,4-β-Glucanase Gene Controls Seed Coat Permeability in Soybean. PLoS ONE 2015, 10, e0128527. [Google Scholar] [CrossRef]
- Wood, J.A.; Knights, E.J.; Choct, M. Morphology of Chickpea Seeds (Cicer arietinum L.): Comparison of desi and kabuli Types. Int. J. Plant. Sci. 2011, 172, 632–643. [Google Scholar] [CrossRef]
- Kader, M.A. A Comparison of Seed Germination Calculation Formulae and the Associated Interpretation of Resulting Data. J. Proc. R. Soc. N. S. Wales 2005, 138, 65–75. [Google Scholar]
- Ranal, M.A.; Santana, D.G. How and why to measure the germination process? Rev. Bras. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Soukup, A.; Tylová, E. Essential Methods of Plant Sample Preparation for Light Microscopy. In Plant Cell Morphogenesis, Methods in Molecular Biology; Žárský, V., Cvrčková, F., Eds.; Humana Press: Totowa, NJ, USA, 2014; pp. 1–23. [Google Scholar] [CrossRef]
- Rasband, W.S.; ImageJ, U.S. National Institutes of Health, Bethesda, MD, USA. 1997–2018. Available online: https://imagej.nih.gov/ij/ (accessed on 26 October 2021).
- Vu, D.T.; Velusamy, V.; Park, E. Structure and chemical composition of wild soybean seed coat related to its permeability. Pak. J. Bot. 2014, 46, 1847–1857. [Google Scholar]
- Meyer, C.J.; Steudle, E.; Peterson, C.A. Patterns and kinetics of water uptake by soybean seeds. J. Exp. Bot. 2006, 58, 717–732. [Google Scholar] [CrossRef] [Green Version]
- Koizumi, M.; Kikuchi, K.; Isobe, S.; Ishida, N.; Naito, S.; Kano, H. Role of Seed Coat in Imbibing Soybean Seeds Observed by Micro-magnetic Resonance Imaging. Ann. Bot. 2008, 102, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.H.; Fortune, J.A.; Gallagher, J. Anatomical structure and nutritive value of lupin seed coats. Aust. J. Agric. Res. 2001, 52, 985. [Google Scholar] [CrossRef]
- Mullin, W.J.; Xu, W. A study of the intervarietal differences of cotyledon and seed coat carbohydrates in soybeans. Food Res. Int. 2000, 33, 883–891. [Google Scholar] [CrossRef]
- Mullin, W.J.; Xu, W. Study of Soybean Seed Coat Components and Their Relationship to Water Absorption. J. Agric. Food Chem. 2001, 49, 5331–5335. [Google Scholar] [CrossRef]
- Shao, S.; Meyer, C.J.; Ma, F.; Peterson, C.A.; Bernards, M.A. The outermost cuticle of soybean seeds: Chemical composition and function during imbibition. J. Exp. Bot. 2007, 58, 1071–1082. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Sekizaki, H.; Yang, Z.; Sawa, S.; Pan, J. Phenolics in the Seed Coat of Wild Soybean (Glycine soja) and Their Significance for Seed Hardness and Seed Germination. J. Agric. Food Chem. 2010, 58, 10972–10978. [Google Scholar] [CrossRef] [PubMed]
- Ma, F. Cracks in the Palisade Cuticle of Soybean Seed Coats Correlate with their Permeability to Water. Ann. Bot. 2004, 94, 213–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hradilová, I.; Duchoslav, M.; Brus, J.; Pechanec, V.; Hýbl, M.; Kopecký, P.; Smržová, L.; Štefelová, N.; Vaclávek, T.; Bariotakis, M.; et al. Variation in wild pea (Pisum sativum subsp. elatius) seed dormancy and its relationship to the environment and seed coat traits. PeerJ 2019, 7, e6263. [Google Scholar] [CrossRef] [Green Version]
- Kedaffrec, E.; Nordborg, M. The maternal environment interacts with genetic variation in regulating seed dormancy in Swedish Arabidopsis Thaliana. PLoS ONE 2017, 12, e0190242. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.J.; Tan, D.Y.; Baskin, C.C.; Baskin, J.M. Effect of Seed Position on Parental Plant on Proportion of Seeds Produced with Nondeep and Intermediate Physiological Dormancy. Front. Plant Sci. 2017, 8, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyatt, J.E. Seed coat and water absorption properties of seed of near-isogenic snap bean lines differing in seed coat color. J. Am. Soc. Hortic. Sci. 1977, 102, 478–480. [Google Scholar]
- Werker, E.; Marbach, I.; Mayer, A.M. Relation Between the Anatomy of the Testa, Water Permeability and the Presence of Phenolics in the Genus Pisum. Ann. Bot. 1979, 43, 765–771. [Google Scholar] [CrossRef]
- Kantar, F.; Pilbeam, C.J.; Hebblethwaite, P.D. Effect of tannin content of faba bean (Vicia faba) seed on seed vigour, germination and field emergence. Ann. App. Biol. 1996, 128, 85–93. [Google Scholar] [CrossRef]
- Caldas, G.V.; Blair, M.W. Inheritance of seed condensed tannins and their relationship with seed-coat color and pattern genes in common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2009, 119, 131–142. [Google Scholar] [CrossRef]
- Díaz, A.M.; Caldas, G.V.; Blair, M.W. Concentrations of condensed tannins and anthocyanins in common bean seed coats. Food Res. Int. 2010, 43, 595–601. [Google Scholar] [CrossRef]
- Legesse, N.; Powel, A.A. Relationship between the development of seed coat pigmentation, seed coat adherence to the cotyledons and the rate of imbibition during the maturation of grain legumes. Seed Sci. Technol. 1996, 24, 23–32. [Google Scholar]
- Lamichaney, A.; Kudekallu, S. Differences in seed vigour traits between desi (pigmented) and kabuli (non-pigmented) ecotypes of chickpea (Cicer arietinum) and its association with field emergence. JEB 2017, 38, 735–742. [Google Scholar] [CrossRef]
- Singh, U.; Manohar, S.; Singh, A.K. The anatomical structure of desi and kabuli seed coats. Int. Chickpea Newsl. 1984, 10, 26–27. [Google Scholar]
- Sastry, D.; Upadhyaya, H.D.; Gowda, C.L.L. Determination of physical properties of chickpea seeds and their relevance in germplasm collections. Indian J. Plant. Genet. Resour. 2014, 27, 1–9. [Google Scholar]
- Dattatreya, A.M.; Nanjegowda, D.K.; Viswanath, P. Microscopic detection of adulteration of Bengal gram (Cicer arietinum) flour with other legume flour based on the seed testa macrosclereids. J. Food Sci. Technol. 2011, 48, 114–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zablatzká, L.; Balarynová, J.; Klčová, B.; Kopecký, P.; Smýkal, P. Anatomy and Histochemistry of Seed Coat Development of Wild (Pisum sativum subsp. elatius (M. Bieb.) Asch. et Graebn. and Domesticated Pea (Pisum sativum subsp. sativum L.). Int. J. Mol. Sci. 2021, 22, 4602. [Google Scholar] [CrossRef]
- Reeve, R.M. Ontogeny of the sclereids in the integument of Pisum sativum L. Am. J. Bot. 1946, 33, 806–816. [Google Scholar] [CrossRef]
- Spurný, M. Changes in the permeability of the seed coat in connection with the development of suberin adcrustations of the macrosclereids from the seed coat of the pea (Pisum sativum L.). Flora 1964, 154, 547–567. [Google Scholar] [CrossRef]
- Cavazza, L. Recherches sur l’impermeabilite des grains dures chez les legumineuses. Ber. Schweiz Bot. Ges. 1950, 60, 596–610. [Google Scholar]
- Hamly, D.H. Softening of the Seeds of Melilotus alba. Bot. Gaz. 1932, 93, 345–375. [Google Scholar] [CrossRef]
- Bevilacqua, L.R.; Roti-Michelozzi, G.; Modenesi, P. The watertight dormancy of Melilotus alba seeds: Further observations on the palisade cell wall. Can. J. Bot. 1989, 67, 3453–3456. [Google Scholar] [CrossRef]
- Martens, H.; Jakobsen, H.B.; Lyshede, O.B. Development of the strophiole in seeds of white clover (Trifolium repens L.). Seed Sci. Res. 1995, 5, 171–176. [Google Scholar] [CrossRef]
- Manning, J.C.; van Staden, J. The development and ultrastructure of the testa and tracheid bar in Erythrina lysistemon Hutch. (Leguminosae: Papilionoideae). Protoplasma 1985, 129, 157–167. [Google Scholar] [CrossRef]
- Wood, J.A.; Tan, H.-T.; Collins, H.M.; Yap, K.; Khor, S.F.; Lim, W.L.; Xing, X.; Bulone, V.; Burton, R.A.; Fincher, G.B.; et al. Genetic and environmental factors contribute to variation in cell wall composition in mature desi chickpea (Cicer arietinum L.) cotyledons: Variation in chickpea cotyledon composition. Plant Cell Environ. 2019, 41, 2195–2208. [Google Scholar] [CrossRef] [Green Version]
- McCartney, L.; Knox, J.P. Regulation of pectic polysaccharide domains in relation to cell development and cell properities in the pea testa. J. Exp. Bot. 2002, 53, 707–713. [Google Scholar] [CrossRef] [Green Version]

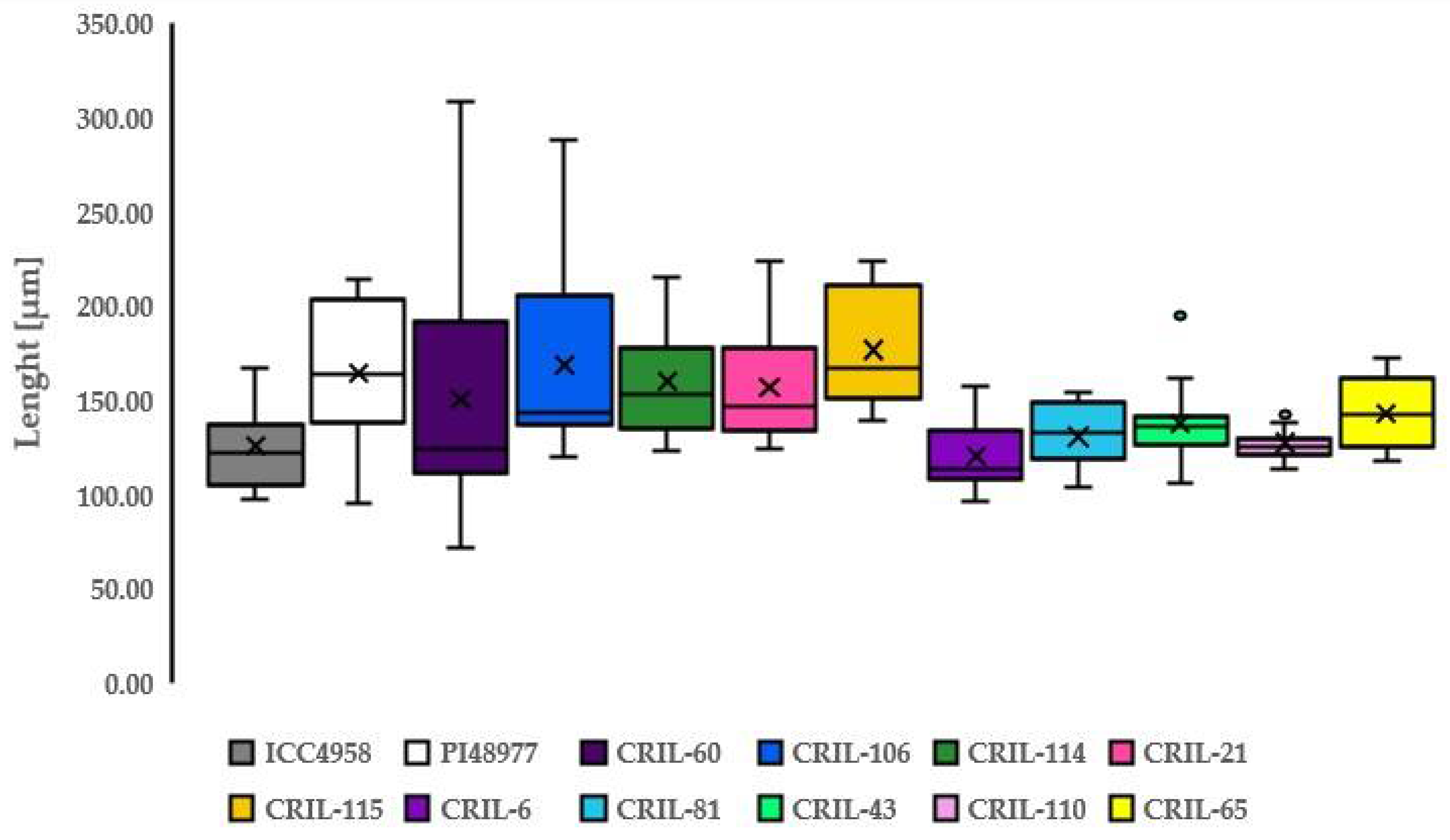
| ICC4958 | Length [µm] | PI48977 | Length [µm] | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| 1 | 143.57 | 22.41 | 1 | 177.45 | 41.25 |
| 2 | 114.78 | 14.81 | 2 | 142.88 | 55.14 |
| 3 | 113.12 | 19.00 | 3 | 155.39 | 15.04 |
| 4 | 134.59 | 31.41 | 4 | 169.49 | 36.84 |
| 5 | 121.40 | 16.10 | 5 | 178.47 | 31.48 |
| Mean | 125.49 | Mean | 164.73 | ||
| SD | 21.94 | SD | 35.30 | ||
| CRIL2-60 | CRIL2-106 | CRIL2-114 | CRIL2-21 | CRIL2-115 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Length [µm] | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| 1 | 99.45 | 23.56 | 162.82 | 37.65 | 149.47 | 13.06 | 143.18 | 8.71 | 158.73 | 7.89 |
| 2 | 230.50 | 82.61 | 184.61 | 90.85 | 158.44 | 34.61 | 172.62 | 44.74 | 210.90 | 17.58 |
| 3 | 124.70 | 17.28 | 160.84 | 41.91 | 155.68 | 15.23 | 191.57 | 28.57 | 141.56 | 3.42 |
| 4 | 129.33 | 26.52 | 151.54 | 19.06 | 174.75 | 42.70 | 148.85 | 6.63 | 167.34 | 16.26 |
| 5 | 183.94 | 67.08 | 182.44 | 75.41 | 161.39 | 44.80 | 130.45 | 3.54 | 205.71 | 19.45 |
| Mean | 150.97 | 168.45 | 159.94 | 157.33 | 176.85 | |||||
| SD | 65.55 | 51.72 | 29.17 | 30.59 | 30.50 | |||||
| CRIL2-6 | CRIL2-81 | CRIL2-43 | CRIL2-110 | CRIL2-65 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Length [µm] | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| 1 | 120.22 | 15.45 | 120.49 | 14.33 | 142.62 | 11.63 | 128.93 | 9.82 | 137.26 | 7.23 |
| 2 | 117.60 | 15.59 | 131.89 | 25.30 | 127.08 | 8.67 | 139.30 | 9.00 | 131.47 | 14.36 |
| 3 | 111.71 | 17.59 | 142.02 | 7.76 | 165.35 | 27.95 | 122.70 | 5.16 | 158.57 | 14.21 |
| 4 | 132.94 | 24.57 | 139.90 | 18.41 | 129.22 | 8.83 | 123.88 | 1.88 | 165.65 | 7.63 |
| 5 | 120.34 | 7.77 | 119.85 | 13.37 | 124.28 | 16.58 | 122.45 | 7.70 | 122.88 | 7.00 |
| Mean | 120.56 | 130.83 | 137.71 | 127.45 | 143.17 | |||||
| SD | 16.11 | 17.23 | 20.96 | 9.03 | 14.00 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sedláková, V.; Hanáček, P.; Grulichová, M.; Zablatzká, L.; Smýkal, P. Evaluation of Seed Dormancy, One of the Key Domestication Traits in Chickpea. Agronomy 2021, 11, 2292. https://doi.org/10.3390/agronomy11112292
Sedláková V, Hanáček P, Grulichová M, Zablatzká L, Smýkal P. Evaluation of Seed Dormancy, One of the Key Domestication Traits in Chickpea. Agronomy. 2021; 11(11):2292. https://doi.org/10.3390/agronomy11112292
Chicago/Turabian StyleSedláková, Veronika, Pavel Hanáček, Marie Grulichová, Lenka Zablatzká, and Petr Smýkal. 2021. "Evaluation of Seed Dormancy, One of the Key Domestication Traits in Chickpea" Agronomy 11, no. 11: 2292. https://doi.org/10.3390/agronomy11112292
APA StyleSedláková, V., Hanáček, P., Grulichová, M., Zablatzká, L., & Smýkal, P. (2021). Evaluation of Seed Dormancy, One of the Key Domestication Traits in Chickpea. Agronomy, 11(11), 2292. https://doi.org/10.3390/agronomy11112292







