Fusarium Secondary Metabolite Content in Naturally Produced and Artificially Provoked FHB Pressure in Winter Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Field Trials
2.2. Inoculum Preparation and Inoculation Procedure
2.3. Fusarium General Resistance and Type I Resistance
2.4. Mycotoxin Analysis
2.5. Statistical Analysis
3. Results
3.1. Fusarium General Resistance and Type I Resistance
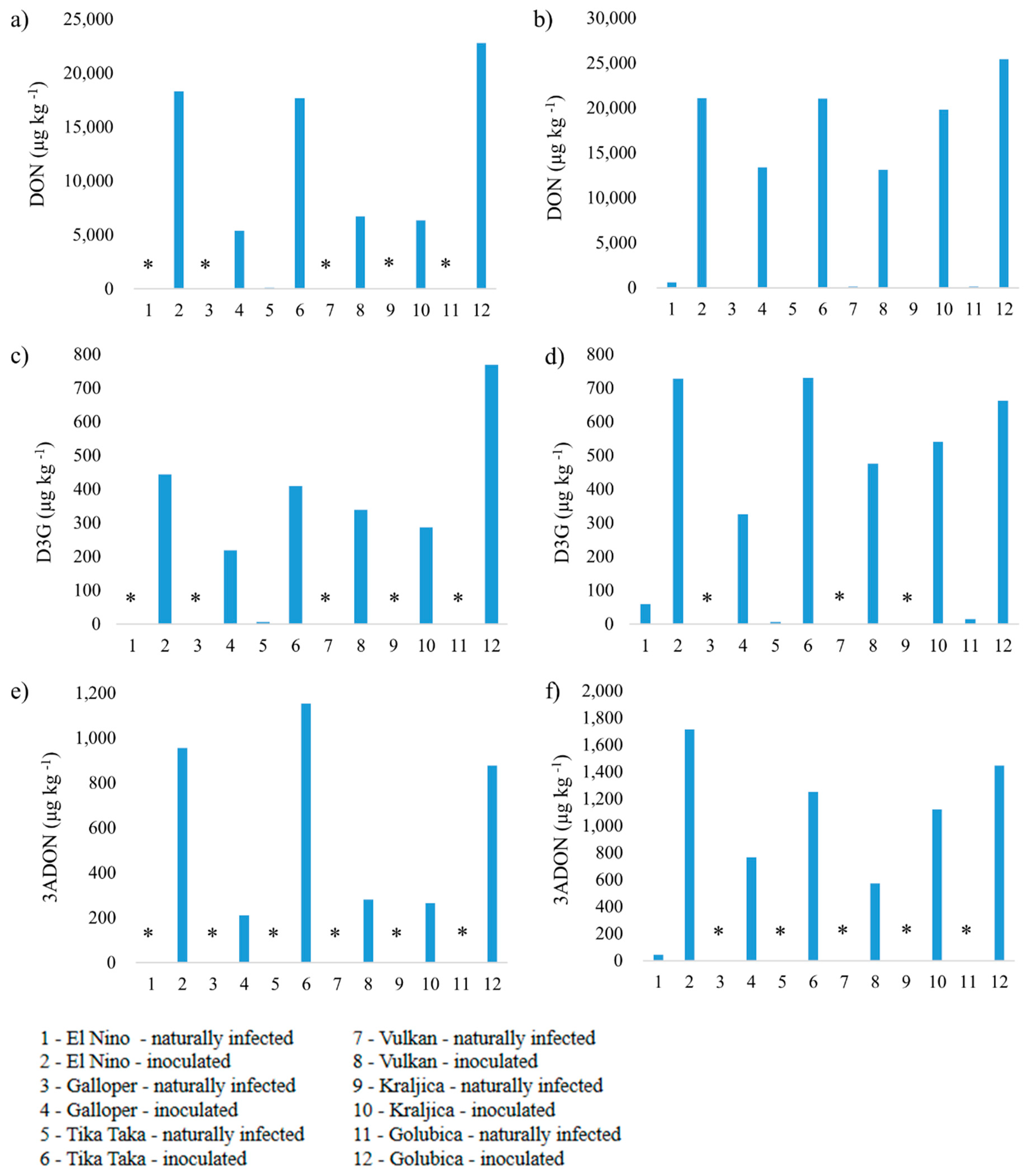
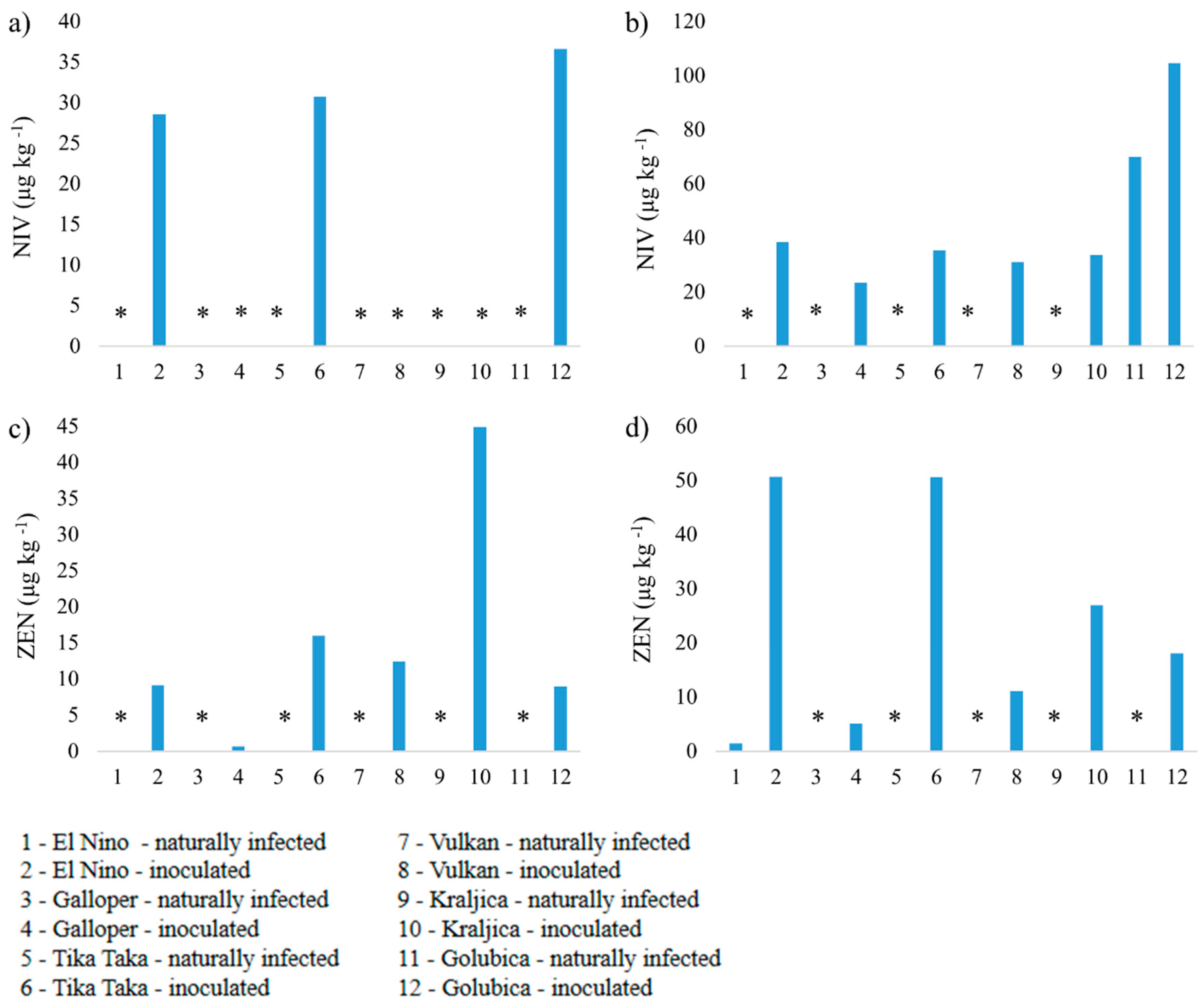
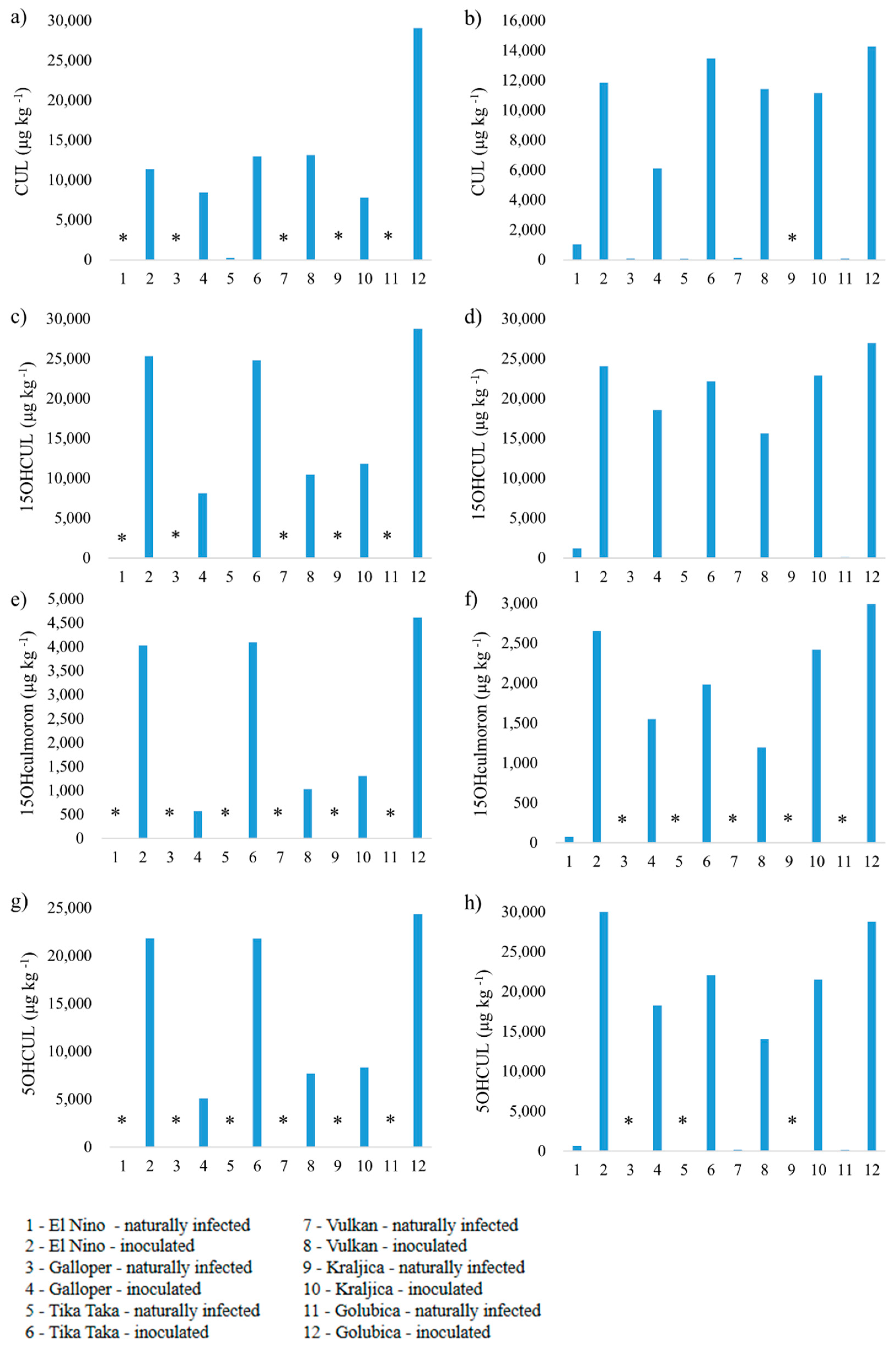
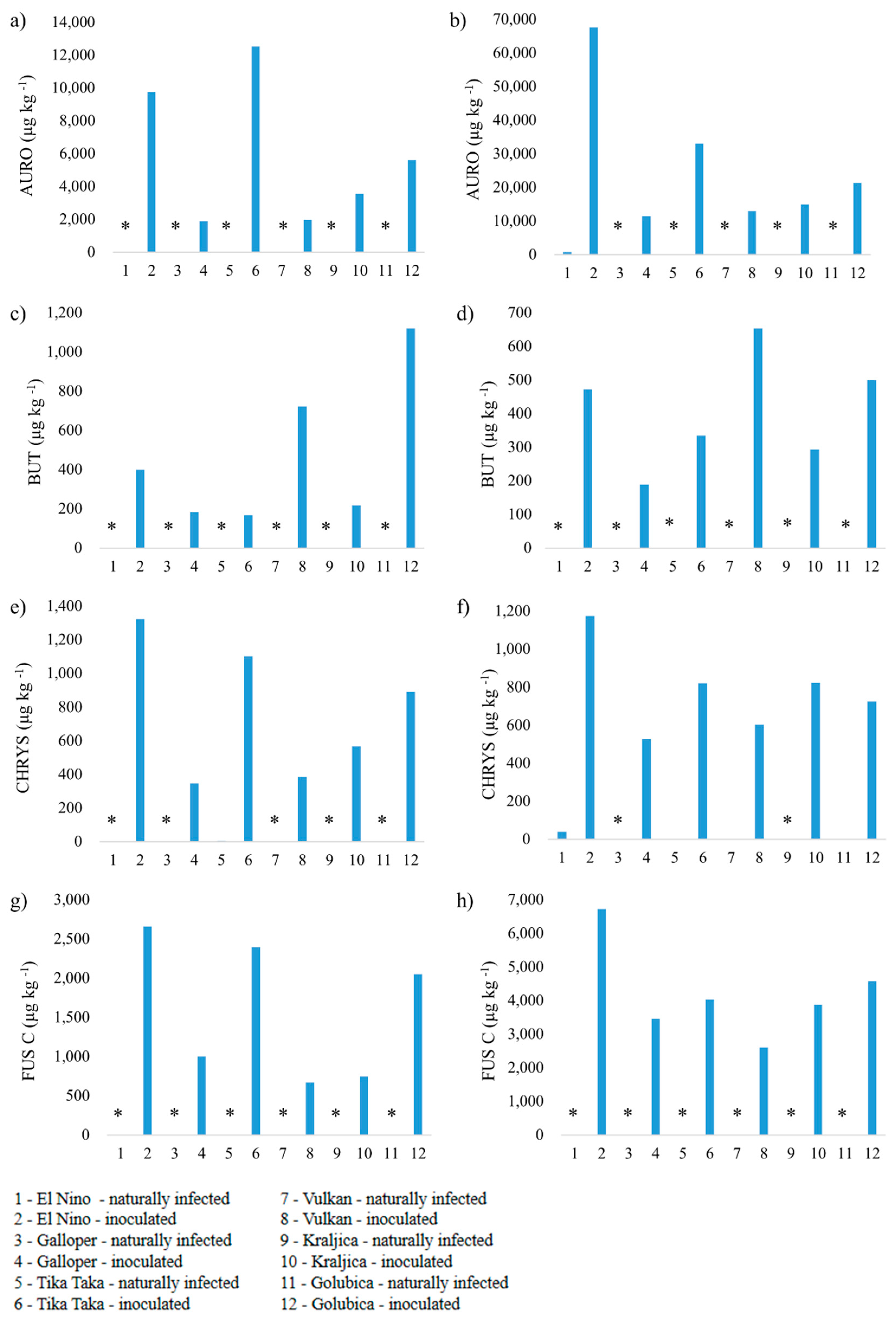
3.2. Mycotoxin Analysis
3.2.1. Deoxynivalenol, Deoxynivalenol-3-glucoside and 3-Acetyldeoxynivalenol
3.2.2. Nivalenol and Zearalenone
3.2.3. Culmorin, 15-Hydroxyculmorin, 15-Hydroxyculmoron and 5-Hydroxyculmorin
3.2.4. Aurofusarin, Butenolide, Chrysogin and Fusarin C
3.3. ANOVA and Correlation Analysis
4. Discussion
4.1. Deoxynivalenol, Deoxynivalenol-3-glucoside and 3-Acetyldeoxynivalenol
4.2. Nivalenol and Zearalenone
4.3. Culmorin, 15-Hydroxyculmorin, 15-Hydroxyculmoron and 5-Hydroxyculmorin
4.4. Aurofusarin, Butenolide, Chrysogin and Fusarin C
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef]
- Osborne, L.E.; Stein, J.M. Epidemiology of Fusarium head blight on small-grain cereals. Int. J. Food Microbiol. 2007, 119, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Popovski, S.; Celar, F.A. The impact of environmental factors on the infection of cereals with Fusarium species and mycotoxin production—A review. Acta Agric. Slov. 2013, 101, 105–116. [Google Scholar] [CrossRef]
- Spanic, V.; Marcek, T.; Abicic, I.; Sarkanj, B. Effects of Fusarium Head Blight on Wheat Grain and Malt Infected by Fusarium culmorum. Toxins 2018, 10, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beccari, G.; Arellano, C.; Covarelli, L.; Tini, F.; Sulyok, M.; Cowger, C. Effect of wheat infection timing on Fusarium head blight causal agents and secondary metabolites in grain. Int. J. Food Microbiol. 2019, 290, 214–225. [Google Scholar] [CrossRef]
- Spanic, V.; Lemmens, M.; Drezner, G. Morphological and molecular identification of Fusarium species associated with head blight on wheat in East Croatia. Eur. J. Plant. Pathol. 2010, 128, 511–516. [Google Scholar] [CrossRef]
- Desjardins, A.E. Fusarium Mycotoxins: Chemistry, Genetics, and Biology, 1st ed.; American Phytopathological Society (APS Press): St. Paul, MN, USA, 2006. [Google Scholar]
- Mielniczuk, E.; Skwaryło-Bednarz, B. Fusarium Head Blight, Mycotoxins and Strategies for Their Reduction. Agronomy 2020, 10, 509. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.M.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Cooke, B.M.; Doohan, F.M.; Brennan, J.; Monaghan, S.; Moretti, A.; Mule, G.; et al. Relationship Between the Fungal Complex Causing Fusarium Head Blight of Wheat and Environmental Conditions. Phytopathology 2008, 98, 69–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czaban, J.; Wróblewska, B.; Sułek, A.; Mikos-Szymańska, M.; Boguszewska, E.; Podolska, G.; Nieróbca, A. Colonisation of winter wheat grain by Fusarium spp. and mycotoxin content as dependent on a wheat variety, crop rotation, a crop management system and weather conditions. Food Addit. Contam. Part A 2015, 32, 874–910. [Google Scholar] [CrossRef]
- Bernhoft, A.; Torp, M.; Clasen, P.-E.; Løes, A.-K.; Kristoffersen, A.B. Influence of agronomic and climatic factors on Fusarium infestation and mycotoxin contamination of cereals in Norway. Food Addit. Contam. Part A 2012, 29, 1129–1140. [Google Scholar] [CrossRef] [Green Version]
- Hope, R.; Aldred, D.; Magan, N. Comparison of environmental profiles for growth and deoxynivalenol production by Fusarium culmorum and F. graminearum on wheat grain. Lett. Appl. Microbiol. 2005, 40, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Mesterhazy, A. Types and components of resistance to Fusarium head blight of wheat. Plant. Breed 1995, 114, 377–386. [Google Scholar] [CrossRef]
- Cuomo, C.A.; Güldener, U.; Xu, J.-R.; Trail, F.; Turgeon, B.G.; Di Pietro, A.; Walton, J.D.; Ma, L.-J.; Baker, S.; Rep, M.; et al. The Fusarium graminearum Genome Reveals a Link Between Localized Polymorphism and Pathogen Specialization. Science 2007, 317, 1400–1402. [Google Scholar] [CrossRef] [Green Version]
- Woelflingseder, L.; Warth, B.; Vierheilig, I.; Schwartz-Zimmermann, H.; Hametner, C.; Nagl, V.; Novak, B.; Šarkanj, B.; Berthiller, F.; Adam, G.; et al. The Fusarium metabolite culmorin suppresses the in vitro glucuronidation of deoxynivalenol. Arch. Toxicol. 2019, 93, 1729–1743. [Google Scholar] [CrossRef] [Green Version]
- Pestka, J.J. Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef]
- Pasquali, M.; Giraud, F.; Brochot, C.; Cocco, E.; Hoffmann, L.; Bohn, T. Genetic Fusarium chemotyping as a useful tool for predicting nivalenol contamination in winter wheat. Int. J. Food Microbiol. 2010, 137, 246–253. [Google Scholar] [CrossRef]
- Bretz, M.; Knecht, A.; Göckler, S.; Humpf, H.-U. Structural elucidation and analysis of thermal degradation products of the Fusarium mycotoxin nivalenol. Mol. Nutr. Food Res. 2005, 49, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.; Proctor, R. Molecular biology of Fusarium mycotoxins. Int. J. Food Microbiol. 2007, 119, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a Fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef]
- Kovač, T.; Borišev, I.; Kovač, M.; Lončarić, A.; Kenjerić, F.Č.; Djordjevic, A.; Strelec, I.; Ezekiel, C.N.; Sulyok, M.; Krska, R.; et al. Impact of fullerol C60(OH)24 nanoparticles on the production of emerging toxins by Aspergillus flavus. Sci. Rep. 2020, 10, 725–810. [Google Scholar] [CrossRef]
- Wipfler, R.; McCormick, S.P.; Proctor, R.H.; Teresi, J.M.; Hao, G.; Ward, T.J.; Alexander, N.J.; Vaughan, M.M. Ward Synergistic Phytotoxic Effects of Culmorin and Trichothecene Mycotoxins. Toxins 2019, 11, 555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghebremeskel, M.; Langseth, W. The occurrence of culmorin and hydroxy-culmorins in cereals. Mycopathology 2001, 152, 103–108. [Google Scholar] [CrossRef]
- Farber, J.; Scott, P.; Fusarin, C. Fusarium, 1st ed.; Chełkowski, J., Ed.; Elsevier BV: Amsterdam, The Netherlands, 1989; Volume 2, pp. 41–52. [Google Scholar]
- Woelflingseder, L.; Adam, G.; Marko, D. Suppression of Trichothecene-Mediated Immune Response by the Fusarium Secondary Metabolite Butenolide in Human Colon Epithelial Cells. Front. Nutr. 2020, 7, 127. [Google Scholar] [CrossRef]
- Jarolim, K.; Wolters, K.; Woelflingseder, L.; Pahlke, G.; Beisl, J.; Puntscher, H.; Braun, D.; Sulyok, M.; Warth, B.; Marko, D. The secondary Fusarium metabolite aurofusarin induces oxidative stress, cytotoxicity and genotoxicity in human colon cells. Toxicol. Lett. 2018, 284, 170–183. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Sulyok, M.; Stadler, D.; Steiner, D.; Krska, R. Validation of an LC-MS/MS-based dilute-and-shoot approach for the quantification of > 500 mycotoxins and other secondary metabolites in food crops: Challenges and solutions. Anal. Bioanal. Chem. 2020, 412, 2607–2620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Commission. COMMISSION DECISION of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of result. Off. J. Eur. Union L. 2002, 221, 8–36. [Google Scholar]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. In Mycotoxins in Plant Disease, 1st ed.; Logrieco, A., Bailey, J.A., Corazza, L., Cooke, B.M., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 611–624. [Google Scholar]
- Spanic, V.; Lemmens, M.; Drezner, G.; Mesterházy, Á. Variability in components of fusarium head blight resistance among wheat genotypes. Cereal Res. Commun. 2013, 41, 420–430. [Google Scholar] [CrossRef]
- Stanciu, O.; Banc, R.; Cozma, A.; Filip, L.; Miere, D.; Mañes, J.; Loghin, F. Occurence of Fusarium Mycotoxins in Wheat from Europe—A Review. Acta Univ. Cibiniensis. Ser. E Food Technol. 2015, 19, 35–60. [Google Scholar] [CrossRef] [Green Version]
- Mortensen, A.; Granby, K.; Eriksen, F.D.; Cederberg, T.L.; Friis-Wandall, S.; Simonsen, Y.; Broesbøl-Jensen, B.; Bonnichsen, R. Levels and risk assessment of chemical contaminants in byproducts for animal feed in Denmark. J. Environ. Sci. Health Part B 2014, 49, 797–810. [Google Scholar] [CrossRef]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current Situation of Mycotoxin Contamination and Co-occurrence in Animal Feed—Focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef] [Green Version]
- Hollingsworth, C.R.; Motteberg, C.D.; Wiersma, J.V.; Atkinson, L.M. Agronomic and Economic Responses of Spring Wheat to Management of Fusarium Head Blight. Plant. Dis. 2008, 92, 1339–1348. [Google Scholar] [CrossRef]
- Gautam, P.; Dill-Macky, R. Fusarium head blight development and trichothecene accumulation in point inoculated Fusarium infected wheat heads. World Mycotoxin J. 2012, 5, 45–55. [Google Scholar] [CrossRef]
- Francl, L. Development of Fusarium head blight in relation to environment and inoculum. In Proceedings National Fusarium Head Blight Forum; Michigan State University, University Printing: East Lansing, MI, USA, 1998; pp. 26–27. [Google Scholar]
- Van der Fels-Klerx, H.; de Rijk, T.; Booij, C.; Goedhart, P.W.; Boers, E.; Zhao, C.; Waalwijk, C.; Mol, H.G.J.; van der Lee, T. Occurrence of Fusarium Head Blight species and Fusarium mycotoxins in winter wheat in the Netherlands in 2009. Food Addit. Contam. Part A 2012, 29, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Stanciu, O.; Juan, C.; Miere, D.; Loghin, F.; Mañes, J. Occurrence and co-occurrence of Fusarium mycotoxins in wheat grains and wheat flour from Romania. Food Control. 2017, 73, 147–155. [Google Scholar] [CrossRef]
- European Commission (EC). Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union L. 2006, 364, 5–24. [Google Scholar]
- Pleadin, J.; Vahčić, N.; Perši, N.; Ševelj, D.; Markov, K.; Frece, J. Fusarium mycotoxins’ occurrence in cereals harvested from Croatian fields. Food Control. 2013, 32, 49–54. [Google Scholar] [CrossRef]
- Kovač, M.; Šubarić, D.; Bulaić, M.; Kovač, T.; Šarkanj, B. Yesterday masked, today modified; what do mycotoxins bring next? Arch. Ind. Hyg. Toxicol. 2018, 69, 196–214. [Google Scholar] [CrossRef] [Green Version]
- Berthiller, F.; Krska, R.; Domig, K.J.; Kneifel, W.; Juge, N.; Schuhmacher, R.; Adam, G. Hydrolytic fate of deoxynivalenol-3-glucoside during digestion. Toxicol. Lett. 2011, 206, 264–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryła, M.; Ksieniewicz-Woźniak, E.; Waśkiewicz, A.; Szymczyk, K.; Jędrzejczak, R. Natural Occurrence of Nivalenol, Deoxynivalenol, and Deoxynivalenol-3-Glucoside in Polish Winter Wheat. Toxins 2018, 10, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dall’Asta, C.; Dall’Erta, A.; Mantovani, P.; Massi, A.; Galaverna, G. Occurrence of deoxynivalenol and deoxynivalenol-3-glucoside in durum wheat. World Mycotoxin J. 2013, 6, 83–91. [Google Scholar] [CrossRef]
- Lemmens, M.; Steiner, B.; Sulyok, M.; Nicholson, P.; Mesterhazy, A.; Buerstmayr, H. Masked mycotoxins: Does breeding for enhanced Fusarium head blight resistance result in more deoxynivalenol-3-glucoside in new wheat varieties? World Mycotoxin J. 2016, 9, 741–754. [Google Scholar] [CrossRef]
- Miedaner, T.; Reinbrecht, C.; Lauber, U.; Schollenberger, M.; Geiger, H.H. Effects of genotype and genotype-environment interaction on deoxynivalenol accumulation and resistance to Fusarium head blight in rye, triticale, and wheat. Plant. Breed. 2001, 120, 97–105. [Google Scholar] [CrossRef]
- Zhao, J.; Cheng, T.; Xu, W.; Han, X.; Zhang, J.; Zhang, H.; Wang, C.; Fanning, S.; Li, F. Natural co-occurrence of multi-mycotoxins in unprocessed wheat grains from China. Food Control. 2021, 130, 108321. [Google Scholar] [CrossRef]
- Tucker, J.R.; Badea, A.; Blagden, R.; Pleskach, K.; Tittlemier, S.A.; Fernando, W.G.D. Deoxynivalenol-3-Glucoside Content Is Highly Associated with Deoxynivalenol Levels in Two-Row Barley Genotypes of Importance to Canadian Barley Breeding Programs. Toxins 2019, 11, 319. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.D.; Greenhalgh, R.; Wang, Y.; Lu, M. Trichothecene Chemotypes of Three Fusarium Species. Mycologia 1991, 83, 121–130. [Google Scholar] [CrossRef]
- Amarasinghe, C.C.; Fernando, W.G.D. Comparative Analysis of Deoxynivalenol Biosynthesis Related Gene Expression among Different Chemotypes of Fusarium graminearum in Spring Wheat. Front. Microbiol. 2016, 7, 1229. [Google Scholar] [CrossRef]
- Cowger, C.; Arrellano, C. Plump Kernels with High Deoxynivalenol Linked to Late Gibberella zeae Infection and Marginal Disease Conditions in Winter Wheat. Phytopathology 2010, 100, 719–728. [Google Scholar] [CrossRef] [Green Version]
- Spanic, V.; Zdunic, Z.; Drezner, G.; Sarkanj, B. The Pressure of Fusarium Disease and Its Relation with Mycotoxins in the Wheat Grain and Malt. Toxins 2019, 11, 198. [Google Scholar] [CrossRef] [Green Version]
- Brennan, J.M.; Fagan, B.; van Maanen, A.; Cooke, B.M.; Doohan, F.M. Studies on in vitro Growth and Pathogenicity of European Fusarium fungi. Eur. J. Plant. Pathol. 2003, 109, 577–587. [Google Scholar] [CrossRef]
- Juan, C.; Ritieni, A.; Mañes, J. Occurrence of Fusarium mycotoxins in Italian cereal and cereal products from organic farming. Food Chem. 2013, 141, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Klarić, M.; Cvetnić, Z.; Pepeljnjak, S.; Kosalec, I. Co-occurrence of Aflatoxins, Ochratoxin A, Fumonisins, and Zearalenone in Cereals and Feed, Determined by Competitive Direct Enzyme-Linked Immunosorbent Assay and Thin-Layer Chromatography. Arch. Ind. Hyg. Toxicol. 2009, 60, 427–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jestoi, M. EmergingFusarium-Mycotoxins Fusaproliferin, Beauvericin, Enniatins, and Moniliformin—A Review. Crit. Rev. Food Sci. Nutr. 2008, 48, 21–49. [Google Scholar] [CrossRef] [PubMed]
- Gareis, M.; Zimmermann, C.; Schothorst, R.; Paulsch, W.; Vidnes, A.; Bergsten, C.; Paulsen, B.; Brera, C.; Miraglia, M.; Grossi, S.; et al. Collection of Occurrence Data of Fusarium toxins in Food and Assessment of Dietary Intake by the Population of EU Member States. 2001. Available online: http://europa.eu.int/comm/food/fs/scoop/task3210.pdf (accessed on 20 May 2021).
- Rotter, R.G.; Trenholm, H.L.; Prelusky, D.B.; Hartin, K.E.; Thompson, B.K.; Miller, J.D. A preliminary examination of potential interactions between deoxynivalenol (DON) and other selected Fusarium metabolites in growing pigs. Can. J. Anim. Sci. 1992, 72, 107–116. [Google Scholar] [CrossRef]
- Weber, J.; Vaclavikova, M.; Wiesenberger, G.; Haider, M.; Hametner, C.; Fröhlich, J.; Berthiller, F.; Adam, G.; Mikula, H.; Fruhmann, P. Chemical synthesis of culmorin metabolites and their biologic role in culmorin and acetyl-culmorin treated wheat cells. Org. Biomol. Chem. 2018, 16, 2043–2048. [Google Scholar] [CrossRef] [Green Version]
- Uhlig, S.; Eriksen, G.S.; Hofgaard, I.S.; Krska, R.; Beltrán, E.; Sulyok, M. Faces of a Changing Climate: Semi-Quantitative Multi-Mycotoxin Analysis of Grain Grown in Exceptional Climatic Conditions in Norway. Toxins 2013, 5, 1682–1697. [Google Scholar] [CrossRef] [PubMed]
- Kifer, D.; Sulyok, M.; Jakšić, D.; Krska, R.; Klarić, M. Šegvić Fungi and their metabolites in grain from individual households in Croatia. Food Addit. Contam. Part. B 2021, 14, 98–109. [Google Scholar] [CrossRef]
- Kotik, A.N.; Trufanova, V.A. Detection of naphtoquinone fusariotoxin aurofusarin in wheat. Mikol. Fitopatol. 1998, 32, 58–61. [Google Scholar]
- Spanic, V.; Katanic, Z.; Sulyok, M.; Krska, R.; Puskas, K.; Vida, G.; Drezner, G.; Šarkanj, B. Multiple Fungal Metabolites Including Mycotoxins in Naturally Infected and Fusarium-Inoculated Wheat Samples. Microorganisms 2020, 8, 578. [Google Scholar] [CrossRef] [Green Version]
- Beccari, G.; Colasante, V.; Tini, F.; Senatore, M.; Prodi, A.; Sulyok, M.; Covarelli, L. Causal agents of Fusarium head blight of durum wheat (Triticum durum Desf.) in central Italy and their in vitro biosynthesis of secondary metabolites. Food Microbiol. 2018, 70, 17–27. [Google Scholar] [CrossRef]
- Frandsen, R.J.; Schütt, C.; Lund, B.W.; Staerk, D.; Nielsen, J.; Olsson, S.; Giese, H. Two Novel Classes of Enzymes Are Required for the Biosynthesis of Aurofusarin in Fusarium graminearum. J. Biol. Chem. 2011, 286, 10419–10428. [Google Scholar] [CrossRef] [Green Version]
- Malz, S.; Grell, M.N.; Thrane, C.; Maier, F.J.; Rosager, P.; Felk, A.; Albertsen, K.S.; Salomon, S.; Bohn, L.; Schäfer, W.; et al. Identification of a gene cluster responsible for the biosynthesis of aurofusarin in the Fusarium graminearum species complex. Fungal Genet. Biol. 2005, 42, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cela, E.; Kiaitsi, E.; Medina, A.; Sulyok, M.; Krska, R.; Magan, N. Interacting Environmental Stress Factors Affects Targeted Metabolomic Profiles in Stored Natural Wheat and That Inoculated with F. graminearum. Toxins 2018, 10, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wollenberg, R.D.; Saei, W.; Westphal, K.R.; Klitgaard, C.S.; Nielsen, K.L.; Lysøe, E.; Gardiner, D.M.; Wimmer, R.; Sondergaard, T.E.; Sørensen, J.L. Chrysogine Biosynthesis Is Mediated by a Two-Module Nonribosomal Peptide Synthetase. J. Nat. Prod. 2017, 80, 2131–2135. [Google Scholar] [CrossRef] [PubMed]

| Variety | AUDPC for General | AUDPC for General | AUDPC for Type I | AUDPC for Type I |
|---|---|---|---|---|
| Resistance Osijek ± SD | Resistance Tovarnik ± SD | Resistance Osijek ± SD | Resistance Tovarnik ± SD | |
| El Nino | 137.3 ± 10.75 | 212.5 ± 142.5 | 244 ± 40 | 421 ± 212.5 |
| Galloper | 1.3 ± 0 | 17.5 ± 9.5 | 33.1 ± 16.63 | 87.4 ± 25.85 |
| Tika Taka | 42.7 ± 11.35 | 69.8 ± 7.25 | 215.3 ± 74.7 | 137.6 ± 41.95 |
| Vulkan | 35.8 ± 6.25 | 33.8 ± 5.25 | 119.9 ± 11.35 | 50.6 ± 4.1 |
| Kraljica | 71.5 ± 23.5 | 18.3 ± 1.75 | 216.5 ± 80.5 | 80.1 ± 10.4 |
| Golubica | 103.8 ± 22.75 | 93 ± 7 | 222.3 ± 29.2 | 111.3 ± 8.3 |
| Source of Variation | Df | F-Value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DON | D3G | 3ADON | NIV | ZEN | CUL | 15-OHCUL | 15-OH Culmoron | 5-OHCUL | AURO | BUT | CHRYS | FUS C | ||
| Location | 1 | 3.43 ns | 3.52 ns | 4.93 * | 8.98 ** | 1.46 ns | 0.51 ns | 1.1 ns | 0.42 ns | 3.68 ns | 4.71 ns | 0.17 ns | 0.01 ns | 9.16 ** |
| Treatment | 1 | 87.93 *** | 105.19 *** | 54.8 *** | 13.3 ** | 18.07 *** | 63.33 *** | 124.29 *** | 43.92 *** | 84.31 *** | 11.25 *** | 33.95 *** | 98.56 *** | 44.54 *** |
| Variety | 5 | 2.14 ns | 2.03 ns | 1.89 ns | 4.62 ** | 1.26 ns | 1.65 ns | 2.13 ns | 1.85 ns | 2.07 ns | 1.12 ns | 1.97 ns | 2.66 ns | 1.03 ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunic, K.; Kovac, T.; Loncaric, A.; Babic, J.; Sulyok, M.; Krska, R.; Drezner, G.; Spanic, V. Fusarium Secondary Metabolite Content in Naturally Produced and Artificially Provoked FHB Pressure in Winter Wheat. Agronomy 2021, 11, 2239. https://doi.org/10.3390/agronomy11112239
Sunic K, Kovac T, Loncaric A, Babic J, Sulyok M, Krska R, Drezner G, Spanic V. Fusarium Secondary Metabolite Content in Naturally Produced and Artificially Provoked FHB Pressure in Winter Wheat. Agronomy. 2021; 11(11):2239. https://doi.org/10.3390/agronomy11112239
Chicago/Turabian StyleSunic, Katarina, Tihomir Kovac, Ante Loncaric, Jurislav Babic, Michael Sulyok, Rudolf Krska, Georg Drezner, and Valentina Spanic. 2021. "Fusarium Secondary Metabolite Content in Naturally Produced and Artificially Provoked FHB Pressure in Winter Wheat" Agronomy 11, no. 11: 2239. https://doi.org/10.3390/agronomy11112239









